Key Teaching Points.
-
•
Although typical atrial flutter is the predominant form of macroreentrant tachycardia in patients without history of cardiac intervention, rare complicated situations such as the present dual-loop biatrial tachycardia may be observed.
-
•
Even when counterclockwise rotation around the tricuspid annulus in the right atrium has been demonstrated, cautious observation of activation sequence in both atria is still critical to discover the unusual complex situation.
-
•
Detailed mapping with a 3-dimensional system is helpful to visualize the possible propagation of reentries and understand underlying substrate. Multiple entrainment mapping and measurement may further support the final diagnosis, show the relation between these 2 reentries, and suggest ablation strategy.
Introduction
While typical atrial flutter (AFL) is often associated with anatomical or functional conduction block along the physiological structures, atypical AFL or macroreentrant atrial tachycardia frequently occurs in patients with structural heart disease, after open cardiac surgery or ablation for atrial fibrillation. The complex lesions contribute to the substrate of atrial macroreentry. Dual-loop atrial reentry was first described in patients who had previously undergone closure of atrial septal defects and the tachycardias were intra-atrial reentries.1, 2 There are only sporadic reports of biatrial tachycardias postsurgery or postablation.3, 4 We present a unique case of a dual-loop biatrial macroreentrant tachycardia, which developed in a patient without structural abnormality or previous cardiac interventions.
Case report
A 69-year-old man with a history of coronary heart disease, but neither myocardial infarction nor history of surgery or ablation, was referred for ablation of persistent AFL. The patient had been in persistent AFL for 21 months. Electrical conversion had been attempted twice with recurrence in a short period, and metoprolol was taken to control the heart rate. Positive P waves presented in leads II (with a tiny negative initial deflection), III, aVF, and V1 on the surface electrocardiogram (Figure 1A). The activation pattern shown by a 20-pole catheter positioned around the tricuspid annulus with distal poles in the coronary sinus (CS) suggested a counterclockwise AFL in the right atrium (RA) but almost simultaneous activation in the CS. The tachycardia cycle length (TCL) was 270 ms (Figure 1B and C). Biatrial 3-dimensional electroanatomical mapping (CARTO 3, Biosense Webster, Irvine, CA) also suggested a cavotricuspid isthmus (CTI)-dependent AFL in the RA, while a craniocaudal activation pattern on the posterior wall of the left atrium (LA) was observed (Figure 2). Entrainment mapping demonstrated differences in postpacing interval (PPI) and a TCL of 0–30 ms at the CTI; lateral, anterior, and septal walls of the RA; and the roof and septal wall of the LA, but > 30 ms at the LA lateral wall and CS. This confirmed that a roof-dependent AFL surrounding the right pulmonary veins (RPV) existed concomitantly with the CTI-dependent AFL and used the interatrial septum as a common pathway (Figure 2). Double potentials were found at the RA posterior wall and inferior septum, the LA anterolateral wall and septum, and the junction between the RPV and LA roof. An area of lower voltage (<0.5 mV) was observed at the posterior LA septum and roof. Accurate measurement of the relative activation time of both sides of the septum demonstrated that the LA septum was activated 40–50 ms earlier than the RA septum inferiorly but 15–50 ms later superiorly, which suggested that it took more time for the reentry in the LA than that in the RA to propagate through the septum. The differences in activation time were more significant in the posterior part than the anterior part of the septum. At the anterosuperior septum, the activation time of both sides was consistent with the CTI-dependent AFL (Figure 3A and B). These observations suggest that the reentries in the LA and RA may rotate separately and involve the left and right septum, respectively. Ablation at the CTI resulted in a sudden alteration of the activation sequence in the RA without cessation of the tachycardia or change of TCL (Figure 1D). Repeated mapping of the LA demonstrated that the same roof-dependent AFL was still present. However, the anterosuperior LA septum was activated much later than previously (Figure 3C and D). This indicated that the anterior septum was driven passively by the RA reentry before this was eliminated and that it was activated by wavefront propagation of the roof-dependent reentry after elimination. Finally, linear ablation of the LA roof and isolation of the RPV led to termination and noninducibility of tachycardia (Figure 1E).
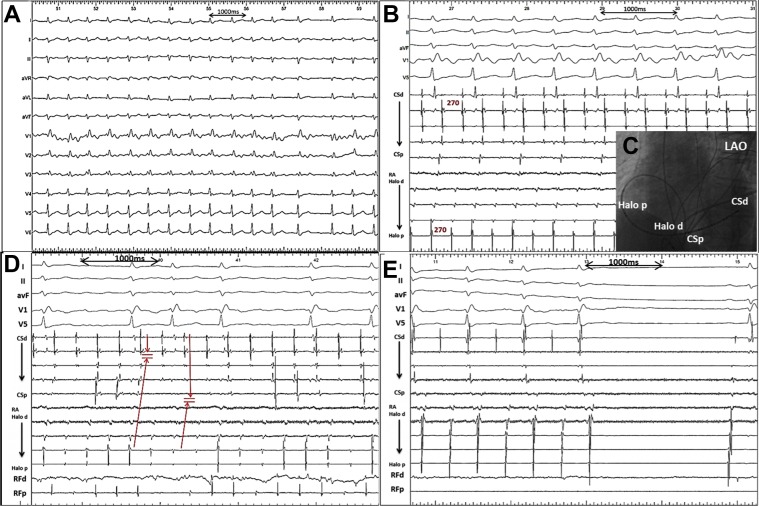
Figure 1.
