Abstract
Ochrobactrum spp. are ubiquitous bacteria attracting growing attention as important members of microbiomes of plants and nematodes and as a source of enzymes for biotechnology. Strain Ochrobactrum sp. A44T was isolated from the rhizosphere of a field-grown potato in Gelderland, the Netherlands. The strain can interfere with quorum sensing (QS) of Gram-negative bacteria through inactivation of N-acyl homoserine lactones (AHLs) and protect plant tissue against soft rot pathogens, the virulence of which is governed by QS. Phylogenetic analysis based on 16S rRNA gene alone and concatenation of 16S rRNA gene and MLSA genes (groEL and gyrB) revealed that the closest relatives of A44T are O. grignonense OgA9aT, O. thiophenivorans DSM 7216T, O. pseudogrignonense CCUG 30717T, O. pituitosum CCUG 50899T, and O. rhizosphaerae PR17T. Genomes of all six type strains were sequenced, significantly expanding the possibility of genome-based analyses in Ochrobactrum spp. Average nucleotide identity (ANIb) and genome-to-genome distance (GGDC) values for A44T and the related strains were below the single species thresholds (95% and 70%, respectively), with the highest scores obtained for O. pituitosum CCUG 50899T (87.31%; 35.6%), O. rhizosphaerae PR17T (86.80%; 34.3%), and O. grignonense OgA9aT (86.30%; 33.6%). Distinction of A44T from the related type strains was supported by chemotaxonomic and biochemical analyses. Comparative genomics revealed that the core genome for the newly sequenced strains comprises 2731 genes, constituting 50–66% of each individual genome. Through phenotype-to-genotype study, we found that the non-motile strain O. thiophenivorans DSM 7216T lacks a cluster of genes related to flagella formation. Moreover, we explored the genetic background of distinct urease activity among the strains. Here, we propose to establish a novel species Ochrobactrum quorumnocens, with A44T as the type strain (= LMG 30544T = PCM 2957T).
Introduction
Ochrobactrum spp., together with the closely related Brucella, Agrobacterium, and Rhizobium genera, belong to the class of Alphaproteobacteria [1–3]. Although the genus is most often associated with O. anthropi [4] and O. intermedium [1], which cause opportunistic infections in humans, the bacteria from the Ochrobactrum spp. genus adapted to a variety of environmental niches and can be found in soil [5], wastewater [6], in association with plants [6,7], and animals [8,9]. The ability of Ochrobactrum spp. members to utilize xenobiotic compounds led to their exploration as potential bioremediation agents [10–13] or a source of enzymes for the biotech industry [14,15]. Ochrobactrum spp. are also of interest as plant beneficial bacteria [16,17]. The plant-derived strains, such as O. lupini LUP21T [7] and O. cytisi ESC1T [18], are able to nodulate roots to fix nitrogen, underlining their close association with the host plants. Currently, the Ochrobactrum genus comprises 18 species [19] (Fig 1A). However, scientific data concerning the majority of the strains is limited to information of taxonomic value.
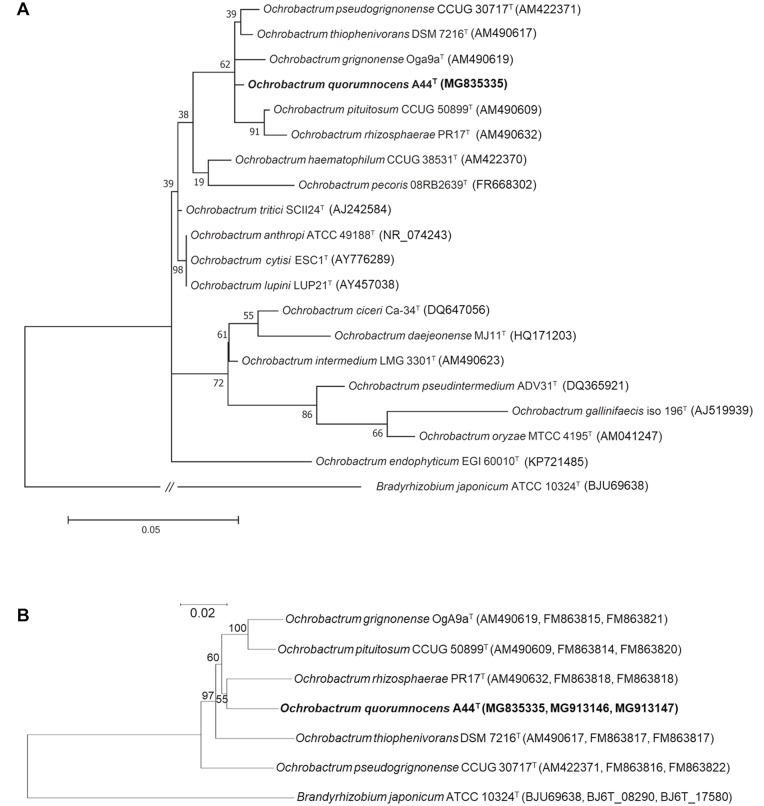
Fig 1. Phylogenetic trees depicting the phylogenetic position of Ochrobactrum sp. A44T strain among other members of the Ochrobactrum genus.
(A) Molecular phylogenetic analysis of partial (1335 bp) 16S rRNA gene sequences by the maximum likelihood method based on the Tamura 3-parameter model. Analyses were conducted in MEGA7. The tree with the highest log likelihood (-3900.57) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. (B) Dendrogram based on MLSA performed on concatenated nucleotide sequences of three genes: 16S rRNA gene (1335 bp), groEL (1165 bp), and gyrB (1012 bp). The tree was obtained using the neighbor-joining algorithm. Bootstrap values based on 1000 replicates are shown at the nodes. Bradyrhizobium japonicum ATCC 10324T was used as an outgroup. Bar indicates number of substitutions per site. Accession numbers of gene sequences are shown in brackets.
