Abstract
The caffeine metabolic ratio is an established marker for cytochrome P450 (CYP) 1A2 activity. Optimal sample size calculation for clinical pharmacokinetic xenobiotic–caffeine interaction studies requires robust estimates of interindividual and intraindividual variation in this ratio. Compared with interindividual variation, factors contributing to intraindividual variation are less defined. An exploratory analysis involving healthy nonsmoking non‐naïve caffeine drinkers (1–3 cups/day; 12 men, 12 women) administered caffeine (160 mg) on five occasions evaluated the effects of CYP1A2 induction status (based on genotype) and other factors on intraindividual variation in CYP1A2 activity. Results were compared with those from previous studies. Regardless of whether a hyperinducer (CYP1A2*1A/*1F or CYP1A2*1F/*1F) or normal metabolizer (CYP1A2*1A/*1A,CYP1A2*1C/*1F, or CYP1A2*1C*1F/*1C*1F), sex, age, oral contraceptive use by women, and smoking status, intraindividual variation was ≤30%. A value of 30% is proposed for optimal design of pharmacokinetic xenobiotic–caffeine interaction studies. Prospective studies are needed for confirmation.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ Optimal sample size calculation for cytochrome P450 (CYP)–mediated pharmacokinetic xenobiotic–drug interaction studies requires robust estimates of both interindividual and intraindividual variation in enzyme activity. Relative to interindividual variation in CYP1A2 activity, commonly assessed by the caffeine metabolic ratio, factors contributing to intraindividual variation are less defined.
what question did this study address?
✓ An exploratory analysis involving a well‐characterized cohort of 24 nonsmoking volunteers administered caffeine on five separate occasions was conducted to investigate the effects of CYP1A2 induction status (based on genotype) and other key factors on the intraindividual variation in CYP1A2 activity.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
✓ Intraindividual variation in the caffeine metabolic ratio, as assessed by the coefficient of variation, was generally ≤30%, regardless of CYP1A2 induction status, sex, and oral contraceptive use by women.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
✓ An intraindividual variation of 30% is proposed for calculating the optimal sample size needed to detect a change in the caffeine metabolic ratio for CYP1A2‐mediated xenobiotic–drug interaction studies. Prospective studies are needed to confirm this observation.
Cytochrome P450 (CYP) 1A2 is considered one of the “big 5” CYPs (CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A), which collectively are responsible for ≈90% of the oxidative metabolism of drugs.1 Caffeine is a widely used and sensitive in vivo CYP1A2 index substrate because of rapid and nearly complete absorption into the systemic circulation upon oral administration (oral bioavailability, ~99%) and extensive hepatic metabolic clearance.2, 3, 4 CYP1A2‐mediated demethylation of caffeine to the primary metabolite, paraxanthine, represents >80% of total caffeine clearance; other primary clearance pathways include demethylation to theobromine and theophylline (Figure 1).5, 6 The caffeine metabolic ratio, based on plasma concentration at a single timepoint or area under the concentration–time curve (AUC) and of the mass excreted into urine over a certain time interval, is a commonly used marker of CYP1A2 activity.7, 8, 9, 10
Figure 1.
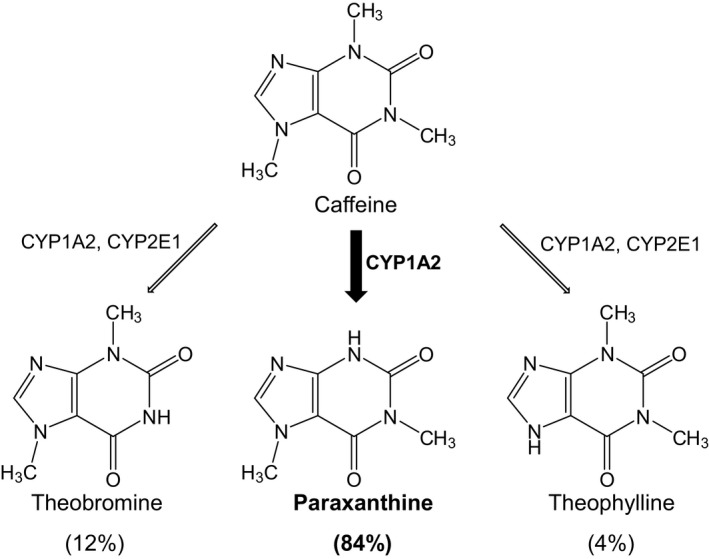
Primary metabolic pathways of caffeine.
As with all CYP index substrates, robust estimates of both interindividual and intraindividual variation in CYP1A2 activity are needed to determine optimal sample size for clinical pharmacokinetic xenobiotic–caffeine interaction studies.4, 11 Multiple factors contribute to the large interindividual variation in CYP1A2 activity (5‐ to 15‐fold) among various populations12 and include sex (activity generally higher in men compared with women), smoking (CYP1A2 inducer), concomitant medications (e.g., oral contraceptives, which are CYP1A2 inhibitors), and genetic polymorphisms. Common CYP1A2 haplotypes include CYP1A2*1A, CYP1A2*1C, CYP1A2*1F, and CYP1A2*1K.12, 13 CYP1A2*1A is considered the reference haplotype. CYP1A2*1C is defined by the single‐nucleotide polymorphism (SNP) −3860G>A (rs2069514); CYP1A2*1F is defined by the SNP −163C>A (rs762551); and CYP1A2*1K is defined by the SNPs −739T>G (rs2069526), −729C>T (rs12720461), and −163C>A (rs762551).13, 14 Haplotype frequencies vary widely with ethnicity. For example, the frequency of CYP1A2*1C ranges from 0.4% to 1% in whites to 7% in blacks or African Americans to 6–25% in Asians,14 whereas that for CYP1A2*1F ranges from ≥0.5% in whites and African Americans to <8% in Asians.15, 16
Compared with CYP1A2*1A, CYP1A2*1C and CYP1A2*1K are associated with decreased CYP1A2 activity in vivo. CYP1A2*1F is generally associated with increased activity because of increased inducibility or “hyperinducibility.”13 For example, both smoking17, 18, 19, 20 and heavy caffeine consumption (≥3 cups/day)21 were associated with higher CYP1A2 activity (by 30–70%) in CYP1A2*1F*1F carriers vs. CYP1A2*1A*1F and CYP1A2*1A*1A carriers. Compared with the interindividual variation, published studies with respect to the intraindividual variation in CYP1A2 activity are few (Table 1). These studies collectively addressed the effects of smoking status, age, and sex using either urinary or plasma ratios at a single timepoint. The effects of common genetic CYP1A2 polymorphisms and use of oral contraceptives by women were not considered. In addition, caffeine consumption habit was not reported.
