Abstract
Neonatal sleep is a crucial state that involves endogenous driven brain activity, important for neuronal survival and guidance of brain networks. Sequential EEG-sleep analysis in preterm infants provides insights into functional brain integrity and can document deviations of the biologically pre-programmed process of sleep ontogenesis during the neonatal period.
Visual assessment of neonatal sleep-EEG, with integration of both cerebral and non-cerebral measures to better define neonatal state, is still considered the gold standard. Electrographic patterns evolve over time and are gradually time locked with behavioural characteristics which allow classification of quiet sleep and active sleep periods during the last 10 weeks of gestation. Near term age, the neonate expresses a short ultradian sleep cycle, with two distinct active and quiet sleep, as well as brief periods of transitional or indeterminate sleep. Qualitative assessment of neonatal sleep is however challenged by biological and environmental variables that influence the expression of EEG-sleep patterns and sleep organization. Developing normative EEG-sleep data with the aid of automated analytic methods, can further improve our understanding of extra-uterine brain development and state organization under stressful or pathological conditions. Based on those developmental biomarkers of normal and abnormal brain function, research can be conducted to support and optimise sleep in the NICU, with the ultimate goal to improve therapeutic interventions and neurodevelopmental outcome.
Keywords: Brain maturation, Sleep ontogenesis, Preterm neonate, EEG-monitoring, Sleep-wake cycle
1. Introduction
Intensive monitoring of the vulnerable preterm and critically ill term neonate, has been increasingly complemented with bed-side neuromonitoring to achieve optimal insight into neurological well-being [1–3]. Serial electro-encephalography (EEG) monitoring over time can document actual normal or altered brain function and provides insight into the progress of brain maturation during this period of intensive neonatal care [1,4,5], with the ultimate goal to improve therapeutic interventions and long-term neurodevelopmental outcome.
Neonates spend most of their time resting in the sleeping state. Previous research has highlighted the important role of neonatal sleep as a state that involves endogenous driven brain activity, crucial for neuronal survival and guidance of brain networks [6–9] and relate the impact of sleep on cognitive, psychomotor and behavioural development in both animal [10,11] as well as human studies [12–16]. Moreover, sleep ontogenesis is a specific, pre-programmed process of the maturing brain that manifests itself within a certain time window that begins in utero with rapid and major changes during the neonatal periods and infancy, and more subtle changes throughout childhood [11,17]. It's a complex and highly regulated neurologic function requiring the integration of different brain networks, influenced by the interplay between genetic endowment and environmental inputs, which allows for manifestations of neuroplasticity [6,8].
Qualitative assessment of neonatal sleep is therefore an essential and valuable measure of functional brain integrity [11,18], and recognition of sleep-wake states can be useful in the day-to-day monitoring, for assessing optimal periods for feeding and neonatal care, to support and optimise sleep in the NICU [19], as well as give insight in the sleep architecture of infants with sleep related problems (e.g. increased risk of sudden infant death syndrome, sleep apnoea).
This review discusses sleep in the preterm and term neonate and its relationship to brain development, measured with video-EEG polysomnography. We preface this with a short overview of monitoring tools and focus on the maturation of electrographic patterns. To summarize, we assess what is currently known regarding qualitative and quantitative sleep-EEG assessment in the neonatal period.
2. Sleep monitoring
2.1. Monitoring techniques
Conventional video-EEG-polysomnography studies are using a combination of behavioural and EEG characteristics for visual sleep-wake scoring to differentiate recognizable sleep EEG patterns in the preterm and full-term neonate, since neither of these characteristics alone are considered as gold standard [17,20,21]. To yield high quality EEG-polysomnography recordings and document specific temporal and regional electrographic changes in brain activity, a reduced 10–20 position with 9 electrodes is preferred (e.g. 2 frontal Fp1–Fp2 or F3–F4, 3 central C3–C4–Cz, 2 temporal T3–T4, 2 occipital O1–O2, reference and ground electrode). Non-cerebral physiological behaviours essential for sleep analysis are motility patterns, cardiorespiration and eye movements. Transcutaneous pO2/CO2 measurements are in general not applied, although major fluctuations can affect brain activity and can be considered in specific conditions [17,22]. The length of recording should cover at least a complete sleep cycle, with preferably a minimum of 3–4 h to take into account the ultradian cycle, however which remain still too restricted to accurately document the periodicity and stability of the developing circadian rhythm [23].
However, this might be too intrusive for the critical, sick extreme preterm infant. In this context, there is an increasing interest to distract sleep activity unobtrusively based on a variety of related techniques such as trend analysis of limited channel amplitude-EEG (aEEG) and heart rate variability [24–27].
2.2. Visual sleep scoring
Electrographic patterns are known to evolve over time, thereby reflecting the development of the brain cortex. It is well known that there are important differences in the development of the cerebral cortex if one compares the foetal and preterm brain [28]. Therefore, it is difficult to talk about a ‘normal’ ex utero brain development. However, assessment of serial multichannel EEG of preterm infants without major brain lesions who develop normally, is generally accepted as the closest approximation of normal functional brain maturation [29,30]. The postmenstrual age (PMA = gestational age at birth plus the weeks postpartum) should be known to assess functional brain maturity and the actual developmental age of the brain, which can be estimated within two weeks of the actual age for the preterm infant (e.g. < 37 weeks) and within one week for the full-term and post-term infant (respectively 37–44 weeks of PMA, and 44–48 weeks PMA). Larger deviations, are considered abnormal or ‘dysmature’ and if permanent, associated with increased risk of abnormal neurodevelopmental outcome [31–34].
Recently, the American Academy of Sleep Medicine renewed their recommendations for neonatal EEG sleep scoring in infants zero to two months of age [35]. In contrast to what has been reported by other experts in the field [11,17,29,30], they recommend only to score the EEG pattern as discontinuous or continuous rather than identifying EEG patterns according to different sleep states, due to the complexity of the EEG patterns and high variability in scoring among raters.
Studies which assess inter-rater agreement of visual EEG-sleep scoring in preterm and term neonates are scarce. Stefanski et al. [36] found an overall agreement of 87% for scoring EEG sleep patterns, whereas the agreement was 10% lower for coding behavioural patterns, and decreased with lower GA. In our own cohort of preterm infants (PMA 27–41 weeks), we found a Cohen's Kappa of 0.93 (95% CI: 0.90–0.95) for QS versus non-QS classified epochs, with the lowest Kappa value for 27–31 weeks PMA [37]. Nevertheless, scoring all different sleep states will be more challenging and induce higher disagreement when characterizing immature sleep patterns, since strict criteria for scoring sleep in preterm infants are lacking [38].
3. Development of neonatal sleep
3.1. Sleep EEG patterns according to age (Figs. 1–8)
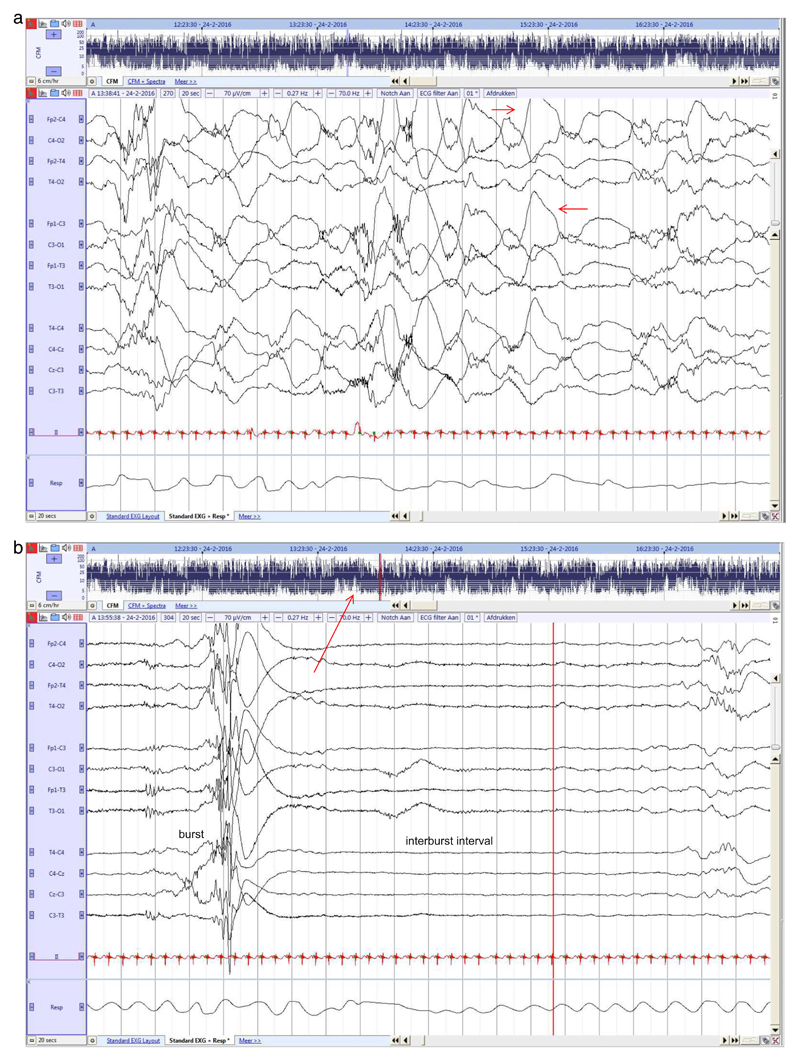
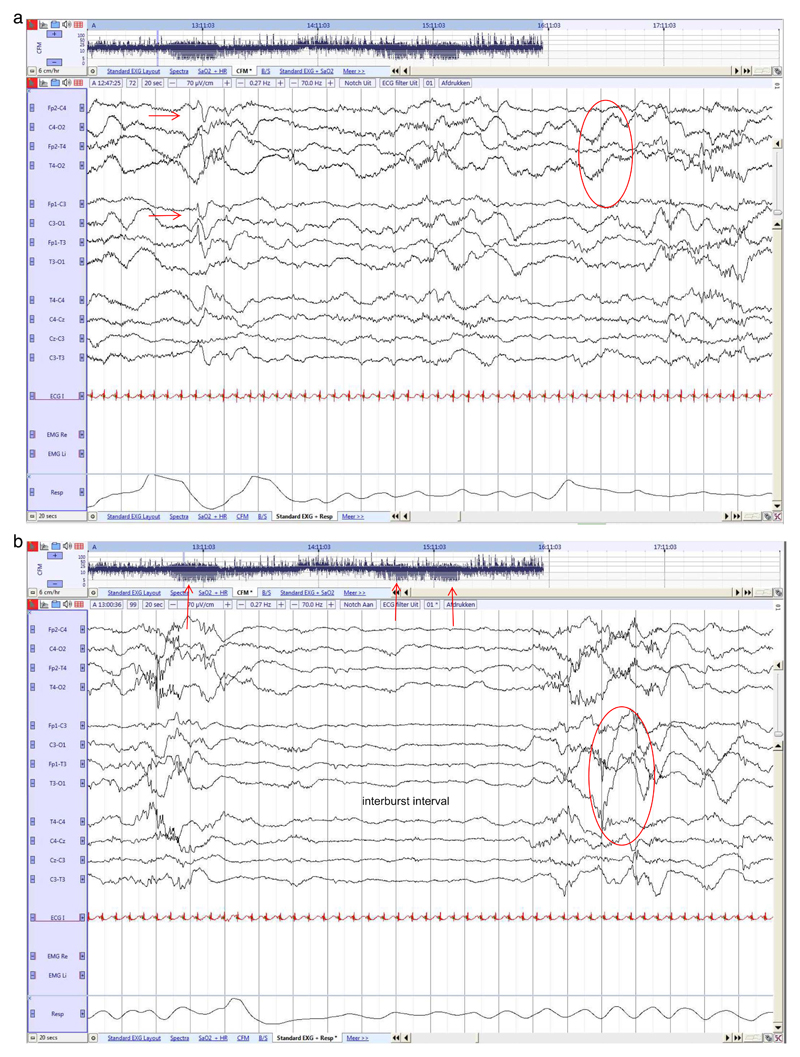
Fig. 1. Preterm neonate (25 2/7 weeks GA), recorded at 25 3/7 PMA.
