Abstract
The Chicago Sanitary and Ship Canal (CSSC) links the Great Lakes to the Mississippi River starting in downtown Chicago. In addition to storm water, the CSSC receives water from Chicago’s wastewater treatment plant (WWTP). Such effluents are known to be sources of organic pollutants to water and sediment. Therefore in 2013, we collected 10 sediment samples from the CSSC and measured the concentrations of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), brominated flame retardants, and organophosphate esters (OPEs). Geometric mean concentrations of the summed concentrations of 16 PAHs ranged from 11,000 to 420,000 ng/g dw, with the highest concentrations located at each end of the canal. Total PCB concentrations had a geometric mean of 1,400 ± 500 ng/g dw. Brominated flame retardants were separated into two groups: polybrominated diphenyl ethers (PBDEs) and non-PBDEs. Concentrations of PBDEs and those of the non-PBDE flame retardants had a geometric average of 83 ± 19 and 7.0 ± 5.8 ng/g dw, respectively. The summed concentrations of 8 OPEs ranged from 470 to 2,800 ng/g dw, with the highest concentration detected at a site located downstream of the Stickney water reclamation plant. Using ANOVA results, some hypotheses on sources to the CSSC could be formulated: Downtown Chicago is probably a source of PAHs, the Cal-Sag Channel may be a source of PCBs, and neither the WWTP nor the Cal-Sag Channel seem to be significant sources of brominated flame retardants or OPEs.
Keywords: Sediment, Polycyclic aromatic hydrocarbons, Polychlorinated biphenyls, Polybrominated diphenyl ethers, Organophosphate esters
Graphical Abstract

1. Introduction
There are three paths by which water can leave Lake Michigan. The first is through the Straits of Mackinac to Lake Huron, from which water exits through the St. Clair and Detroit Rivers to Lake Erie. The second path is through the Chicago River that flows into the Chicago Sanitary and Ship Canal (CSSC), which in turn connects to the Des Plaines River and eventually to the Illinois River. The third path is through the Calumet River which flows into the Calumet-Saganashkee Channel (Cal-Sag Channel), which merges with the CSSC. The CSSC is a man-made, 45 km long canal that was designed to receive the storm water, sewage, and other wastewater generated by greater Chicago and redirect it away from Lake Michigan, which is Chicago’s drinking water source. In effect, the CSSC is a connection between Lake Michigan and the Mississippi River by way of Chicago’s residents.
The CSSC is about 7 m deep and 35–75 m wide, and it has a flow rate of 7.4 × 106 m3/day1 which is only a small percent of the total flow out of the Great Lakes – about 5.7 × 108 m3/day1 at the St. Lawrence River. The flow rate in the CSSC is controlled by three locks, one at each end of the CSSC and one on the Calumet River. These locks also allow barge traffic, carrying crude materials, manufactured goods, coal, petroleum, and chemicals,2 in and out of the CSSC/Cal-Sag system. The CSSC is part of the Chicago wastewater system, and the outflow of the Stickney water reclamation plant enters the canal about 18 km from Lake Michigan with an maximum discharge flow rate of 4.5 × 106 m3/day which makes the Stickney plant the world’s largest wastewater treatment plant.3
Wastewater treatment plants (WWTPs) are known to be sources of pollutants, many of which are considered persistent organic pollutants (POPs), such as polychlorinated biphenyls (PCBs),4 polycyclic aromatic hydrocarbons (PAHs),4 polybrominated diphenyl ethers (PBDEs),5,6,7,8 non-PBDE brominated flame retardants,5,6,9 and organophosphate ester flame retardants (OPEs).5,6,10,11 For example, the summed concentrations of 21 PAHs in the influent and effluent of a WWTP in Montreal were 1550 and 420 ng/L, respectively; therefore, this plant had a 73% removal rate (percentage of compound in the effluent compared to the influent) of PAHs.4 On the other hand, the Marine Park WWTP in Washington state measured the concentrations of tris(2-chloroethyl)phosphate (TCEP, an OPE) to be 960 and 810 ng/L in the influent and effluent, respectively, which means there was only a 16% removal rate of TCEP in this plant.5 Because of these variations in the removal rates of different compounds in different WWTPs, the Metropolitan Water Reclamation District of Greater Chicago is tasked with monitoring the water and sediment quality in the CSSC.12 However, they only measure selected, high priority organic pollutants, including PAHs, pesticides, and PCBs.
In this study, we have measured the concentrations of several persistent organic pollutants in sediment from the CSSC with the goal of determining if the city, the Stickney water reclamation plant, the Cal-Sag Channel, or some other human related activity was a source of these compounds. We have focused on the following compounds: polycyclic aromatic hydrocarbons (PAHs) [specifically fluorene, phenanthrene, anthracene, fluoranthene, pyrene, retene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, benzo[e]pyrene, indeno[1,2,3-cd]pyrene, dibenzo[a,h]anthracene, benzo[ghi]perylene, and coronene], polychlorinated biphenyls (PCBs) [congeners 1–209], 15-brominated flame retardants (BFRs) [specifically the polybrominated diphenyl ethers (congeners 7, 17, 28, 47, 49, 85, 99, 100, 153, 154, 206–209, PBDEs), hexabromobenzene (HBB), 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB), hexabromocyclododecane (measured as the sum of the α, β, and γ isomers, HBCDs), 1,2-bis(2,4,6-tribromophenoxy)ethane (BTBPE), and decabromodiphenylethane (DBDPE)], and organophosphate esters (OPEs) [specifically tris(2-chloroethyl)phosphate (TCEP), tris(1-chloro-2-propyl)phosphate (TCIPP), triphenyl phosphate (TPHP), tris(2-butoxyethyl) phosphate (TBOEP), 2-ethylhexyl diphenyl phosphate (EHDP), tris(2-ethylhexyl) phosphate (TEHP), tris(2-isopropylphenyl) phosphate (TIPPP), and tris(4-tert-butylphenyl) phosphate (TBPP)]. Concentrations, relationships among these levels, and their spatial distributions are presented and discussed.