Table 1.
Intraindividual variability in the caffeine metabolic ratio reported in the literature
| Race/ethnicitya | Sex | Smoker? | Age (y) | No. of subjects | Caffeine formulation (caffeine content) | Metabolic ratio | No. of replicates within a subject | Interval of replicate measurement | CV (%) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Description | Mean ± SD | Mean (range) | ||||||||
| Japanese | M | No | 30.4 (17‐46)b | 5 | Instant coffee (caffeine content NS) | Urinary (17U+ PX)/CAF, 4–5 h collection interval | 8.7 | 2 | 11 mo | 27.5 (0.7–66.7) | 31 |
| F | No | 31.7 (23–36)b | 3 | 2.8 | 59.5 (41.6–84.5) | ||||||
| M | Yes | 35.3 (25–50)b | 4 | 11.6 | 17.6 (6.9–37.6) | ||||||
| White | M | No | (22–61)b | 10 | Brewed coffee (~200 mg) | Urinary PX/CAF, 4–5 h collection interval | 4.5 ± 1.7 | 3 | 1 wk | 24.3 (3.0–71.5) | 28 |
| F | No | 9 | 5.5 ± 1.5 | 26.2 (4.2–60.4) | |||||||
| NS | M | No | 34.8 ± 7.9 | 10 | NoDoze (2 mg/kg) | Urinary (1U+1X+AFMU)/17U, 14–15 h collection interval | 9.4 ± 10.2 | 6 | 18.6 (4.5–45.3) | 29 | |
| F | No | 38.2 ± 9.3 | 10 | 5.7 ± 2.3 | ~2 wk | 20.7 (8.7–49.3) | |||||
| NS | M | No | Young subjects | 34 | Koffein “Dak” (200 mg) | Plasma PX/CAF at 6 h | 0.75 ± 0.22 | 2 | 18 (1–45) | 32 | |
| M | Yes | 8 | 1.6 ± 0.7 | 12–14 wk | 16 (2–46) | ||||||
| NS | NS | No | 25 ± 0.3 | 12 | Instant coffee (150 mg) | Plasma PX/CAF at 5 h | 0.45 ± 0.19 | 5 | 3 wk | 16.2 (6.9–25.7) | 33 |
| Yes | 4 | 0.76 ± 0.21 | 21.8 (18.9–26.5) | ||||||||
| No | 70 ± l .7 | 13 | 0.32 ± 0.14 | 16.4 (8.6–32.9) | |||||||
| Yes | 3 | 0.45 ± 0.07 | 15.3 (10.4–18.5) | ||||||||
AFMU, 5‐acetylamino‐6‐formylamino‐3‐methyluracil; CAF, caffeine; CV, coefficient of variation; F, female; M, male; NS, not specified; PX, paraxanthine; 1U, 1‐methyl uric acid; 17U, 1,7‐dimethyluric acid; 1X, 1‐methylxanthine. aThe studies from references 29, 32, and 33 were conducted in the United States, Denmark, and France, respectively. bMean (range) or (range).
Caffeine, as brewed coffee, carbonated beverage, and energy drink, is heavily consumed worldwide. Approximately 50% of adults in the United States alone consume 1–3 cups of coffee/day.22, 23 Based on safety concerns with energy drinks and other nonconventional forms of caffeine, a previous study compared the pharmacokinetics of caffeine under different drink temperature (hot vs. cold), rate of consumption (2 vs. 20 minutes), and vehicle (coffee vs. energy drink) conditions.24 Each of 24 nonsmoking, non‐naïve, caffeine‐drinking (1–3 cups/day) volunteers was administered 160 mg caffeine, as coffee or energy drink, under the various conditions. The pharmacokinetics of caffeine did not differ among conditions, indicating no significant impact of temperature, consumption rate, and vehicle.24 Based on the aforementioned gaps in the literature, the objective of the present study was to assess the effects of CYP1A2 induction status (based on genotype), sex, and oral contraceptive use by women on both the interindividual and intraindividual variation in the caffeine metabolic ratio. Plasma (0–8 hours) and DNA were obtained from this well‐characterized cohort of participants. Results from this exploratory analysis provide a robust estimate of intraindividual variation in CYP1A2 activity for optimal design of clinical xenobiotic–caffeine interaction studies.