a. Rudimentary state differentiation with more continuous and discontinuous EEG. High voltage central (red arrow) and temporal delta activity during more continuous EEG, lasting 20 s.
b. Period of high discontinuity, with long IBIs alternated with high amplitude burst activity. Fluctuation of rudimentary state can also be observed in the aEEG trends (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 8. Term neonate (41 2/7 weeks GA), recorded at 42 3/7 weeks PMA.
High voltage slow wave sleep at QS onset.
The development of different behavioural states in humans is species-specific and can be divided in quiet sleep (QS or non-REM sleep (NREM)), active sleep (AS or Rapid-Eye Movement (REM)), and wakefulness. These behavioural states are usually defined by a combination of behavioural, cardiorespiratory and EEG state specific criteria which emerge coherently over time, concurrent with the process of rapid brain maturation during foetal and early life [39].
Ultrasound observations have documented a rest-activity pattern in foetuses as young as 20–28 weeks' gestation [39], in line with Scher et al. and Vecchierini et al. who described rudimentary sleep states in the stabilized, extremely premature infant, as early as 24 weeks [40,41] (Fig. 1a & b). A more significant organization of sleep occurs from 28 to 29 weeks PMA [6,11]. Deeper brain nuclei modulate the first reflections of two distinct sleep stages in the cortical activity and the differentiation between ‘active’ and ‘quiet sleep’ based on different grades of EEG discontinuity, can be made. However, these differences in EEG characteristics are only subtle and in preterm infants < 30 weeks, there is still the greatest uncertainty in classifying sleep. Indeed, when both behavioural and EEG criteria are required for state definition, more immature infants will have increased ‘Indeterminate Sleep’ (IS). These segments are sleeping periods (eyes are closed) but in which behavioural and clinical EEG features do not correspond to a definite AS or QS segment, indicating the immaturity of the organization of each parameter to represent distinct sleep states in the very premature infant [11,42], resulting in an augmentation of IS and lower levels of AS or QS. Transitional sleep (TS) is seen as a temporary presentation of IS, usually during transitions between AS and QS, when typical criteria for a specific behavioural state are vague.
From 30 to 31 weeks onwards, physical parameters of rapid eye movement, body movement and respiration are gradually integrated and time-locked with more specific recognizable EEG patterns for QS and AS [11,17,29,43], with increasing concordance over age [20,44]. This give rise to a less chaotic and more predictable stable sleep wake organization, modulated by the developing afferent connections from the deeper brain nuclei (e.g. thalamo-cortical connections, hypothalamus, hippocampus) [11,43,45]. Rapid eye movements are the main characteristic features associated with AS and a (semi)-continuous background EEG pattern of mainly synchronous occipital delta activity, together with increased facial and head movements and irregular heart rate and respiration (Fig. 2a). The EEG background pattern during QS is highly discontinuous with bursts of high amplitude ≥ 3 s and periods of hypoactivity or electrographic quiescence (= interburst intervals or IBIs) ≤ 15–20s, associated with a more regular cardiorespiratory pattern and less body movements (Fig. 2b). From 32 weeks onwards, AS is defined as periods of continuous tracing with no discontinuity for ≥ 60s and < 30s of micro-arousal (Fig. 3a) and QS is defined as discontinuous tracing with bursts of activity ≥ 3 s, and periods IBIs of ≤ 15 s in all EEG channels [19,29], with specific spatial and temporal organization of EEG features (Fig. 3b). A further decrease in amplitude during AS (Fig. 4a) and decrease in IBI during QS is seen from 34 weeks (Fig. 4b) [4]. QS periods are defined as still discontinuous or semi-continuous in infants of 35–36 weeks GA and can be differentiated of AS with continuous tracing, episodes of REM and specific age-related transients. A detailed overview with a summary of cerebral and non-cerebral features for EEG sleep state scoring in preterm infants is presented in Table 1.
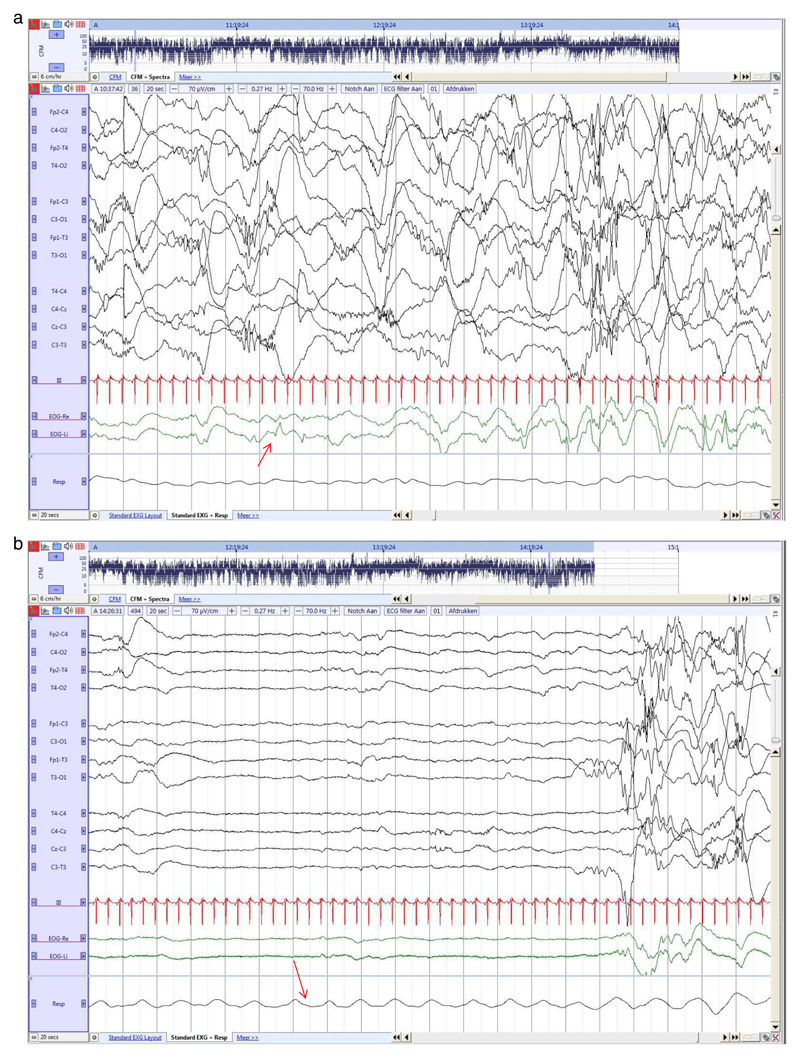
Fig. 2. Preterm neonate (26 3/7 weeks GA), recorded at 30 2/7 weeks PMA.
a. Continuous EEG activity associated with REM (red arrow).