A44T is a potato rhizosphere isolate, obtained in 2002 from the experimental field in Bennekom, the Netherlands, and studied for its ability to inactivate N-acyl homoserine lactones (AHLs) [20] via AHL-acylase activity [21]. The AHLs are small molecules produced, secreted, and auto-sensed by many Gram-negative bacteria in a regulatory mechanism called quorum sensing (QS). In QS, the expression of target genes is modulated in the presence of a threshold concentration of signal molecules in the immediate environment of the cell [22,23]. QS is known to govern vital metabolic processes in the AHL-utilizing bacteria, including production of virulence factors and biofilm formation. This enables strains like A44T to deplete the pool of AHLs and interfere with QS of other species of scientific and applicational interest [24,25]. It was shown that through inactivation of AHLs, A44T is able to attenuate the QS-dependent virulence of Pectobacterium parmentieri SCC3193 [26] and P. carotovorum EC71 [27] and hence protect the plant tissue from soft rot caused by these pathogens [20].
In this study, we establish the taxonomic position of Ochrobactrum sp. strain A44T using sequence-based, genome-scale calculations, supported by the analysis of chemotaxonomic markers and phenotypic assays. Based on the results, we propose delineation of a novel species Ochrobactrum quorumnocens sp. nov. with A44T (= LMG 30544T = PCM 2957T) as a type strain. Furthermore, we perform comparative genome analysis of A44T and the related Ochrobactrum spp. and use the genomic data to link chosen differential phenotypes for the studied group to their genetic background. The only study involving genetic analysis of Ochrobactrum spp. concerns variation among isolates of Ochrobactrum intermedium and was presented by Aujoulat et al. [28] and Kulkarmi et al. [29]. To our knowledge, this is the first attempt of such analyses for other members of this genus.
Results and discussion
16S rRNA gene analysis and MLSA
First, we performed phylogenetic analysis of A44T and the type strains of all 18 validly published Ochrobactrum spp. (Fig 1A; S1A and S1B Fig). Based on 16S rRNA gene sequence analysis, the closest relatives of A44T were O. thiophenivorans DSM 7216T, isolated from wastewater in Germany [6], O. pseudogrignonense CCUG 30717T, isolated from a blood sample in Sweden [30], O. grignonense OgA9aT, obtained from bulk soil in France [5,30], O. rhizosphaerae PR17T, originating from the roots of potato grown in Austria [6], and O. pituitosum CCUG 50899T, isolated from industrial environment in Sweden [31] (Fig 1A; S1 Table). It was already shown that the listed A44T-related strains cluster on a separate branch of the phylogenetic tree of Ochrobactrum spp. [30]. To obtain higher phylogenetic resolution within this group, we employed Multilocus Sequence Analysis (MLSA) [32] based on the concatenated sequences of 16S rRNA, gyrB, and groEL genes, which suggest that the closest relative of A44T is O. rhizosphaerae PR17T (Fig 1B).
Genome sequencing and genome-based phylogeny
At the time of this study, genome sequences of only two out of eighteen Ochrobactrum sp. type strains were publicly available, namely O. anthropi ATCC 49188T (CP000758.1-CP000763.1) and O. intermedium LMG 3301T (ACQA00000000.1), thereby limiting the application of the whole-genome resolution tools in Ochrobactrum taxonomy, as well as comparative genomics. Hence, we obtained the genome sequences of A44T and the five closely related type strains of the Ochrobactrum genus: O. grignonense OgA9aT, O. thiophenivorans DSM 7216T, O. pseudogrignonense CCUG 30717T, O. pituitosum CCUG 50899T, and O. rhizosphaerae PR17T. Complete genome sequence of A44T (DDBJ/ENA/GenBank accessions of four replicons: CP022602.1-CP022605.1) was obtained by hybrid assembly of data from Illumina HiSeq2500 and PacBio RS (BaseClear B.V., the Netherlands). Draft genome sequences of O. rhizosphaerae PR17T (NNRK00000000.1), O. thiophenivorans DSM 7216T (NNRJ00000000.1), O. grignonense OgA9aT (NNRL00000000.1), and O. pseudogrignonense CCUG 30717T (NNRM00000000.1) were obtained using Illumina HiSeq2500, and the draft genome sequence of O. pituitosum CCUG 50899T (PYSY00000000.2) was generated with the use of a combination of Illumina MiniSeq and Oxford Nanopore MinION platforms. With the exception of O. pituitosum CCUG 50899T, all sequences were annotated using the IGS Annotation Engine (Institute for Genome Sciences, University of Maryland School of Medicine, USA) [33]. Basic features of the obtained genomes are presented in S2 Table.
The complete genome of A44T consists of four replicons: the main chromosome (2585.393 kbp; CP022604.1), with classical replication system, a chromid (2008.185 kbp; CP022603.1) carrying a plasmid-type replication system and the genes essential for the basic metabolism [34], [35], and two plasmids pOqn1 (1032.012 kbp; CP022605.1) and pOqn2 (19.701 kbp; CP022602.1). Genomes comprising several replicons were also reported for O. anthropi ATCC 49188T [36] and the non-type O. pituitosum strain AA2 [37]. Interestingly, pOqn2 from A44T, as well as the pOAN03 from O. anthropi ATCC 49188T [36], lacks the typical replication/partition plasmid characteristics. Presence of multiple replicons, including chromids and megaplasmids, is widespread among the members of Alphaproteobacteria [35].
Out of the total of 5272 open reading frames comprising the genome of A44T, 3329 (57.3%) have assigned function, 1443 (24.9%) were considered conserved hypothetical, and the remaining were either of unknown function: 546 (9.4%) or unclassified: 480 (8.3%). Detailed role category breakdown for all ORFs of A44T genome is given in the S4 Table. Metabolic maps created for the strains are available from the Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://www.genome.jp/kegg/) [38], organism number T05040.