Methods
Pharmacokinetic study
Plasma and whole blood were obtained from a previous study involving 24 healthy volunteers (12 men) who were nonsmoking, non‐naïve caffeine drinkers (1–3 caffeine‐containing beverages/day on average), aged 18 to 30 years; 16 subjects were self‐identified as white, six as Asians, and two as Pacific Islanders.24 In brief, the pharmacokinetics of a single oral dose of caffeine (160 mg), administered in the form of instant coffee or a sugar‐free energy drink, were compared with respect to temperature and rate of consumption: arm A, hot coffee consumed over 20 minutes; arm B, cold coffee consumed over 2 minutes; arm C, cold coffee consumed over 20 minutes; arm D, energy drink consumed over 2 minutes; and arm E, energy drink consumed over 20 minutes. Study arms were separated by at least 3 days. Before the first treatment, subjects were asked to abstain from caffeine ~24 hours before caffeine administration. DNA was extracted from whole blood using the QIAamp DNA blood mini kit (Qiagen, Germantown, MD) and stored at −80°C pending analysis for the CYP1A2 SNPs rs2069514, rs2069526, rs762551, and rs12720461 by Genelex Labs (Seattle, WA).
Analysis of plasma for caffeine and paraxanthine
Plasma was analyzed for caffeine as described using a Xevo G2S QToF mass spectrometer (Waters, Millford, MA) coupled to an ultra–high‐pressure liquid chromatographic system (Waters).24 Plasma was analyzed for paraxanthine in a similar manner using d3‐paraxanthine (Toronto Research Chemicals, Toronto, ON, Canada) as the internal standard. Analytes were separated via an Acquity HSS T3 column (2.1 × 100 mm, 1.8 μm) (Waters). The mass spectrometer was operated in positive ion mode using m/z values of 181.070 and 184.091 for paraxanthine and d3‐paraxanthine, respectively. The limit of quantification was 39 ng/mL.
Pharmacokinetic and statistical analysis
The pharmacokinetics of caffeine were determined via a noncompartmental methods as described24 using Phoenix WinNonlin, version 6.4 (Certara, St. Louis, MO). The concentrations of caffeine and paraxanthine at 4 hours (C4 h,CAF and C4 h,PX, respectively) were obtained directly from the plasma concentration–time profiles. Areas under the curves from time 0 to 8 hours for caffeine and paraxanthine (AUC0‐8 h,CAF and AUC0‐8 h,PX, respectively) were calculated using the trapezoidal method with linear interpolation. Caffeine metabolic ratios based on C4 h and AUC0‐8 h were calculated using molar concentrations.
Caffeine metabolic ratios were log transformed before statistical analysis. Repeated‐measures analysis of variance was used to test for differences in the mean C4 h,PX/C4 h,CAF and AUC0‐8 h, PX/AUC0‐8 h,CAF ratios among the five treatment arms; P < 0.05 was considered significant. The 24 subjects were grouped with respect to sex, CYP1A2 induction status, and oral contraceptive use by six of the women to make comparisons. Within each study arm, the Wilcoxon‐Mann‐Whitney U‐test was used to test for differences in mean C4 h,PX/C4 h,CAF and AUC0‐8 h, PX/AUC0‐8 h,CAF ratios; P < 0.05 was considered significant. Interindividual variability in CYP1A2 activity was calculated as the coefficient of variation (CV), the ratio of SD/mean, for each metabolic ratio within a treatment arm. Intraindividual variability in CYP1A2 activity was calculated as the CV for each ratio among the five treatment arms within each subject. Differences in interindividual and intraindividual variability between two groups were compared using the Wilcoxon‐Mann‐Whitney U‐test; P < 0.05 was considered significant.
Results
CYP1A2 genotype
Using the PharmGKB summary for CYP1A2 as a guide,13 subjects with genotypes CYP1A2*1F/*1F and CYP1A2*1A/*1F were designated hyperinducers (n = 15), and subjects with genotypes CYP1A2*1A/*1A, CYP1A2*1C/*1F, or CYP1A2*1C*1F/*1C*1F were designated normal metabolizers (n = 9) (Table 2).
Table 2.
CYP1A2 induction status and genotype for 24 healthy volunteers
| Induction Status | Genotype | SNPa | Sex (n) | Race (n) | |
|---|---|---|---|---|---|
| −3860G>A | −163C>A | ||||
| N | *1A/*1A | GG | CC | M (1), F (3) | White (3), Asian (1) |
| N | *1C/*1F | GA | CA | M (1), F (1) | Asian (1), PI (1) |
| N | *1C*1F/*1C*1F | AA | AA | M (2), F (1) | Asian (2), PI (1) |
| H | *1A/*1F | GG | CA | M (6), F (5) | White (9), Asian (2) |
| H | *1F/*1F | GG | AA | M (2), F (2) | White (4) |
F, female; H, hyperinducer; M, male; N, normal metabolizer; SNP, single‐nucleotide polymorphism; PI, Pacific Islander.
aAll subjects harbored the T allele for −739T>G (rs2069526) and the C allele for −729C>T (rs12720461).
Caffeine metabolic ratios
The pharmacokinetics of caffeine were consistent with those determined in the previous study in which a different pharmacokinetic analysis program was used.24 Paraxanthine concentrations increased, reaching a plateau at ≈4 hours that remained until the last plasma collection at 8 hours (data not shown). Plasma caffeine metabolic ratios based on C4 h and AUC0‐8 h ranged from 0.12–0.96 and 0.12–0.79, respectively, with all treatment arms and subjects combined (n = 120 total measurements for each ratio); there was a strong correlation between the two ratios (r = 0.97). The mean C4 h ratios did not differ among treatment arms with all subjects combined (0.37–0.41). When stratified by sex, the C4 h ratio was significantly higher in men, by ~40%, compared with women (P < 0.01) with all subjects and treatment arms combined; if the six women taking oral contraceptives were excluded, the difference was 24% (P < 0.03) (Table 3). Within each treatment arm, a sex difference was detected (P < 0.05) (Table 3, Figure 2) but was not detected if the oral contraceptive users were excluded. Within women in each treatment arm, the C4 h ratio tended to be lower in oral contraceptive users compared with nonusers (P > 0.07). The mean interindividual CV was significantly lower in men, by 44%, compared with women (P < 0.02); a sex difference was not detected if the oral contraceptive users were excluded (Table 3). Comparisons using the AUC0‐8 h ratio were similar to those using the C4 h ratio.