b. Discontinuous EEG (tracé discontinu) during QS, no REM and more regular breathing (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
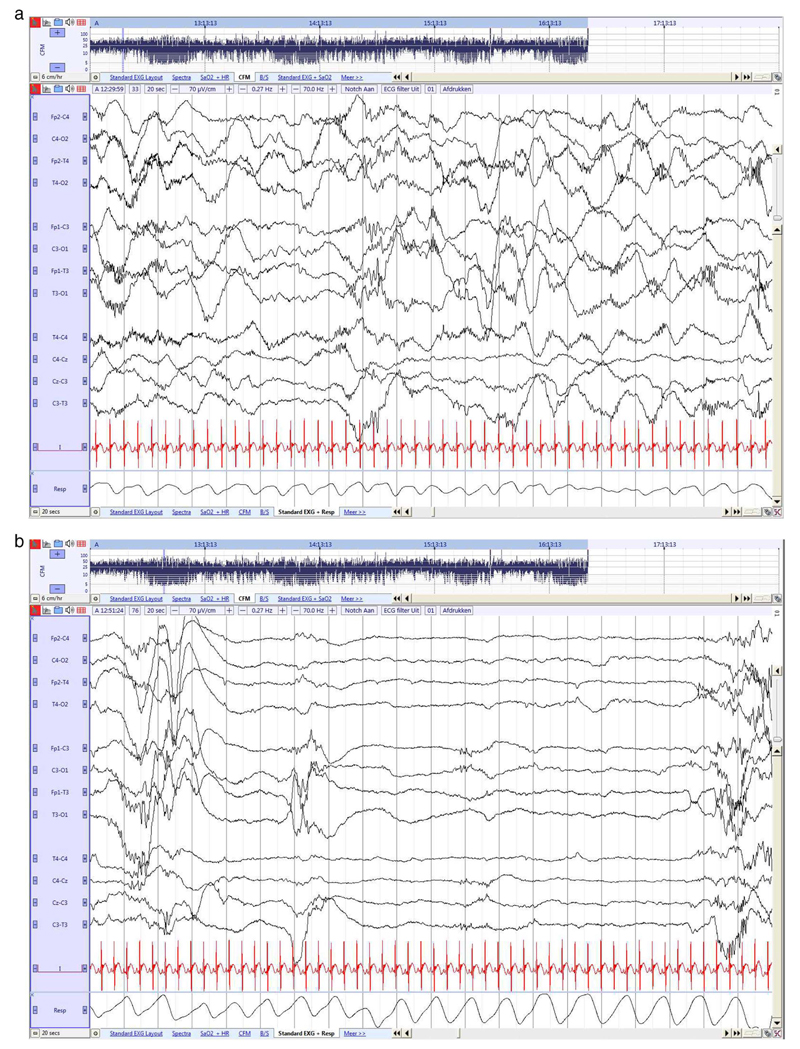
Fig. 3. Preterm neonate (30 4/7 weeks GA), recorded at 32 3/7 weeks PMA.
a. Continuous tracing during AS. Delta waves with superimposed fast rhythms (delta brushes) in both temporal and occipital brain regions. Irregular breathing pattern. Sleep state organization is now clearly visible in the aEEG trend.
b. Preterm neonate (30 4/7 weeks GA), recorded at 32 3/7 weeks PMA. Discontinuous tracing during QS, with IBI's ≤ 15 s. More regular breathing pattern. Temporal theta activity mainly during QS and occipital delta brushes.
Fig. 4. Preterm neonate (29 4/7 weeks GA), recorded at 34 4/7 weeks PMA.
a. Continuous tracing during AS. Clear decrease in amplitude. Delta waves with superimposed fast rhythms (delta brushes) in the occipital brain regions (red circle). Immature frontal transient (symmetrical) (red arrow). Irregular breathing pattern.
b. Tracé discontinu during QS, with IBI's ≤ 10s. More regular breathing pattern.
Temporal theta decreased during QS and occipital delta brushes (red circle). Three periods of tracé discontinu can be observed in the aEEG trend. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1. Summary of cerebral and non-cerebral features for sleep state scoring and sleep organization in preterm infants.
| GA in weeks | 24–26 | 27–28 | 29–30 | 31–32 | 33–34 | 35–36 |
|---|---|---|---|---|---|---|
| Organization of behavioural state1 | Rudimentary state with REM2 and No REM periods | AW: mainly artefacts AS with REM QS IS |
AW: mainly artefacts ↑3 AS with REM QS IS |
AW: mainly artefacts ↑ QW AS with REM QS IS |
AW: mainly artefacts QW: ↑ AS with REM QS IS ≥36: AS1-QS-AS2 |
|
| EEG background activity during sleep states | Inconsistently: Eye movements: less discontinuous, higher amplitude No eye movements: discontinuous, IBIs up to 30s, lower amplitude |
AS with REM: discontinuous or semi-continuous, QS –without REM: bursts of activity ≥3 s, IBIs< 20–30s |
AS with REM: (semi) continuous QS -without REM: bursts ≥3 s, IBIs ≤15–20s |
QW: continuous AS with REM: continuous QS - No REM: bursts ≥3 s, IBIs ≤10–15 s |
QW: continuous AS1 with REM: higher amplitude, continuous AS2 with REM: lower amplitude, faster frequency continuous activity QS-TD – without REM: (semi) discontinuous IBIs < 10s & amplitude 15-25 μV |
|
| State specific temporal and spatial organization | No state specific organization Monorhythmic alpha and theta activity in occipital regions | =STOP4 rhythm | Temporal theta in QS ↑ Synchronous delta numerous in AS Amplitude ↓ |
Delta brushes occipital and temporal Delta waves with theta numerous in AS Temporal theta (sawtooth) AS/QS |
Delta brushes occipital, ↓ in amplitude Temporal theta (sawtooth) mainly in QS and ↓ Immature frontal transients in AS |
Diffuse theta waves AS2 > AS1 Diffuse delta waves in QS, occipital and numerous in AS1 > AS2 Immature frontal transients in AS1 Onset of QS: asynchrony |
Non-cerebral features
|
AS: ± QS: − AS: irregular QS: irregular or more regular Not discriminant AS ~ face and body, segmental myoclonus or generalized myoclonic, tonic posturing / |
AS: + QS: − |
AS: + QS: − AS: variability CR rate QS: ↓ variability CR rate AS: + +, ↑ head & facial movements QS:↓ small and large body movements AS: − QS:±(not consistent, absent in ~20% of QS) |
AS: + QS: − AS: variability CR rate QS: ↓ variability CR rate AS: + + +, ↑ head & facial movements QS:↓ small and large body movements AS: − QS:±(not consistent, absent in ~20% of QS) |
AS: + + QS: − AS: variability CR rate QS: ↓ variability CR rate AS: + + +, ↑ head & facial movements QS:↓ smaller, segmental body movements AS: − QS:±(not consistent, absent in ~20% of QS) |
|
|
Cycle length (min) Range (mean ± SD) | ||||||
| [43] | > 27 weeks | 10–59.6 (39.7 ± 18.6) | 5–84.6 (30.0 ± 27.6) 9.3–71.3 (35.0 ± 17.2) |
|||
| [41] | 9–55 | 10–59 | 4–43 | |||
| [40] | > 25 weeks | 37–100 (68 ± 19-) |
< 30 weeks | |||
| [37] | > 27 weeks | 1 0.6–90.2 (32 ± 18) | 5.8–83 (31.6 ± 10.6) |
5.9–83.9 (39.5 ± 14.8) |
6.3–109 (50.9 ± 19.2) |
|
| Provoked EEG reactivity | No reactivity on EEG during gentle tactile stimulation Inconsistently and variable in expression |
→ → |
AS: transient decrease in EEG amplitude QS: transient continuous EEG |
AS: transient decrease in activity QS: appearance of continuous high amplitude slow wave activity |
||
State coded by the concordance of cerebral and non-cerebral features according to the gestational age. Adapted from: [29,9,41,17,30,35].
Abbreviations: AW= Active Wake, AS= Active Sleep, QS = Quiet Sleep, QW: Quiet Wake, IS: Indeterminate sleep, TST= Total Sleep Time, QS-TD: Quiet Sleep, Tracé Discontinu, IBI= Interburst Interval, CR: cardiorespiratory rate.
↑: increase, ↓: decrease, >: more.
STOP rhythm: spontaneous theta activity in the occipital regions in the premature neonate.
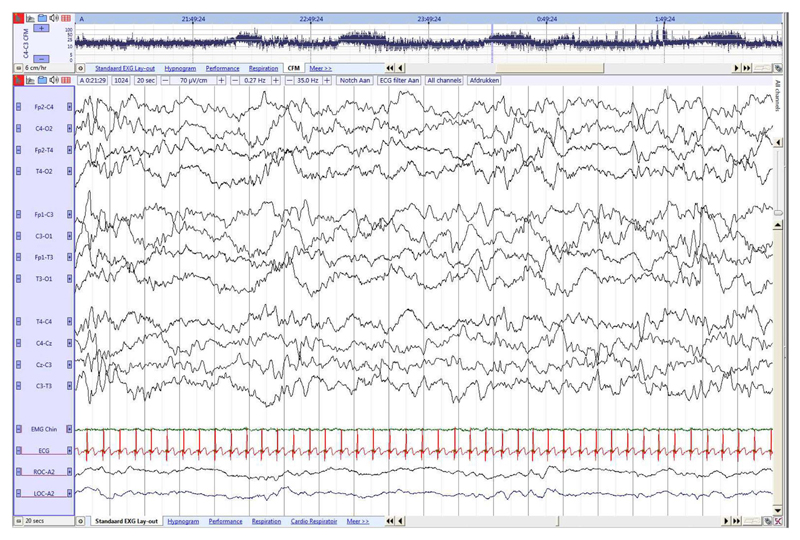
As more complex sleep stages follow the growth of major cortical afferent connections, the organization of the four neonatal sleep stages (two AS and two QS periods) and wakefulness are established after 36 weeks [17,46]. The background pattern during AS or REM is continuous with mixed (M) frequency activity as sleep onset AS 1 (Fig. 5), and a low voltage irregular (LVI) pattern AS 2 (Fig. 6) following QS [29,30,35]. Delta brushes are predominantly occipital in AS 1 whereas AS 2 contains more rapid, lower amplitude theta and alpha activity. From 37 to 38 weeks, a new sleep developmental trajectory during QS is expressed, with the emerging of ‘Tracé Alternant’ (TA) (Fig. 7), with the length of bursts equal to the length of IBIs, initiated by a brief ‘High Voltage Slow Wave’ (HVS, continuous diffuse high voltage delta with occipital and central predominance) QS pattern (Fig. 8). This leads to a globally more continuous EEG and the relative change in discontinuity between QS and non-QS states is only minor [37,47]. A regular respiratory rate and no REMs are the most consistent non-cerebral features associated with QS. Chin EMG activity, tonic between QS and low between AS, can be present however this parameter might be absent or unreliable to observe until 48–50 weeks GA [35].Table 2 summarizes the EEG and non-cerebral features used in literature to score sleep in (near) term neonates [17,29–31,48].