A: Twelve-lead electrocardiogram showed persistent atrial flutter (AFL) with positive P waves in leads II (with a tiny negative initial deflection), III, aVF, and V1; negative P waves in lead aVR; and almost isoelectric P waves in leads I, aVL, and V2–V6. B: Intracardiac electrograms of the AFL recorded from a 20-electrode catheter from the lateral wall of the right atrium (RA) to the distal coronary sinus (CS). The tachycardia cycle length was 270 ms. The activation sequence in the RA was consistent with a counterclockwise direction while the activation in the CS was almost simultaneous. C: Fluoroscopic image. D: Ablation at the cavotricuspid isthmus led to a sudden alteration of atrial activation sequence, although the tachycardia continued. E: Isolation of the right pulmonary veins and linear ablation of the left atrial roof led to termination of the tachycardia and restoration of sinus rhythm. CSd = distal of the coronary sinus; CSp = proximal of the coronary sinus; Halo d = distal of the RA part of the 20-electrode catheter; Halo p = proximal of the RA part of the 20-electrode catheter; LAO = left anterior oblique.
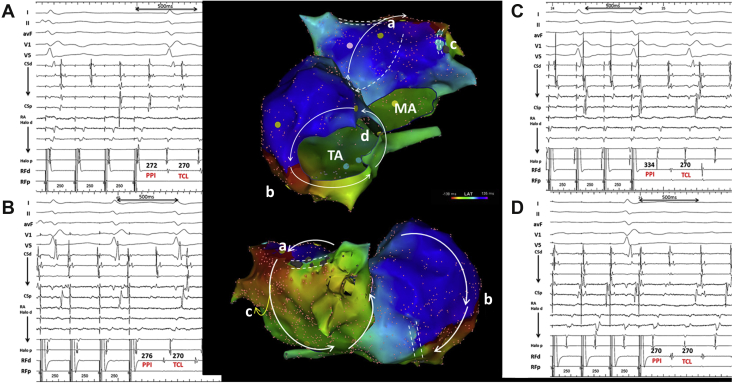
Figure 2.
Activation map indicating the cavotricuspid isthmus–dependent reentry in the right atrium (RA) and roof-dependent reentry in the left atrium (LA) (middle upper panel: anterior view; middle lower panel: posterior view; white arrows indicate the directions of propagation of the reentrant circuits). Entrainment pacing and further measurements support the existence of concomitant reentrant circuits with postpacing interval (PPI)–tachycardia cycle length (TCL) ≤ 30 ms at the LA roof (A), RA lateral wall along the tricuspid annulus (B), and interseptum (D), while a PPI–TCL at the LA lateral wall is much longer (C). MA = mitral annulus; TA = tricuspid annulus.
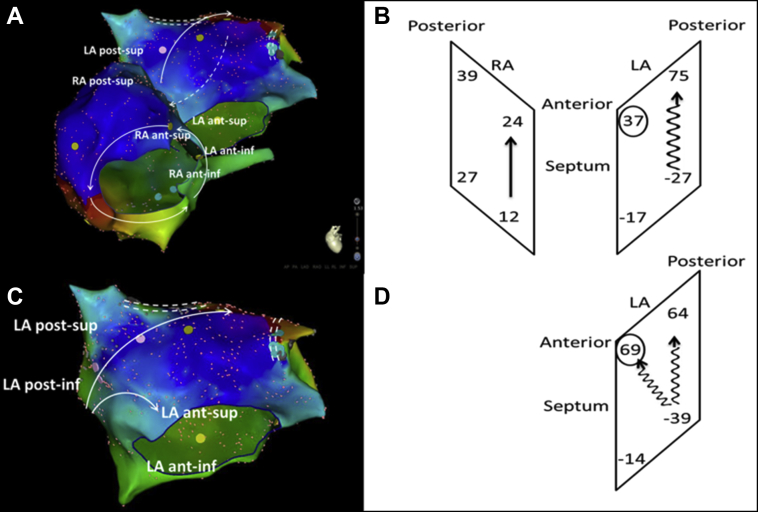
Figure 3.