Table 3.
Caffeine metabolic ratios and interindividual variability within each treatment arm
| Treatment arm | Metabolic ratio (mean ± SD) | CV (%) | ||
|---|---|---|---|---|
| C4 h,PX/C4 h,CAF | AUC0–8 h,PX/AUC0–8 h,CAF | C4 h,PX/C4 h,CAF | AUC0–8 h,PX/AUC0–8 h,CAF | |
| Men (n = 12) | ||||
| A | 0.44 ± 0.14 | 0.40 ± 0.13 | 31.3 | 31.5 |
| B | 0.44 ± 0.12 | 0.39 ± 0.10 | 26.5 | 24.8 |
| C | 0.48 ± 0.13 | 0.45 ± 0.14 | 27.6 | 31.2 |
| D | 0.46 ± 0.16 | 0.42 ± 0.14 | 34.7 | 33.1 |
| E | 0.50 ± 0.17 | 0.45 ± 0.11 | 32.9 | 25.4 |
| All armsa | 0.46 ± 0.14 | 0.42 ± 0.12 | 30.6 ± 3.50 | 29.2 ± 3.82 |
| Women (n = 12) | ||||
| A | 0.32 ± 0.12 | 0.30 ± 0.10 | 37.5 | 32.7 |
| B | 0.30 ± 0.13 | 0.28 ± 0.10 | 44.5 | 37.3 |
| C | 0.31 ± 0.15 | 0.30 ± 0.13 | 47.3 | 44.3 |
| D | 0.32 ± 0.16 | 0.29 ± 0.11 | 51.2 | 38.8 |
| E | 0.33 ± 0.13 | 0.32 ± 0.11 | 40.4 | 35.9 |
| All armsa | 0.32 ± 0.14b | 0.30 ± 0.11b | 44.2 ± 5.44c | 37.8 ± 4.28c |
| OC users (all arms)a | 0.26 ± 0.11d | 0.25 ± 0.09d | 44.8 ± 22.8 | 41.5 ± 14.6 |
| Non‐OC users (all arms)a | 0.37 ± 0.14e | 0.34 ± 0.11e | 39.1 ± 5.44 | 33.0 ± 3.45 |
AUC, area under the concentration–time curve; CAF, caffeine; CV, coefficient of variation; OC, oral contraceptive; PX, paraxanthine.
aData represent mean ± SD of five arms. b P < 0.01 compared with men (Wilcoxon‐Mann‐Whitney U‐test). c P < 0.02 compared with men (Wilcoxon‐Mann‐Whitney U‐test). d P < 0.0001 compared with men (Wilcoxon‐Mann‐Whitney U‐test). e P < 0.03 compared with men (Wilcoxon‐Mann‐Whitney U‐test).
Figure 2.
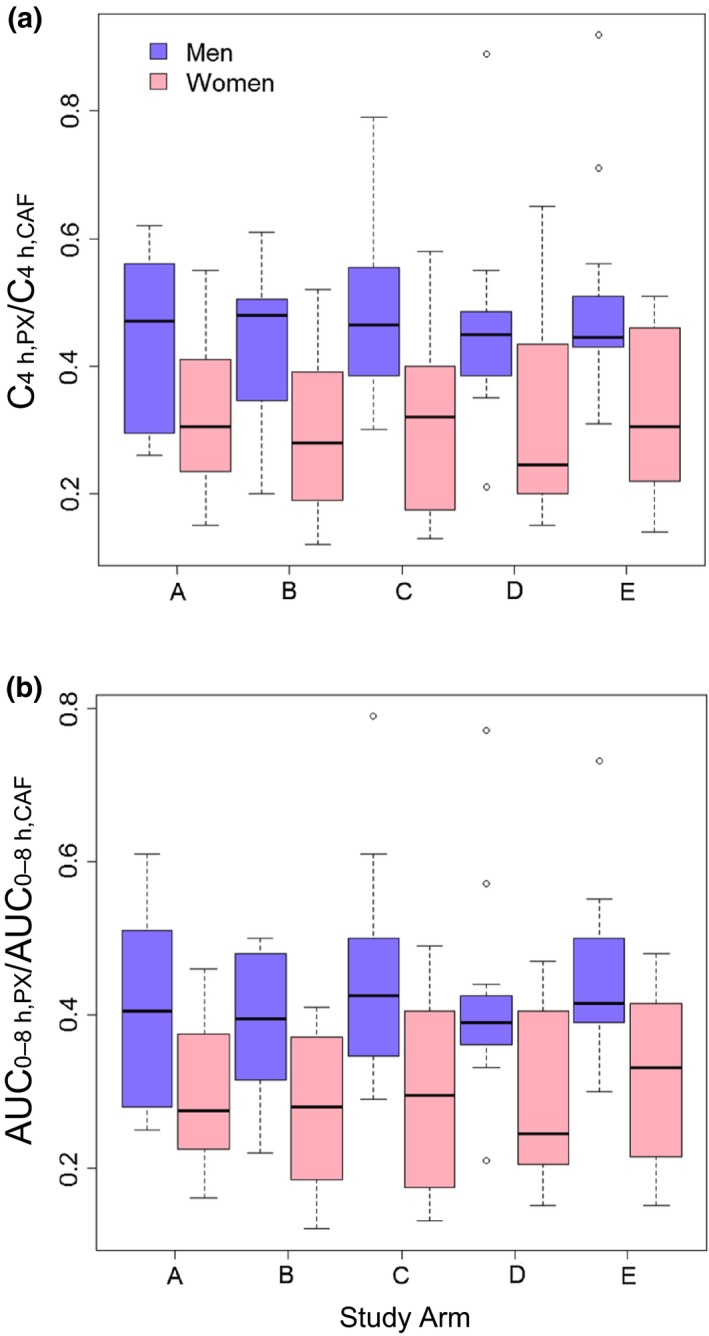
Box‐and‐whisker plots of C4 h, PX/C4 h, CAF (a) and AUC 0‐8 h, PX/AUC 0‐8 h, CAF (b) ratios for each study arm among men and women. Arm A, hot coffee consumed over 20 minutes; arm B, cold coffee consumed over 2 minutes; arm C, cold coffee consumed over 20 minutes; arm D, energy drink consumed over 2 minutes; and arm E, energy drink consumed over 20 minutes. Lines inside the boxes denote medians, ends of boxes denote 25th and 75th percentiles, whiskers denote 1.5 times the interquartile distance, and circles denote outliers. A sex difference was detected (P < 0.05; Wilcoxon‐Mann‐Whitney U‐test) within each treatment arm when oral contraceptive–using women were included.