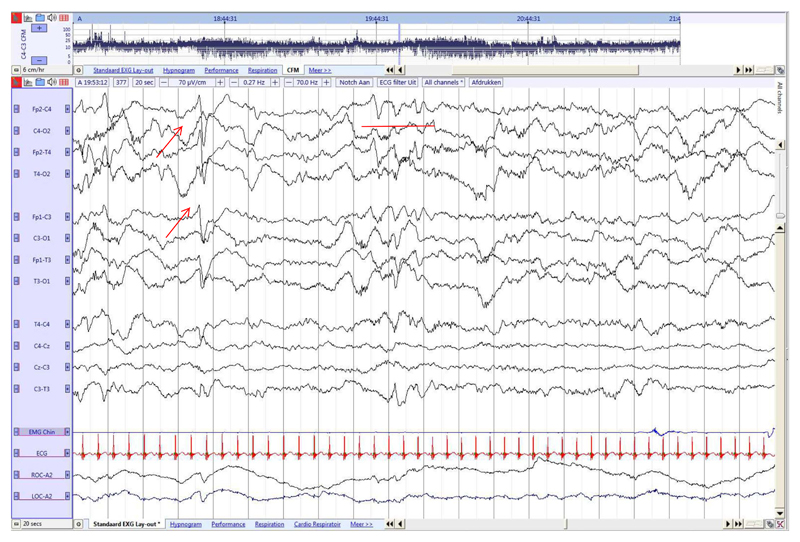
Fig. 5. Late term neonate (36 weeks GA), recorded at 38 6/7 weeks PMA.
Continuous activity with mixed frequencies during AS1 associated with REM, with higher amplitude and lower frequencies than during AS2. Frontal transients (red arrow) and slow anterior dysrhythmia in frontal regions (red line). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
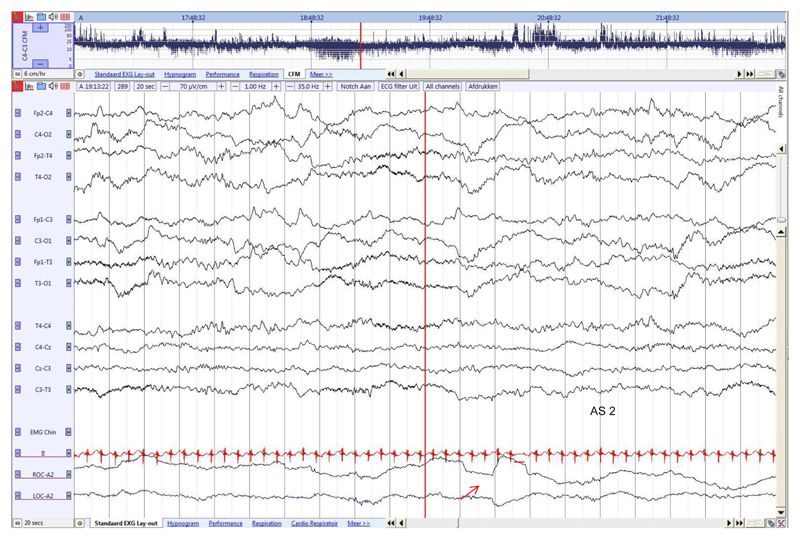
Fig. 6. Preterm neonate (29 4/7 weeks GA), recorded at 36 3/7 weeks PMA.
Continuous tracing with low voltage irregular pattern during AS2 and REM (red arrow). EMG was omitted from this figure due to technical artefact. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
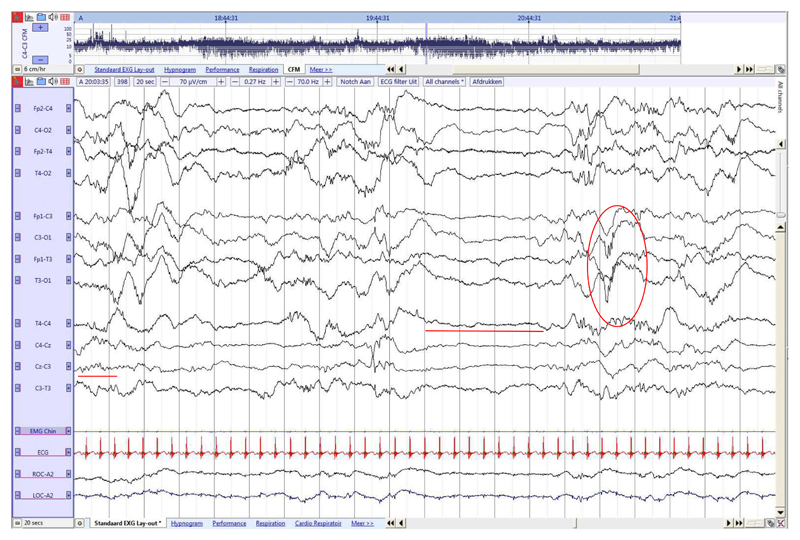
Fig. 7. Late term neonate (36 weeks GA), recorded at 38 6/7 weeks PMA.
Tracé alternant activity during QS. Length bursts = length IBIs, IBI amplitude ≥ 25 μV (red line). Delta brushes only in QS (red circle) and rolandic theta (red line). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2. Summary of cerebral and non-cerebral features for sleep state scoring and sleep organization in term neonates.
| GA in weeks | 37–38 | 39–40 | 41–44 | 43–48 |
|---|---|---|---|---|
| Organization of behavioural state1 | AW, QW, AS1, AS2, QS-HVS, QS-TA, IS, TS2 | |||
| Proportion of time spent in any state according to age based on average cycle length of 50–60 min | AS: 50–60% QS: 30–40% IS: 10–15% |
AS1: 25–35% QS-HVS: 3–5% QS-TA: 30% AS2: 15–25% IS: 10–15% |
AS1: 25–35% QS-HVS: 15–20% QS-TA: 15–20% AS2: 15–25% IS: 10–15% |
AS1: 25–35% QS-HVS ~ NREM: 45% AS2: 10–15% IS: 10–15% |
| EEG background activity during sleep states | AW/QW/AS2 low voltage irregular pattern AS1 mixed frequencies, higher amplitude QS-TA: length IBI = length bursts ~6 s, IBI amplitude ≥25 μV |
AW/QW/AS2 low voltage irregular pattern AS1 mixed frequencies, higher amplitude QS-HVS: high voltage, slow waves, occipital predominance QS-TA: length IBI = length bursts ~6 s, IBI amplitude > 25 μV |
AW/QW/AS2 low voltage irregular pattern AS1 mixed frequencies, higher amplitude QS-HVS: high voltage, slow waves, occipital predominance ↑ QS-TA:↓ |
AW/QW/AS2 low voltage irregular pattern AS1 mixed frequencies, higher amplitude QS-HVS: High voltage, slow waves |
| State specific temporal and spatial organization | Rolandic theta waves in AS2 > AS1 Delta brushes occipital in AS & QS Diffuse delta waves QS Frontal transient, slow anterior dysrhythmia AS 1, transition QS AS: synchronous Onset of QS: asynchrony |
Sharp rolandic alpha & heta bursts in QS Delta brushes occipital ↓, only in QS Frontal transient, slow anterior dysrhythmia AS 1, transition QS AS: synchronous QS: synchronous |
Sharp rolandic alpha & heta bursts in QS Delta brushes disappeared in all states Frontal transient, slow anterior dysrhythmia AS 1, transition QS AS: synchronous QS: synchronous |
QS-HVS: sporadic spindle like activity |
Non-cerebral features
|
AS: + + + QS: − AS: variability CR rate QS: ↓ variability CR rate AS: + + +, ↓ head & facial movements QS:↓ smaller, segmental body movements (10% of QS) AS: − QS: ± (absent in ~20% of QS) |
AS: + + + QS: − AS: variability CR rate QS: ↓ variability CR rate AS: + + +, ↓ head & facial movements QS:↓ smaller, segmental body movements (3% of QS) AS: − QS: ± (absent in ~20% of QS) |
AS: + + QS: − AS: variability CR rate QS: ↓ variability CR rate AS: + + +, ↓ head & facial movements QS:↓ smaller, segmental body movements (3% of QS) AS: − QS: ± (absent in ~20% of QS) ↓ |
|
State coded by the concordance of cerebral and non-cerebral features according to the gestational age. Adapted from: [35,9,17,29,30].
Abbreviations: AW =Active Wake, AS= Active Sleep, QS =Quiet Sleep, QW: Quiet Wake, IS: Indeterminate Sleep, TS: Transitional sleep HVS: High voltage slow wave sleep, TA: Tracé Alternant, REM =Rapid Eye Movements, IBI= Interburst Interval, CR: cardiorespiratory rate.
Significant re-organization of sleep state occurs after 48 weeks GA, with transition from neonatal to infant sleep and change in terminology from AS to REM and QS to NREM [35]. The TA pattern will disappear and will be replaced completely by HVS and midline central sleep spindle activity will appear during NREM. Sleep onset will be more likely in NREM [17,35].
