Abstract
Members of the transient receptor potential (TRP) family of cation conducting channels are found in several tissues and cell types where they have different physiological functions. The canonical TRP channel 6 (TRPC6) is present on the platelet membrane and appears to participate in calcium influx during platelet activation. However, limited information is available on the importance of TRPC channels in megakaryocytes (MKs), the precursor cells of platelets. We determined the mRNA and protein expression of TRPC family members and investigated the role of TRPC6 for proliferation and differentiation of human MKs derived from CD34+ progenitor cells. TRPC6 transcripts were highly expressed during the differentiation of MKs and TRPC6 protein was detectable in MK cytoplasm by confocal staining. TRPC6 channel activity was modulated by pharmacological approaches using flufenamic acid (FFA) for activation and SKF96365 for inhibition. Upon FFA stimulation in MKs, an increase in intracellular calcium was observed, which was blocked by SKF96365 at 10 μM concentration. Incubation of MKs with SKF96365 resulted in a reduction in thrombopoietin-stimulated cell proliferation. Our results suggest a role of TRPC6 in calcium homeostasis during MK development, particularly for cell proliferation.
Keywords: calcium, cell proliferation, megakaryocyte, non-store-operated calcium entry, platelet, transient receptor potential C6
Introduction
The super-family of transient receptor potential (TRP) channels consists of several functionally versatile cation-conducting protein channels. The first TRP gene was described in Drosophila (Montell and Rubin, 1989). In mammals, 28 TRP channels have been identified and are grouped into six sub-families based on sequence homology: TRPC (Canonical), TRPV (Vanilloid), TRPM (Melastatin), TRPA (Ankyrin), TRPML (MucoLipin) and TRPP (Polycystin) (Venkatachalam and Montell, 2007). TRP channels consist of six putative transmembrane domains (S1–S6) with a cation-permeable pore-forming loop between S5 and S6 and intracellular N- and C-termini (Owsianik et al., 2006). The channels assemble as homo- or hetero-multimers within each sub-family to form a variety of uniquely functional cation channels (Venkatachalam and Montell, 2007). TRP channels participate in many biological processes including sensory, homeostatic and motile functions. A great deal on TRP channel expression and functions in various tissues is still unknown. However, there are indications that they have a prominent role in the regulation of intracellular calcium (Ca2+) in excitable and non-excitable cells. The TRPC members are involved in G-protein-coupled receptor (GPCR) signalling and phospholipase (PLC) activation. Growth factors, hormones and neurotransmitters are reported to increase TRPC activity independent of PLC (Abramowitz and Birnbaumer, 2009). Members of the TRP family are found on stem cells such as mesenchymal stem cells (Torossian et al., 2010), haematopoietic stem cells (Park et al., 2011), myoblasts (Woo et al., 2010) and neural progenitor cells (Paez et al., 2011).
It is well known that platelet responses during haemostasis depend on an elevation in intracellular calcium concentration ([Ca2+]i). Ca2+ transport is achieved through several calcium permeable channels in the platelet membrane (Varga-Szabo et al., 2009). Several studies have shown a role of TRPCs in platelet calcium homeostasis (Hassock et al., 2002; Jardin et al., 2009). TRPC6 has been proposed to be the major store-independent channel in human platelets (Hassock et al., 2002), which is also highly expressed in mature human (den Dekker et al., 2001b) and murine megakaryocytes (MKs; Carter et al., 2006). MKs develop from pluripotent haematopoietic stem cells (HSCs), which proliferate and terminally differentiate to yield functional platelets. MK maturation is a unique developmental process where immature MKs undergo endomitosis that amplifies DNA, resulting in polyploidy. Cytoplasmic maturation involves the formation of an extensive demarcation membrane system (DMS), a continuous network of specialised membranes within the cytoplasm, and synthesis of numerous platelet-specific α and dense granules (Hartwig and Italiano, 2003). Megakaryopoiesis also comprises the synthesis of proteins necessary for platelet formation and function, including surface receptors and platelet granule components. Mature MKs are fully equipped with the elements necessary for platelet biogenesis via proplatelet formation and finally platelet release (Patel et al., 2005).
TRPC6 has been identified in immature CD34+ cells. Functional characterisation suggests a role in haematopoiesis such as activation of calcium-dependent transcription factors (Park et al., 2011). It has been observed that erythropoietin-induced changes in cytoplasmic calcium influence the proliferation and differentiation of erythroid progenitors and precursors (Miller et al., 1988). Recently, it has been found that TRPC3 channel activation by erythropoietin is modulated by TRPC6 in human erythroid precursors, thereby regulating intracellular calcium required for erythroid growth (Hirschler-Laszkiewicz et al., 2009). As TRPC6 can be activated by growth factors and is also expressed in immature CD34+ cells, it is possible that this channel also plays a role in modulating calcium influx in MKs, and influences proliferation and cell growth. Recent research by our group in TRPC6-deficient mice showed that platelets lacking TRPC6 display normal activation responses to most platelet agonists in vitro and maintain normal haemostatic and thrombotic function in vivo (Ramanathan et al., 2012). In addition, these mice exhibit normal platelet count and size, indicating that this channel protein is dispensable for the development of MKs and release of platelets in mice. In contrast, several studies with washed human platelets illustrate a compelling role for TRPC6 in calcium influx and platelet function (Jardin et al., 2009; Harper and Sage, 2010; Dionisio et al., 2011).
