Abstract
OBJECTIVE.
To assess antimicrobial utilization before and after a change in urine culture ordering practice in adult intensive care units (ICUs) whereby urine cultures were only performed when pyuria was detected.
DESIGN.
Quasi-experimental study
SETTING.
A 700-bed academic medical center
PATIENTS.
Patients admitted to any adult ICU
METHODS.
Aggregate data for all adult ICUs were obtained for population-level antimicrobial use (days of therapy [DOT]), urine cultures performed, and bacteriuria, all measured per 1,000 patient days before the intervention (January–December 2012) and after the intervention (January–December 2013). These data were compared using interrupted time series negative binomial regression. Randomly selected patient charts from the population of adult ICU patients with orders for urine culture in the presence of indwelling or recently removed urinary catheters were reviewed for demographic, clinical, and antimicrobial use characteristics, and pre- and post-intervention data were compared.
RESULTS.
Statistically significant reductions were observed in aggregate monthly rates of urine cultures performed and bacteriuria detected but not in DOT. At the patient level, compared with the pre-intervention group (n = 250), in the post-intervention group (n = 250), fewer patients started a new antimicrobial therapy based on urine culture results (23% vs 41%, P = .002), but no difference in the mean total DOT was observed.
CONCLUSION.
A change in urine-culture ordering practice was associated with a decrease in the percentage of patients starting a new antimicrobial therapy based on the index urine-culture order but not in total duration of antimicrobial use in adult ICUs. Other drivers of antimicrobial use in ICU patients need to be evaluated by antimicrobial stewardship teams.
Bacteriuria is a prevalent condition, often difficult to distinguish from urinary tract infection (UTI) in hospitalized patients.1–3 Bacteriuria is also associated with overuse of antimicrobials and is therefore a potential target for antimicrobial stewardship intervention.4–7
Although the presence of pyuria (ie, white blood cells [WBC] in the urine) is not equivalent to UTI,8 the absence of pyuria is valuable in excluding UTI with a negative predictive value of 100% in trauma intensive care unit (ICU) patients, according to one study.9 A review of ordering practices at our hospital showed that providers frequently order urine cultures as part of “fever packs” in critically ill patients, either without an accompanying urinalysis or in the absence of pyuria. Therefore, an initiative aimed at reducing unnecessary urine cultures was implemented in all adult ICUs as follows: A new urine-culture ordering practice known as reflex urine culture was introduced whereby a urinalysis was performed first and urine culture was performed reflexively only if there was evidence of pyuria (ie, urine WBC count >10 per high power field [hpf]).
We hypothesized that this change in ordering practice would reduce the number of unnecessary urine cultures performed, thereby decreasing the detection and treatment of bacteriuria, and ultimately reduce overall antimicrobial use. The objective of this study was to evaluate changes in antimicrobial utilization following the implementation of the reflex urine culture protocol.
METHODS
Study Design and Setting
We conducted a retrospective, quasi-experimental study examining the rates of antimicrobial utilization before the initiation of the reflex urine culture protocol (January– December 2012) and after the initiation of the reflex urine culture protocol (January–December 2013). We evaluated data routinely collected for patient care, infection control surveillance and pharmacy surveillance, in all 7 adult ICUs at our 700-bed tertiary care academic hospital.
Intervention: Reflex Urine Culture Protocol
A ‘reflex’ urine culture protocol was introduced in all adult ICUs in January 2013. The protocol was initially implemented manually by ICU nurses and healthcare providers who checked the results of urinalyses and sent urine samples for culture only if the urine WBC count was >10 per high power field (hpf). In July 2013, an electronic order for the reflex urine culture was created to automate this process at the laboratory level. When the reflex urine culture order is selected, 2 urine tubes are collected simultaneously, 1 for urinalysis and 1 for urine culture. A urinalysis is performed first, and a urine WBC count >10 per hpf generates an order for urine culture visible only to the microbiology lab. If the WBC count is ≤10 per hpf, the urine sample for culture is discarded. Providers may still order regular urinalysis and urine culture (and not reflex urine culture) at their discretion (eg, in immunocompromised patients or patients undergoing urologic procedures).