Because the metabolic ratio did not differ between the various conditions, intraindividual variation in CYP1A2 activity was calculated using the metabolic ratio obtained from the five arms for each subject. A sex difference was not detected in intraindividual CV for the C4 h ratio (Table 4). Within the 16 white subjects, the intraindividual CV for the C4 h ratio was comparable between men and women (data not shown). When stratified by induction status based on CYP1A2 genotype (hyperinducer or normal metabolizer), the C4 h ratio did not differ with all subjects and treatment arms combined (data not shown) and within each treatment arm (Figure 3). The intraindividual CV for the C4 h ratio did not differ between hyperinducers and normal metabolizers for all subjects (Table 4) and for white subjects (data not shown). Comparisons using the AUC0‐8 h ratio were similar to those using the C4 h ratio except the AUC‐based intraindividual CV within the 16 white subjects was significantly lower in men, by 44%, compared with women (P = 0.03; data not shown).
Table 4.
Intraindividual variability in caffeine metabolic ratio stratified by sex, oral contraceptive use by women, induction status, and race
| Subjectsa | No. of subjects | CV (%) (mean ± SD) | |
|---|---|---|---|
| C4 h,PX/C4 h,CAF | AUC0–8 h,PX/AUC0–8 h,CAF | ||
| Men | 12 | 19.1 ± 5.7 | 17.1 ± 6.9 |
| Women | 12 | 26.4 ± 16.6 | 23.1 ± 12.4 |
| Women using OCs | 6 | 34.6 ± 19.0 | 30.1 ± 13.2 |
| Women not using OCs | 6 | 18.3 ± 9.1 | 16.2 ± 7.1 |
| Hyperinducers | 15 | 25.8 ± 14.7 | 23.2 ± 11.2 |
| Normal metabolizers | 9 | 17.8 ± 6.2 | 15.1 ± 6.2 |
| White subjects | 16 | 24.3 ± 15.0 | 20.8 ± 12.0 |
| Nonwhite subjects | 8 | 19.8 ± 5.7 | 18.9 ± 6.2 |
| All subjects | 24 | 22.8 ± 12.7 | 20.1 ± 10.3 |
AUC, area under the concentration–time curve; CAF, caffeine; CV, coefficient of variation; OC, oral contraceptive; PX, paraxanthine.
aA significant difference was not detected between subjects when stratified by sex, OC use, induction status, and race.
Figure 3.
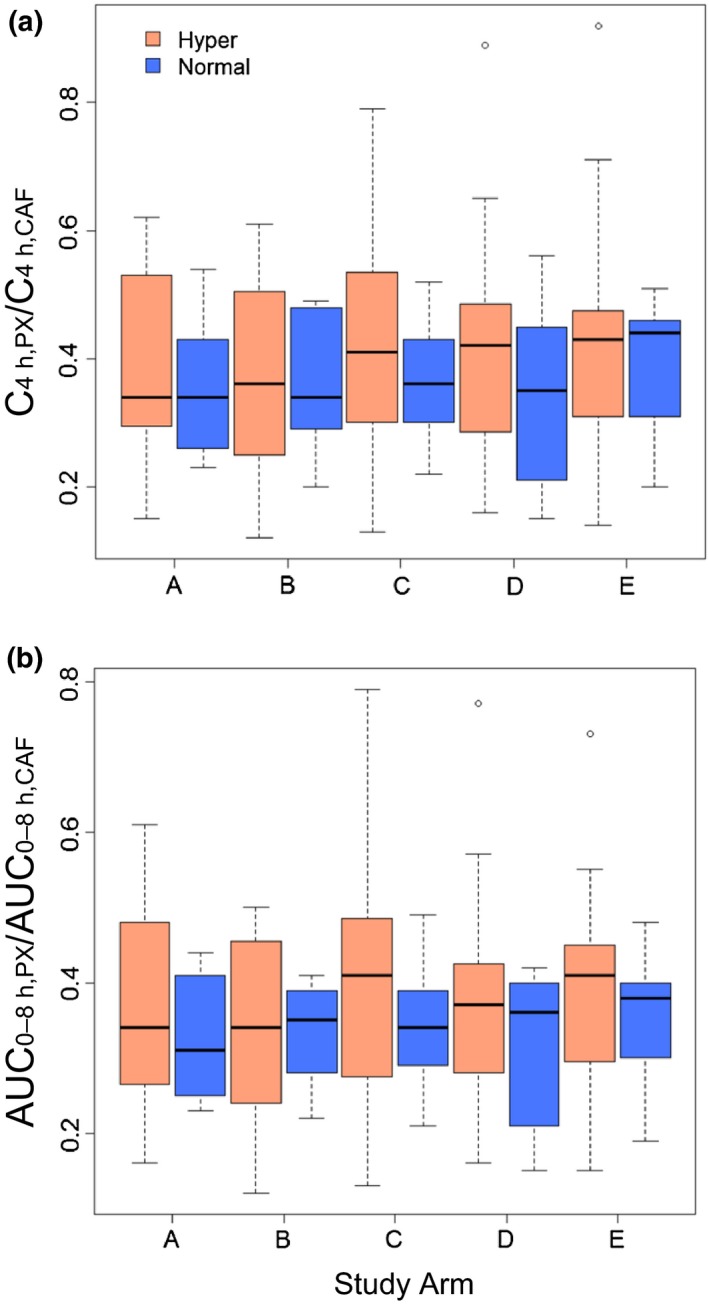
Box‐and‐whisker plots of C4 h, PX/C4 h, CAF (a) and AUC 0‐8 h, PX/AUC 0‐8 h, CAF (b) ratios for each study arm among CYP1A2 hyperinducers (Hyper) and normal metabolizers (Normal). Arm A, hot coffee consumed over 20 minutes; arm B, cold coffee consumed over 2 minutes; arm C, cold coffee consumed over 20 minutes; arm D, energy drink consumed over 2 minutes; and arm E, energy drink consumed over 20 minutes. Lines inside the boxes denote medians, ends of boxes denote 25th and 75th percentiles, whiskers denote 1.5 times the interquartile distance, and circles denote outliers. No differences were detected within each treatment arm (P > 0.05; Wilcoxon‐Mann‐Whitney U‐test).