EEG arousals, lasting for 3 s to 1 min, are characterized by a desynchronization or change in the EEG pattern, and are usually associated with muscle activity, body activity, alterations in the respiratory pattern, and/or eye opening. Longer events (> 1 min) are scored as ‘awakening’. Neonates are considered ‘asleep’ when eyes remain closed ≥3 min [19,35], however others define a behavioural state to be present when features are observed for > 1 min [30]. During wakefulness (W), eyes are open, with general body movements and high muscle tone, heart rate and respiratory pattern are irregular and a low voltage, irregular or mixed EEG pattern (LVI or M) with frequent movement artefacts is presented on EEG monitoring.
4. Qualitative assessment of neonatal sleep
4.1. Sleep state and sleep wake cycling across early brain development
A rough, rudimentary sleep wake cycle, based on alternating periods with and without eye movements related to EEG discontinuity, have been described in preterm infants of 24–30 weeks of GA (Table 1) [40,41]. In our own cohort with PMA between 27 and 31 weeks, we found a slightly shorter cycle duration between two successive QS periods based on quantitative analysis (for detailed description of this study group see [37]). This is comparable to results of visual EEG-assessment of Curzi Dascalavoa et al., and coherent with the observation of a shorter cycle duration reported by Vecchierini et al. [44]. Nevertheless, there is still a notable wide range in cycle duration. Methodological differences in study conditions, impede exact comparison, however the presence of ‘alternating vigilance states’ in clinically stable preterms, is evident [49] and can also be captured in a time-compressed presentation with aEEG [24,25]. Although the fluctuation in aEEG trend is considered a surrogate marker for normal brain function and a delayed appearance of sleep cycles is also associated with developmental impairment [26,50,51], qualitative sleep state interpretation with aEEG in preterm infants < 30 weeks is more complicated due to higher amounts of IS [36,43]. For a more qualitative assessment of preterm sleep, the integration of both cerebral and noncerebral measures is required which can be observed in more detail with video-PSG monitoring.
Studies investigating sleep in utero and low risk premature infants found that the amount of AS predominates in the last 10 weeks of gestation [6,43,52,53]. Endogenous neuronal activation, occurring during AS, provides the growth of neural networks (e.g. neurosensory systems) in preterm and term infants that have limited waking experiences. Mirmiran et al. [11] investigated sleep behaviour in 96 low risk preterm infants < 30 weeks GA with sequential polygraphic and behavioural measurements and found a significant age effect for both the amount of QS and IS. There was a significant decrease in time spent in IS from 50% at 30–31 weeks to 20% at term age. This was accompanied by an increase of QS during this period of brain maturation when those brain areas are prepared for environmental stimuli. Percentages of QS and wakefulness will further rise up to 3 months of age, accompanied by a further decline of the time in AS (Table 3) [11,17,54]. In line with those findings, we found an increase in cycle length and mean QS time from 33 weeks PMA to term age in a group of 26 premature infants with GA < 32 weeks and normal neurodevelopmental outcome at 24 months who had sequential 4 h–EEG recordings over time (Table 1) [37]). At 33–34 weeks PMA, mean QS time was 15.1 ± 3 min (range 9–32 min), at 35–36 weeks PMA mean QS time was 19 ± 3.6 min (range 4–34 min), at 37–38 weeks PMA mean QS time was 18.8 ± 3.6 min (range 5–33 min) and at 38–40 weeks PMA mean QS time was 20.3 ± 3.3 min (range 7–37 min) which is comparable with the findings of Mirmiran et al. [11].
Table 3. Development of sleep states according to the gestational age in preterm and full-term neonates.
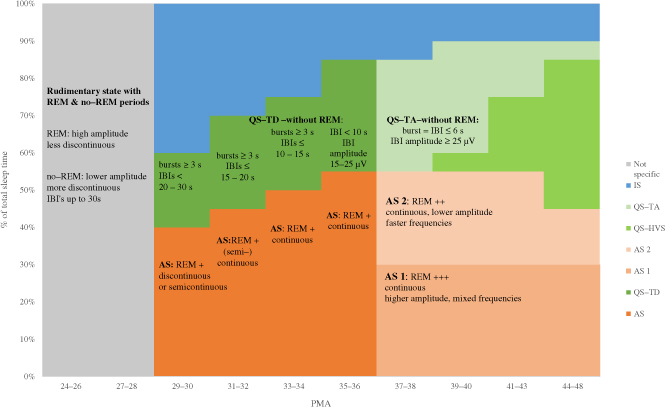 |
Near term age, the neonate expresses a clear ultradian sleep cycle across the day of 50–60 min (range 30–70 min), alternating with waking episodes in a 3–4 h cycle [17,35]. In contrast to older infants, sleep onset in neonates is predominantly with an AS segment (AS 1). An overview of the full-term sleep cycle is presented in Table 4. Only a small amount, 10–15% (10 min) of the total sleep time, is TS or IS in the healthy term infant [17,35] and this will be considered abnormal if abundant in the term neonate [30,42,55].
Table 4. Full-term sleep cycle average ± 50–60 min.
 |
4.2. Sleep quality
Interpretation of sleep states remains a significant methodological challenge depending on the selected variables, maturity of the infants (biological variability), severity of illness of the infant and different environmental conditions (e.g. kangaroo care, light and sound stimulus, prone position, length of recording), which can all influence the quality of sleep expression, and the time variation of the SWC [19,54,56–59].
Several studies have focused on AS and its role in early brain development, nevertheless the importance of QS and conservation of a qualitative sleep-wake cycle cannot be overstated. Sleep has a vital function and AS provides crucial input for the development of long-term circuitry, proper sleep cycles and QS/NREM sleep are essential for the preservation of brain plasticity and the consolidation of those processes [6,11]: the progression from QS to AS is important for memory processing, and hippocampal processing of external stimuli occurs during QS [60].
Moreover, it has been hypothesized that mainly QS may be more responsive to stress factors (e.g. environmental stress related to longer extrauterine life) compared to other states and that the brain adapts to those conditions with an altered expression of state organization [61]. Scher et al. have reported changes in sleep architecture with longer sleep cycles and more sustained QS periods in preterm infants compared to matched full-terms at the same PMA, suggesting an acceleration in their development [57,61,62]. Some diurnal differences in the expressions of EEG sleep-wake patterns, triggered by the developing circadian rhythm [63] and environmental influences were also documented by Biagioni et al. and others [11,64]. Few awakenings during the morning hours and more QS patterns during the day-time were found in the preterms whereas QS patterns were equally distributed in day and night-time near term age.
Skin-to skin-care (SSC) is associated with less arousals and an increased percentage of QS in preterm infants when also exposed to low ambient light levels, which may indicate better sleep conditions [19,65]. Welch et al. found that frontal brain activity (EEG power) increased both during QS and AS when preterm infants had SSC [66,67]. The beneficial effect of all those adaptations regarding better and more stable state organization looks promising and favours the major improvements that have been made in the developmental care interventions of those infants [68,69], but the prolonged benefits on long-term neurodevelopmental outcome, still needs to be confirmed.
In the neurologically compromised neonate, physiological parameters as well as the EEG background will undergo noticeable alterations (e.g. hypoxic ischemic encephalopathy, intraventricular haemorrhage, seizures and sedative medication). The EEG-defined sleep states may no longer meet the criteria previously defined [11,42,55] and different grades of background depression with alterations over the whole full-term sleep cycle can be observed. In maximal depression of brain function in neonates, sleep cycles completely disappear and state differentiation is no longer possible in the acute stage. The background pattern is severely suppressed with extreme low voltage activity or with no discernible cerebral activity. Heart rate variability is decreased. In marked depression, there is a burst-suppression pattern (an invariant and unreactive pattern of bursts of paroxysmal activity alternating with periods of marked voltage attenuation), and sleep cycles are also abolished. In moderate depression, the background is depressed with low voltage and discontinuous activity, and the state-EEG relation and sleep cycles are grossly disturbed. A discontinuous pattern can be seen in both QS and AS, although less so in the latter. HVS and M patterns are no longer seen [55].
In neonates with mild depression of brain function, sleep cycles and state-EEG relation are mildly disturbed but still can be identified. QS will be replaced by IS or a discontinuous pattern and HVS will disappear and AS will have more LVI patterns as mixed frequency. In minimal depression, some EEG background abnormalities will only be apparent in QS, making the EEG more discontinuous and markedly asynchronous, whereas sleep cycles and sleep state are maintained, making QS an intriguing clinical marker for assessing more subtle alterations in brain function [11,55,70]. Not only the severity of background suppression and related to this, the quality of sleep states and cycles, but also the timing of ‘re-appearance’ and ‘stability’ of sleep wake cycles have been correlated with long-term outcomes [31,42,55,71].
5. Automated sleep-EEG analysis
Automated detection of sleep periods and quantification of sleep may document alterations in cortical function during extra-uterine brain development and accelerate the more subjective visual assessment.
Detection of sleep is, however, challenging during this period of rapid brain maturation, because of the biological and technical variability in EEG background patterns. Moreover, multiple sleep-EEG studies indicate that newborn state recognition is more comprehensively assessed when integrating both cerebral and non-cerebral measures. Previous attempts were either based on single channel EEG in very preterm infants < 32 weeks [49,72,73] or focused on the neonatal, term EEG [62,74–76] or mainly determined quantitative features based on visual pre-selection of sleep states [5,70,77–80]. Quantitative EEG-features that are most related with brain maturation and applied to sleep in previous studies, are spectral analysis (which decomposes the EEG signal into its constituent frequency components) [23,79,81] and methods to define EEG discontinuity [4,49,82,83]. Recently, more interest is directed to the analysis of functional brain connectivity and non-linear methods using complexity analysis, since those methods can document physiological behaviours as a measure of the state of a dynamical process [65]. Koolen et al. showed that the synchrony between cortical activations in QS periods, based on the ‘Activation Synchrony Index’ (ASI), significantly increased between 30 and 40 weeks PMA [5,79]. Tokariev et al. provided a benchmarking study that functional brain connectivity develops rapidly and is related to vigilance states in human term neonates [84]. All together, these studies provide important developmental features of functional brain maturation related to sleep states, however, current procedures for automated sleep detection, which allow subsequent quantification, are limited. To bridge this gap, our group developed an automated QS detection algorithm that performs robustly over a wide PMA range [37]. Using this automated approach, quantification of the electrocortical activity during QS was performed, as a gateway to assess the quality of brain maturation in preterm infants in future studies and allow for further exploration of the relationship between cerebral activity, brain development, and neurodevelopmental outcome. In line with this, Koolen et al. [85] used a combination of EEG features to classify EEG epochs as AS and QS across preterm brain development and provided a sleep probability trend.