2. Materials and Methods
2.1. Sampling.
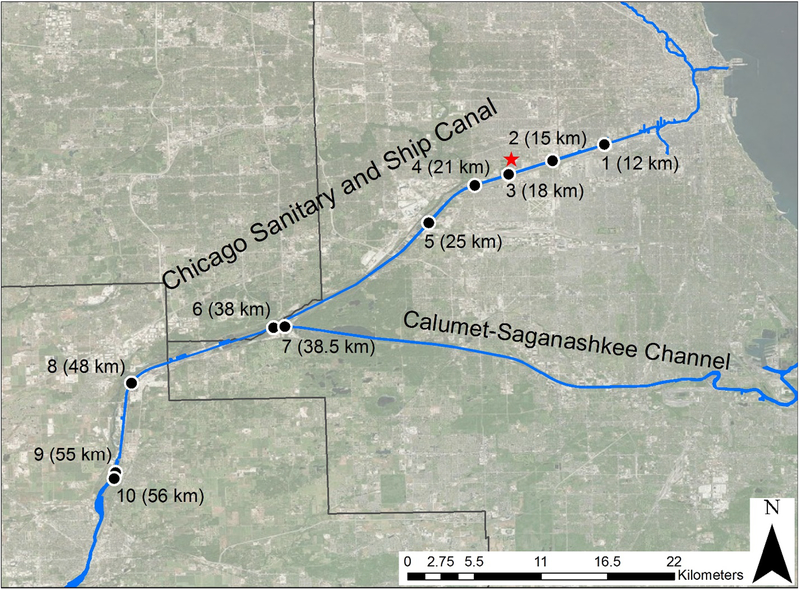
Sampling generally followed the protocol outlined by Martinez et al.13 Surficial sediment samples were obtained on 19 June 2013 in the Chicago Sanitary and Ship Canal from the Metropolitan Water Reclamation District of Greater Chicago’s boat Pollution Control 100. Ten samples, the locations of which are shown in Figure 1 with site numbers and distances from the start of the Chicago River at Lake Michigan (in river km), were collected with a standard Ponar dredge sampler (clamshell-bucket). Table 1 gives the geographical coordinates of each site and the percent total organic carbon converted from loss on ignition.14 Site 7 is located up the Cal-Sag Channel just before it merges with the CSSC, and site 6 is slightly downstream from this point. Using these two sites, we hoped to estimate the load of pollutants entering the CSSC from the Cal-Sag Channel. Sediment samples were extracted with suitable solvents, cleaned up by liquid-solid column chromatography, and analyzed by gas chromatography (GC) coupled with mass spectrometry (MS) and tandem mass spectrometry GC/MS/MS. Details on these procedures are given in the supporting information, along with the quality assurance and quality control protocols.
Figure 1:
Map of the sampling sites on the Chicago Sanitary and Ship Canal (CSSC) in Chicago, Illinois. Sampling sites are labeled with a black dot with their corresponding site number and distance (in river km) from the start of the Chicago River at Lake Michigan. Latitudes and longitudes for each site are given in Table 1. The red star is the Stickney water reclamation plant. The black straight lines represent the county borders; the largest one is Cook County
Table 1:
Site numbers, distance from the start of the Chicago River at Lake Michigan (in river km), percent total organic carbon (TOC), latitude and longitude, and geometric mean concentrations of each compound or compound group (in ng/g dw). All values represent triplicate measurements; however, total PCBs concentrations are based on single measurements except for site 8 which was analyzed trice. ANOVA results are also given for each row; concentrations that share a letter are not significantly different (P < 5%).
| Site # (Fig. 1) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distance (km) | 12 | 15 | 18 | 21 | 25 | 38 | 38.5 | 48 | 55 | 56 | ||||||||||
| TOC (%) | 3.58 | 18.68 | 1.75 | 8.27 | 2.95 | 3.86 | 3.10 | 5.25 | 5.33 | 4.11 | ||||||||||
| Latitude, Longitude | 41.8319, 87.7041 | 41.8198, 87.7425 | 41.8096, 87.7752 | 41.8016, 87.8003 | 41.7737, 87.8346 | 41.6956, 87.9501 | 41.6964, 87.9418 | 41.6545, 88.0558 | 41.5876, 88.0675 | 41.5830, 88.0687 | ||||||||||
| Phen. | 65,000 | a | 31,000 | ab | 4,300 | cde | 35,000 | ab | 6,800 | bcd | 2,200 | de | 1,900 | de | 950 | e | 8,300 | bcd | 18,000 | abc |
| Fluoran. | 96,000 | a | 70,000 | ab | 6,200 | de | 34,000 | abcd | 12,000 | bcde | 5,900 | de | 4,600 | e | 1,700 | e | 9,700 | cde | 47,000 | abc |
| Pyrene | 66,000 | a | 51,000 | a | 4,400 | bc | 27,000 | ab | 9,400 | abc | 4,900 | bc | 4,700 | bc | 1,300 | c | 12,000 | ab | 65,000 | a |
| total PAHs | 420,000 | a | 340,000 | ab | 26,000 | de | 170,000 | abc | 58,000 | cde | 30,000 | de | 22,000 | de | 11,000 | e | 68,000 | bcd | 270,000 | abc |
