Abstract
Introduction
Following the release of the Centers for Disease Control and Prevention guidelines in 2016, institutions are encouraged to have controlled substance agreements that require the use of drug screens for appropriate opioid prescribing. Correct evaluation of urine drug tests (UDTs) is essential for appropriate chronic prescribing of controlled substances. Anticipating the increase in use of these tests, our institution developed and implemented an educational program to improve knowledge of and comfort level with UDT interpretation.
Methods
The educational program was 30 minutes in duration and consisted of a PowerPoint presentation followed by informal discussion. All internal medicine and medicine/pediatrics residents were encouraged to attend. A survey assessing knowledge of and comfort level with interpreting UDTs before, immediately after, and 2 months following the educational program was used as an assessment tool.
Results
A total of 44 out of 76 residents at our institution attended the educational program. The majority (81.8%) had no prior education on UDT interpretation; however, most (97.7%) stated they interpreted UDTs monthly, and 22.7% had refused refills within the prior month based on UDT results. Change in residents' knowledge and change in residents' comfort were both found to be significantly increased following the educational program (p < .0001 for both variables).
Discussion
Significant increases in both comfort with and knowledge of UDT interpretation occurred following the educational program provided for medical residents, which supports its expansion to other institutions as an easy and effective means of promoting appropriate UDT evaluation.
Keywords: Opioid-Related Disorders, Opioid Abuse, Urine Drug Test, Urine Drug Screen, Pharmacist Education
Educational Objectives
By the end of this educational program, learners will be able to:
-
1.
Describe the two main screening methods employed for urine drug tests (UDTs).
-
2.
Identify ways to assess a patient's urine sample for adulteration, substitution, or dilution.
-
3.
Recognize common medication causes for UDT false positives.
-
4.
Apply knowledge gained to correctly interpret a patient's UDT results.
Introduction
Drug overdoses and deaths continue to increase in the United States. In 2015, drug overdoses accounted for 52,404 deaths in the U.S., including 33,091 (63.1%) that involved an opioid.1 The rise in opioid-related deaths has been directly linked to an increase in opioid prescriptions, with opioid prescriptions and opioid-related deaths both quadrupling since 1999.2,3 The abuse of controlled substances is generally related to their euphoric and pain-relieving effects.4
In 2016, concerns about an opioid abuse epidemic in the U.S. led the Centers for Disease Control and Prevention (CDC) to release recommendations for safe prescribing of opioids.5 These guidelines recommend the use of urine drug tests (UDTs) before starting opioid therapy and then at least annually if the prescription is continued. UDTs assess a patient's urine sample for the presence of certain illegal drugs and prescription medications by detecting controlled substances and their metabolites via immunoassay and mass spectrometry. UDTs are a useful tool as they are easily administered and are effective in detecting commonly abused medications.6 With these CDC recommendations, the use of UDTs is expected to increase. However, UDTs can be difficult to interpret and are subject to misinterpretation as other medications taken by patients may cause false positive results if initial immunoassay results are not confirmed with mass spectrometry.7 Variability in the metabolism of specific medications such as oxycodone and fentanyl resulting in negative opioid screening and lack of urine sample validation through urinalysis are another common source of misinterpretation.7 Correct interpretation of UDTs is essential for safe and appropriate long-term prescribing of controlled substances.
In July 2016, following the newly released CDC guidelines, the outpatient clinics (OPCs) within our health system updated their controlled substance agreement in order to align with the new CDC recommendations. This agreement affects all controlled substance prescriptions, including opioids as well as other medication classes monitored by the Drug Enforcement Administration. Some examples of medications included in this agreement are amphetamines, barbiturates, benzodiazepines, and methadone. All clinic patients prescribed controlled substances for more than 90 days must sign one of these agreements. The agreement requires that the patient have a negative UDT for illicit substances or controlled substances not being prescribed and a positive UDT for any controlled substance(s) being prescribed. Our medical group–affiliated OPC is impacted by this agreement. Within the OPC, medical residents serve as primary care providers for over 5,000 patients, 40% of whom are currently prescribed a controlled substance. Clinical pharmacists are also on staff in the OPC caring for patients and serving as a resource for residents and their patients. Following revisions to the controlled substance agreement, pharmacists within the OPC received many questions about the correct interpretation of UDTs, indicating a gap in knowledge and a need for resident education.