6. Future directions
Studies of qualitative and quantitative assessment of preterm sleep have not been widely reported and improvements in neonatal medicine (Kangaroo Care, Newborn Individualized Developmental Care and Assessment Program (NIDCAP), non-invasive ventilation [86]) over the last decade have changed the sleeping conditions of those vulnerable preterm infants and ask for a more recent and updated assessment of preterm and neonatal sleep to complement those data. Further attempts to fully automate EEG sleep wake classification, may not only improve the analytic accuracy to develop normative data across different centres, of interest to compare subgroup populations at high risk for impaired brain function, but may also be useful in the day to day monitoring. A bedside tool, minimal invasive, which facilitate the recognition of sleep states, can guide and improve developmental clinical care.
7. Conclusion
Sequential EEG-sleep analysis during the neonatal period provides crucial insights into functional brain integrity and documents deviations of the biologically pre-programmed process of sleep ontogenesis. Visual assessment of neonatal sleep-EEG, with integration of both cerebral and non-cerebral measures to better define neonatal state, is considered the gold standard, however future studies on inter-rater agreement are definitely needed to improve its validity.
Developing qualitative and normative data with the aid of automated signal processing approaches, can further improve our understanding of extra-uterine brain development under stressful or pathological conditions and the impact of neonatal sleep on later neurocognitive function.
Acknowledgments
The authors would like to thank the parents and infants involved in this study and the staff at the UZ Leuven NICU.
Funding
This research was funded by the Wellcome Trust Centre [grant number 098461/Z/12/Z] (Sleep, Circadian Rhythms & Neuroscience Institute), the RCUK Digital Economy Programme [grant number EP/G036861/1] (Oxford Centre for Doctoral Training in Healthcare Innovation), IWT [grant number TBM 110697-NeoGuard], Bijzonder Onderzoeksfonds KU Leuven (BOF): The effect of perinatal stress on the later outcome in preterm babies [grant number C24/15/036], iMinds Medical Information Technologies (SBO-2016), Belgian Federal Science Policy Office, IUAP, [grant number P7/19] (Dynamical systems, control and optimization, 2012–2017), and ERC Advanced Grant: BIOTENSORS [grant number 339804].
Abbreviations
- AS
active sleep
- QS
quiet sleep
- IS
indeterminate sleep
- TS
transitional sleep
- SWC
sleep-wake cycle
- IBI
interburst interval
- REM
rapid-eye movement
- NREM
non rapid-eye movement
- PMA
postmenstrual age
- GA
gestational age
- HVS
high voltage slow wave sleep
- LVI
low voltage irregular pattern
- M
mixed frequencies
- W
wakefulness
- NIDCAP
Newborn Individualized Developmental Care
- SSC
skin-to-skin care
- aEEG
amplitude EEG
Footnotes
Conflict of interest
None
Contributor Information
Anneleen Dereymaeker, Department of Development and Regeneration, University Hospitals Leuven, Neonatal Intensive Care Unit, KU Leuven (University of Leuven), Leuven, Belgium.
Kirubin Pillay, Institute of Biomedical Engineering (IBME), Department of Engineering Science, University of Oxford, Oxford, United Kingdom.
Jan Vervisch, Department of Development and Regeneration, University Hospitals Leuven, Neonatal Intensive Care Unit, KU Leuven (University of Leuven), Leuven, Belgium; Department of Development and Regeneration, University Hospitals Leuven, Child Neurology, KU Leuven (University of Leuven), Leuven, Belgium.
Maarten De Vos, Institute of Biomedical Engineering (IBME), Department of Engineering Science, University of Oxford, Oxford, United Kingdom.
Sabine Van Huffel, KU Leuven (University of Leuven), Department of Electrical Engineering-ESAT, Division Stadius, Leuven, Belgium Imec, Leuven, Belgium.
Katrien Jansen, Department of Development and Regeneration, University Hospitals Leuven, Neonatal Intensive Care Unit, KU Leuven (University of Leuven), Leuven, Belgium; Department of Development and Regeneration, University Hospitals Leuven, Child Neurology, KU Leuven (University of Leuven), Leuven, Belgium.
Gunnar Naulaers, Department of Development and Regeneration, University Hospitals Leuven, Neonatal Intensive Care Unit, KU Leuven (University of Leuven), Leuven, Belgium.
References
- [1].Iyer KK, Roberts JA, Hellstrom-Westas L, Wikstrom S, Hansen Pupp I, Ley D, et al. Cortical burst dynamics predict clinical outcome early in extremely preterm infants. Brain. 2015;138:2206–2218. doi: 10.1093/brain/awv129. [DOI] [PubMed] [Google Scholar]
- [2].Malk K, Metsaranta M, Vanhatalo S. Drug effects on endogenous brain activity in preterm babies. Brain Dev. 2014;36:116–123. doi: 10.1016/j.braindev.2013.01.009. [DOI] [PubMed] [Google Scholar]
- [3].Benders MJ, Palmu K, Menache C, Borradori-Tolsa C, Lazeyras F, Sizonenko S, et al. Early brain activity relates to subsequent brain growth in premature infants. Cereb Cortex. 2015;25:3014–3024. doi: 10.1093/cercor/bhu097. [DOI] [PubMed] [Google Scholar]
- [4].Dereymaeker A, Koolen N, Jansen K, Vervisch J, Ortibus E, De Vos M, et al. The suppression curve as a quantitative approach for measuring brain maturation in preterm infants. Clin Neurophysiol. 2016;127:2760–2765. doi: 10.1016/j.clinph.2016.05.362. [DOI] [PubMed] [Google Scholar]
- [5].Koolen N, Dereymaeker A, Rasanen O, Jansen K, Vervisch J, Matic V, et al. Early development of synchrony in cortical activations in the human. Neuroscience. 2016;322:298–307. doi: 10.1016/j.neuroscience.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Graven S. Sleep and brain development. Clin Perinatol. 2006;33:693–706. doi: 10.1016/j.clp.2006.06.009. (vii) [DOI] [PubMed] [Google Scholar]
- [7].Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP. A functional role for REM sleep in brain maturation. Behav Brain Res. 1995;69:1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- [8].Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- [9].Mirmiran M, R A. Role of REM sleep in brain development and plasticity. In: Maquet P, Smith C, Stickgold R, editors. Sleep and Brain Plasticity. Oxford University Press; New York: 2003. pp. 181–187. [Google Scholar]
- [10].Mirmiran M, Scholtens J, van de Poll NE, Uylings HB, van der Gugten J, Boer GJ. Effects of experimental suppression of active (REM) sleep during early development upon adult brain and behavior in the rat. Brain Res. 1983;283:277–286. doi: 10.1016/0165-3806(83)90184-0. [DOI] [PubMed] [Google Scholar]
- [11].Mirmiran M, Maas YG, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev. 2003;7:321–334. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- [12].Touchette E, Petit D, Seguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30:1213–1219. doi: 10.1093/sleep/30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lam P, Hiscock H, Wake M. Outcomes of infant sleep problems: a longitudinal study of sleep, behavior, and maternal well-being. Pediatrics. 2003;111:e203–e207. doi: 10.1542/peds.111.3.e203. [DOI] [PubMed] [Google Scholar]
- [14].Ednick M, Cohen AP, McPhail GL, Beebe D, Simakajornboon N, Amin RS. A review of the effects of sleep during the first year of life on cognitive, psychomotor, and temperament development. Sleep. 2009;32:1449–1458. doi: 10.1093/sleep/32.11.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Arditi-Babchuk H, Feldman R, Eidelman AI. Rapid eye movement (REM) in premature neonates and developmental outcome at 6 months. Infant Behav Dev. 2009;32:27–32. doi: 10.1016/j.infbeh.2008.09.001. [DOI] [PubMed] [Google Scholar]
- [16].Scher MS, Steppe DA, Banks DL. Prediction of lower developmental performances of healthy neonates by neonatal EEG-sleep measures. Pediatr Neurol. 1996;14:137–144. doi: 10.1016/0887-8994(96)00013-6. [DOI] [PubMed] [Google Scholar]
- [17].Scher MS. Ontogeny of EEG-sleep from neonatal through infancy periods. Sleep Med. 2008;9:615–636. doi: 10.1016/j.sleep.2007.08.014. [DOI] [PubMed] [Google Scholar]
- [18].Mirmiran M. The function of fetal/neonatal rapid eye movement sleep. Behav Brain Res. 1995;69:13–22. doi: 10.1016/0166-4328(95)00019-p. [DOI] [PubMed] [Google Scholar]