In this study, we evaluated whether TRPC6 is of importance for the proliferation and development of human MKs. We could observe an increasing expression of TRPC6 transcripts and TRPC6 protein synthesis during the development of MKs, and we found that calcium influx is mediated by this channel in human MKs.
Materials and methods
Materials
Acetoxymethyl esters of Fluo-4 and Fura Red were from Invitrogen/Molecular Probes (Lofer, Austria). Rabbit anti-TRPC6 antibody was from Alomone labs, Jerusalem, Israel. Flufenamic acid (FFA) was obtained from Sigma. SKF96365. HCl was purchased from Calbiochem. All cytokines were purchased from Miltenyi Biotec.
Cell culture
Following informed consent umbilical cord blood (CB) was collected from healthy newborns and was used within 4 h after delivery. Mononuclear blood cells were separated by centrifugation on a layer of Ficoll-Paque Leucosep (Greiner Bio-One GmbH, Austria). Human CD34+ stem cells were isolated using the CD34 microbead isolation kit from Miltenyi Biotec (Germany) according to the manufacturer’s instructions. The CD34+ cells were cultured in serum-free medium (Stempro34, Invitrogen) containing interleukin-3 (IL-3) and stem cell factor (SCF) at 1 ng/mL and thrombopoietin (TPO) at 50 ng/mL, with medium changes twice weekly. After the first 6 days of culture, the growth medium was supplemented with only TPO at 50 ng/mL. The study was approved by the Ethics Committee of the Medical University of Vienna, Austria.
Flow cytometry
Cells were re-suspended in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and 2 mM EDTA. Then, they were stained with FITC-conjugated anti-human CD41a and CD61 (eBioscience), allophycocyanin-conjugated anti-human CD42b (Becton Dickinson) or isotype-matched IgGs (negative controls). Data acquisition was performed on a FACSCalibur (Becton Dickinson) and analysed using FlowJo (TreeStar).
Immunofluorescence
Cultured cells were centrifuged onto adhesive microscopic glass slides and fixed with 4% paraformaldehyde. Following permeabilisation by treatment with 0.1% Triton X-100 and blocking with 3% BSA in PBS, cells were incubated with FITC-conjugated anti-CD61 and rabbit anti-TRPC6 (Alomone Labs). Labelling with TRPC6 was followed by incubation with AlexaFluor 555 (Molecular Probes). Cells were mounted using ProlongGold anti-fade reagent containing 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes). Images were acquired and analysed using the Carl Zeiss LSM 700 laser scanning microscope.
Quantitative real-time PCR
Total RNA was isolated from CB-MKs using the RNeasy Mini Kit (QIAGEN). Up to 1 μg of total RNA was reverse transcribed using MultiScribe reverse transcriptase (Applied Biosystems). The first-strand cDNA was used as a template in quantitative PCR (qPCR), using the EvaGreen Master Mix (Bio-rad). The cycling programme was set as follows: denaturation at 95°C for 3 min, followed by 45 cycles of 95°C for 10 s and 55°C for 45 s. Primers used for qPCR of TRPC ion channels were spanning at least one intron. The Abelson (Abl) and the GAPDH genes were used as internal controls. Primer sequences are listed in Table 1.
Table 1. Primer sequences used in qPCR.
| Gene | Primers | |
|---|---|---|
| hTRPC1 | Fwd 5′–3′ | TGC AGC TTC TTT TGG ACT ACG |
| Rev 5′–3′ | CGA TGA GCA GCT AAA ATG ACA | |
| hTRPC3 | Fwd 5′–3′ | GAT CGC ACC TTG CAG CAG GC |
| Rev 5′–3′ | AGG CAT TGA ACA CAA GCA GAC CC | |
| hTRPC6 | Fwd 5′–3′ | AAA CGC TCC AGA GTG GTG AT |
| Rev 5′–3′ | GGA GAG AAG TTG CTG TTG GC | |
| hTRPC7 | Fwd 5′–3′ | ACA AGT GGT GGC CTT CAG AC |
| Rev 5′–3′ | CGT TGG CTG GCA GAA TGT AT | |
| hABL1 | Fwd 5′–3′ | TGT ATG ATT TTG TGG CCA GTG GAG |
| Rev 5′–3′ | GCC TAA GAC CCG GAG CTT TTC A | |
| hGAPDH | Fwd 5′–3′ | TCA AGG GCA TCC TGG GCT ACA CTG AG |
| Rev 5′–3′ | TGA CAA AGT GGT CGT TGA GGG CAA TG |
Measurement of intracellular-free calcium
Free cytosolic calcium in CB-MKs was measured by time-dependent flow cytometry. Aliquots containing 2 × 105 MK cells cultured for 12 days were washed and incubated with acetoxymethylester-derivatives of Fluo-4 (1 μM) and Fura Red (2 μM) at 37°C for 30 min in calcium-free buffer. Following removal of unbound fluorescent dyes, cells were re-suspended in buffer containing calcium at 1.8 mM and basal calcium levels were determined. Then, agonists and inhibitors were added and data acquisition was continued. Fluo-4 signals were acquired in the FL-1 channel and Fura Red in the FL-3 channel. Fluorescence ratio and kinetics of calcium influx analysis was performed using FlowJo (TreeStar).