There have been several MedEdPORTAL publications over the previous 10 years outlining educational resources for substance abuse and mitigation; however, they do not expand on the outcomes and retention of education provided.8–11 Current literature in MedEdPORTAL mainly focuses on qualitative screening for substance abuse, with no publications centered on teaching appropriate UDT utilization and interpretation. In addition, the majority of these publications were released prior to 2016 and therefore do not reflect the recently implemented CDC guideline recommendations.
To address the knowledge gap at our institution and in response to the lack of published educational materials on UDTs, we developed an interactive educational program consisting of a PowerPoint lecture with discussion provided by a clinical pharmacist to internal medicine (IM) and medicine/pediatrics residents (PGY 1-PGY 4). The benefits of education by a clinical pharmacist within a resident training program have long been established and include residents' increased satisfaction with their education, improved pharmacotherapy knowledge, and better collaboration amongst disciplines.12–15 Interprofessional collaboration was an additional benefit of our educational program; however, education outlined in the program could be provided by any educator with background in appropriate UDT interpretation. The educational program was offered to IM and medicine/pediatrics residents but was developed to be broad enough to be given to any residency specialty, medical students, or other health professionals. We hypothesized that the educational program would improve residents' knowledge of UDTs, as well as their comfort with utilizing them, while educating on the importance of collaborative interprofessional care. The education provided was also evaluated to determine the utility of the clinical pharmacist–led educational program within an OPC on the comfort level with and correct clinical interpretation of UDTs.
Methods
All medical residents (as of July 2016) in an IM or medicine/pediatrics residency at Beaumont Hospital, Royal Oak campus, were invited to attend the educational program. IM and medicine/pediatrics residents rotate through various hospital specialties on a monthly basis at our institution. They rotate through the OPC every 4 months, and during that time, they spend their entire month in the clinic seeing a variety of patients and disease states, including patients prescribed chronic controlled substances. Because of this schedule, our educational programs were given in 4 separate months to allow for participation of all IM and medicine/pediatrics residents as they rotated through the OPC. To incorporate the programs into the residents' educational curriculum, they were conducted in 30-minute time slots reserved for weekly OPC lectures. This allowed the lecture to be given to a smaller number of residents, which enabled better facilitation of informal discussion following the program.
The 30-minute educational program was developed and given by a clinical pharmacist. The PowerPoint presentation (Appendix A) included information on the following topics: types of UDTs; initial and confirmatory urine testing; sample adulteration, dilution, and substitution; and potential causes of false positives for the major medication classes screened for in UDTs. Of these topics, the majority of the PowerPoint lecture was devoted to education on the main medication classes commonly screened for in UDTs and potential causes of false positives. These topics were identified in tertiary literature and through discussion with our toxicology department as areas where adequate knowledge was critical for appropriate UDT interpretation.
Materials needed for the program included the following:
-
•
AV equipment and a computer to present the didactic presentation.
-
•
Hard copies of the preeducation survey (Appendix B).
-
•
Hard copies of the immediate posteducation survey for residents (Appendix C).
-
•
Hard copies of the quick reference tool Appendix D.
To ensure adequate preparation for the informal group discussions during and following the program, the clinical pharmacist providing the education met with our hospital's toxicology department director in addition to reviewing primary and tertiary literature on UDTs and their interpretation. Guidance for adequate preparation was given to the clinical pharmacist by other OPC clinical pharmacists as well as the toxicology department director.