We performed a whole-genome phylogenetic analysis for A44T, the other five newly-sequenced strains, and the type strains of O. anthropi and O. intermedium. Highest similarity scores based on ANIb and Genome-to-Genome Distance Calculator 2.0 (GGDC) were obtained for O. pituitosum CCUG 50899T (ANIb = 87.31%, GGDC = 35.6%), followed by O. rhizosphaerae PR17T (ANIb = 86.80%, GGDC = 34.3%), O. grignonense OgA9aT (ANIb = 86.30%, GGDC = 33.6%), O. pseudogrignonense CCUG 30717T (ANIb = 82.23%, GGDC = 26.5%), O. thiophenivorans DSM 7216T (ANIb = 81.04%, GGDC = 25.3%), O. anthropi ATCC 49188T (ANIb = 77.51%, GGDC = 23%), and O. intermedium LMG 3301T (ANIb = 77.26%, GGDC = 22.5%) (Table 1). None of the obtained values exceeded the single species thresholds recommended for ANIb (95%) and GGDC (70%), indicating that A44T should be classified as a novel species. For comparison, ANIb and GGDC values calculated for the type strains of O. pituitosum vs O. grignonense were 91.52% and 47.90%, respectively.
Table 1. Average nucleotide identity (ANI) values, digital DNA-DNA hybridization (dDDH) and G+C content (mol%) for the genome sequences of O. quorumnocens A44T and the related strains.
| Species | Strain | GenBank Accession | Genome assembly level (contig no.) | Sequence lenght (Mbp) | G+C (mol%) | GGDCA (%) | ANIb (%) |
|---|---|---|---|---|---|---|---|
| O. quorumnocens | A44T | CP022602.1—CP022605.1 | complete (4) | 5.65 | 53.16 | - | - |
| O. pituitosum | CCUG 50899T | PYSY00000000.2 | draft (10) | 5.52 | 53.40 | 35.6B | 87.31 |
| O. rhizosphaerae | PR17 T | NNRK00000000.1 | draft (36) | 4.90 | 53.01 | 34.3 | 86.80 |
| O. grignonense | OgA9aT | NNRL00000000.1 | draft (169) | 4.84 | 54.15 | 33.6 | 86.30 |
| O. pseudogrignonense | CCUG 30717T | NNRM00000000.1 | draft (53) | 5.53 | 53.99 | 26.5 | 82.23 |
| O. thiophenivorans | DSM 7216T | NNRJ00000000.1 | draft (77) | 4.36 | 51.65 | 25.3 | 81.04 |
| O. anthropi | ATCC 49188T | CP000758.1 –CP000763.1 | complete (6) | 5.20 | 56.13 | 23 | 77.51 |
| O. intermedium | LMG 3301T | ACQA00000000.1 | draft (4) | 4.72 | 57.7 | 22.5 | 77.26 |
A genome-to-genome distance, a form of digital DNA-DNA hybridization
B the highest of the calculated values are shown in bold.
Until June 2018, sixty six genome assemblies for strains identified as belonging to the Ochrobactrum genus were available in GenBank (NCBI). Twenty two of the sequenced strains were classified only to the genus level. Others were assigned to certain species, however, most of them without documented phylogenetic studies. We used JSpeciesWS (http://jspecies.ribohost.com/jspeciesws/) to calculate ANIb value between the genome of A44T and all 65 assemblies in order to find other strains potentially representing O. quorumnocens sp. nov. No other representatives of O. quorumnocens were found based on this search. The highest ANIb score (87.54%) was obtained between A44T and strain SJY1 (AZRT01000000), designated as O. rhizosphaerae (S3 Table). Interestingly, the A44T is also very closely related (ANIb>87%) to a set of Ochrobactrum spp. isolates recently obtained from the nematode Caenorhabditis elegans dwelling on a rotten apple (S3 Table). Data obtained in this work can significantly facilitate assigning those isolates to the respective species. We have also found that the ANIb value for O. rhizosphaerae SJY1 and PR17T, the O. rhizosphaerae type strain, is 86.10%, thus below the single species threshold. ANIb calculation for O. rhizosphaerae strain SJY1 and O. pituitosum CCUG 50899T suggests that SJY1 should be classified as O. pituitosum (ANIb = 96.97%).
Comparative genome analysis
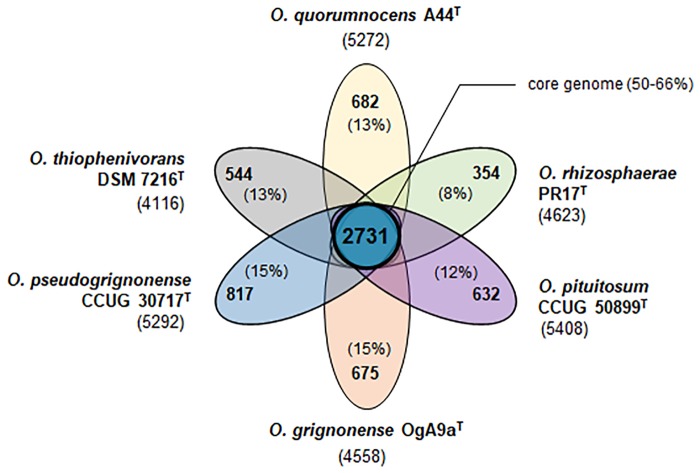
Obtaining genome sequences of A44T and the 5 related type strains (O. pituitosum CCUG 50899T, O. rhizosphaerae PR17T, O. grignonense OgA9aT, O. pseudogrignonense CCUG 30717T, and O. thiophenivorans DSM 7216T) enabled us to perform comparative genome analyses using EDGAR [39]. Sequences uniformly annotated with Prokka [40] were used as the input files. Core genome for the six newly sequenced strains consists of 2731 coding sequences, making up 50–66% of each individual genome and 27% of the pan-genome (10296 CDSs) (Fig 2; S5 Table). The set of unique genes harbored by each strain varies from 354 for O. rhizosphaerae PR17T to 817 for O. pseudogrignonense CCUG 30717T (8–15%) (S6–S11 Tables—supplementary online content for the amino acid sequences). The remaining genes (32–46%) are shared by a subset of strains. Whereas hypothetical proteins constitute 25% of the core genome, this fraction is significantly enriched within the singletons subsets, ranging from 59% in O. thiophenivorans DSM 7216T to 80% in O. rhizosphaerae PR17T. The fact that each type strain of the analyzed group contributed new genes to the pan-genome suggests that the pan-genome of Ochrobactrum spp. is “open” (as defined by Guimarães et al. [41]). However, this hypothesis needs to be confirmed on a broader pool of Ochrobactrum spp. genomes, as their availability in public databases continuously increases.