Cell proliferation assay
Ten days old MK cells were seeded at 2 × 104 cells per well in a 96-well plate. Cells were treated with SKF96365 and incubated at 37°C for 48 h. After 44 h of incubation, MTS (Promega) was added, and absorbance was measured at 490 nm.
Statistical analysis
Results from at least three experiments are presented as mean ± SD. Statistical differences were assessed by unpaired 2-tailed Student t-test. P-values <0.05 were considered statistically significant.
Results
Development of MKs from haematopoietic progenitors
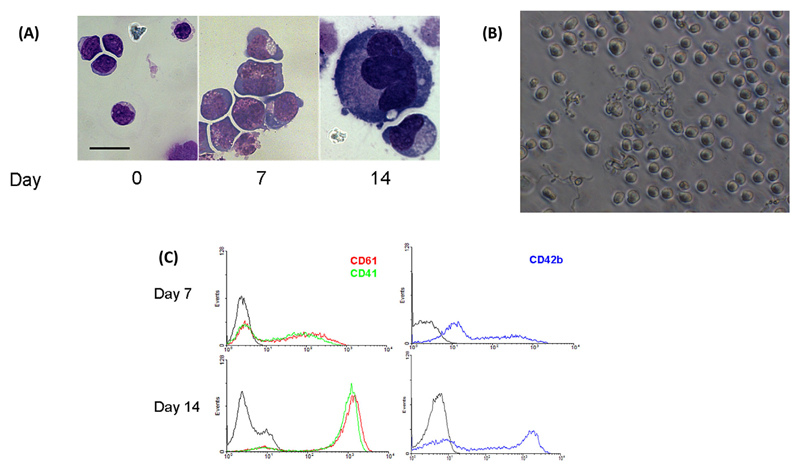
Following cultivation in serum-free media in the presence of 50 ng/mL TPO, CD34+ haematopoietic progenitor cells presented as undifferentiated mononuclear MK precursors with little but highly basophilic cytoplasm (Figure 1A) on day 7. Upon further cultivation until day 14, cells matured and increased in cell size, and large cells with increased cytoplasmic volume exhibiting two or more nuclear lobes could be observed. Between days 12 and 14, cells displayed cytoplasmic extensions, which resembled proplatelet formation (Figure 1B). The average number of cells with visible proplatelet extensions in a given visual field, excluding pseudopod-like structures, was 17 ± 4.2%. To confirm differentiation to megakaryocytic cells, the expression of surface glycoproteins was determined (Figure 1C). On day 7, flow cytometry showed 56.1 ± 12% cells positive for CD61 and 52.8 ± 13.8% cells positive for CD41a (mean ± SEM of three independent experiments). After 14 days, 95.8 ± 1.7% cells were CD61 and 94.6 ± 1.4% cells were CD41 positive. CD42b was expressed on 61.0 ± 9.2% and 91.4 ± 2.2% cells on days 7 and 14, respectively.
Figure 1. Development and characterisation of megakaryocytes.
(A) Morphology of MKs derived from cord blood CD34+ cells observed at different days by light microscopy after May-Grunwald staining. Scale bar represents 20 μM. (B) Proplatelet forming cells on day 12 of culture. (C) Surface marker expression in developing MKs stained with anti-CD61 (red), CD41 (green) and CD42b (blue) human antibodies on days 7 and 14 of culture. Grey line represents isotype control.
mRNA and protein expression of TRPC channels in developing MKs
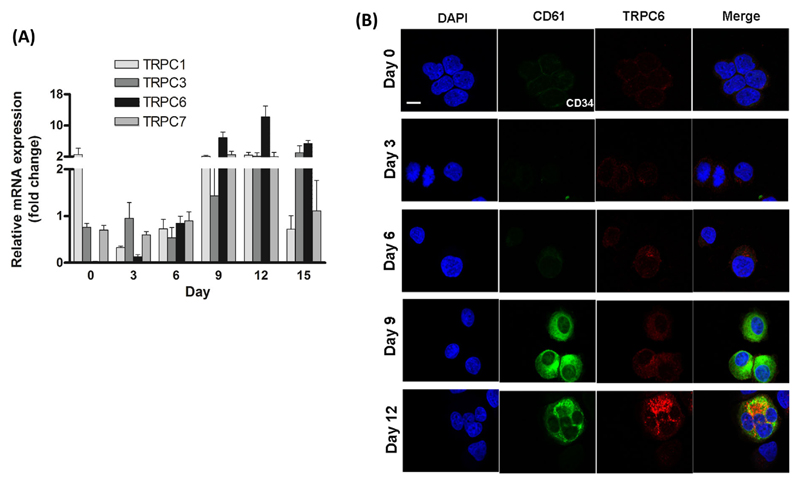
We observed the expression of transcripts of the TRPC family in developing MKs by qPCR (shown in Figure 2A). Compared to CD34+ cells on day 0, transcript expression of TRPC6 increased 250- to 300-fold, of TRPC1 1.6- to 1.8-fold, of TRPC3 6.2- to 8.9-fold and of TRPC7 7.7 to 8.3-fold after 12 days. Although TRPC1, TRPC3 and TRPC7 levels raised relatively quickly and were higher than those of TRPC6 on day 3, mRNA levels of TRPC6 increased significantly in the later phase of MK maturation. Interestingly, we could observe small amounts of TRPC6 protein already in immature MKs by confocal microscopy. This indicates an early but low expression of TRPC6 in cultures. The protein concentration increased upon MK maturation (Figure 2).