Outcomes, Data Collection, and Statistical Analysis
This study consisted of 2 distinct components:
1. Population-level analysis.
In the objective of this part of the study, we assessed the population-level impact of this intervention in all adult ICU patients. Aggregate data on the total numbers of urine cultures performed, bacteriuria detected, and antimicrobial use for all patients admitted to any of the 7 adult ICUs between January 1, 2012, and December 1, 2013, were obtained in an automated manner from the hospital’s Infection Prevention and Pharmacy surveillance databases.
The primary outcome was antimicrobial utilization measured using days of therapy (DOT) per 1,000 patient days. For DOT, each antimicrobial administered was counted separately for each day of administration, regardless of number of doses (eg, a patient on both ciprofloxacin and vancomycin for the same 2 days would have a DOT of 4).10 We included the following antimicrobials in the calculation of DOT: formulary carbapenems (doripenem, ertapenem, meropenem), ceftriaxone, fluoroquinolones (ciprofloxacin, levofloxacin), gentamicin, and piperacillin/tazobactam. These antimicrobials were selected because they represent the most commonly used agents for the empiric treatment of UTI in our ICUs and are included in UTI treatment protocols. To determine the intervention’s impact on the frequency of urine culturing and positive urine cultures, the following were measured as intermediate outcome variables: rates of urine cultures performed, defined as number of urine cultures processed by the microbiology lab per 1,000 patient days, and rates of bacteriuria (including funguria), defined as number of positive urine cultures (ie, bacterial or yeast colony count >104 per mL) per 1,000 patient days.
We performed interrupted time series analyses to evaluate changes in monthly rates for antimicrobial utilization, urine cultures performed, and bacteriuria in the pre- and post-intervention periods. Negative binomial regression was used because of overdispersed data for all outcomes. An additional analysis was performed by dividing the post-intervention time period into 2 subperiods: January–July 2013 (when reflex urine culture protocol was implemented but was performed manually), and August–December 2013 (when the protocol was automated). Regression models for all outcomes included a term for secular time (in months), an indicator for pre-intervention period vs post-intervention period, and an indicator-by-time interaction terms, with the period January– December 2012 serving as the reference period. Therefore, we were able to evaluate the trend (slope) in monthly incidence rate prior to the intervention, the overall level change immediately following the intervention (intercept change), and the difference in monthly incidence rate trend between post- and pre-intervention periods (slope change) for each outcome.
3. Patient-level analysis.
To understand the impact of the change in culture ordering practice in a subgroup of patients in whom the intervention was directly applied, we randomly selected 500 patients who had a urine culture ordered, 250 each in both the pre- and post-intervention periods. These patients were selected from the population of patients ≥18 years, had been admitted to an ICU at the time of the index urine-culture order, and had had an indwelling urinary catheter or catheter removed within 48 hours of urine culture. Patients with recent or current urinary catheters were selected because of the higher frequency of bacteriuria and/or UTI compared with non-catheterized patients.11 For the post-intervention period, we selected patients from those hospitalized between August 1 and December 31, 2013, because prior to July 23, the reflex urine culture protocol was a manual process and it was not feasible to distinguish between patients for whom regular vs reflex urine cultures were ordered.
We performed chart reviews for demographic and clinical characteristics of patients. We evaluated any changes in antimicrobial therapy in response to the index urine-culture rder (regular or reflex) by classifying each patient’s antimicrobial status at the time the urine culture was ordered and by whether or not new antimicrobial(s) were started or changed based on the result (positive, negative, or missing urine culture). For each patient, we calculated the combined DOT for all antibiotics as well as the length of therapy (LOT).10 Each day counted only once regardless of the number of anti-microbials administered (eg, a patient on both ciprofloxacin and vancomycin for the same 2 days would have a DOT = 4 and an LOT = 2). Total DOT and LOT were calculated for both the index ICU stay and hospital stay. To evaluate the impact of the intervention if 100% urine culture orders were processed via the reflex urine culture protocol (efficacy analysis), we calculated DOT and LOT for only the subset of patients in the post-intervention period for whom index urine cultures had been ordered via the reflex protocol, compared with all patients in the pre-intervention period. Comorbidity was measured by the Charlson comorbidity index12 using International Classification of Disease, 9th revision (ICD-9) discharge codes from the hospital’s Oracle-based clinical data repository.