- [19].Ludington-Hoe SM, Johnson MW, Morgan K, Lewis T, Gutman J, Wilson PD, et al. Neurophysiologic assessment of neonatal sleep organization: preliminary results of a randomized, controlled trial of skin contact with preterm infants. Pediatrics. 2006;117:e909–e923. doi: 10.1542/peds.2004-1422. [DOI] [PubMed] [Google Scholar]
- [20].Dos Santos AA, Khan RL, Rocha G, Nunes ML. Behavior and EEG concordance of active and quiet sleep in preterm very low birth weight and full-term neonates at matched conceptional age. Early Hum Dev. 2014;90:507–510. doi: 10.1016/j.earlhumdev.2014.06.014. [DOI] [PubMed] [Google Scholar]
- [21].Grigg-Damberger M, Gozal D, Marcus CL, Quan SF, Rosen CL, Chervin RD, et al. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med. 2007;3:201–240. [PubMed] [Google Scholar]
- [22].Granot S, Meledin I, Richardson J, Friger M, Shany E. Influence of respiratory acidosis and blood glucose on cerebral activity of premature infants. Pediatr Neurol. 2012;47:19–24. doi: 10.1016/j.pediatrneurol.2012.03.018. [DOI] [PubMed] [Google Scholar]
- [23].Scher MS, Steppe DA, Banks DL, Guthrie RD, Sclabassi RJ. Maturational trends of EEG-sleep measures in the healthy preterm neonate. Pediatr Neurol. 1995;12:314–322. doi: 10.1016/0887-8994(95)00052-h. [DOI] [PubMed] [Google Scholar]
- [24].Hellstrom-Westas L, Rosen I, Svenningsen NW. Cerebral function monitoring during the first week of life in extremely small low birthweight (ESLBW) infants. Neuropediatrics. 1991;22:27–32. doi: 10.1055/s-2008-1071411. [DOI] [PubMed] [Google Scholar]
- [25].Kuhle S, Klebermass K, Olischar M, Hulek M, Prusa AR, Kohlhauser C, et al. Sleep-wake cycles in preterm infants below 30 weeks of gestational age. Preliminary results of a prospective amplitude-integrated EEG study. Wien Klin Wochenschr. 2001;113:219–223. [PubMed] [Google Scholar]
- [26].Kidokoro H, Kubota T, Hayashi N, Hayakawa M, Takemoto K, Kato Y, et al. Absent cyclicity on aEEG within the first 24 h is associated with brain damage in preterm infants. Neuropediatrics. 2010;41:241–245. doi: 10.1055/s-0030-1270479. [DOI] [PubMed] [Google Scholar]
- [27].Werth J, Atallah L, Andriessen P, Long X, Zwartkruis-Pelgrim E, Aarts RM. Unobtrusive sleep state measurements in preterm infants - a review. Sleep Med Rev. 2017;32:109–122. doi: 10.1016/j.smrv.2016.03.005. [DOI] [PubMed] [Google Scholar]
- [28].Lefevre J, Germanaud D, Dubois J, Rousseau F, de Macedo Santos I, Angleys H, et al. Are developmental trajectories of cortical folding comparable between cross-sectional datasets of fetuses and preterm newborns? Cereb Cortex. 2016;26:3023–3035. doi: 10.1093/cercor/bhv123. [DOI] [PubMed] [Google Scholar]
- [29].Andre M, Lamblin MD, d'Allest AM, Curzi-Dascalova L, Moussalli-Salefranque F, SNT T, et al. Electroencephalography in premature and full-term infants. Developmental features and glossary. Neurophysiol Clin. 2010;40:59–124. doi: 10.1016/j.neucli.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [30].Tsuchida TN, Wusthoff CJ, Shellhaas RA, Abend NS, Hahn CD, Sullivan JE, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society Critical Care Monitoring Committee. J Clin Neurophysiol. 2013;30:161–173. doi: 10.1097/WNP.0b013e3182872b24. [DOI] [PubMed] [Google Scholar]
- [31].Holmes GL, Lombroso CT. Prognostic value of background patterns in the neonatal EEG. J Clin Neurophysiol. 1993;10:323–352. doi: 10.1097/00004691-199307000-00008. [DOI] [PubMed] [Google Scholar]
- [32].Le Bihannic A, Beauvais K, Busnel A, de Barace C, Furby A. Prognostic value of EEG in very premature newborns. Arch Dis Child Fetal Neonatal Ed. 2012;97:F106–F109. doi: 10.1136/adc.2010.204735. [DOI] [PubMed] [Google Scholar]
- [33].Hayashi-Kurahashi N, Kidokoro H, Kubota T, Maruyama K, Kato Y, Kato T, et al. EEG for predicting early neurodevelopment in preterm infants: an observational cohort study. Pediatrics. 2012;130:e891–e897. doi: 10.1542/peds.2012-1115. [DOI] [PubMed] [Google Scholar]
- [34].Nunes ML, Khan RL, Gomes Filho I, Booij L, da Costa JC. Maturational changes of neonatal electroencephalogram: a comparison between intra uterine and extra uterine development. Clin Neurophysiol. 2014;125:1121–1128. doi: 10.1016/j.clinph.2013.10.049. [DOI] [PubMed] [Google Scholar]
- [35].Grigg-Damberger MM. The visual scoring of sleep in infants 0 to 2 months of age. J Clin Sleep Med. 2016;12:429–445. doi: 10.5664/jcsm.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stefanski M, Schulze K, Bateman D, Kairam R, Pedley TA, Masterson J, et al. A scoring system for states of sleep and wakefulness in term and preterm infants. Pediatr Res. 1984;18:58–62. [PubMed] [Google Scholar]
- [37].Dereymaeker A, Pillay K, Vervisch J, Van Huffel S, Naulaers G, Jansen K, et al. An automated quiet sleep detection approach in preterm infants as a gateway to assess brain maturation. Int J Neural Syst. 2017;1 doi: 10.1142/S012906571750023X. 750,023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hoppenbrouwers T, Hodgman J, Arakawa K, Geidel SA, Sterman MB. Sleep and waking states in infancy: normative studies. Sleep. 1988;11:387–401. doi: 10.1093/sleep/11.4.387. [DOI] [PubMed] [Google Scholar]
- [39].Nijhuis JG, Prechtl HF, Martin CB, Jr, Bots RS. Are there behavioural states in the human fetus? Early Hum Dev. 1982;6:177–195. doi: 10.1016/0378-3782(82)90106-2. [DOI] [PubMed] [Google Scholar]
- [40].Scher MS, Johnson MW, Holditch-Davis D. Cyclicity of neonatal sleep behaviors at 25 to 30 weeks' postconceptional age. Pediatr Res. 2005;57:879–882. doi: 10.1203/01.PDR.0000157678.84132.A8. [DOI] [PubMed] [Google Scholar]
- [41].Vecchierini MF, d'Allest AM, Verpillat P. EEG patterns in 10 extreme premature neonates with normal neurological outcome: qualitative and quantitative data. Brain Dev. 2003;25:330–337. doi: 10.1016/s0387-7604(03)00007-x. [DOI] [PubMed] [Google Scholar]
- [42].Kidokoro H, Inder T, Okumura A, Watanabe K. What does cyclicity on amplitude-integrated EEG mean? J Perinatol. 2012;32:565–569. doi: 10.1038/jp.2012.25. [DOI] [PubMed] [Google Scholar]
- [43].Curzi-Dascalova L, Figueroa JM, Eiselt M, Christova E, Virassamy A, d'Allest AM, et al. Sleep state organization in premature infants of < 35 weeks' gestational age. Pediatr Res. 1993;34:624–628. doi: 10.1203/00006450-199311000-00013. [DOI] [PubMed] [Google Scholar]
- [44].Scher MS, Steppe DA, Dokianakis SG, Guthrie RD. Maturation of phasic and continuity measures during sleep in preterm neonates. Pediatr Res. 1994;36:732–737. doi: 10.1203/00006450-199412000-00008. [DOI] [PubMed] [Google Scholar]
- [45].Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20–45 weeks' gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [46].Lamblin MD, Andre M, Challamel MJ, Curzi-Dascalova L, d'Allest AM, De Giovanni E, et al. Electroencephalography of the premature and term newborn. Maturational aspects and glossary. Neurophysiol Clin. 1999;29:123–219. doi: 10.1016/s0987-7053(99)80051-3. [DOI] [PubMed] [Google Scholar]
- [47].Vanhatalo S, Kaila K. Development of neonatal EEG activity: from phenomenology to physiology. Semin Fetal Neonatal Med. 2006;11:471–478. doi: 10.1016/j.siny.2006.07.008. [DOI] [PubMed] [Google Scholar]
- [48].Koolen N, Dereymaeker A, Rasanen O, Jansen K, Vervisch J, Matic V, et al. Interhemispheric synchrony in the neonatal EEG revisited: activation synchrony index as a promising classifier. Front Hum Neurosci. 2014;8:1030. doi: 10.3389/fnhum.2014.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Palmu K, Kirjavainen T, Stjerna S, Salokivi T, Vanhatalo S. Sleep wake cycling in early preterm infants: comparison of polysomnographic recordings with a novel EEG-based index. Clin Neurophysiol. 2013;124:1807–1814. doi: 10.1016/j.clinph.2013.03.010. [DOI] [PubMed] [Google Scholar]
- [50].Klebermass K, Olischar M, Waldhoer T, Fuiko R, Pollak A, Weninger M. Amplitude-integrated EEG pattern predicts further outcome in preterm infants. Pediatr Res. 2011;70:102–108. doi: 10.1203/PDR.0b013e31821ba200. [DOI] [PubMed] [Google Scholar]
- [51].Natalucci G, Rousson V, Bucher HU, Bernet V, Hagmann C, Latal B. Delayed cyclic activity development on early amplitude-integrated EEG in the preterm infant with brain lesions. Neonatology. 2013;103:134–140. doi: 10.1159/000345202. [DOI] [PubMed] [Google Scholar]
- [52].Mirmiran M, Koster-Van Hoffen GC, Bos NP. Circadian rhythm generation in the cultured suprachiasmatic nucleus. Brain Res Bull. 1995;38:275–283. doi: 10.1016/0361-9230(95)00100-s. [DOI] [PubMed] [Google Scholar]