| total PCBs | 1,600 | 5,000 | 1,200 | 2,000 | 1,900 | 1,200 | 4,900 | 1,100 | 1,300 | 69 | ||||||||||
| BDE-47 | 16 | a | 14 | a | 13 | a | 25 | a | 20 | a | 14 | a | 2.0 | b | 25 | a | 14 | a | 2.4 | b |
| BDE-99 | 23 | ab | 21 | ab | 7.6 | ab | 18 | ab | 19 | ab | 15 | ab | 2.5 | bc | 35 | a | 18 | ab | 0.79 | c |
| BDE-209 | 130 | a | 180 | a | 2.2 | b | 44 | a | 66 | a | 57 | a | 2.3 | b | 210 | a | 140 | a | 2.9 | b |
| total PBDEs | 190 | a | 240 | a | 30 | bc | 120 | ab | 130 | ab | 100 | ab | 8.8 | c | 310 | a | 200 | a | 6.4 | c |
| HBB | 0.11 | a | 0.13 | a | 0.07 | a | 0.18 | a | 0.05 | a | 0.07 | a | 0.20 | a | 0.08 | a | ||||
| EH-TBB | 5.6 | ab | 3.9 | b | 2.2 | bc | 4.8 | b | 4.7 | b | 2.5 | bc | 1.3 | bc | 17 | a | 5.4 | ab | 0.75 | c |
| HBCDs | 3.9 | ab | 5.6 | a | 0.13 | c | 1.3 | abc | 1.3 | abc | 1.9 | abc | 0.18 | bc | 5.9 | a | 2.6 | abc | ||
| BTBPE | 2.2 | ab | 3.0 | ab | 0.16 | e | 0.50 | cde | 1.0 | bcd | 1.1 | bc | 0.26 | de | 6.3 | a | 2.7 | ab | 0.19 | e |
| DBDPE | 2.8 | a | 3.0 | a | 1.2 | a | 0.99 | a | 3.0 | a | 0.65 | a | 3.0 | a | 2.0 | a | ||||
| TCEP | 41 | ab | 44 | a | 18 | bcd | 33 | abc | 16 | cd | 17 | cd | 13 | d | 31 | abcd | 33 | abc | ||
| TCIPP | 130 | ab | 140 | ab | 46 | cd | 190 | a | 60 | cd | 57 | cd | 25 | d | 82 | bc | 130 | ab | 36 | d |
| TPHP | 40 | ab | 63 | ab | 43 | ab | 170 | a | 26 | ab | 54 | ab | 18 | b | 22 | b | 22 | b | 18 | b |
| TBOEP | 500 | cd | 690 | bc | 510 | cd | 1,600 | a | 460 | cde | 440 | cde | 320 | e | 580 | bc | 830 | b | 330 | de |
| EHDP | 260 | b | 290 | b | 210 | b | 600 | a | 210 | b | 140 | c | 690 | a | 130 | c | 210 | b | 28 | d |
| TEHP | 190 | ab | 190 | ab | 29 | d | 73 | c | 89 | c | 110 | bc | 110 | bc | 250 | ab | 190 | ab | 32 | d |
| TIPPP | 84 | cd | 120 | cd | 29 | e | 110 | cd | 85 | cd | 74 | d | 270 | a | 140 | bc | 220 | ab | 4.7 | f |
| TBPP | 130 | ab | 130 | ab | 14 | cd | 57 | ab | 49 | abc | 34 | bcd | 9.3 | d | 100 | ab | 150 | a | 9.2 | d |
| total OPEs | 1,400 | bcd | 1,700 | b | 910 | d | 2,800 | a | 990 | cd | 990 | cd | 1,400 | bc | 1,300 | bcd | 1,800 | b | 470 | e |
3. Results and Discussion
To simplify our data, we have focused on compounds that were detected in more than 50% of the samples and that were found in less than 15% of the blanks. Concentrations, given as geometric means, with their standard errors and detection frequencies, for each compound at the 10 sites are given in Tables S1 and S2. Concentrations are expressed as ng of compound per gram of dry sediment (ng/g dw). Correlation statistics for all compounds are given in Table S3; for the remainder of this manuscript, a P value equal or less than 5% is considered to be statistically relevant. Throughout this discussion, we will focus on phenanthrene, fluoranthene, pyrene, total PAHs (the sum of all 16 PAHs), total PCBs (the sum of all 209 congeners except PCB-14, 166 and 204), BDE-47, BDE-99, BDE-209, total PBDEs (sum of BDE-7, 17, 28, 47, 49, 85, 99, 100, 153, 154, and 206–209), HBB, EH-TBB, HBCDs, BTBPE, DBDPE, TCEP, TCIPP, TPHP, TBOEP, EHDP, TEHP, TIPPP, TBPP, and total OPEs (sum of TCEP, TCIPP, TPHP, TBOEP, EHDP, TEHP, TIPPP, and TBPP). A condensed table giving the geometric means of these compounds and the results of an ANOVA using the logarithmically transformed concentrations is given in Table 1. For ANOVA results, concentrations that share a letter are not significantly different.