Fig 2. Flower plot depicting the core genome (in the center) and strain-specific genes (in the petals) for the group of six analyzed Ochrobactrum spp. type strains.
Accession numbers for the sequences can be found in Table 2. Comparative genome analysis was performed using EDGAR. Total number of coding sequences identified by Prokka annotation tool is given next to designations of the particular strains.
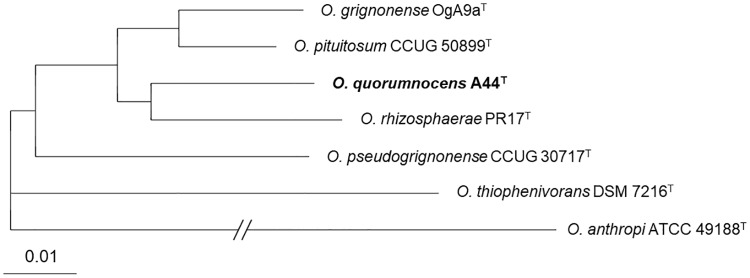
Amino acid sequences encoded by 2604 core genes established for A44T, its 5 closest relatives, and O. anthropi ATCC 49188T were used to construct a core genome-based phylogenetic tree. The analysis was performed with EDGAR [40]. According to this approach, grouping of A44T, O. rhizosphaerae PR17T, O. grignonense OgA9aT, CCUG 50899T, O. pseudogrignonense CCUG 30717T, and O. thiophenivorans DSM 7216T was analogous to that obtained by MLSA, with O. rhizosphaerae PR17T indicated as the closest relative of A44T (Fig 3).
Fig 3. Core genome-based phylogenetic tree for A44T and the related Ochrobactrum sp. type strains.
The tree was built using EDGAR based 2604 genes per genome (862702 amino acid residues per genome), concatenated to one multiple alignment. The scale indicates phylogenetic distance by substitution events.
Analysis of chemotaxonomic markers
Sequence-based study was complemented by chemotaxonomic analyses and phenotypic assays. Bacterial fatty acid methyl esters (FAMEs) were obtained according to Bligh and Dyer [42]. Composition of FAMEs obtained for A44T, O. pituitosum CCUG 50899T, O. rhizosphaerae PR17T, O. grignonense OgA9aT, O. pseudogrignonense CCUG 30717T, and O. anthropi ATCC 49188T are shown in S12 Table.
FAME profiles for these closely related species were similar, with small species-specific differences. Major FAMEs produced by A44T were: C18:19 (73.3%), C18:0 (12.4%), C16:0 (7.8%), and, at lower percentage, methyl cis-9,10-methyleneoctadecanoate (4%), C16:19 (1.9%), and C17:0 (0.6%).
The whole-cell MALDI-TOF mass spectrometry profiles were performed for A44T and the related strains: O. rhizosphaerae PR17T, O. grignonense OgA9aT, O. pituitosum CCUG 50899T, O. pseudogrignonense CCUG 30717T, O. thiophenivorans DSM 7216T, and O. anthropi ATCC 49188T. The tested strains presented similar peak patterns with subtle species-specific differences (S2 Fig). S13 Table summarizes the obtained m/z values and indicates distinctiveness of A44T from the closely related species.
Biochemical and phenotypic traits
A set of phenotypic assays was performed for A44T and the closely related strains: O. rhizosphaerae PR17T, O. grignonense OgA9aT, O. pituitosum CCUG 50899T, O. pseudogrignonense CCUG 30717T, O. thiophenivorans DSM 7216T. Comparison of biochemical traits, determined with GEN III MicroPlates (Biolog, USA), revealed twelve differences between A44T and O. rhizosphaerae PR17T, twelve between A44T and O. grignonense OgA9aT, fifteen for A44T and O. thiophenivorans DSM 7216T, sixteen for A44T and O. pituitosum CCUG 50899T, and seventeen for A44T and O. pseudogrignonense CCUG 30717T (S14 Table). Moreover, with the API 20NE test (bioMérieux, France), we determined that A44T is negative for the production of urease, unlike the urease-positive O. thiophenivorans DSM 7216T, and is unable to reduce nitrates to nitrites, as opposed to O. grignonense OgA9aT and O. pseudogrignonense CCUG 30717T. Within the Ochrobactrum genus, urease activity can vary between species and should not be considered as a criterion for genus identification [43]. In this work, strain A44T, along with the type strains of O. pseudogrignonense CCUG 30717T, O. rhizosphareae PR17T, O. grignonense OgA9aT, and O. pituitosum CCUG 50899T, was shown to be urease-negative on API strips and in the urea-indole medium. On the contrary, strain O. thiophenivorans DSM 7216T, for which no previous data concerning urease activity had been available, gave a positive reaction in the urease assays (S3 Fig).
Motility of the strain, its closest relatives, and O. anthropi ATCC 49188T, was assessed in glass tubes assay after 4 days incubation at 28°C. Strain A44T was found to be motile under all tested conditions. The same was observed for O. rhizosphaerae PR17T and O. grignonense OgA9aT, whereas O. pseudogrignonense CCUG 30717T was motile only in the presence of casamino acids, and O. thiophenivorans DSM 7216T was immotile irrespective of the tested conditions. O. anthropi ATCC 49188T was motile only in the presence of glucose (with and without casamino acids) but not in glycerol, unless supplemented with casamino acids (S15 Table).