An educational program was given monthly for 4 consecutive months, and residents rotating through the OPC were informed of the program and given an initial survey to complete immediately prior to attending. This preeducation survey assessed the residents' baseline knowledge of and comfort with interpreting UDTs. To do this, the survey tested the residents' general knowledge of UDTs and their ability to apply this knowledge through case-based questions. Residents were not expected or required to have any previous education on or knowledge of UDT interpretation. Immediately following survey completion, residents attended the PowerPoint lecture and participated in open discussion at the end of the program. As participation in this education was optional, the attendance at each program varied; however, thee were never more than 20 residents. The program was immediately followed by a posteducation survey, postsurvey 1, which was given to all residents in attendance and collected prior to residents leaving the program. Due to time constraints in the residents' conference schedule, total time allotted to this educational program was 30 minutes, leading to our use of a didactic lecture as opposed to other instructional models. In the time frame, the program was divided to allow for group discussion during and following the PowerPoint lecture as well as time for completion of surveys. A second posteducation survey, postsurvey 2, was administered to residents 2 months following the program they attended to assess retention of the education provided.
Below is an example time line for the 30-minute educational program:
-
•
12:00–12:05 pm: introduction to the educational program and time to complete the preeducational survey to assess baseline knowledge of and comfort with UDTs.
-
•
12:05–12:25 pm: didactic PowerPoint presentation.
-
•
12:25–12:28 pm: time for questions and discussion following the PowerPoint presentation.
-
•
12:28–12:30 pm: time to complete postsurvey 1 to assess knowledge of and comfort with UDTs following the educational program.
Responses to the presurvey, postsurvey 1, and postsurvey 2 were linked between residents, and any duplicate results from the same resident were excluded. The clinical pharmacist conducting the educational program distributed all surveys in person, and all surveys used were identical.
Using a pre- and postsurvey model, we compared residents' comfort with and knowledge of UDTs to evaluate the effectiveness of the educational program. The primary outcomes were medical residents' knowledge of and comfort with UDT interpretation before and after an educational program given by a clinical pharmacist. Secondary outcomes evaluated were the composite impact of clinical pharmacist UDT education on medical resident knowledge of and comfort with UDT interpretation, level of knowledge retention 2 months following UDT education given by a clinical pharmacist, and differences in comfort with and knowledge of UDTs by residency year.
All surveys were paper based, and residents were not required to participate; however, their response to the surveys was used as consent for inclusion in evaluation of the education provided. Data analysis was conducted with the help of a biostatistician from our research institute. All pre- and postsurvey responses were linked between the same resident. Questions included in the surveys explored residents' comfort with UDTs, as well as their knowledge of UDTs and their appropriate case-based interpretation. Resident background experiences with UDTs were also collected.
Results
Seventy-six IM and medicine/pediatrics residents were invited to participate in the educational program. Of the 76 residents eligible, 44 (57.9%) attended an educational program. All residents participating completed the presurvey and postsurvey 1, with 22 (50.0%) residents completing and returning postsurvey 2. The majority attending were IM residents, with an even distribution between residency years (Table 1). Of the 44 residents, 36 (81.8%) stated they had no prior education on UDTs or appropriate UDT interpretation (Table 2). The eight (18.2%) residents with prior education reported having received their training in medical school or through lectures and podcasts. The majority of the residents reported interpreting between one and 10 UDTs monthly (93.2%), and 10 (22.7%) residents had refused patient refills based on UDT results in the prior month.