Pre- and post-intervention patient group characteristics were compared using the Student t test for continuous variables and χ2 test for categorical variables. To assess potential variables associated with antimicrobial use, multivariate linear regression was used to model antimicrobial DOT (for the index hospital stay), including terms for age, sex, type of urine culture (regular vs reflex), ICU location of index urine culture, number of other urine cultures performed, and number of blood cultures performed. All analyses were performed with an α value of 0.05 using Stata 12 (College Station, TX). We estimated that with a sample size of 250 per group, the minimum difference in DOT (or LOT) that we would be able to detect between groups would be 6 days, with 80% power.
RESULTS
Population-Level Analysis
There were a total of 47,129 ICU patient days in the pre-intervention period during which 6,048 urine cultures were performed and 1,685 were positive (bacteriuria). The post-intervention period consisted of 48,589 ICU patient days with 2,856 urine cultures performed and 1,039 cases of bacteriuria.
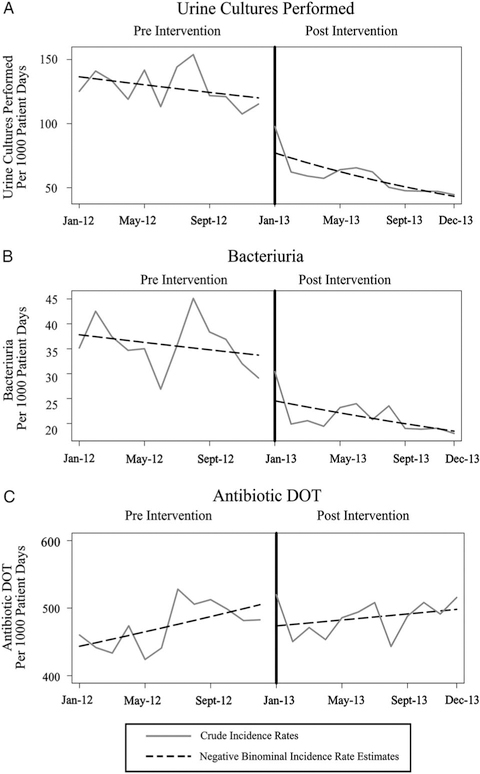
The overall incidence rate of urine cultures performed was 139 urine cultures per 1,000 patient days pre-intervention and 93 cultures per 1,000 patient days post-intervention (89 in January–July 2013, and 93 in August–December 2013). Prior to the intervention, from January–December 2012, there was a significant 2% month-to-month decrease in the rates of urine cultures performed (P < .05). Compared with the pre-intervention period, there was a 30% decrease in the rate of urine cultures performed immediately following the intervention (P < .001), followed by a 6% relative decrease in the month-to-month trend in January–December 2013 (P < .05) (Figure 1A).
FIGURE 1.
Crude and predicted estimates for monthly rates of urine cultures performed, (B) bacteriuria, and (C) antimicrobial days of therapy, before and after the change in urine-culture ordering practice.
The overall incidence rate of bacteriuria was 37 positive urine cultures per 1,000 patient days in the pre-intervention period and 27 positive urine cultures per 1,000 patient days in the post-intervention period (27 in January–July 2013, and 29 in August–December 2013). Prior to the intervention, from January–December 2012, there was no significant month-to-month change in the bacteriuria rate. A 28% decrease in the bacteriuria rate occurred immediately following the intervention (P < .001), but no significant relative change in the month-to-month trend in January–December 2013 (Figure 1B).
The overall utilization of selected urinary antimicrobials was 449 DOT per 1,000 patient days during the pre-intervention period and 425 DOT per 1,000 patient days during the post-intervention period (423 in January–July 2013, and 426 in August–December 2013). Prior to the intervention, from January–December 2012, there was a significant 1% month-to-month increase in the antimicrobial utilization rate (P < .01). Compared with the pre-intervention period, there was no significant change in the antimicrobial utilization rate either immediately following the intervention or in the relative month-to-month trend in January–December 2013 (Figure 1C).