We verified the growth ability of A44T, the five related strains: O. rhizosphaerae PR17T, O. grignonense OgA9aT, O. pituitosum CCUG 50899T, O. pseudogrignonense CCUG 30717T, O. thiophenivorans DSM 7216T, and O. anthropi ATCC 49188T in a range of temperatures varying from 7 up to 42°C. Following 7 days of incubation on LB agar plates, all tested strains showed growth at 7, 10, 20, 28, and 37°C, but not at 42°C. However, species-specific differences were observed at 37°C, triggering us to perform detailed growth rate comparison in LB for this temperature. Results revealed that the strains isolated from clinical samples, namely O. anthropi ATCC 49188T and O. pseudogrignonense CCUG 30717T, showed the highest growth rate at 37°C among the tested strains. Strains A44T and O. grignonense OgA9aT grew well, O. pituitosum CCUG 50899T and O. thiophenivorans DSM 7216T grew moderately, and O. rhizosphaerae PR17T showed weak growth at 37°C (S4 Fig). When grown for 5 days in the LB medium containing varying concentrations of NaCl, all tested strains could grow in medium containing up to 4.5% NaCl (S16 Table). However, the growth of A44T, O. pseudogrignonense CCUG 30717T, O. thiophenivorans DSM 7216T, and O. anthropi ATCC 49188T was less affected by the increasing concentration of NaCl than it was in the case of other investigated strains. Only those four microorganisms showed any growth at 6% of NaCl, with O. anthropi being the most resistant to salinity. According to the results from GEN III MicroPlates (Biolog, USA), none of the tested strains (viz. A44T, O. grignonense OgA9aT, O. thiophenivorans DSM 7216T, O. pseudogrignonense CCUG 30717T, O. pituitosum CCUG 50899T, O. rhizosphaerae PR17T) could grow at pH 5, and all strains, apart from O. rhizosphaerae PR17T, could grow at pH 6 (S14 Table).
Considering that A44T was previously studied for its ability to inactivate the AHL signal molecules, we verified the potential of the related Ochrobactrum sp. type strains to inactivate AHLs. Interestingly, O. rhizosphaerae PR17T was found to be unable to interfere with quorum sensing. Using a modified assay as described by Jafra and van der Wolf [44] and the AHL biosensor E. coli pSB401 [45], we showed that O. rhizosphaerae PR17T cannot inactivate C6-HSL—one of the AHLs efficiently inactivated by A44T, O. pituitosum CCUG 50899T, O. grignonense OgA9aT, O. pseudogrignonense CCUG 30717T, and O. thiophenivorans DSM 7216T (Fig 4).
Fig 4. Inactivation of C6-HSL by O. quorumnocens A44T and the type strains of the related Ochrobactrum spp.
Error bars in the graph indicate standard deviation values for the mean values of two independent experiments. RLU—relative luminescence of E. coli [pSB401] biosensor. O. quorum.—O. quorumnocens A44T, O. rhizos.—O. rhizosphaerae PR17T, O. grignon.—O. grignonense OgA9aT, O. pseudogr.—O. pseudogrignonense CCUG 30717T, O. thioph.—O. thiophenivorans DSM 7216T, O. pituit.—O. pituitosum CCUG 50899T, O. anth.—O. anthropi ATCC 49188T, ref.—reference sample to which no potential C6-HLS-degrading agent was added.
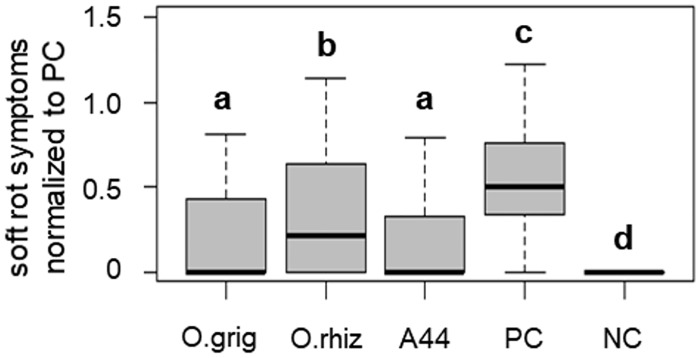
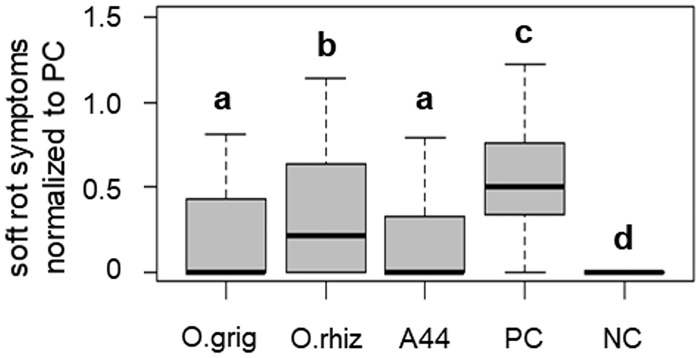
In line with this finding, strain O. rhizosphaerae PR17T showed a significantly lower potential to attenuate soft rot caused by P. parmentieri SCC3193 when compared to A44T, as demonstrated in a potato tuber slices assay conducted according to Jafra et al. [20] (Fig 5).
Fig 5. Soft rot symptoms on potato tuber slices inoculated with plant pathogen P. parmentieri SCC 3193 alone (PC) and co-inoculated with P. parmentieri and the respective Ochrobactrum spp.
O. grig—O. grignonense OgA9aT, O. rhiz—O. rhizosphaerae PR17T, and A44 —O. quorumnocens A44T; NC—no pathogen control. In the box plots, the bold lines represent median values, whiskers indicate extreme values and boxes determine the inter-quartile range (Q1–Q3). Groups significantly different (α<0.05) from one another are marked with different letters (a-d).
List of the selected phenotypic traits which can be used for efficient discrimination between A44T and the related strains is given in Table 2.