Table 1. Distribution of Residents by Specialty and Practice Year.
| Residency Year | No. (%) |
|---|---|
| Internal medicine | |
| PGY 1 | 16 (36.4) |
| PGY 2 | 15 (34.1) |
| PGY 3 | 9 (20.5) |
| Total | 40 (90.9) |
| Medicine/pediatrics | |
| PGY 1 | 2 (4.6) |
| PGY 2 | 0 (0) |
| PGY 3 | 1 (2.3) |
| PGY 4 | 1 (2.3) |
| Total | 4 (9.1) |
Table 2. Baseline Medical Resident Assessment From Presurvey Responses (N = 44).
| Demographic Variable | No. Residents (%) |
|---|---|
| Previous education on UDTs | |
| No | 36 (81.8) |
| Yes | 8 (18.2) |
| Setting of prior education (n = 8) | |
| Medical school | 6 (13.6) |
| Lecture | 1 (2.3) |
| Lecture and podcasts | 1 (2.3) |
| Average number of UDTs interpreted monthly | |
| None | 1 (2.3) |
| 1–10 | 41 (93.2) |
| 10–20 | 2 (4.6) |
| Residents refusing controlled substance refills to a patient in the past month based on UDT results | |
| No | 34 (77.3) |
| Yes | 10 (22.7) |
| Resident confidence level in UDT interpretation | |
| Not confident | 3 (6.8) |
| Somewhat confident | 10 (22.7) |
| Neutral | 22 (50.0) |
| Confident | 9 (20.5) |
| Very confident | 0 (0) |
Abbreviation: UDT, urine drug test.
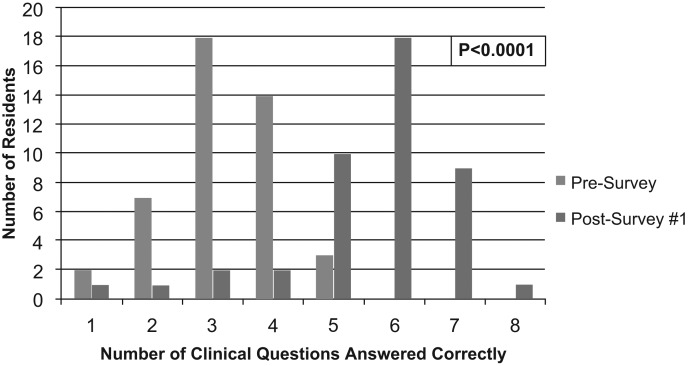
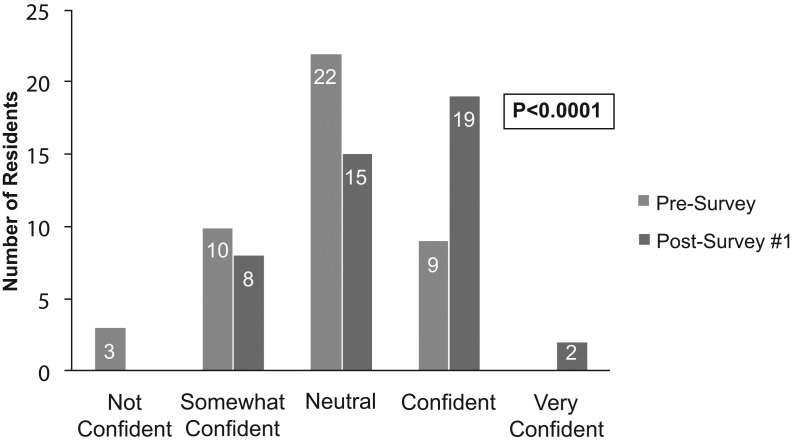
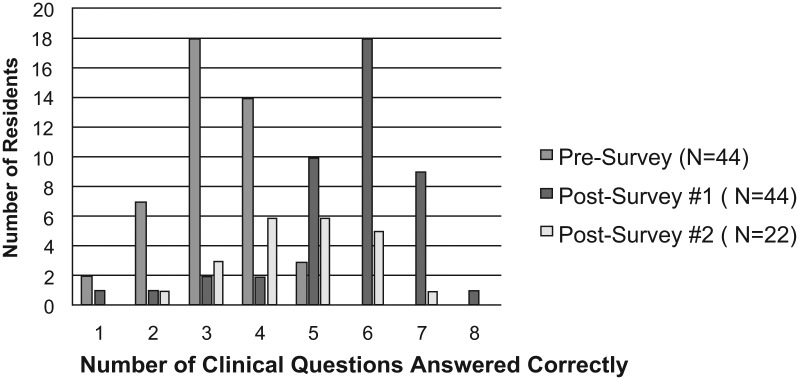
The educational program aimed to evaluate Kirkpatrick's training levels 1 and 2, reaction and learning. Both the primary outcome of impact on resident knowledge and the impact on resident comfort level were found to be significantly improved following education provided by a clinical pharmacist (comparison between presurvey and postsurvey 1 resulted in p < .0001 for both variables; Figure 1 & Figure 2). On postsurvey 1, no residents stated they were not confident in UDT interpretation, and the majority answered more than five questions correctly. Assessment of knowledge between specific presurvey and postsurvey questions found the majority to be significantly improved following education (Table 3). The only question on which residents' performance diminished following education was question 10 (presurvey)/11 (postsurvey). Of the 22 postsurvey 2s collected, there was a decrease in the number of questions answered correctly compared to postsurvey 1, but knowledge did remain improved from baseline (presurvey; Figure 3). Lastly, when looking at whether there was any variance in knowledge and comfort between residency years, no significant difference was found for knowledge, but there was an increase in confidence seen in residents further along in their educational program.
Figure 1. Comparison of knowledge based on frequency of questions answered correctly between the presurvey (Mdn = 3.0) and postsurvey 1 (Mdn = 6.0, p < .0001). Exact marginal homogeneity was assessed to determine statistical significance through the use of Monte Carlo simulations.