Discussion
Caffeine, consumed worldwide as a beverage, is a sensitive index CYP1A2 substrate4 commonly used to evaluate the CYP1A2 inhibitory or inductive effects of new molecular entities and other xenobiotics, including herbal and other natural products.25, 26 The optimal sample size calculation for clinical pharmacokinetic xenobiotic–drug interaction studies requires robust estimates of both interindividual and intraindividual variation in the primary end point, which for CYP‐mediated interactions typically involves a validated urinary or plasma metabolic ratio of the index substrate. Sex, smoking status, concomitant oral contraceptive use by women, and genetic polymorphisms are established factors that influence variation in the caffeine metabolic ratio.
Relative to estimates of interindividual variation, estimates of intraindividual variation in the caffeine metabolic ratio are few (Table 1). In addition, none of those studies assessed the effects of CYP1A2 induction status and oral contraceptive use by women and did not report whether the subjects were habitual caffeine consumers, the latter of which reflect at least half of the US and other populations.22, 23, 27 Based on these knowledge gaps, the objective of this study was to revisit the intraindividual variation in the caffeine metabolic ratio by assessing the effects of CYP1A2 induction status (based on genotype) and other key factors. The analysis involved plasma and DNA specimens obtained from a previous study involving 24 nonsmoking, non‐naïve, caffeine‐drinking healthy volunteers whose caffeine pharmacokinetics were determined on five separate occasions.24 Because the pharmacokinetics did not differ under the various conditions (drink temperature, consumption time, and caffeine vehicle), this cohort of subjects was representative of a major segment of the population.
Among the 24 subjects, the caffeine metabolic ratio, based on either C4 h or AUC0‐8 h, varied from four‐ to six fold within each treatment arm. This interindividual variability was near the low end of the 5‐ to‐15‐fold reported range,12 which likely reflected the relatively homogeneous nature of the study subjects. When stratified by sex, the mean metabolic ratios were higher in men compared with women, by ~40% (Table 3). Oral contraceptive use by women, which has been reported to inhibit CYP1A2 activity,15, 17 may contribute to this sex difference. In the current study, oral contraceptive users tended to have lower metabolic ratios compared with nonusers, which further explains the observation in the previous study24 that caffeine apparent oral clearance was lower in oral contraceptive users compared with nonusers (0.8 vs. 1.3 mL/min per kg). If the nonusers were excluded from the analysis, a sex difference was not detected within each treatment arm, consistent with a previous report.17 Interindividual variability was greater in women compared with men (~40% vs. 30%) (Table 3); again, if the oral contraceptive users were excluded, a difference was not detected. Although in agreement with the previous study that involved a much larger sample size (>200 subjects),17 these observations contradict those from two other equally large studies showing that oral contraceptive use did not contribute to the sex difference in CYP1A2 activity. Reasons for these discrepancies may include a relatively understudied ethnic group with respect to variation in CYP1A2 activity (Turkish)18 and enrollment of smokers only,19 the latter of which suggests that smoking may mask the CYP1A2 inhibitory effects of oral contraceptives.
The intraindividual variability in the caffeine metabolic ratio was assessed by the CV, which was calculated from the five replicates obtained from each subject. Among all subjects, the CV was ≈20% (Table 4). When stratified by sex, induction status, oral contraceptive use by women, or race, the CV was generally no more than 30%; the only exception was the C4 h‐based CV of 35% in women taking oral contraceptives. Although the value was higher in women compared with men (~25% vs. 18%), a sex difference was not detected, even if extreme values for each sex were excluded. Similar trends were observed for the white subjects (data not shown). These data are consistent with previous reports involving nonsmoking subjects, most presumed to be white28, 29 (Table 1). Within women, use of oral contraceptives did not affect intraindividual CV significantly. With respect to CYP1A2 induction status, the mean metabolic ratios did not differ between hyperinducers and normal metabolizers (~0.39 vs. ~0.35; Figure 3), yet the intraindividual CV was higher in hyperinducers compared with normal metabolizers (25% vs. 16%; Table 4). Similar trends were observed comparing only the homozygous CYP1A2*1F carriers with noncarriers (data not shown). These data indicate that common CYP1A2 genotypes are not robust predictors of CYP1A2 phenotype (Table 2), consistent with previous reports.10, 21
Heavy coffee consumption (≥3 cups/day) was shown to be associated with higher CYP1A2 activity in nonsmoking white subjects.21 The previous studies (Table 1) did not report caffeine consumption habit; thus, the effects of caffeine consumption on the intraindividual CV could not be assessed. All subjects in the current work were known to be non‐naïve caffeine drinkers (1–3 cups/day). Because the CV was similar to that for the previous studies, moderate caffeine consumption is an unlikely contributor to intraindividual variation in CYP1A2 activity.