Table 2. Phenotypic traits for differentiation between O. quorumnocens sp. nov. and the related Ochrobactrum spp. type strains.
| Trait | 1A | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Carbon source utilization | ||||||
| Citric acid | - | + | + | + | + | - |
| d-maltose | - | + | + | - | - | - |
| Sucrose | - | + | + | - | - | - |
| l-rhamnose | + | + | + | + | + | - |
| l-histidine | + | +/- | + | + | + | - |
| Pectin | - | + | + | - | - | - |
| Glucoic acid | +/- | + | + | + | - | +/- |
| Bromo-succinic acid | + | + | +/- | - | + | +/- |
| Other | ||||||
| Reduction of nitrates to nitrites | - | - | - | + | + | - |
| Urease | - | - | - | - | - | + |
| Inactivation of C6-HSL | + | + | - | + | + | + |
| Growth at 37°C in LB | good | moderate | weak | good | very good | moderate |
| Motility | + | + | + | + | +/-B | - |
A 1—O. quorumnocens A44T, 2—O. pituitosum CCUG 50899T, 3—O. rhizosphaerae PR17T, 4—O. grignonense OgA9aT, 5—O. pseudogrignonense CCUG 30717T, 6—O. thiophenivorans DSM 7216T
B motile in the presence of amino acids, immotile in the absence of amino acids in minimal medium with glycerol as a sole carbon source (details in Supplementary data, S3 Table)
All strains were non-hemolytic on Columbia blood agar (BTL, Poland). Following 19 h of incubation of GEN III plates at 28°C, all strains were negative for the utilization of d-cellobiose, d-raffinose, α-d-lactose, d-melibiose, fusidic acid, d-serine, minocyclin, p-hydroxy-phenylacetic acid and did not show growth in the presence of 8% NaCl and at pH 5. In contrast, all strains metabolized α-d-glucose, d-mannose, d-fructose, d-galactose, d-fucose, L-fucose, 1% sodium lactate, d-arabitol, L-alanine, L-glutamic acid, d-galacturonic acid, d-galactonic acid lactone, d-glucuronic acid, and grew in the presence of rifamycin SV, vancomycin, troleandomycin, guanidine HCl, tetrazolium violet, tetrazolium blue, L-lactic acid, L-malic acid, lithium chloride, potassium tellurite, acetoacetic acid, acetic acid, aztreonam.
Determining the genetic background of distinct urease activity and motility among strains
It is often found in literature that the presented results of genome analyses or genome comparisons are limited to ‘dry’ numeric data sets. To go a small step beyond this scenario, we investigated the genetic background for two phenotypes with differentiating values for the studied group of Ochrobactrum spp. strains.
Urease-related genes
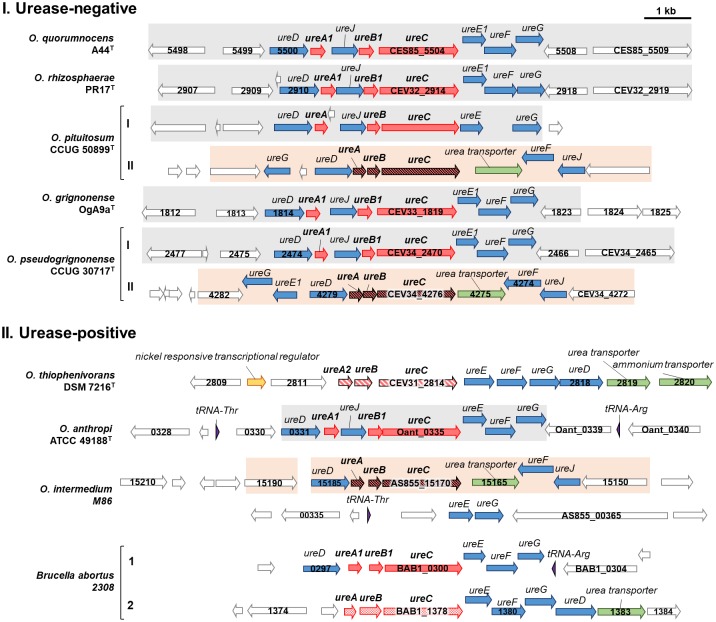
Ureases are enzymes which catalyze the hydrolysis of urea to ammonia and carbon dioxide. Bacterial ureases are complex nickel-dependent enzymes, the synthesis of which requires three structural genes, ureABC, encoding gamma, beta, and alpha subunits, respectively, and four accessory genes, ureDEFG, involved in the protein assembly. We investigated the presence and the relative position of the ureABCDEFG genes in the six newly-sequenced Ochrobactrum spp. genomes. We found that all analyzed strains, irrespective of being urease-positive or -negative, possessed homologues of genes from the ure operon. However, the organization and the genomic context of the urease clusters showed high diversity (Fig 6).
Fig 6. Clusters encoding the homologues of urease genes in O. quorumnocens A44T, the closely related Ochrobactrum type strains, and O. anthropi ATCC 49188T, O. intermedium M86, and Brucella abortus 2308.
The clusters were divided into two groups according to their origin from urease-negative (I) and urease-positive (II) strains, as determined in a diagnostic urease assay. ORF colors represent: structural genes (red), accessory genes (blue), transporter genes (green), nickel metabolism related genes (yellow), tRNA encoding regions (violet). Differentiating design patterns were used for the structural genes ureC, ureA, and ureB to indicate different/similar alleles. Matching background colors were used for clusters/cluster regions showing structural resemblance. ORF numbers represent the respective loci in the genomes of the analyzed strains.
Among the urease-negative strains, O. quorumnocens A44T, O. rhizosphaerae PR17T, and O. grignonense OgA9aT had a single urease cluster (further referred to as type I) with a similar gene organization. In this type of cluster, apart from ureABCDEFG, also the ureJ gene, encoding transmembrane hydrogenase/urease accessory protein of unclear function, was identified [46]. O pituitosum CCUG 50899T and O. pseudogrignonense CCUG 30717T were found to have two urease clusters—one of type I and a second, distinct operon (type II). The type II cluster had a different organization of genes and contained an urea transporter. For the structural proteins UreA, UreB and UreC, the identity was higher for homologues derived from the same cluster type, although from different strains, than between homologues from different cluster types. For instance the identity of protein homologues for type I clusters from of A44T and O. pseudogrignonense CCUG 30717T was 98% for UreC and 95% for both UreA and UreB, in comparison to 70%, 71% and 66%, respectively, for the homologues of these proteins from clusters I and II of O. pseudogrignonense.