3.1. Summary of Polycyclic Aromatic Hydrocarbons.
PAHs are formed by the incomplete combustion of carbon-based fuels, and many PAH are carcinogenic or mutagenic. Their levels in the environment are generally correlated with human population density.15,16 In the CSSC sediment samples we have studied here, PAHs had the highest detection frequencies and concentrations of all the compounds we measured. Concentrations of total PAHs ranged from 11,000 to 420,000 ng/g dw with the highest levels at both ends of the canal and the lowest at site 8. The concentrations and ANOVA results for total PAHs are shown in Table 1. The most abundant individual compounds were fluoranthene, pyrene, and phenanthrene contributing on average 20 ± 0.6, 17 ± 0.7, and 12 ± 0.8%, respectively to the total PAH load. This profile has been seen previously in sediment and effluent samples.4,12 As seen in Table S3, concentrations of the individual PAHs correlated well with each other, except for retene, which is usually associated with biomass burning.17 The PAH levels reported here are similar to those reported by the Metropolitan Water Reclamation District of Great Chicago in 2006 for the same sampling area.12 They reported the total concentration of 12 PAHs, and these geometric means ranged from 15,000–156,000 ng/g dw. At the New Bedford Harbor in Massachusetts, a Superfund site mainly due to PCB contamination, the mean concentration of 16 PAHs ranged from 14,400 to 902,000 ng/g dw.18
3.2. Summary of Polychlorinated Biphenyls.
PCBs were manufactured from 1929 until 1979, when they were banned due to their toxicity and environmental persistence. PCBs were used in numerous industrial and commercial applications such as transformers, capacitors, thermal insulation, and plastics. Due to their widespread use, their improper disposal, and their persistence, PCBs have become a worldwide contaminant and were one of the first 12 substances listed under the Stockholm Convention.19 The average detection frequency for the individual 209 congeners was 91%. Concentrations of total PCBs can be found in Table 1 and had a geometric average of 1,400 ± 500 ng/g dw with a range of 69 to 5,000 ng/g dw; individual congener concentrations can be found in Table S2. It is worth noting that only site 8 was analyzed in triplicate for PCBs. Because the variance detected at this site was minimal, PCB concentrations at the remaining sites were only measured once. The two highest concentrations were at sites 2 and 7. The Metropolitan Water Reclamation District of Great Chicago reported PCB levels in 2006 for the same sampling area, but their concentrations were in terms of four different Aroclor mixtures. Geometric mean concentrations of Aroclor 1254, 1248, 1260, and 1016 were 1,500, 2,800, 680, 180 ng/g dw, respectively, and are similar to the levels we found.12 Sediment in the Indiana Harbor and Ship Canal, designated as an area of concern due to contamination of heavy metals, PAHs, and PCBs, had an average total PCB concentration of 8,700 ng/g dw;13 these levels are the same order of magnitude as the most contaminated site in the CSSC.
3.3. Summary of Brominated Flame Retardants.
BFRs are commonly used in commercial and consumer products to slow the spread of fires. For the CSSC sediment samples, geometric mean concentrations and ANOVA results for BDE-47, 99, 209, total PBDE, HBB, EH-TBB, HBCDs, BTBPE and DBDPE are given in Table 1. PBDEs constituted a high portion of BFRs used, but because of their presence in the environment, PBDEs have been withdrawn from the market.20 Of the 37 PBDE congeners measured in this study, 14 had an average detection frequency of > 50%. Sites 1, 2, 4, 5, 6, 8, and 9 had the highest levels of total PBDEs, with a geometric average of 170 ± 17 ng/g dw, and these concentrations were not statistically distinguishable from one another. The sites 3, 7, and 10 had the lowest levels of total PBDEs, averaging 12 ± 20 ng/g dw, and these concentrations were not statistically distinguishable from one another.
Overall, the most abundant congener was BDE-209 (52 ± 4% of total PBDEs) followed by BDE-47 and 99 at 19 ± 3 and 16 ± 1%, respectively. This congener profile agrees with other sediment studies.6,7,21,22 Concentrations of BDE-99 correlated well with those of BDE-47 and 209. As seen in Table S3, total PBDE concentrations correlated well with all PBDE congeners except BDE-7, 17, 28, and 49. A previous study reported sediment concentrations of BDE-209 in Arkansas up to 34,000 ng/g dw for sites near the major PBDE manufacturing facilities.23 In addition, PBDEs were measured in sediment from the River Besòs in Spain, which is heavily populated and industrialized, at levels ranging up to 810 ng/g.6 Both of these levels are higher than those we measured here in the CSSC.
Between 2004 and 2013, PBDE were taken off the flame retardant market by their manufacturers and replaced by other highly brominated compounds.20 We detected several of these non-PBDE brominated flame retardants (HBB, EH-TBB, HBCDs, BTBPE, and DBDPE) in the CSSC sediment but at much lower levels compared to the PBDEs. Concentrations of HBB were statistically indistinguishable at all sites and averaged 0.11 ± 0.02 ng/g dw (Table 1). Sediment samples from Japan in 1982 had HBB concentrations up to 4.3 ng/g dw.24 EH-TBB was the most abundant non-PBDE brominated flame retardant at all sites except at site 2 and 6. EH-TBB concentrations were significantly higher at sites 1, 8, and 9; the EH-TBB concentrations at all of the other sites were not statistically different than one another and averaged 2.7 ± 0.41 ng/g dw. EH-TBB concentrations correlated well with those of total PBDEs. At site 2, the most abundant non-PBDE brominated flame retardant was HBCDs, and with the exception of sites 3 and 7, its concentrations did not vary significantly with location. At these other sites, the average HCBD concentration was 2.9 ± 0.61 ng/g dw. HBCD concentrations correlated well with those of total PBDEs and EH-TBB.