Figure 2. Comparison of comfort level in residents between the presurvey and postsurvey 1. McNemar's test was used to assess exact marginal homogeneity to determine statistical significance.
Figure 3. Comparison of knowledge retention based on frequency of questions answered correctly between presurvey, postsurvey 1, and postsurvey 2.
Table 3. Correct Response Rates of Individual Questions Between Presurvey and Postsurvey 1.
| No. (%) Correct | |||
|---|---|---|---|
| Questiona | Presurvey | Postsurvey 1 | p |
| 7 | 15 (34.1) | 42 (95.5) | <.0001 |
| 8 | 1 (2.3) | 9 (20.5) | <.0001 |
| 9 | 18 (40.9) | 36 (81.8) | .0001 |
| 10 | 24 (54.6) | 11 (25.0) | .0016 |
| 11 | 2 (4.6) | 32 (72.7) | <.0001 |
| 12 | 36 (81.8) | 39 (88.6) | .317 |
| 13 | 12 (27.3) | 38 (86.4) | <.0001 |
| 14 | 33 (75.0) | 39 (88.6) | .058 |
Question number given is for the presurvey. See Appendix B for question texts.
Discussion
Recent CDC recommendations have already led to a significant increase in UDT use at our practice site, and a similar increase is expected at other clinics prescribing controlled substances. This increase warrants evaluation of provider ability to correctly interpret these test results. Our didactic lecture and discussion program was created to address the educational need, and in 30 minutes, residents were able to increase both their knowledge of and comfort with UDT interpretation. Verbal feedback received from residents and other providers in the clinic was positive. Prior to our education, the majority of residents included in the program at our institution reported interpreting at least one UDT monthly; however, they had a low level of confidence in and knowledge of UDT assessment. Most residents also reported having no prior education on UDTs. Those with prior education had mainly received instruction in medical school, despite previously published research showing that medical school lectures alone, without appropriate evaluation of their ability to improve clinical interpretation, may not adequately educate providers to identify and treat substance abuse.16
The evaluation of the survey results highlights the need for improved provider education on UDT ordering and interpretation to appropriately monitor patients prescribed chronic controlled substance therapy. In addition to prior studies, our program has also demonstrated a benefit from clinical pharmacist–led education on UDT interpretation.12–15 The clinical pharmacist providing the education was able to identify multiple gaps in resident knowledge of appropriate UDT interpretation. Some of the gaps included the majority of residents at our institution being unaware that poppy seeds could cause false positives in UDTs and that oxycodone and fentanyl would not cause a positive opiate screen. Residents also did not know what to assess in a urinalysis to determine the validity of a urine sample. Presence of detection time variation even within the same medication class was another area in which residents' knowledge was improved.