We compared the organization of the urease clusters of the O. quorumnocens A44T and the related strains to that of three urease-positive clinical isolates: O. anthropi ATCC 49188T, O. intermedium M86, and Brucella abortus 2308 (Fig 5). O. anthropi ATCC 49188T harbors a single cluster resembling that of type I, however settled in a different genetic context. In O. intermedium M86, single homologues of the ure genes could be found as well, as reported earlier by Kulkarni et al. [47]. We found that the ure operon in O. intermedium M86 is similar to that of type II from O. pituitosum CCUG 50899T and O. pseudogrignonense CCUG 30717T, yet deprived of the ureE and ureG accessory genes. The missing genes could be located in a remote region of the genome. Neither cluster type I nor II of the studied Ochrobactrum spp. strains resembled the two urease clusters present in B. abortus 2308 [48]. Difference between the urease type from O. intermedium M86 and that of Brucella sp. has been previously reported by [49].
O. thiophenivorans DSM 7216T, the only urease positive strain of the close relatives of A44T harbors a single urease cluster, however of an entirely different type than those observed for the other analyzed Ochrobactrum spp. (Fig 5). We found that the protein encoded by the ureA allele from O. thiophenivorans DSM 7216T, while showing >58% identity to that of other Ochrobactrum spp. strains, shows high homology to that of a particular group of saline water-isolated alphaproteobacterial strains: Pseudoruegeria sp. SK021 (query coverage 91%, identity 91%), Martelella mediterranea (91%, 89%) and Nitratireductor sp. OM-1 (91%, 88%) [50]. Further analysis revealed that the nearly 7 kb fragment containing the urease cluster from O. thiophenivorans (nine genes, from ureA2 to the ammonium transporter encoding gene), shows 91% query coverage and 78% identity at the nucleotide level with an analogous cluster from Pseudoruegeria sp. SK021 (MTBG01000044.1: 6099–12600), isolated from the North Sea sediment [51]. Thus, it is highly possible that the unique, in terms of Ochrobactrum spp., urease cluster was obtained by O. thiophenivorans via horizontal gene transfer.
We may only hypothesize that O. quorumnocens A44T and the related Ochrobactrum spp. are urease-negative despite harboring urease-encoding operons due to the point mutations, frameshifts, or deletions/insertions that have accumulated in the (ancestor) clusters. The activity of urease results in increase in pH due to production of ammonia. For human pathogens such as Helicobacter pylori, Yersinia enterocolitica, and Brucella sp. urease activity increases the survival rate in the acidic environment of the host’s gastric tract [48,51,52]. Analogous function was proposed for ureases produced by clinical isolates of Ochrobactrum spp. which, like H. pylori, can also be found in urease-positive gastric biopsies [47, 53]. Also, the non-pathogenic soil microorganisms including Rhizobium spp. [54] produce ureases. Mineralization of nitrogen from urea by the soil microbiota makes it more accessible to the plants and is essential for completing the nitrogen cycle. This includes both the naturally occurring urea and the urea-based fertilizers—the most widely used nitrogen fertilizers in agriculture (http://faostat.fao.org). Gram-negative bacteria can obtain nitrogen both from organic compounds, such as amino acids, and from inorganic ammonia—the decomposition product of urea [55].
Flagella-related genes
O. thiophenivorans DSM 7216T was shown to be non-motile, both in the original study [6] and under the conditions tested in this work. To investigate the genetic background of this phenotype, we searched the genomes of all six strains for genes related to flagella formation [56]. Our results showed that O. thiophenivorans DSM 7216T lacks 12 out of 25 investigated genes, 11 of which were among the 24 genes present in every other strain (Table 3).
Table 3. Flagella-related genes in O. quorumnocens A44T and the related Ochrobactrum spp.
| Gene symbol | Gene Name/Annotation | Gene locus in a given strain | |||||
|---|---|---|---|---|---|---|---|
| 1A | 2 | 3 | 4 | 5 | 6 | ||
| flagellar biosynthesis, FliO family protein | CES85_1156 | PYSY02000001.1: 1264838 to 1266090 B | CEV32_0466 | CEV33_0058 | CEV34_3015 | CEV31_2471 | |
| flagellin N-methylase family protein | NF | PYSY02000001.1: 1736305 to 1736751 | CEV32_0985 | CEV33_0518 | NF | CEV31_0389 | |
| putative flagellar export protein FliJ | CES85_1827 | PYSY02000001.1: 1864435 to 1864827 | CEV32_1112 | CEV33_0638 | CEV34_0632 | CEV31_0503 | |
| flgD Ig-like domain protein | NF | NF | NF | NF | CEV34_0645 | NF | |
| fliN | flagellar motor switch protein FliN | CES85_5570 | PYSY02000002.1:1006599 to 1006919 | CEV32_2976 | CEV33_1682 | CEV34_2409 | CEV31_4116 |
| flagellar motor switch FliM family protein | CES85_5572 | PYSY02000002.1: 1005195 to 1006133 | CEV32_2978 | CEV33_1684 | CEV34_2407 | CEV31_4118 | |
| flgF | flagellar basal-body rod protein FlgF | CES85_5575 | PYSY02000002.1:1002650 to 1003381 | CEV32_2981 | CEV33_1688 | CEV34_2403 | CEV31_4122 |
| fliI | flagellar protein export ATPase FliI | CES85_5576 | PYSY02000002.1:1001304 to 1002646 | CEV32_2982 | CEV33_1689 | CEV34_2402 | CEV31_4123 |
| flgB | flagellar basal-body rod protein FlgB | CES85_5578 | PYSY02000002.1:1000168 to 1000548 | CEV32_2984 | CEV33_1691 | CEV34_2400 | CEV31_4125 |