Figure 2. Expression of TRPC6 in developing MKs.
(A) Relative mRNA expression profile of TRPC transcripts on days 0, 3, 6, 9, 12 and 15 as determined by qPCR. Gene expression relative to the housekeeping gene Abelson (Abl) was determined using the comparative Δ Ct method, mean ± SEM, n = 3. (B) Confocal microscopy of TRPC6 protein in human MKs determined on days 0, 3, 6, 9 and 12. Cells were stained with DAPI (blue), CD61 (green) and TRPC6 (red). Scale bar represents 10 μM. Images are representative of four independent cultures.
Calcium entry and proliferation of MKs
We investigated calcium entry from the extracellular medium by time-dependent flow cytometry using the calcium-binding fluorescent dyes Fluo-4 and Fura Red and FFA. FFA directly and specifically activates TRPC6 while inhibiting other non-selective TRPC channels and has been used as a pharmacological tool to investigate Ca2+ signalling via TRPC6 channels (Foster et al., 2009). The ratio of fluorescence signals indicates changes in intracellular calcium ([Ca2+]i).
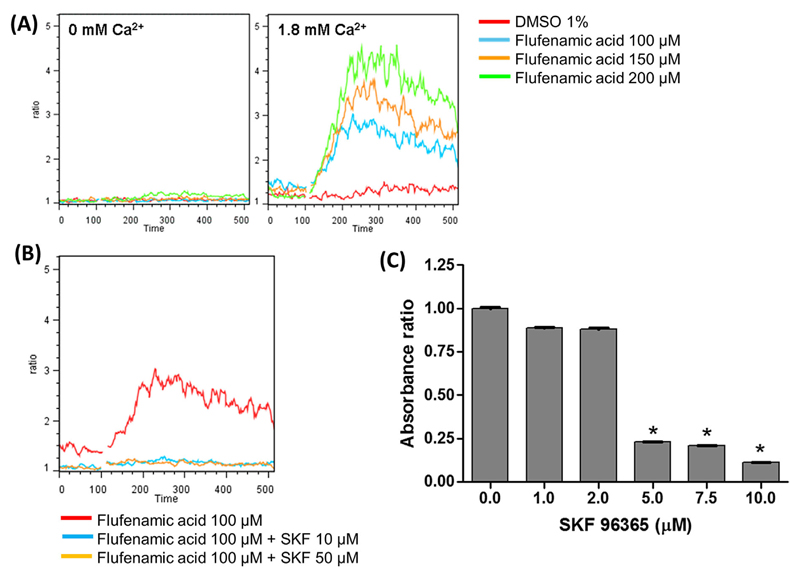
In a calcium-free buffer (0 mM Ca2+), FFA did not induce a change in [Ca2+]i (Figure 3A, left) indicating that FFA does not activate Ca2+ release from intracellular stores and does not participate in store-operated calcium entry (SOCE). Even at 200 μM concentration, FFA did not induce a Ca2+ release from intracellular stores. In contrast, in the presence of external Ca2+ (1.8 mM Ca2+), activation of cells by FFA resulted in a dose-dependent increase in intracellular Ca2+ (Figure 3A, right). This suggests that the response to FFA is due to non-store-operated calcium entry (non-SOCE) mediated by the non-selective TRPC6 cation channel.
Figure 3. Induction of Ca2+ influx in MKs and effect of SKF96365 on MK cell proliferation.
(A) Ca2+ influx was determined by time-dependent flow cytometry using flufenamic acid (FFA) at different concentrations in day 12 MKs in Ca2+-free buffer (0 mM Ca2+), left, and in the presence of external Ca2+ (1.8 mM Ca2+), right. The change in fluorescence signals of the Ca2+-binding dyes, Fluo-4 and Fura Red was acquired in the FL-1 and FL-3 channels, respectively, and the ratio (FL-1/FL-3) is presented as a function of time. The curves are representative of three independent experiments. (B) MK cells at day 12 were pre-incubated with SKF96365 at 10 and 50 μM for 3 min and then activated with 100 μM FFA in the presence of external Ca2+. (C) MK cells on day 10 were cultured for 48 h in the presence of 50 ng/mL TPO with increasing concentrations of SKF96365. Cell proliferation was determined using the MTT assay. Data shown are the mean ± SD of triplicate measurements compared to the control, 0 μM SKF96365. * P-value < 0.05.