The most frequently detected non-PBDE brominated flame retardant was BTBPE with a detection frequency of 97%. The concentrations of BTBPE were highly variable, ranging from 0.16 ng/g dw at site 3 to 6.3 ng/g dw at site 8, and these concentrations correlated well with those of total PBDEs, EH-TBB, and HBCDs. López et al.25 reported levels of BTBPE in river sediments in the Netherlands up to 0.3 ng/g dw. Decabromodiphenylethane (DBDPE) was detected in 70% of the CSSC samples and was the most abundant non-PBDE brominated flame retardant at site 6. Its concentrations did not statistically differ among sites (Table 1), and its overall average concentration was 2.0 ± 0.26 ng/g dw. The levels of DBDPE correlated well with those of total PBDEs, HBB, and HBCDs. The average concentration of DBDPE is similar to those reported in lakes located about 200 km away from its manufacturing facilities in Arkansas (1.7–3.0 ng/g dw).23 Cristale and co-workers6 also report that DBDPE was found in sediment samples in Spain, but HBB, EH-TBB, and BTBPE were not detected there. Overall, the significant correlations between the concentrations of these compounds in the CSSC sediments and those of total PBDEs indicates that these chemicals probably have similar sources.
3.4. Summary of Organophosphate Esters.
OPEs have many applications.26,27 The two chlorinated OPEs, TCEP and TCIPP, are used as flame retardants, and many others are used as plasticizers for flexible and rigid polyurethane foams. TPHP is mainly used as plasticizer in PVC, but it has also been used as a flame retardant and in hydraulic fluids. TBOEP is widely used in floor waxes and as a plasticizer in many types of PVC products. EHDP is used in hydraulic fluids and as a plasticizer in food packaging. TEHP has been used as a flame retardant, plasticizer, and fungicide. Less is known about the uses of TIPPP and TBPP, but they are used as plasticizers and flame retardants, respectively.
Geometric mean concentrations and ANOVA results for TCEP, TCIPP, TPHP, TBOEP, EHDP, TEHP, TIPPP, TBPP, and total OPEs are given in Table 1. Of the OPEs discussed here the only ones that were detected less frequently than 100% were the two halogenated OPEs (TCEP and TCIPP) and TEHP (at an overall detection frequency of 87, 93, and 97%, respectively). All of the others were present in all 30 samples. The levels of total OPEs are an order of magnitude higher than those of total PBDEs. This concentration difference in OPEs verses PBDEs has been previously seen in sediment analyzed from Spain.22 The most abundant OPEs were TBOEP and EHDP, as seen in the OPE profile (Figure S1) contributing on average 46 ± 2 and 19 ± 2%, respectively to the total OPE load. This agrees with a previous study which found that TBOEP was the most abundant OPE detected in sediments.11,28
Concentrations of TBOEP and EHDP ranged from 320–1,600 and 28–690 ng/g dw, respectively, and were highly variable among sites. Concentrations of TBOEP correlate well with those of TCIPP, TPHP, and total OPEs (Table S3). Concentrations of EHDP only correlated well with those of total OPEs. The least abundant OPE was TCEP with an average concentration of 26 ± 3.0 ng/g dw, and the levels of TCEP were the only ones to correlate well with those of the all the PAHs. The concentrations of TCIPP, the other chlorinated OPE, correlated well with those of TCEP. Concentrations for TEHP and TBPP both correlated with those of total PBDEs. The concentrations of TEHP ranged from 29–250 ng/g dw, and those of TBPP ranged from 9.2–150 ng/g dw. The concentrations of TIPPP ranged from 4.7–270 ng/g dw but did not correlate to any other compound measured. A recent review summarizes OPE levels measured in a variety of matrices including sediment samples, and the authors discuss the wide range of concentrations published for sediment samples.27 The highest reported values were for sediment samples in Norway, where, for the total of 8 OPEs, the concentrations ranged from 7,500 to 34,000 ng/g.29 The lowest reported levels were for Taihu Lake in China with a range of 3.4–14 ng/g.28 Our observed concentration are about in the middle of these previously measured values.
3.5. Spatial Distributions.
For spatial analyses, results from an ANOVA were used, and these results are given in Table 1 for each compound. Graphs of concentrations (in ng/g dw) versus distance from the start of the Chicago River at Lake Michigan (in river km) are given in Figures 2 and 3 for total PAHs, PCBs, PBDEs, and OPEs, respectively. For comparison Figure S2 shows the concentrations of each group of compounds in normalized to grams organic carbon (ng/g oc) versus the distance from the start of the Chicago River at Lake Michigan. With this analysis, we hoped to determine if the city of Chicago, the Stickney water reclamation plant, the Cal-Sag Channel, or some other human related activity was the source of these pollutants to the canal. The effluent of the WWTP enters the canal near site 3, and the Cal-Sag Chanel enters at sites 6 and 7. With the exception of total PAH, none of the significant spatial variations that we observed (see discussion above) could be related to either the WWTP or to the Cal-Sag Chanel.