The additional analysis did not reveal any significant differences in the interpretations of the effects when considering the post-intervention period as a single time period or 2 separate time periods corresponding to the manual vs automated electronic intervention described above.
Patient-Level Analysis
A total of 500 patients (250 each in the pre- and post- intervention groups) were assessed. Comparing the post- and pre-intervention periods, there was a lower proportion of male patients (57% vs 64%) and more trauma ICU patients (37% vs 26%). There were no differences between the pre-intervention and post-intervention groups in age, Charlson comorbidity score, length of ICU stay and hospital stay, duration of urinary catheterization, proportion of patients already receiving antimicrobials at the time of the index urine culture, proportion of patients with at least 1 blood culture performed, or the mean number of blood cultures per patient (Table 1).
TABLE 1.
Demographic, Clinical, and Urine Culture Characteristics of Random Samples of Adult ICU Patients with Orders for Urine Cultures, Pre- and Post-Implementation of a Reflex Urine Culture Protocol
| Characteristic | Pre-intervention, (n = 250) | Post-intervention (n = 250) | P Value |
|---|---|---|---|
| Age, y, mean (SD) | 57.4 (18) | 57.7 (17) | .85 |
| Sex, No. (% male) | 161 (64) | 139 (56) | .04 |
| Charlson comorbidity index, mean (SD) | 2.55 (2.1) | 2.42 (2.5) | .48 |
| ICU type, No. (%) | |||
| Medical | 63 (25) | 51 (20) | .09 |
| Cardiac | 15 (6) | 13 (5) | |
| Cardiac surgical | 30 (12) | 24 (10) | |
| Trauma | 66 (26) | 93 (37) | |
| Surgical | 41 (16) | 28 (11) | |
| Neurocare | 35 (14) | 41 (16) | |
| Hospital length of stay, d, mean (SD) | 24.4 (22.7) | 21.7 (17.5) | .13 |
| ICU length of stay, d, mean (SD) | 16.2 (15.2) | 15.3 (12.3) | .49 |
| Duration of catheterization, d, mean (SD) | 14.9 (15.4) | 12.6 (12.4) | .06 |
| On any antibiotic at index urine culture, No. (%) | 116 (46) | 127 (51) | .32 |
| Type of index urine culture, No. (%) | |||
| Regular | 250 (100) | 74 (30) | <.001 |
| Reflex | 0 (0) | 176 (70) | |
| WBC/hpf of index urinalysis, No. (%) | |||
| Not sent | 29 (12) | 20 (8) | .29 |
| ≤10 per hpf | 138 (55) | 152 (61) | |
| >10 per hpf | 83 (33) | 78 (31) | <.001 |
| Result of index urine culture | |||
| Negative | 173 (69) | 70 (28) | |
| Positive | 77 (31) | 40 (16) | |
| Not applicable, culture not performed | 0 (0) | 140 (56) | |
| Another urine culture performed | 178 (71) | 131 (52) | <.001 |
| No. of urine cultures per patient,a mean (SD) | 2.6 (3.3) | 1.0 (1.5) | <.001 |
| Any blood culture performed, No. (%) | 226 (90) | 227 (91) | .88 |
| No. of blood cultures per patient,a mean (SD) | 3.5 (3.4) | 3.2 (3.3) | .45 |
NOTE. ICU, Intensive care unit; hpf, high power field; SD, standard deviation; IQR, interquartile range.
No. of cultures per patient were for the entire hospital stay.
During the pre-intervention period, all 250 patient urine cultures were ordered as regular urine cultures, whereas in the post-intervention period, 74 (30%) of urine cultures were ordered as regular cultures and 176 (70%) were ordered as reflex urine cultures. Approximately 30% of patients in both groups had pyuria. The mean number of urine cultures performed per patient was significantly less in the post-intervention group (1, standard deviation [SD], 0.8) compared with pre-intervention group (2.6; SD, 2.2; P < .001). The proportion of patients detected to have bacteriuria was significantly less in the post-intervention group (pre-intervention, 77 of 250, 31%; post-intervention, 40 of 250, 16%; P < .05) (Table 1).