In addition to the aforementioned factors that could contribute to the intraindividual variation in the caffeine metabolic ratio, other factors, such as the time interval between replicate measurements, plasma vs. urinary ratios, age, and smoking status, likely contribute. However, with the exception of the one study involving a few Japanese subjects, the intraindividual CV reported in the previous studies was always <30% (Table 1). This observation, combined with those in the current study (Table 4), suggest 30% intraindividual variation may be the most appropriate for optimal sample size calculation for CYP1A2‐mediated xenobiotic–caffeine interaction studies.
There are limitations to the current work. First, because this study was exploratory, firm conclusions cannot be made about the effects of CYP1A2 induction status, sex, and oral contraceptive use by women on the intraindividual variation in the caffeine metabolic ratio. Based on the current data (Table 4) and assuming a type I error of 0.05 and a power of 0.80, 33 and 21 subjects per group would be needed to detect a difference in intraindividual variation based on C4 h,PX/C4 h,CAF and AUC0‐8 h,PX/AUC0‐8 h,CAF, respectively, between hyperinducers and normal metabolizers. Second, a limited number of CYP1A2 genotypes were determined. Forty‐one CYP1A2 haplotypes have been reported to date;13 thus, investigation of other haplotypes is warranted to improve the understanding of the CYP1A2 genotype–phenotype relationship. Third, most study subjects were self‐identified as white. CYP1A2 activity has been reported to be higher in white individuals compared with Asians and African Americans.17, 30 Whether different frequencies of multiple genotypes across race/ethnicity influence intraindividual variation remains to be determined. Fourth, although CYP1A2 activity did not differ among the different treatment conditions, drink temperature, consumption rate, and vehicle may have confounded the assessment of intraindividual variability.
In summary, this exploratory study demonstrated that the intraindividual variation in CYP1A2 activity in a well‐characterized group of young nonsmoking, non‐naïve, caffeine‐drinking healthy volunteers was generally no more than 30%, regardless of CYP1A2 induction status, sex, and oral contraceptive use by women. These results add to information from previous studies (Table 1) in which intraindividual variability in CYP1A2 activity, stratified by smoking status, sex, and age, was generally <30%. Collectively, a value of 30% is proposed for optimal design of clinical pharmacokinetic xenobiotic–caffeine interaction studies. Prospective studies are needed to confirm these observations.
Funding
This work was supported in part by the National Institutes of Health National Center for Complementary and Integrative Health (U54 AT008909) and the American Beverage Association.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
D.D.T., J.R.W., and M.F.P. wrote the manuscript; D.D.T., J.R.W., and M.F.P. designed the research; D.D.T., S.N., J.R.W., and M.F.P. performed the research; D.D.T., S.N., J.R.W., and M.F.P. analyzed the data; S.N. contributed new reagents/analytical tools.
Acknowledgment
The authors thank Gang Chen (Department of Pharmaceutical Sciences, Washington State University) for analyzing the plasma samples for paraxanthine and Brandon T. Gufford (Clinical Pharmacology, Medical & Scientific Affairs, Covance, Inc.) for helpful discussions. M.F.P. dedicates this article to David P. Paine.
References
- 1. Zanger, U.M. , Turpeinen, M. , Klein, K. & Schwab, M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal. Bioanal. Chem. 6, 1093–1108 (2008). [DOI] [PubMed] [Google Scholar]
- 2. Kalow, W. & Tang, B.K. The use of caffeine for enzyme assays: a critical appraisal. Clin. Pharmacol. Ther. 5, 503–514 (1993). [DOI] [PubMed] [Google Scholar]
- 3. Fredholm, B.B. , Battig, K. , Holmen, J. , Nehlig, A. & Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1, 83–133 (1999). [PubMed] [Google Scholar]
- 4. Food and Drug Administration Center for Drug Evaluation and Research . Clinical Drug Interaction Studies—Study Design, Data Analysis, and Clinical Implications Guidance for Industry (Draft Guidance) (US Food and Drug Administration, Silver Spring, MD, 2017). [Google Scholar]
- 5. Perera, V. , Gross, A.S. & McLachlan, A.J. Measurement of CYP1A2 activity: a focus on caffeine as a probe. Curr. Drug Metab. 5, 667–678 (2012). [DOI] [PubMed] [Google Scholar]
- 6. Heckman, M.A. , Weil, J. & de Gonzalez Mejia, E . Caffeine (1, 3, 7‐trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 3, R77–R87 (2010). [DOI] [PubMed] [Google Scholar]
- 7. Krul, C. & Hageman, G. Analysis of urinary caffeine metabolites to assess biotransformation enzyme activities by reversed‐phase high‐performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1, 27–34 (1998). [DOI] [PubMed] [Google Scholar]
- 8. Perera, V. , Gross, A.S. , Xu, H. & McLachlan, A.J. Pharmacokinetics of caffeine in plasma and saliva, and the influence of caffeine abstinence on CYP1A2 metrics. J. Pharm. Pharmacol. 9, 1161–1168 (2011). [DOI] [PubMed] [Google Scholar]
- 9. Derungs, A. et al Effects of cytochrome P450 inhibition and induction on the phenotyping metrics of the Basel cocktail: a randomized crossover study. Clin. Pharmacokinet. 1, 79–91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang, L. et al Association between common CYP1A2 polymorphisms and theophylline metabolism in non‐smoking healthy volunteers. Basic Clin. Pharmacol. Toxicol. 4, 257–263 (2013). [DOI] [PubMed] [Google Scholar]
- 11. European Medicines Agency, Committee for Human Medicinal Products Guideline on the investigation of drug interactions. (European Medicines Agency, London: 2012). [Google Scholar]
- 12. Faber, M.S. , Jetter, A. & Fuhr, U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin. Pharmacol. Toxicol. 3, 125–134 (2005). [DOI] [PubMed] [Google Scholar]
- 13. PharmGKB . CYP1A2<https://www.pharmgkb.org/gene/PA27093> (2018) Accessed January 12, 2018.