To confirm that the Ca2+ flux mediated by FFA occurred via a member of the TRPC family, we used SKF96365, a synthetic organic broad range inhibitor that interferes with second-messenger-activated and store-operated Ca2+ channels and blocks TRPC channels. When cells were preincubated with SKF96365 at 10 and 50 μM concentration before the addition of FFA, the increase in fluorescence was significantly reduced (Figure 3B), demonstrating that FFA activates one or more non-selective TRPC channels. The treatment of proliferating MKs with SKF96365 had a noticeable physiologic effect. It reduced the proliferation of MKs in a dose-dependent manner, 50% reduction in cell numbers at 5 μM and higher concentrations, suggesting that TRPC channels could be involved in MK proliferation (Figure 3C). Interestingly, CD61+ cells separated from the same cultures were not affected by SKF96365 in the concentration range of 1–12.5 μM, whereas CD61-negative cells, representing immature, proliferating MKs showed a continuous reduction of cell numbers with increasing concentrations of SKF96365 (data not shown). These results indicate that SKF cytotoxicity is minimal in the concentration range that we used in the assay and the results are indicative of reduced cell proliferation.
Discussion
CD34+ cells isolated from human umbilical CB and cultured in the presence of TPO have been successfully used to study megakaryopoiesis in vitro. The two-step culture method in this study with the removal of IL-3 and SCF after the first week of culture and supplementation with TPO alone for the remaining culture period resulted in several fold increase in megakaryocyte numbers as also reported by others (Majka et al., 2001; Sun et al., 2004). Recent studies have shown that polyploidy and cytoplasmic maturation are independent processes and CB-derived MKs are capable of reaching full MK maturation (Liu et al., 2011). Even though the CB-derived MKs showed low DNA content with small size indicating primitive MK progenitors, the cells displayed all surface phenotype characteristics of mature MKs as expected from previous observations (Mattia et al., 2002).
It has been shown that murine and human MKs and platelets contain TRPC mRNA (den Dekker et al., 2001b; Carter et al., 2006). The expression of TRPC1, TRPC3, TRPC6 and TRPC7 mRNAs varied between immature and mature MKs depending on the exposure to TPO. These results suggest that TRPC channels may be involved in initiating and maintaining thrombopoietin-induced Ca2+-mediated differentiation. We observed during the first incubation period a modest decrease in mRNA abundance for TRPC1, which then gradually increased and maintained a constant mRNA level throughout the differentiation period. TRPC3 and -7 displayed similar changes in their mRNA expression pattern throughout the MK differentiation process. Only TRPC6 showed significant changes in mRNA levels on days 6, 9, 12 and 15 compared to baseline expression on day 0 (P < 0.01). TRPC6 was abundant in proliferating MKs, whereas its expression was very low during the early stages of commitment and differentiation. We found higher levels of TRPC6 in CB-derived MKs compared to other members of the TRPC family (den Dekker et al., 2001b; Carter et al., 2006; Lim et al., 2008). To our knowledge, this is the first time that TRPC6 mRNA has been quantified in differentiating MKs. The presented confocal images corroborated the mRNA results for TRPC6 expression. TRPC6 protein initially showed low expression, which impressively increased from days 6 to 9 and maintained high-expression levels until the late stages of differentiation. Thus, the mRNA and protein expression for TRPC6 coincides, demonstrating that TRPC6 is increased in a timely manner in developing MKs. Our findings may be important as TRPC6 could represent a reliable marker for TPO-induced human MK differentiation.
Our finding of TRPC3 mRNA in primary human MKs is new and may be important. Until now, only the megakaryocytic cell lines MEG01, Dami and HEL have been shown to express TRPC3 (Berg et al., 1997; den Dekker et al., 2001b), while TRPC3 protein expression in human platelets has been reported (Zbidi et al., 2009). Our observation of increasing concentrations of TRPC3 transcripts in developing human MKs corroborates and extends the reported data. We observed that TRPC7 expression followed a similar profile as TRPC3 in developing MKs. Numaga et al. described TRPC7 to be constitutively active and susceptible to negative regulation by extracellular Ca2+. The authors speculated that the physiological importance of TRPC7 in a native environment may lie in Ca2+ signalling (Numaga et al., 2007). However, until now, the exact role of the TRPC7 protein and its function in platelets remains elusive. Although we did not observe TRPC4 mRNA expression in our cells culture system, the protein has been detected by immunoblotting (Wakabayashi et al., 2006) and immuno-fluorescence (Liu et al., 2008) in human platelets. Hassock et al. (2002) observed only TRPC1 and six proteins in platelet membrane lysates, whereas other studies have reported the presence of TRPC3, 4 and 5 (Wakabayashi et al., 2006; Liu et al., 2008; Zbidi et al., 2009) protein expression in human platelets. These differences maybe be due to the different antibodies used by each group and the level of expression of the proteins in platelets.
Interestingly, a strong increase of the mRNA levels during further development of the MKs is only observed for TRPC6. We hypothesise that this upregulation of TRPC6 in the mature MKs occurs to enable Ca2+ signalling in human platelets. Indeed, a dual role for TRPC6 has been described. Although the assembly of TRPC6 and TRPC3 in resting and diacylglycerol (DAG)-activated platelets allows non-SOCE, the interaction of TRPC6 with the Orai1-STIM1 complex TRPC6 seems to play a role in SOCE (Jardin et al., 2009).