Retention results from postsurvey 2 demonstrated that more than 2 months following the educational program, residents' knowledge of UDTs diminished; however, it did remain improved over baseline. This demonstrates that our formal PowerPoint lecture was not enough to sustain a high level of knowledge of UDT interpretation. From this evaluation, we realized that to help sustain the education provided, those ordering UDTs needed access to a quick reference tool and the ability to consult with pharmacists at their institution and/or toxicology departments conducting their UDTs. As the quick reference tool is not a fully comprehensive resource, we believe access to personnel with expertise (either in person or over the phone) is still critical for difficult or complex cases. Overwhelmingly positive verbal feedback from the residents also indicated that the program was well received and helped to highlight the importance of interprofessional practice.
Our educational program did have limitations. First, it was strictly formal in nature, consisting of a didactic PowerPoint lecture. The lack of interactive case-based application to solidify knowledge within the educational program may have led to the decreased knowledge retention seen in our evaluation. In the future, the PowerPoint lecture could be modified to have patient cases integrated throughout to better facilitate discussion within the program and promote increased knowledge retention. Expansion of the educational program time frame would be warranted to successfully accomplish this. Of note, both the clinical pharmacist and the toxicology department director felt topics covered within the lecture were adequate and not an area where expansion was needed. Also, all surveys utilized included identical questions to allow direct comparison, which could have led to recall bias. There may have been potential for education variability and retention between programs 1–4 as well, due to discussion that occurred within the different resident groups. Additionally, question 10 may have been unfair, based on the number of correct responses declining following education. This may have been the result of methamphetamine and amphetamine being presented together during UDT education. In the future, more time within the educational program should be taken to explain the difference between these two compounds. There was also poor follow-up with residents for postsurvey 2. As postsurvey 2 was difficult to collect at exactly 2 months posteducation, some were received more than 2 months following the educational program attended by the resident. This may have resulted in an exaggeration of the lowered retention results seen with postsurvey 2. However, the results reflected a more realistic retention as residents were expected to retain knowledge gained regardless of when they had completed postsurvey 2. Finally, residents were not monitored while they completed postsurvey 2, so even though they were encouraged to complete all questions independently, they had the opportunity to ask peers for help or search for answers.
Overall, a significant increase in both comfort with and knowledge of UDT interpretation occurred following an educational program provided by a clinical pharmacist. Previously published trials have shown a need for UDT education, and the surveys we utilized in this program echoed this conclusion.17,18 In total, the educational program provided was only 30 minutes in duration but had a large impact on posteducation knowledge and comfort. The educational program also allowed residents from different specialties and varied levels of prior education on UDTs to gain knowledge in a low-pressure setting. We did find a decrease in knowledge retained in the months following the UDT education. To address this, a quick reference tool has been developed with the toxicology department to be disseminated to all medical group–affiliated clinic providers and residents. We will also be working with the residency program coordinators to incorporate the education into the residents' annual general training. Both interventions are aimed at capturing the remaining residents who were unable to attend the original educational programs. This project has the potential to serve as a rationale for expansion of education on UDTs to include not only residents but also medical students, pharmacists, and any health care providers prescribing under a controlled substances agreement or utilizing UDTs for their patients. Such expansion could be easily adopted as a quick and effective means of promoting appropriate trainee education and patient care.
Appendices
A. UDT PowerPoint.ppt
B. UDT Presurvey.doc
C. UDT Postsurvey.doc
D. UDT Quick Reference.doc
All appendices are peer reviewed as integral parts of the Original Publication.
Acknowledgements
Charity Chen, a biostatistician from the Beaumont Hospital Research Institute, assisted with statistical analysis of data.