The proportion of patients (not previously on antimicrobials) started on a new antimicrobial based on the result of the index urine-culture order was significantly lower in the post-intervention group: 28 of 123 (23%) compared to 55 of 134 (41%) in the pre-intervention group (P = .002). There was no difference in the mean total DOT or LOT during the index ICU stay or hospital stay between the pre- and post-intervention groups, even when restricting the analysis to patients with only reflex urine culture orders in the post-intervention group, and whether including or excluding antifungal therapy. There was no significant difference between groups in the proportion of patients with lab-confirmed C. difficile infection (diagnosed after index urine culture) (Table 2).
TABLE 2.
Characteristics of Antimicrobial Utilization and Clostridium difficile Infection in Random Sample of Adult ICU Patients with Orders for Urine Cultures, Pre- and Post-Reflex Urine Culture Protocol
| Pre-intervention (n = 250) | Post-intervention (n = 250) | P value | |||
|---|---|---|---|---|---|
| Antibiotic changes in response to index urine culture, No. (%) | |||||
| Not on antibiotic, new antibiotic started | 55 (22) | 28 (11) | .002a | ||
| Not on antibiotic, no new antibiotic started | 79 (32) | 95 (38) | |||
| On antibiotic, changed based on culture result | 41 (16) | 37 (15) | .30b | ||
| On antibiotic, no change | 75 (30) | 90 (36) | |||
| Antibiotic utilization | Pre-intervention (n = 250) | Post-intervention (n = 250) | P Valuec | Post-Intervention (Reflex Urine Culture only, n = 176) | P Valued |
| Days of therapy (hospital stay), mean (SD) | 26.8 (37.3) | 26.6 (45.5) | .98 | 22.3 (29.7) | .19 |
| Days of therapy (ICU stay), mean (SD) | 18.2 (23.8) | 17.5 (21.6) | .75 | 17.8 (22.4) | .86 |
| Length of therapy (hospital stay), mean (SD) | 15.3 (17.3) | 14.9 (26.9) | .85 | 12.6 (13.8) | .09 |
| Length of therapy (ICU stay), mean (SD) | 14.4 (18.0) | 13.3 (15.4) | .50 | 13.6 (16.7) | .67 |
| C. difficile infection after index urine culture, No. (%) | 19 (8) | 14 (6) | .37 | 12 (7) | .76 |
NOTE. ICU, intensive care unit; SD, standard deviation.
P value derived from χ2 test of equal proportion between those “not on antibiotic, new antibiotic started” vs “not on antibiotic, no new antibiotic started.”
P value derived from χ2 test of equal proportion between those “on antibiotic, changed based on any culture result” vs “on antibiotic, no change.”
P value derived from unpaired t test between pre- and post-intervention groups.
P value derived from unpaired t test between pre-intervention group and those patients having an index reflex urine culture in the post-intervention period.
Adjusting for age, sex, type of index urine culture (reflex vs regular), Charlson comorbidity score, and ICU location at the time of the index urine culture, there was an increase of 2 DOT (95% confidence interval [CI], 1.5–2.6) for every additional urine culture performed, and an increase of 2 DOT (95% CI, 1.7–2.6) for every additional blood culture performed (both P < .001). Relative to the medical ICU, being in the neurocare ICU or the trauma ICU at the time of index urine culture was associated with 8 fewer DOT (95% CI, 4.6–11.7; P < .001) and 7 fewer DOT (95% CI, 3.0–12.4; P < .05), respectively. There was no significant difference for surgical, cardiac, or cardiac surgical ICU DOT compared with medical ICU DOT. The effect of reflex vs regular urine culture was not significant in this model.
DISCUSSION
The introduction of a urine-culture ordering practice in which a urine culture is conditionally performed only in the presence of pyuria was associated with significant and immediate reductions in the rates of urine cultures performed and detected bacteriuria among adults in ICUs. Among patients with orders for urine culture, there was a significant decrease in the proportion of patients detected to have bacteriuria, and therefore newly started on antimicrobial therapy after introduction of the reflex urine culture protocol. However, this decrease was not accompanied by a decrease in the overall antimicrobial utilization in this population.