We could show that in human, MKs FFA does not induce store-release and does not contribute to SOCE. From our data, we can conclude that the Ca2+ influx seen in MKs in the presence of FFA is through non-selective cation channels, possibly mainly due to TRPC6. The significant reduction of MK proliferation by SKF96365 that we observed suggests a possible involvement of TRPC channels in TPO-induced MK progenitor proliferation. It is reported that TPO is capable of inducing Ca2+ entry in stem cells and (im) mature MKs (den Dekker et al., 2001a) distinct from SOCE currents. It has been found upregulated in PDGF-stimulated pulmonary vascular smooth muscle cell proliferation (Yu et al., 2003). Deregulated TRPC6 expression resulting in cell cycle progression and cancer development has also been described in oesophageal carcinoma (Ding et al., 2010a) and glioma (Ding et al., 2010b). These findings indicate an important role for TRPC6 in cell proliferation of different cell types supporting our observations in MKs.
Proplatelet formation in vivo is an essential process for platelet release and maintenance of blood platelet numbers. MK-specific ADP-mediated GPCR signalling leading to intracellular calcium release and calcium influx was shown to be important for proplatelet formation in vitro (Di Buduo et al., 2014). Orai1 and Stim1 mediate SOCE in platelets but the Orai1 and Stim1 knockout mice display normal peripheral platelet counts (Grosse et al., 2007; Braun et al., 2009). TRPC1 and TRPC6 knockout mice also have normal platelet numbers (Varga-Szabo et al., 2008; Ramanathan et al., 2012). However, as human and murine platelets express different isoforms of Orai, Stim and TRPC, a single knockdown is unexpected to affect all MK and platelet functions. In this regard, platelet counts in the Orai1−/−, Trpc6−/− double knockout mice were also not affected (Chen et al., 2014) but these mice show reduced intracellular Ca2+ store content, whereas platelets lacking only Orai1 or TRPC6 had normal Ca2+ levels in their stores. This finding suggests a combined effort of SOCE and non-SOCE channels in regulating MK and platelet calcium homeostasis for a variety of functions. We did not study proplatelet formation in our cultures in the presence of SKF96365 in detail. As the ECM component plays an essential role in MK adhesion and proplatelet formation, it is possible that in this context, pharmacological inhibitors of calcium signalling may lead to defective proplatelet formation.
We have demonstrated that TRPC6 exclusively mediates DAG-induced Ca2+ entry in murine platelets (Ramanathan et al., 2012). We found a defective DAG-induced Ca2+ entry but intact store-operated calcium signalling in TRPC6−/− platelets. The absence of TRPC6 did not affect in vitro functional assays. In addition, TRPC6−/− mice had normal platelet numbers and platelet size. Thus, in mice, TRPC6 apparently does not influence the development of murine MKs. A study of platelet function in TRPC6-deficient mice performed by the group of Paez Espinosa at the same time revealed a role for TRPC6 in haemostasis and thrombus formation (Paez Espinosa et al., 2012). The authors reported that TRPC6−/− mice displayed prolonged bleeding time and delayed vessel occlusion following FeCl3-induced injury of the carotid artery compared to wild-type littermates. The controversial results of this study and ours could be possibly due to technical differences such as the type of anaesthetic used for the tail bleeding experiment and the strength of the stimulus during occlusive thrombus formation. Harper et al. (2013) provided some insights into the role of TRPC channels in platelet physiology. Their study demonstrates that platelet activation by thrombin in combination with collagen results in sustained Ca2+ signalling involving the store-independent TRPC3 and TRPC6 channels. The increased levels of [Ca2+]i result in high phosphatidylserine (PS) exposure on the platelets supporting thrombus formation. In the absence of TRPC6, PS exposure on platelets and thrombus formation may be impaired in specific in vivo circumstances.
In conclusion, we suggest that TRPC6 is important for MKs during early development. The time-dependent increase in TRPC6 expression is maybe a useful and reliable marker during TPO-induced human MK differentiation. The impaired proliferation of MKs upon inhibition of TRPC channels suggests their possible participation in the proliferation of human MK progenitors.
Acknowledgements and funding
All confocal images were taken at the Core Facility Imaging at the Medical University of Vienna. This work was supported by the Austrian Science Fund (FWF), the Cell Communication in Health and Disease (CCHD) PhD programme at the Medical University of Vienna and a grant from the Society of Thrombosis and Haemostasis Research (GTH).