Disclosures
None to report.
Funding/Support
None to report.
Ethical Approval
Reported as not applicable.
References
- 1.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50–51):1445–1452. https://doi.org/10.15585/mmwr.mm655051e1 [DOI] [PubMed] [Google Scholar]
- 2.CDC WONDER website. https://wonder.cdc.gov/ Updated January 10, 2018.
- 3.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 4.Center for Behavioral Health Statistics and Quality. Key Substance Use and Mental Health Indicators in the United States: Results From the 2015 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2016. [Google Scholar]
- 5.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645. https://doi.org/10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8(4):408–416. https://doi.org/10.1007/s13181-012-0274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66–76. https://doi.org/10.4065/83.1.66 [DOI] [PubMed] [Google Scholar]
- 8.Truncali A, McNeely J, Huben L, Kerr D, Naegle M, Gourevitch M. Screening for substance abuse: good idea or not ready for prime time? MedEdPORTAL. 2011;7:9012 https://doi.org/10.15766/mep_2374-8265.9012 [Google Scholar]
- 9.Monteiro K, Dumenco L, Collins S, et al. Substance use disorder training workshop for future interprofessional health care providers. MedEdPORTAL. 2017;13:10576 https://doi.org/10.15766/mep_2374-8265.10576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klapheke M, Pasarica M. Opioid risk mitigation strategies and overdose resuscitation. MedEdPORTAL. 2017;13:10621 https://doi.org/10.15766/mep_2374-8265.10621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yelen M, Anderson L, Wright K, Fleming M. Using SPs to teach about patients at risk for drug abuse. MedEdPORTAL. 2014;10:9907 https://doi.org/10.15766/mep_2374-8265.9907 [Google Scholar]
- 12.Jarrett JB, Lounsbery JL, D'Amico F, et al. Clinical pharmacists as educators in family medicine residency programs: a CERA study of program directors. Fam Med. 2016;48(3):180–186. [PubMed] [Google Scholar]
- 13.Wilkening GL, Gannon JM, Ross C, et al. Evaluation of branched-narrative virtual patients for interprofessional education of psychiatry residents. Acad Psychiatry. 2017;41(1):71–75. https://doi.org/10.1007/s40596-016-0531-1 [DOI] [PubMed] [Google Scholar]
- 14.Jorgenson D, Muller A, Whelan AM, Buxton K. Pharmacists teaching in family medicine residency programs: national survey. Can Fam Physician. 2011;57(9):e341–e346. [PMC free article] [PubMed] [Google Scholar]
- 15.Jorgenson D, Muller A, Whelan AM. Pharmacist educators in family medicine residency programs: a qualitative analysis. BMC Med Educ. 2012;12:74 https://doi.org/10.1186/1472-6920-12-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming M, Barry K, Davis A, Kropp S, Kahn R, Rivo M. Medical education about substance abuse: changes in curriculum and faculty between 1976 and 1992. Acad Med. 1994;69(5):362–369. https://doi.org/10.1097/00001888-199405000-00009 [DOI] [PubMed] [Google Scholar]
- 17.Reisfield GM, Webb FJ, Bertholf RL, Sloan PA, Wilson GR. Family physicians' proficiency in urine drug test interpretation. J Opioid Manag. 2007;3(6):333–337. https://doi.org/10.5055/jom.2007.0022 [DOI] [PubMed] [Google Scholar]
- 18.Levy S, Harris SK, Sherritt L, Angulo M, Knight JR. Drug testing of adolescents in ambulatory medicine: physician practices and knowledge. Arch Pediatr Adolesc Med. 2006;160(2):146–150. https://doi.org/10.1001/archpedi.160.2.146 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. UDT PowerPoint.ppt
B. UDT Presurvey.doc
C. UDT Postsurvey.doc
D. UDT Quick Reference.doc
All appendices are peer reviewed as integral parts of the Original Publication.