Prior studies have shown some effectiveness of interventions to reduce unnecessary antimicrobial therapy for bacteriuria in various settings.13–16 In a quasi-experimental study using an educational memorandum placed in patient charts, Linares et al15 found a 65% relative reduction in antimicrobial days for asymptomatic bacteriuria and culture-negative pyuria among patients with abnormal urinalysis or urine culture results in a Veterans’ Affairs hospital. Similarly, another quasi-experimental study assessing the impact of infectious diseases consultation on bacteriuria treatment in a geriatric hospital reported a reduction in total antimicrobial days of therapy following the intervention.13 A cluster-randomized trial utilizing a multifaceted diagnostic and treatment algorithm for suspected UTI in long-term care facilities found a reduction in the number of antimicrobial prescriptions for UTI but no difference in the total antimicrobial use per 1,000 resident days between the intervention and usual care groups, similar to our study.16
The ordering of urine cultures and treatment of patients with positive urine cultures, even with a low clinical suspicion of UTI, could be related to long-standing practices and perceptions17,18 rather than knowledge of clinical guidelines.4 A recent proof-of-concept study found a reduction in treatment of asymptomatic bacteriuria (measured as the proportion of patients treated) after discontinuing the routine reporting of positive urine cultures of non-catheterized patients in their electronic medical record unless requested by clinicians.14 Our intervention is conceptually similar in that the presence of bacteriuria is “hidden” because pyuria-negative urines were not cultured and, therefore, cases of bacteriuria associated with lack of pyuria were not detected. We also found a reduction in the proportion of patients started on antibiotics following the reflex urine culture protocol, but it was not associated with a reduction in the overall antimicrobial use in those patients or in the ICU population as a whole.
Our patient-level analysis provides some insight into factors associated with antimicrobial use. Specifically, the numbers of blood and urine cultures per patient were strongly associated with days of antimicrobial therapy, which likely reflects the fact that suspicion of sepsis frequently drives both culturing and antimicrobial treatment. Given the lack of effect on total antimicrobial utilization (days of therapy) despite fewer patients being started on a new antimicrobial therapy following the index urine culture results, it appears that the treatment of isolated episodes of identified bacteriuria is only a drop in the larger antimicrobial use bucket. While experts have long argued for a change in clinical practice to align both the ordering and interpretation of urine cultures with clinical suspicion for UTI even in critically ill patients,19 this finding has been countered with the importance of early empiric antibiotic therapy in reducing mortality due to sepsis.20,21 In a survey of empiric antimicrobial therapy practice patterns among critically ill surgical patients, deteriorating organ function was associated with a decision to broaden empiric therapy.22 Collectively, our findings underscore the need to understand the complexity of factors that drive antimicrobial use in hospitals, particularly among ICU patients, prior to designing antimicrobial stewardship interventions.
The strengths of our study include a robust analysis of patient-level and population-level outcomes. With one exception,16 previous studies did not assess the overall antimicrobial use (as opposed to only UTI-specific therapy) in their study population, and no study included a population of critically ill patients. The main limitation of this study is that it was performed at a single center and did not have a control group. We were unable to completely account for factors such as other clinical diagnoses that could affect outcomes. Furthermore, this intervention occurred alongside other initiatives aimed at reducing catheter-associated UTI rates at our hospital in 2012–2013, which could have influenced urine culture and bacteriuria rates and is likely reflected in monthly decreases in urine cultures performed even prior to introduction of the reflex protocol. However, the significant immediate effect following the reflex urine culture protocol suggests a specific impact of this intervention. In the patient-level comparisons, the pre-intervention group was selected between January and December, whereas the post-intervention group was selected between August and December. It is possible that there is a seasonal difference in antimicrobial use that was not reflected in the overall trends in antimicrobial use. Similarly, although the lack of difference in DOT at the patient level could be a function of sample size, no difference in aggregate DOT rates was observed when including the entire ICU patient population. Finally, while we were unable to systematically study potential harms of undetected bacteriuria, no missed cases of urosepsis were identified through routine infection control surveillance.
In summary, implementation of a reflex urine culture protocol was associated with significant decreases in the rates of urine cultures performed, detected bacteriuria, and the proportion of patients newly started on antibiotics based on urine culture orders among adults admitted to ICUs. However, these changes were not associated with a reduction in overall antimicrobial utilization in this population. Additional strategies in reducing unnecessary antimicrobial use need to be considered.