Abbreviations
- TRPC6
transient receptor potential canonical 6
- MK
megakaryocyte
- FFA
flufenamic acid
- Ca2+
calcium
- SOCE
store-operated calcium entry
- non-SOCE
non-store-operated calcium entry
- DAG
diacylglycerol
References
- Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23(2):297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg LP, Shamsher MK, El-Daher SS, Kakkar VV, Authi KS. Expression of human TRPC genes in the megakaryocytic cell lines MEG01, DAMI and HEL. FEBS Lett. 1997;403(1):83–6. doi: 10.1016/s0014-5793(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, Bosl M, Stoll G, Nieswandt B. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2009;113(9):2056–63. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]
- Carter RN, Tolhurst G, Walmsley G, Vizuete-Forster M, Miller N, Mahaut-Smith MP. Molecular and electrophysiological characterization of transient receptor potential ion channels in the primary murine megakaryocyte. J Physiol. 2006;576(Pt 1):151–62. doi: 10.1113/jphysiol.2006.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Thielmann I, Gupta S, Subramanian H, Stegner D, van Kruchten R, Dietrich A, Gambaryan S, Heemskerk JW, Hermanns HM, Nieswandt B, et al. Orai1-induced store-operated Ca(2+) entry enhances phospholipase activity and modulates canonical transient receptor potential channel 6 function in murine platelets. J Thromb Haemost. 2014;12(4):528–39. doi: 10.1111/jth.12525. [DOI] [PubMed] [Google Scholar]
- den Dekker E, Gorter G, van der Vuurst H, Heemskerk JW, Akkerman JW. Biogenesis of G-protein mediated calcium signaling in human megakaryocytes. Thromb Haemost. 2001a;86(4):1106–13. [PubMed] [Google Scholar]
- den Dekker E, Molin DG, Breikers G, van Oerle R, Akkerman JW, van Eys GJ, Heemskerk JW. Expression of transient receptor potential mRNA isoforms and Ca(2+) influx in differentiating human stem cells and platelets. Biochim Biophys Acta. 2001b;1539(3):243–55. doi: 10.1016/s0167-4889(01)00112-4. [DOI] [PubMed] [Google Scholar]
- Di Buduo CA, Moccia F, Battiston M, De Marco L, Mazzucato M, Moratti R, Tanzi F, Balduini A. The importance of calcium in the regulation of megakaryocyte function. Haematologica. 2014;99(4):769–78. doi: 10.3324/haematol.2013.096859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, He Z, Shi Y, Wang Q, Wang Y. Targeting TRPC6 channels in oesophageal carcinoma growth. Expert Opin Ther Targets. 2010a;14(5):513–27. doi: 10.1517/14728221003733602. [DOI] [PubMed] [Google Scholar]
- Ding X, He Z, Zhou K, Cheng J, Yao H, Lu D, Cai R, Jin Y, Dong B, Xu Y, Wang Y. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J Natl Cancer Inst. 2010b;102(14):1052–68. doi: 10.1093/jnci/djq217. [DOI] [PubMed] [Google Scholar]
- Dionisio N, Albarran L, Berna-Erro A, Hernandez-Cruz JM, Salido GM, Rosado JA. Functional role of the calmodulin- and inositol 1,4,5-trisphosphate receptor-binding (CIRB) site of TRPC6 in human platelet activation. Cell Signal. 2011;23(11):1850–6. doi: 10.1016/j.cellsig.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Foster RR, Zadeh MA, Welsh GI, Satchell SC, Ye Y, Mathieson PW, Bates DO, Saleem MA. Flufenamic acid is a tool for investigating TRPC6-mediated calcium signalling in human conditionally immortalised podocytes and HEK293 cells. Cell Calcium. 2009;45(4):384–90. doi: 10.1016/j.ceca.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Grosse J, Braun A, Varga-Szabo D, Beyersdorf N, Schneider B, Zeitlmann L, Hanke P, Schropp P, Muhlstedt S, Zorn C, Huber M, et al. An EF hand mutation in Stim1 causes premature platelet activation and bleeding in mice. J Clin Invest. 2007;117(11):3540–50. doi: 10.1172/JCI32312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper MT, Sage SO. Src family tyrosine kinases activate thrombin-induced non-capacitative cation entry in human platelets. Platelets. 2010;21(6):445–50. doi: 10.3109/09537104.2010.483295. [DOI] [PubMed] [Google Scholar]
- Harper MT, Londono JE, Quick K, Londono JC, Flockerzi V, Philipp SE, Birnbaumer L, Freichel M, Poole AW. Transient receptor potential channels function as a coincidence signal detector mediating phosphatidylserine exposure. Sci Signal. 2013;6(281):ra50. doi: 10.1126/scisignal.2003701. [DOI] [PubMed] [Google Scholar]
- Hartwig J, Italiano J., Jr The birth of the platelet. J Thromb Haemost. 2003;1(7):1580–6. doi: 10.1046/j.1538-7836.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- Hassock SR, Zhu MX, Trost C, Flockerzi V, Authi KS. Expression and role of TRPC proteins in human platelets: evidence that TRPC6 forms the store-independent calcium entry channel. Blood. 2002;100(8):2801–11. doi: 10.1182/blood-2002-03-0723. [DOI] [PubMed] [Google Scholar]
- Hirschler-Laszkiewicz I, Tong Q, Conrad K, Zhang W, Flint WW, Barber AJ, Barber DL, Cheung JY, Miller BA. TRPC3 activation by erythropoietin is modulated by TRPC6. J Biol Chem. 2009;284(7):4567–81. doi: 10.1074/jbc.M804734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardin I, Gomez LJ, Salido GM, Rosado JA. Dynamic interaction of hTRPC6 with the Orai1-STIM1 complex or hTRPC3 mediates its role in capacitative or non-capacitative Ca(2+) entry pathways. Biochem J. 2009;420(2):267–76. doi: 10.1042/BJ20082179. [DOI] [PubMed] [Google Scholar]