- 14. Thorn, C.F. , Aklillu, E. , Klein, T.E. & Altman, R.B. PharmGKB summary: very important pharmacogene information for CYP1A2. Pharmacogenet. Genomics 1, 73–77 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gunes, A. & Dahl, M.L. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics 5, 625–637 (2008). [DOI] [PubMed] [Google Scholar]
- 16. Aklillu, E. et al Genetic polymorphism of CYP1A2 in Ethiopians affecting induction and expression: characterization of novel haplotypes with single‐nucleotide polymorphisms in intron 1. Mol. Pharmacol. 3, 659–669 (2003). [DOI] [PubMed] [Google Scholar]
- 17. Ghotbi, R. et al Comparisons of CYP1A2 genetic polymorphisms, enzyme activity and the genotype‐phenotype relationship in Swedes and Koreans. Eur. J. Clin. Pharmacol. 6, 537–546 (2007). [DOI] [PubMed] [Google Scholar]
- 18. Gunes, A. et al Influence of genetic polymorphisms, smoking, gender and age on CYP1A2 activity in a Turkish population. Pharmacogenomics 5, 769–778 (2009). [DOI] [PubMed] [Google Scholar]
- 19. Dobrinas, M. et al Impact of smoking, smoking cessation, and genetic polymorphisms on CYP1A2 activity and inducibility. Clin. Pharmacol. Ther. 1, 117–125 (2011). [DOI] [PubMed] [Google Scholar]
- 20. Sachse, C. , Brockmoller, J. , Bauer, S. & Roots, I. Functional significance of a C–>A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br. J. Clin. Pharmacol. 4, 445–449 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Djordjevic, N. , Ghotbi, R. , Jankovic, S. & Aklillu, E. Induction of CYP1A2 by heavy coffee consumption is associated with the CYP1A2 ‐163C>A polymorphism. Eur. J. Clin. Pharmacol. 7, 697–703 (2010). [DOI] [PubMed] [Google Scholar]
- 22. Saad, L . Americans’ coffee consumption is steady, few want to cut back. <http://news.gallup.com/poll/184388/americans-coffee-consumption-steady-few-cut-back.aspx.> (2015). Accessed January 12, 2018.
- 23. Park, S.Y. et al Association of coffee consumption with total and cause‐specific mortality among nonwhite populations. Ann. Intern. Med. 4, 228–235 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. White, J.R. Jr et al Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowly in coffee chilled or hot versus chilled energy drink in healthy young adults. Clin. Toxicol. (Phila) 4, 308–312 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gurley, B.J. , Fifer, E.K. & Gardner, Z. Pharmacokinetic herb‐drug interactions (part 2): drug interactions involving popular botanical dietary supplements and their clinical relevance. Planta Med. 13, 1490–1514 (2012). [DOI] [PubMed] [Google Scholar]
- 26. Izzo, A.A. & Ernst, E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 13, 1777–1798 (2009). [DOI] [PubMed] [Google Scholar]
- 27. Ferdman, R.A . Here are the countries that drink the most coffee—the U.S. isn't in the top 10. <https://www.theatlantic.com/business/archive/2014/01/here-are-the-countries-that-drink-the-most-coffee-the-us-isnt-in-the-top-10/283100/? (2014). Accessed January 12, 2018.
- 28. McQuilkin, S.H. , Nierenberg, D.W. & Bresnick, E. Analysis of within‐subject variation of caffeine metabolism when used to determine cytochrome P4501A2 and N‐acetyltransferase‐2 activities. Cancer Epidemiol. Biomarkers Prev. 2, 139–146 (1995). [PubMed] [Google Scholar]
- 29. Kashuba, A.D. et al Quantitation of three‐month intraindividual variability and influence of sex and menstrual cycle phase on CYP1A2, N‐acetyltransferase‐2, and xanthine oxidase activity determined with caffeine phenotyping. Clin. Pharmacol. Ther. 5, 540–551 (1998). [DOI] [PubMed] [Google Scholar]
- 30. Relling, M.V. , Lin, J.S. , Ayers, G.D. & Evans, W.E. Racial and gender differences in N‐acetyltransferase, xanthine oxidase, and CYP1A2 activities. Clin. Pharmacol. Ther. 6, 643–658 (1992). [DOI] [PubMed] [Google Scholar]
- 31. Nakajima, M. et al Phenotyping of CYP1A2 in Japanese population by analysis of caffeine urinary metabolites: absence of mutation prescribing the phenotype in the CYP1A2 gene. Cancer Epidemiol. Biomarkers Prev. 5, 413–421 (1994). [PubMed] [Google Scholar]
- 32. Damkier, P. & Brosen, K. Quinidine as a probe for CYP3A4 activity: intrasubject variability and lack of correlation with probe‐based assays for CYP1A2, CYP2C9, CYP2C19, and CYP2D6. Clin. Pharmacol. Ther. 2, 199–209 (2000). [DOI] [PubMed] [Google Scholar]
- 33. Simon, T. et al Variability of cytochrome P450 1A2 activity over time in young and elderly healthy volunteers. Br. J. Clin. Pharmacol. 5, 601–604 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]