ACKNOWLEDGMENTS
Financial support. A.D.H. is supported by National Institutes of Health (grant no. 5K24AI079040–05). All other authors report no financial support relevant to this article.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
REFERENCES
- 1.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50:625–663. [DOI] [PubMed] [Google Scholar]
- 2.Kwon JH, Fausone MK, Du H, Robicsek A, Peterson LR. Impact of laboratory-reported urine culture colony counts on the diagnosis and treatment of urinary tract infection for hospitalized patients. Am J Clin Pathol 2012;137:778–784. [DOI] [PubMed] [Google Scholar]
- 3.Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients. Arch Intern Med 2000;160:678–682. [DOI] [PubMed] [Google Scholar]
- 4.Cope M, Cevallos ME, Cadle RM, Darouiche RO, Musher DM, Trautner BW. Inappropriate treatment of catheter-associated asymptomatic bacteriuria in a tertiary care hospital. Clin Infect Dis 2009;48:1182–1188. [DOI] [PubMed] [Google Scholar]
- 5.Dellit TH, Owens RC, McGowan JE Jr., et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44:159–177. [DOI] [PubMed] [Google Scholar]
- 6.Hartley S, Valley S, Kuhn L, et al. Overtreatment of asymptomatic bacteriuria: identifying targets for improvement. Infect Control Hosp Epidemiol 2015;36:470–473. [DOI] [PubMed] [Google Scholar]
- 7.Silver SA, Baillie L, Simor AE. Positive urine cultures: a major cause of inappropriate antimicrobial use in hospitals? Can J Infect Dis Med Microbiol 2009;20:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med 2000;160:673–677. [DOI] [PubMed] [Google Scholar]
- 9.Stovall RT, Haenal JB, Jenkins TC, et al. A negative urinalysis rules out catheter-associated urinary tract infection in trauma patients in the intensive care unit. J Am Coll Surg 2013;217:162–166. [DOI] [PubMed] [Google Scholar]
- 10.Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011;53:1100–1110. [DOI] [PubMed] [Google Scholar]
- 11.Infections Healthcare-associated (HAIs)/Catheter-associated Urinary Tract Infections (CAUTI). Centers for Disease Control and Prevention website http://www.cdc.gov/HAI/ca_uti/uti.html. Published 2015. [Google Scholar]
- 12.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 13.Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc 2008;9:605–609. [DOI] [PubMed] [Google Scholar]
- 14.Leis JA, Rebick GW, Daneman N, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis 2014;58:980–983. [DOI] [PubMed] [Google Scholar]
- 15.Linares LA, Thornton DJ, Strymish J, Baker E, Gupta K. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol 2011;32:644–648. [DOI] [PubMed] [Google Scholar]
- 16.Loeb M, Brazil K, Lohfeld L, et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ 2005;331:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leis JA, Gold WL, Daneman N, Shojania K, McGeer A. Downstream impact of urine cultures ordered without indication at two acute care teaching hospitals. Infect Control Hosp Epidemiol 2013;34:1113–1114. [DOI] [PubMed] [Google Scholar]
- 18.Walker S, McGeer A, Simor AE, Armstrong-Evans M, Loeb M. Why are antibiotics prescribed for asymptomatic bacteriuria in institutionalized elderly people? A qualitative study of physicians’ and nurses’ perceptions. CMAJ 2000;163:273–277. [PMC free article] [PubMed] [Google Scholar]
- 19.Vaisman A, Gold WL, Leis JA. Interpreting positive urine cultures. The authors respond. CMAJ 2013;185:1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackerman MJ, Worster A, Lin D. Interpreting positive urine cultures. CMAJ 2013;185:1526–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellinger RP, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Crit Care Med 2013;41:580. [DOI] [PubMed] [Google Scholar]
- 22.Aarts MA, Granton J, Cook DJ, Bohnen JM, Marshall JC. Empiric antimicrobial therapy in critical illness: results of a surgical infection society survey. Surg Infect (Larchmt) 2007;8:329–336. [DOI] [PubMed] [Google Scholar]