Measurements of the local activation time (LAT) at both sides of septum with location of assessment (left panel) and explanatory sketch (right panel). The left atrial (LA) septum was activated earlier than the right atrium (RA) septum inferiorly (LAT anterior: -17 vs 12 ms, posterior: -27 vs 27 ms) but later superiorly (anterior: 37 vs 24 ms, posterior: 75 vs 39 ms). Conduction time was longer along the LA septum than the RA septum (anterior: 54 vs 12 ms, posterior: 102 vs 12 ms; A, B). Repeated mapping of the LA after the RA reentry was eliminated (C, D) demonstrated that the same roof-dependent atrial flutter was still present, but the anterosuperior LA septum was activated much later than before (LAT 69 vs 37 ms, shown in circles), with the activation time of the posterior LA septum unchanged (103 vs 102 ms).
Discussion
Complex macroreentrant atrial tachycardia is frequently related to structural heart disease, surgery, or ablation lesions. Dual-loop biatrial macroreentrant tachycardia has scarcely been reported3, 4 and also is related to postsurgery state. In this patient with no previous cardiac interventions, the counterclockwise atrial activation surrounding the tricuspid annulus might simply have given an initial impression of typical AFL. However, the positive P waves in leads II, III, and aVF did not fit the typical AFL, and in addition, the activation sequence of the LA did not support a passive bystander role for the LA. On observing this, we used biatrial 3-dimensional and entrainment mapping and finally revealed the dual-loop biatrial reentry. It may therefore be necessary to map both atria for complex atypical AFL cases to clarify the mechanisms involved.
Another interesting phenomenon is the discordant conduction time and direction along the LA and RA septum. In contrast to most interdependent dual-loop reentry (such as 8-figure), this case suggested that the 2 reentries might share the atrial septum pathway but rotate independently. In other words, the atrial septum may not serve as a common isthmus. Targeting of ablation on the anatomically complicated interatrial septum is almost impossible and probably has limited effect, and the tachycardias must be treated separately.5, 6
Finally, double potentials were observed in multiple regions in both atria. The presence of double potentials in the right posterior wall and septum was more likely physiological, in consistency with the anatomical obstacles of the crista terminalis and eustachian ridge, and those in the LA were correspondent to the low voltage area and might relate to pathophysiological changes. These areas with functional block and slow conduction provide the substrate for the formation of the specific dual-loop biatrial reentries. These observations also suggest that underlying pathophysiological changes, which are possibly caused by longstanding persistent AFL and atrial remodeling, may exist even in a heart without prior incision or ablation lesions.
Conclusion
Complex macroreetrant tachycardia can occur in a patient without apparent structural abnormality or cardiac intervention history. Detailed mapping by use of a 3-dimensional system combined with entrainment and analytical measurement could be helpful to establish the final diagnosis.
Footnotes
Dr Jian Chen serves as an advisory consultant for Biosense Webster and Johnson & Johnson. Dr Song-Yun Chu is funded by the CSC scholarship from the Research Council of Norway.
References
- 1.Shah D., Jais P., Takahashi A., Hocini M., Peng J.T., Clementy J., Haissaguerre M. Dual-loop intra-atrial reentry in humans. Circulation. 2000;101:631–639. doi: 10.1161/01.cir.101.6.631. [DOI] [PubMed] [Google Scholar]
- 2.Magnin-Poull I., De Chillou C., Miljoen H., Andronache M., Aliot E. Mechanisms of right atrial tachycardia occurring late after surgical closure of atrial septal defects. J Cardiovasc Electrophysiol. 2005;16:681–687. doi: 10.1046/j.1540-8167.2005.30605.x. [DOI] [PubMed] [Google Scholar]
- 3.Namdar M., Gentil-Baron P., Sunthorn H., Burri H., Shah D. Postmitral valve replacement biatrial, septal macroreentrant atrial tachycardia developing after perimitral flutter ablation. Circ Arrhythm Electrophysiol. 2014;7:171–174. doi: 10.1161/CIRCEP.113.000656. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T., Mitsuhashi T., Fujita H., Momomura S. Dual-loop bi-atrial macroreentrant atrial tachycardia in a patient with modified Cox Maze IV: where is the initial ablation target? J Cardiovasc Electrophysiol. 2016;27:621–622. doi: 10.1111/jce.12881. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura T., Martin R., Denis A. Characteristics of single-loop macroreentrant biatrial tachycardia diagnosed by ultrahigh-resolution mapping system. Circ Arrhythm Electrophysiol. 2018;11:e005558. [Google Scholar]
- 6.Guiraudon G.M., Jones D.L., Skanes A., Tweedie E., Klein G.J. Revisiting right atrial isolation rationale for atrial fibrillation: functional anatomy of interatrial connections. J Interv Card Electrophysiol. 2013;37:267–273. doi: 10.1007/s10840-013-9804-8. [DOI] [PubMed] [Google Scholar]