- [53].Mulder EJ, Visser GH, Bekedam DJ, Prechtl HF. Emergence of behavioural states in fetuses of type-1-diabetic women. Early Hum Dev. 1987;15:231–251. doi: 10.1016/0378-3782(87)90082-x. [DOI] [PubMed] [Google Scholar]
- [54].Hoppenbrouwers T, Hodgman JE, Rybine D, Fabrikant G, Corwin M, Crowell D, et al. Sleep architecture in term and preterm infants beyond the neonatal period: the influence of gestational age, steroids, and ventilatory support. Sleep. 2005;28:1428–1436. doi: 10.1093/sleep/28.11.1428. [DOI] [PubMed] [Google Scholar]
- [55].Watanabe K. Neurophysiological aspects of neonatal seizures. Brain Dev. 2014;36:363–371. doi: 10.1016/j.braindev.2014.01.016. [DOI] [PubMed] [Google Scholar]
- [56].Shany E, Meledin I, Gilat S, Yogev H, Golan A, Berger I. In and ex utero maturation of premature infants electroencephalographic indices. Clin Neurophysiol. 2014;125:270–276. doi: 10.1016/j.clinph.2013.06.185. [DOI] [PubMed] [Google Scholar]
- [57].Scher MS, Steppe DA, Dahl RE, Asthana S, Guthrie RD. Comparison of EEG sleep measures in healthy full-term and preterm infants at matched conceptional ages. Sleep. 1992;15:442–448. doi: 10.1093/sleep/15.5.442. [DOI] [PubMed] [Google Scholar]
- [58].Guyer C, Huber R, Fontijn J, Bucher HU, Nicolai H, Werner H, et al. Very preterm infants show earlier emergence of 24-hour sleep-wake rhythms compared to term infants. Early Hum Dev. 2015;91:37–42. doi: 10.1016/j.earlhumdev.2014.11.002. [DOI] [PubMed] [Google Scholar]
- [59].Scher MS, Jones BL, Steppe DA, Cork DL, Seltman HJ, Banks DL. Functional brain maturation in neonates as measured by EEG-sleep analyses. Clin Neurophysiol. 2003;114:875–882. doi: 10.1016/s1388-2457(03)00026-9. [DOI] [PubMed] [Google Scholar]
- [60].Buzsaki G, D C, Csicsvari J. Maintenance and modification of firing rates and sequences in the hippocampus: does sleep play a role? In: Maquet P, S S, Stickgold R, editors. Sleep and Brain Plasticity. Oxford University Press; New York: 2003. pp. 247–270. [Google Scholar]
- [61].Scher MS, Johnson MW, Ludington SM, Loparo K. Physiologic brain dysmaturity in late preterm infants. Pediatr Res. 2011;70:524–528. doi: 10.1203/PDR.0b013e31822f24af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Scher MS, Dokianakis SG, Sun M, Steppe DA, Guthrie RD, Sclabassi RJ. Computer classification of sleep in preterm and full-term neonates at similar postconceptional term ages. Sleep. 1996;19:18–25. doi: 10.1093/sleep/19.1.18. [DOI] [PubMed] [Google Scholar]
- [63].Rivkees SA. Developing circadian rhythmicity in infants. Pediatr Endocrinol Rev. 2003;1:38–45. [PubMed] [Google Scholar]
- [64].Biagioni E, Boldrini A, Giganti F, Guzzetta A, Salzarulo P, Cioni G. Distribution of sleep and wakefulness EEG patterns in 24-h recordings of preterm and full-term newborns. Early Hum Dev. 2005;81:333–39. doi: 10.1016/j.earlhumdev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [65].Scher MS, Ludington-Hoe S, Kaffashi F, Johnson MW, Holditch-Davis D, Loparo KA. Neurophysiologic assessment of brain maturation after an 8-week trial of skin-to-skin contact on preterm infants. Clin Neurophysiol. 2009;120:1812–1818. doi: 10.1016/j.clinph.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Welch MG, Myers MM, Grieve PG, Isler JR, Fifer WP, Sahni R, et al. Electroencephalographic activity of preterm infants is increased by family nurture intervention: a randomized controlled trial in the NICU. Clin Neurophysiol. 2014;125:675–684. doi: 10.1016/j.clinph.2013.08.021. [DOI] [PubMed] [Google Scholar]
- [67].Baley J, Committee On F, Newborn Skin-to-skin care for term and preterm infants in the neonatal ICU. Pediatrics. 2015;136:596–599. doi: 10.1542/peds.2015-2335. [DOI] [PubMed] [Google Scholar]
- [68].Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry. 2014;75:56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- [69].Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J Pediatr. 2014;164:52–60 (e2). doi: 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shellhaas RA, Burns JW, Barks JD, Chervin RD. Quantitative sleep stage analyses as a window to neonatal neurologic function. Neurology. 2014;82:390–395. doi: 10.1212/WNL.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Osredkar D, Toet MC, van Rooij LG, van Huffelen AC, Groenendaal F, de Vries LS. Sleep-wake cycling on amplitude-integrated electroencephalography in term newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2005;115:327–332. doi: 10.1542/peds.2004-0863. [DOI] [PubMed] [Google Scholar]
- [72].Stevenson NJ, Palmu K, Wikstrom S, Hellstrom-Westas L, Vanhatalo S. Measuring brain activity cycling (BAC) in long term EEG monitoring of preterm babies. Physiol Meas. 2014;35:1493–1508. doi: 10.1088/0967-3334/35/7/1493. [DOI] [PubMed] [Google Scholar]
- [73].Scher MS, Waisanen H, Loparo K, Johnson MW. Prediction of neonatal state and maturational change using dimensional analysis. J Clin Neurophysiol. 2005;22:159–165. [PubMed] [Google Scholar]
- [74].Piryatinska A, Terdik G, Woyczynski WA, Loparo KA, Scher MS, Zlotnik A. Automated detection of neonate EEG sleep stages. Comput Methods Prog Biomed. 2009;95:31–46. doi: 10.1016/j.cmpb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [75].Lofhede J, Thordstein M, Lofgren N, Flisberg A, Rosa-Zurera M, Kjellmer I, et al. Automatic classification of background EEG activity in healthy and sick neonates. J Neural Eng. 2010;7 doi: 10.1088/1741-2560/7/1/016007. 16,007. [DOI] [PubMed] [Google Scholar]
- [76].Krajca V, P S, Mohylová J, Paul K, Gerla V, Lhotská L. Neonatal EEG sleep stages modelling by temporal profiles. In: Díaz RM, F P, Arencibia AQ, editors. Computer Aided Systems Theory - EUROCAST, 4739. Springer Berlin Heidelberg; Berlin, Heidelberg: 2007. pp. 195–201. [Google Scholar]
- [77].Scher MS, Dokianakis SG, Steppe DA, Banks DL, Sclabassi RJ. Computer classification of state in healthy preterm neonates. Sleep. 1997;20:132–141. [PubMed] [Google Scholar]
- [78].Scher MS, Turnbull J, Loparo K, Johnson MW. Automated state analyses: proposed applications to neonatal neurointensive care. J Clin Neurophysiol. 2005;22:256–270. doi: 10.1097/01.wnp.0000161418.87923.10. [DOI] [PubMed] [Google Scholar]
- [79].Tolonen M, Palva JM, Andersson S, Vanhatalo S. Development of the spontaneous activity transients and ongoing cortical activity in human preterm babies. Neuroscience. 2007;145:997–1006. doi: 10.1016/j.neuroscience.2006.12.070. [DOI] [PubMed] [Google Scholar]
- [80].Omidvarnia A, Fransson P, Metsaranta M, Vanhatalo S. Functional bimodality in the brain networks of preterm and term human newborns. Cereb Cortex. 2014;24:2657–2668. doi: 10.1093/cercor/bht120. [DOI] [PubMed] [Google Scholar]
- [81].Niemarkt HJ, Jennekens W, Pasman JW, Katgert T, Van Pul C, Gavilanes AW, et al. Maturational changes in automated EEG spectral power analysis in preterm infants. Pediatr Res. 2011;70:529–534. doi: 10.1203/PDR.0b013e31822d748b. [DOI] [PubMed] [Google Scholar]
- [82].Koolen N, Jansen K, Vervisch J, Matic V, De Vos M, Naulaers G, et al. Line length as a robust method to detect high-activity events: automated burst detection in premature EEG recordings. Clin Neurophysiol. 2014;125:1985–1994. doi: 10.1016/j.clinph.2014.02.015. [DOI] [PubMed] [Google Scholar]
- [83].Niemarkt HJ, Andriessen P, Peters CH, Pasman JW, Zimmermann LJ, Oetomo Bambang S. Quantitative analysis of maturational changes in EEG background activity in very preterm infants with a normal neurodevelopment at 1 year of age. Early Hum Dev. 2010;86:219–224. doi: 10.1016/j.earlhumdev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- [84].Tokariev A, Videman M, Palva JM, Vanhatalo S. Functional brain connectivity develops rapidly around term age and changes between vigilance states in the human newborn. Cereb Cortex. 2016;26:4540–4550. doi: 10.1093/cercor/bhv219. [DOI] [PubMed] [Google Scholar]
- [85].Koolen N, Oberdorfer L, Rona Z, Giordano V, Werther T, Klebermass-Schrehof K, et al. Automated classification of neonatal sleep states using EEG. Clin Neurophysiol. 2017;128:1100–1108. doi: 10.1016/j.clinph.2017.02.025. [DOI] [PubMed] [Google Scholar]
- [86].Collins CL, Barfield C, Davis PG, Horne RS. Randomized controlled trial to compare sleep and wake in preterm infants < 32 weeks of gestation receiving two different modes of non-invasive respiratory support. Early Hum Dev. 2015;91:701–704. doi: 10.1016/j.earlhumdev.2015.09.011. [DOI] [PubMed] [Google Scholar]