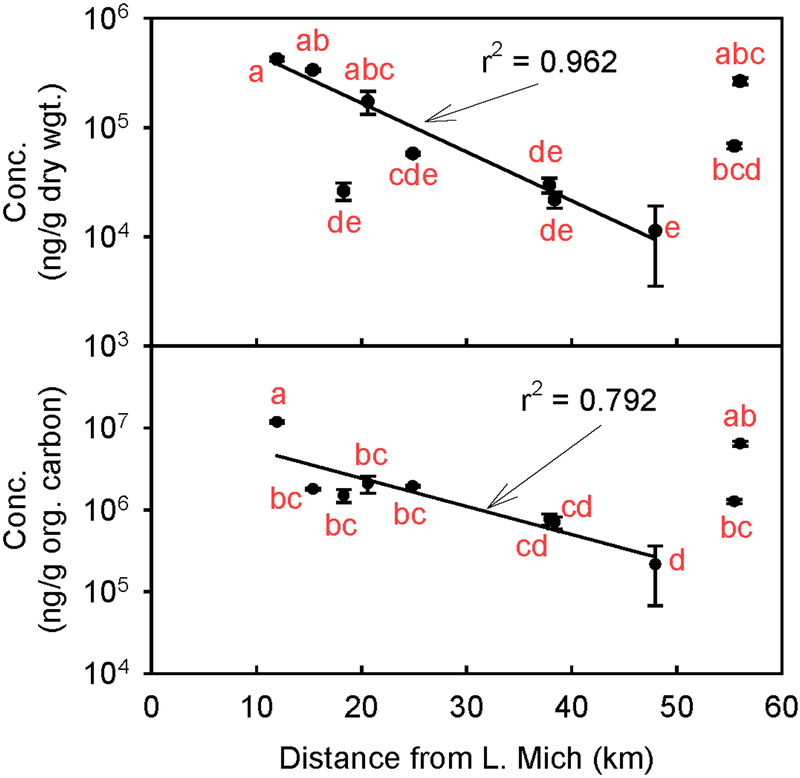
Figure 2:
(Top) Geometric mean concentrations (in ng/g dw) of total PAHs with their corresponding ANOVA results. The line is a regression of the logarithmically transformed concentrations as a function of distance from site 1 to 8, omitting data at site 3; the coefficient of determination is significant at P < 0.01%. (Bottom) Geometric mean concentrations (in ng/g organic carbon) of total PAHs with their corresponding ANOVA results. The line is a regression of the logarithmically transformed concentrations as a function of distance; the coefficient of determination is significant at P = 0.31%. In both cases, the error bars represent standard errors of triplicate measurements, and concentrations sharing the same letter are not significantly different (P < 5%).
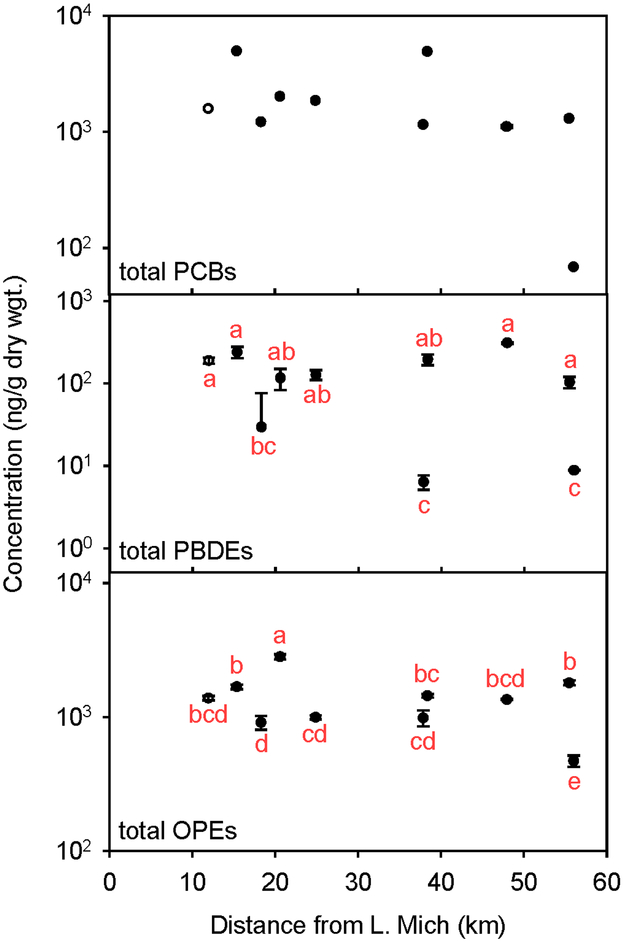
Figure 3:
Concentrations (in ng/g dw) of total PCBs, and geometric mean concentrations (in ng/g dw) of total PBDEs and total OPEs with their corresponding ANOVA results. The error bars represent standard errors of triplicate measurements; however, for total PCBs, only site 8 (48 km) has triplicate measurements. Concentrations sharing the same letter are not significantly different (P < 5%).
As seen in Figure 2 (top), there is a very significant increase in concentrations of PAH in the sediment between sites 3 and 4, but the PAH concentrations at sites 1, 2, 4, and 10 are statistically indistinguishable from one another. Thus, the Stickney water reclamation plant is probably not a source of these compounds. On the other hand, the total PAH concentrations decrease exponentially from sites 1 to 8 (omitting site 3); see the lines in Figure 2. This observation is consistent with a PAH source at the downtown Chicago end of the canal. The relatively high PAH concentrations at sites 9 and 10 are puzzling. These higher levels could be due to more industries and a large amount of barge traffic and dockage at this end of the canal or due to the trapping of sediment just before the downstream dam and lock. The significantly lower levels at site 3 (Figure 2, top) could be related to the inflow of the WWTP causing a temporary dilution of the polluted sediment coming from downtown Chicago, although this effect is not observed with the organic carbon adjusted concentrations (Figure 2, bottom).
A general decrease in PAH concentrations with distance from Chicago was also reported by Mehler and co-workers30 in 2010, who studied the sediment of the CSSC. They reported that the total PAH concentration decreased from 3 mg/g oc (normalized to organic carbon) at the start of the CSSC to 1.3 mg/g oc at the merger of the CSSC and the Cal-Sag Channel. That paper also reported that the total PAH concentration in sediment decreased to 0.4 mg/g oc in Lower Peoria Lake, which is approximately 230 km downstream from Chicago. A similar trend can be seen with sediments taken from Lake Michigan.31 The concentrations of 14 PAHs (summed together) was 400 ng/g dw at a site 8.5 km off shore between Chicago, Illinois and Gary, Indiana, and decreased with increasing distance from shore. It is interesting to note that the total PAH concentration at site 1, nearest Lake Michigan, was about 1,000 times higher than the off-shore level in Lake Michigan. This observation suggests that sediment in the CSSC should not be allowed to leak back into the lake in order to effectively protect Lake Michigan as Chicago’s drinking water source.