- Lim CK, Hwang WY, Aw SE, Sun L. Study of gene expression profile during cord blood-associated megakaryopoiesis. Eur J Haematol. 2008;81(3):196–208. doi: 10.1111/j.1600-0609.2008.01104.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Maier A, Scholze A, Rauch U, Boltzen U, Zhao Z, Zhu Z, Tepel M. High glucose enhances transient receptor potential channel canonical type 6-dependent calcium influx in human platelets via phosphatidylinositol 3-kinase-dependent pathway. Arterioscler Thromb Vasc Biol. 2008;28(4):746–51. doi: 10.1161/ATVBAHA.108.162222. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Italiano J, Jr, Ferrer-Marin F, Gutti R, Bailey M, Poterjoy B, Rimsza L, Sola-Visner M. Developmental differences in megakaryocytopoiesis are associated with up-regulated TPO signaling through mTOR and elevated GATA-1 levels in neonatal megakaryocytes. Blood. 2011;117(15):4106–17. doi: 10.1182/blood-2010-07-293092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka M, Baj-Krzyworzeka M, Kijowski J, Reca R, Ratajczak J, Ratajczak MZ. In vitro expansion of human megakaryocytes as a tool for studying megakaryocytic development and function. Platelets. 2001;12(6):325–32. doi: 10.1080/09537100120068152. [DOI] [PubMed] [Google Scholar]
- Mattia G, Vulcano F, Milazzo L, Barca A, Macioce G, Giampaolo A, Hassan HJ. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood. 2002;99(3):888–97. doi: 10.1182/blood.v99.3.888. [DOI] [PubMed] [Google Scholar]
- Miller BA, Scaduto RC, Jr, Tillotson DL, Botti JJ, Cheung JY. Erythropoietin stimulates a rise in intracellular free calcium concentration in single early human erythroid precursors. J Clin Invest. 1988;82(1):309–15. doi: 10.1172/JCI113588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2(4):1313–23. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Numaga T, Wakamori M, Mori Y. Trpc7. Handb Exp Pharmacol. 2007;179:143–51. doi: 10.1007/978-3-540-34891-7_8. [DOI] [PubMed] [Google Scholar]
- Owsianik G, D’Hoedt D, Voets T, Nilius B. Structure-function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol. 2006;156:61–90. [PubMed] [Google Scholar]
- Paez Espinosa EV, Murad JP, Ting HJ, Khasawneh FT. Mouse transient receptor potential channel 6: role in hemostasis and thrombogenesis. Biochem Biophys Res Commun. 2012;417(2):853–6. doi: 10.1016/j.bbrc.2011.12.058. [DOI] [PubMed] [Google Scholar]
- Paez PM, Fulton D, Spreuer V, Handley V, Campagnoni AT. Modulation of canonical transient receptor potential channel 1 in the proliferation of oligodendrocyte precursor cells by the golli products of the myelin basic protein gene. J Neurosci. 2011;31(10):3625–37. doi: 10.1523/JNEUROSCI.4424-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Pang B, Park SJ, Lee YG, Bae JY, Park S, Kim I, Kim SJ. Identification and functional characterization of ion channels in CD34(+) hematopoietic stem cells from human peripheral blood. Mol Cells. 2011;32(2):181–8. doi: 10.1007/s10059-011-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115(12):3348–54. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan G, Gupta S, Thielmann I, Pleines I, Varga-Szabo D, May F, Mannhalter C, Dietrich A, Nieswandt B, Braun A. Defective diacylglycerol-induced Ca2+ entry but normal agonist-induced activation responses in TRPC6-deficient mouse platelets. J Thromb Haemost. 2012;10(3):419–29. doi: 10.1111/j.1538-7836.2011.04596.x. [DOI] [PubMed] [Google Scholar]
- Sun L, Tan P, Yap C, Hwang W, Koh LP, Lim CK, Aw SE. In vitro biological characteristics of human cord blood-derived megakaryocytes. Ann Acad Med Singapore. 2004;33(5):570–5. [PubMed] [Google Scholar]
- Torossian F, Bisson A, Vannier JP, Boyer O, Lamacz M. TRPC expression in mesenchymal stem cells. Cell Mol Biol Lett. 2010;15(4):600–10. doi: 10.2478/s11658-010-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga-Szabo D, Authi KS, Braun A, Bender M, Ambily A, Hassock SR, Gudermann T, Dietrich A, Nieswandt B. Store-operated Ca(2+) entry in platelets occurs independently of transient receptor potential (TRP) C1. Pflugers Arch. 2008;457(2):377–87. doi: 10.1007/s00424-008-0531-4. [DOI] [PubMed] [Google Scholar]
- Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7(7):1057–66. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi I, Marumo M, Graziani A, Poteser M, Groschner K. TRPC4 expression determines sensitivity of the platelet-type capacitative Ca2+ entry channel to intracellular alkalosis. Platelets. 2006;17(7):454–61. doi: 10.1080/09537100600757489. [DOI] [PubMed] [Google Scholar]
- Woo JS, Cho CH, Kim do H, Lee EH. TRPC3 cation channel plays an important role in proliferation and differentiation of skeletal muscle myoblasts. Exp Mol Med. 2010;42(9):614–27. doi: 10.3858/emm.2010.42.9.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003;284(2):C316–30. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- Zbidi H, Lopez JJ, Amor NB, Bartegi A, Salido GM, Rosado JA. Enhanced expression of STIM1/Orai1 and TRPC3 in platelets from patients with type 2 diabetes mellitus. Blood Cells Mol Dis. 2009;43(2):211–3. doi: 10.1016/j.bcmd.2009.04.005. [DOI] [PubMed] [Google Scholar]