Without replicate measurements of PCBs, we were not able to apply ANOVA techniques to these data; however upon inspection of Figure 3, PCB concentrations do not seem to differ from site to site except for site 10, which is at a lower concentration. Site 7 has one of the highest concentrations of total PCBs (even with normalization to organic carbon; see Figure S2), which suggests that the Cal-Sag Channel, not the WWTP, may be a source of these compounds to the CSSC.
The lack of significant spatial variations of flame retardant concentrations as a function of distance down the CSSC suggests that neither the WWTP nor the Cal-Sag Channel are significant sources of these compounds to the canal (see Figures 3 and S2 for concentration of total PBDEs and ANOVA results verses distance). Previous work has shown that wastewater treatment plants are generally doing a good job of removing flame retardants from the plant’s effluent and that these compounds generally remain in the plant’s sludge. Schreder and La Guardia,5 found that the removal rate for BDE-47, 99, and 209 (summed together) was 86%. Therefore, it was expected that the WWTP in this study would not be a source for PBDEs to the CSSC, and that is what we observed. This is not to say that there are no local sources of flame retardants near the canal itself. For example, there are two industrial areas around site 8 that include a decommissioned coal-fired power plant and a gas refinery. A recent study32 found that coal-fired power generation plants are sources of PBDEs to the atmosphere; thus, it is possible that such a facility could be a source of these compounds to the CSSC.
The spatial variations of total OPE concentrations do not indicate a specific source of these compounds (see Figures 3 and S2 for concentrations of total OPEs and ANOVA results verses distance). The only clear exception is site 10, which showed significantly lower concentrations of all of the OPEs. These data suggest that the Stickney water reclamation plant is not a major source of OPEs to the CSSC and that these compounds are not accumulating behind the lower lock and dam system. It should be noted that not all of the OPEs have the same spatial pattern (see Figure S1 and the ANOVA results in Table 1). As stated before, levels of TCEP follow those of the PAHs; thus, it seems likely that the city of Chicago is more of a source of this particular compound than the WWTP. Interestingly, the concentrations of EHDP and TIPPP were highest at site 7, suggesting that its source is the Cal-Sag Channel; however, EHDP also showed a significantly high concentration at site 4, suggesting that this compound may have two sources. Based on the concentrations and spatial trends of PBDEs and OPEs found in this study, it would be interesting to examine more sediment collected between sites 6 and 9 and further up the Cal-Sag Channel.
Supplementary Material
4. Acknowledgements
We thank the U.S. Environmental Protection Agency’s Great Lakes National Program Office for funding (Grant Number GL-00E00515–0, Todd Nettesheim, project officer), Kevin Romanak (Indiana University) for sampling and laboratory assistance, Kyleigh Kriener (University of Massachusetts - Lowell) for PCB statistical analyses, and the Metropolitan Water Reclamation District of Greater Chicago for use of the boat and for help with sampling. The authors declare no competing financial interests.
Footnotes
Associated Content
Supporting Information:
Additional information noted in the text is available.
6. References
- (1).United States Geological Survey, Surface Water Data for USA: USGS Surface-Water Annual Statistics. (2/5/2015).
- (2).US Army Corps of Engineers, Illinois Waterway Locks & Dams. Rock Island District 2012. [Google Scholar]
- (3).>Metropolitan Water Reclamation District of Greater Chicago. Water reclamation plants. https://www.mwrd.org/irj/portal/anonymous/waterreclamation (1/18/14). [DOI] [PubMed]
- (4).Pham T-T; Proulx S PCBs and PAHs in the Montreal Urban Community (Quebec, Canada) wastewater treatment plant and in the effluent plume in the St Lawrence River. Wat. Res 1997, 31, 1887–1896. [Google Scholar]
- (5).Schreder ED; La Guardia MJ Flame retardant transfers from U.S. households (dust and laundry wastewater) to the aquatic environment. Environ. Sci. Technol 2014, 48, 11575–11583. [DOI] [PubMed] [Google Scholar]
- (6).Cristale J; Vázquez AG; Barata C; Lacorte S Priority and emerging flame retardants in rivers: Occurrence in water and sediment, Daphnia magna toxicity and risk assessment. Environ. Int 2013, 59, 232–243. [DOI] [PubMed] [Google Scholar]
- (7).North KD Tracking polybrominated diphenyl ether releases in a wastewater treatment plant effluent, Palo Alto, California. Environ. Sci. Technol 2004, 38, 4484–4488. [DOI] [PubMed] [Google Scholar]
- (8).Song M; Chu S; Letcher RJ; Seth R Fate, partitioning, and mass loading of polybrominated diphenyl ethers (PBDEs) during the treatment processing of municipal sewage. Environ. Sci. Technol 2006, 40, 6241–6246. [DOI] [PubMed] [Google Scholar]
- (9).Covaci A; Harrad S; Abdallah MA-E; Ali N; Law RJ; Herzke D; de Wit CA Novel brominated flame retardants: A review of their analysis, environmental fate, and behavior. Environ. Int 2011, 37, 532–556. [DOI] [PubMed] [Google Scholar]
- (10).Marklund A; Andersson B; Haglund P Organophosphorus flame retardants and plasticizers in Swedish sewage treatment plants. Environ. Sci. Technol 2005, 39, 7423–7429. [DOI] [PubMed] [Google Scholar]
- (11).Martínez-Carballo E; González-Barreiro C; Sitka A; Scharf S; Gans O Determination of selected organophosphate esters in the aquatic environment of Austria. Sci. Total Environ 2007, 388, 290–299. [DOI] [PubMed] [Google Scholar]
- (12).Metropolitan Water Reclamation District of Greater Chicago. Ambient water quality monitoring in the Chicago, Calumet, and Des Plaines River Systems: A summary of biological, habitat, and sediment quality during 2006. Report No. 09–76. [Google Scholar]
- (13).Martinez A; Norström K; Wang K; Hornbuckle KC Polychlorinated biphenyls in the surficial sediment of Indiana Harbor and Ship Canal, Lake Michigan. Environ. Int 2010, 36, 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Craft CB; Seneca ED; Broone SW Loss on ignition and Kjeldahl digestion for estimating organic carbon and total nitrogen in estuarine marsh soils: Calibration with dry combustion. Estuaries 1991, 14, 175–179. [Google Scholar]
- (15).Liu L-Y; Wang J-Z; Wei G-L; Guan Y-F; Wong CS; Zeng EY Sediment records of polycyclic aromatic hydrocarbons (PAHs) in the continental shelf of China: Implications for evolving anthropogenic impacts Environ. Sci. Technol 2012, 46, 6497–6504. [DOI] [PubMed] [Google Scholar]
- (16).Liu L-Y; Kukučka P; Venier M; Salamova A; Klánová J; Hites RA Differences in spatiotemporal variations of atmospheric PAH levels between North America and Europe: Data from two air monitoring projects. Environ. Int 2014, 64, 48–55. [DOI] [PubMed] [Google Scholar]
- (17).Ramdahl T Retene - a molecular marker of wood combustion in ambient air. Nature 1983, 306, 580–582. [Google Scholar]
- (18).Subedi B; Yun S; Jayaraman S; Bergen BJ; Kannan K Retrospective monitoring of persistent organic pollutants, including PCBs, PBDEs, and polycyclic musks in blue mussels (Mytilus edulis) and sediments from New Bedford Harbor, Massachusetts, USA: 1991–2005. Environ. Monit. Assess 2014, 186, 5273–5284. [DOI] [PubMed] [Google Scholar]
- (19).United States Environmental Protection Agency, Basic Information, Polychlorinated Biphenyls (PCBs). http://www.epa.gov/epawaste/hazard/tsd/pcbs/about.htm (2/19/15).
- (20).Ma Y; Salamova A; Venier M; Hites RA Has the phase-out of PBDEs affected their atmospheric levels? Trends of PBDEs and their replacements in the Great Lakes atmosphere. Environ. Sci. Technol 2013, 47, 11457–11464. [DOI] [PubMed] [Google Scholar]
- (21).La Guardia MJ; Hale RC; Newman B Brominated flame-retardants in Sub-Saharan Africa: Burdens in inland and coastal sediments in the eThekwini Metropolitan Municipality, South Africa. Environ. Sci. Technol 2013, 47, 9643–9650. [DOI] [PubMed] [Google Scholar]
- (22).Cristale J; Lacorte S Development and validation of a multi-residue method for the analysis of polybrominated diphenyl ethers, new brominated and organophosphorus flame retardants in sediment, sludge and dust. J. Chromatogr. A 2013, 1305, 267–275. [DOI] [PubMed] [Google Scholar]
- (23).Wei H; Aziz-Schwanbeck AC; Zou Y; Corcoran MB; Poghosyan A; Li A; Rockne KJ; Christensen ER; Sturchio NC Poly-bromodiphenyl ethers and decabromodiphenyl ethane in aquatic sediment from southern and eastern Arkansas, United States. Environ. Sci. Technol 2012, 46, 8017–8024. [DOI] [PubMed] [Google Scholar]
- (24).Watanabe I; Sakai S Environmental release and behavior of brominated flame retardants. Environ. Int 2003, 29, 665–682. [DOI] [PubMed] [Google Scholar]
- (25).López P; Leonards P; Brandsma SA; de Boer J New brominated flame retardants in Dutch sediment and suspended particulate matter. Organohalogen Compd. 2008, 70, 224–227. [Google Scholar]
- (26).van der Veen I; de Boer J Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [DOI] [PubMed] [Google Scholar]
- (27).Wei G; Li D; Zhuo M Liao Y; Xie Z; Guo T; Li J; Zhang S; Liang Z Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity, and human exposure. Environ. Pollut 2015, 196, 29–46. [DOI] [PubMed] [Google Scholar]
- (28).Cao S; Zeng X; Song H; Li H; Yu Z; Sheng G; Fu J Levels and distributions of organophosphate flame retardants and plasticizers in sediment from Taihu Lake, China. Environ. Toxicol. Chem 2012, 31, 1478–1484. [DOI] [PubMed] [Google Scholar]
- (29).Green N; Schlabach M; Bakke T; Brevik EM; Dye C; Herzke D; Huber S; Plosz B; Remberger M; Schoyen M; Uggerud HT; Vogelsang C Screening of selected metals and new organic contaminants 2007. Norwegian Pollution Control Agency, 2008. [Google Scholar]
- (30).Mehler WT; Maul JD; You J; Lydy MJ Identifying the causes of sediment-associated contamination in the Illinois River (USA) using a whole-sediment toxicity identification evaluation. Environ. Toxicol. Chem 2010, 29, 158–167. [DOI] [PubMed] [Google Scholar]
- (31).Huang L; Chernyak SM; Batterman SA PAHs (polycyclic aromatic hydrocarbons), nitro-PAHs, and hopane and sterane biomarkers in sediments of southern Lake Michigan, USA. Sci. Total Environ 2014, 487, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Dong Y; Fu S; Zhang Y; Nie H; Li Z Polybrominated diphenyl ethers in atmosphere from three different typical industrial areas in Beijing, China. Chemosphere 2015, 123, 33–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.