| flgC | flagellar basal-body rod protein FlgC | CES85_5579 | PYSY02000002.1: 999745 to 1000164 | CEV32_2985 | CEV33_1692 | CEV34_2399 | CEV31_4126 |
| flagellar hook-basal body complex FliE family protein | CES85_5580 | PYSY02000002.1: 999410 to 999745 | CEV32_2986 | CEV33_1693 | CEV34_2398 | CEV31_4127 | |
| flgG | flagellar basal-body rod protein FlgG | CES85_5581 | PYSY02000002.1:998594 to 999383 | CEV32_2987 | CEV33_1694 | CEV34_2397 | CEV31_4128 |
| flgA | flagella basal body P-ring formation protein FlgA | CES85_5582 | PYSY02000002.1: 998004 to 998501 | CEV32_2988 | CEV33_1695 | CEV34_2396 | CEV31_4129 |
| flagellar basal body-associated FliL family protein | CES85_5586 | PYSY02000002.1: 994935 to 995426 | CEV32_2992 | CEV33_1699 | CEV34_2392 | NF | |
| fliP | flagellar biosynthetic protein FliP | CES85_5587 | PYSY02000002.1:994203 to 994938 | CEV32_2993 | CEV33_1700 | CEV34_2391 | NF |
| fliC | flagellin | CES85_5588 | PYSY02000002.1:993034 to 993930 | CEV32_2994 | CEV33_1702 | CEV34_2390 | NF |
| fliF | flagellar M-ring protein FliF | CES85_5590 | PYSY02000002.1:991141 to 992886 | CEV32_2996 | CEV33_1703 | CEV34_2389 | NF |
| flagellar hook-length control FliK family protein | CES85_5594 | PYSY02000002.1:986689 to 988022 | CEV32_3000 | CEV33_1707 | CEV34_2385 | NF | |
| flgE | flagellar hook protein FlgE | CES85_5597 | PYSY02000002.1: 983757 to 984959 | CEV32_3003 | CEV33_1710 | CEV34_2382 | NF |
| flgK | flagellar hook-associated protein FlgK | CES85_5598 | PYSY02000002.1: 982201 to 983655 | CEV32_3004 | CEV33_1711 | CEV34_2381 | NF |
| bacterial flagellin C-terminal helical region family protein | CES85_5599 | PYSY02000002.1: 981149 to 982195 | CEV32_3005 | CEV33_1712 | CEV34_2380 | NF | |
| fliQ | flagellar biosynthetic protein FliQ | CES85_5603 | PYSY02000002.1: 979454 to 979720 | CEV32_3009 | CEV33_1716 | CEV34_2376 | NF |
| fliR | flagellar biosynthetic protein FliR | CES85_5605 | PYSY02000002.1: 976491 to 977258 | CEV32_3011 | CEV33_1718 | CEV34_2374 | NF |
| putative flagellar biosynthesis protein FliR | CES85_5606 | PYSY02000002.1: 976098 to 976483 | CEV32_3012 | CEV33_1719 | CEV34_2373 | NF | |
| flgN family protein | CES85_5608 | PYSY02000002.1: 974784 to 975239 | CEV32_3014 | CEV33_1722 | CEV34_2371 | CEV31_3946 | |
A 1—O. quorumnocens A44T, 2—O. pituitosum CCUG 50899T, 3—O. rhizosphaerae PR17T, 4—O. grignonense OgA9aT, 5—O. pseudogrignonense CCUG 30717T, 6—O. thiophenivorans DSM 7216T
B encoding regions in WGS sequence PYSY00000000,
NF—not found,
The missing genes include fliC encoding flagellin, flgE and flgK encoding hook-associated proteins, and fliF, fliQ, and fliR encoding flagellar biosynthetic proteins. In the motile strains, 11 out of the 12 missing genes occur in a cluster, indicating that the loss of motility by O. thiophenivorans was due to a major deletion of genes essential for flagella formation.
Formal description of Ochrobactrum quorumnocens sp. nov.
Ochrobactrum quorumnocens sp. nov. (quo.rum.nocens L. neut. noun, quorum quorum; L. present participle nocens, from L. verb noceo incapacitating, referring to the fact that the type strain was initially studied for its ability to perform quorum quenching through inactivation of AHL signal molecules). Cells are Gram-negative, short non-spore-forming rods (1.4 μm in length and 0.9 μm in width), occurring singly and motile by a unipolar flagellum (S5 Fig), urease negative, oxidase and catalase positive, and non-hemolytic on Columbia blood agar. Colonies grown on LB agar are of round shape, smooth surface and edges, low-convex, shiny, and exhibit light beige color. After 5 days of growth at room temperature (22°C) on Tryptic Soy Broth agar, the colonies may become light orange. Growth occurs in 0–4.5% (w/v) NaCl (optimum below 3%), at pH 5.5–10, and temperature range of 7–37°C (optimum around 28°C). The strain grows on McConkey agar but does not ferment lactose. The predominant cellular fatty acid is C18:19. In line with the new standards [57], biochemical traits for the formal description of the strain are given in tabular format (S17 Table).
The type strain A44T (= LMG 30544T = PCM 2957T) was isolated from the rhizosphere of a potato plant grown in a field in the Netherlands. Genome sequence of the type strain is available from DDBJ/EMBL/GenBank under the accession CP022602.1-CP022605.1. It comprises a main chromosome (2.59 Mbp), a second chromosome (chromid) (2.01 Mbp), and two plasmids (1.03 Mb and 0.02 Mbp). The average G+C content is 53.16%. A44T is able to inactivate AHLs, which are the signal molecules of many Gram-negative bacteria. The formal proposal of the new species Ochrobactrum quorumnocens sp. nov. is given in S17 Table with the Taxonumber TA00464 (http://imedea.uib-csic.es/dprotologue/).
Conclusions
Ochrobactrum spp. receive growing interest as members of plant and nematode microbiomes and as potent tools in biotechnology. In this study, on the basis of sequence-based and phenotypic analyses, we propose to establish the QS-interfering strain A44T as a type strain of a novel species: Ochrobactrum quorumnocens sp. nov. (Taxonumber TA00464). The strain possesses a multi-replicon genome, characteristic for this genus. Before this study, genomes of only 2 out of 18 Ochrobactrum spp. type strains were available. Obtaining draft genomes of 5 of A44T-related type strains significantly contributes to the application of golden standard, genome-based approaches in taxonomy of this genus, especially in the light of an increasing number of genome assemblies for new strains, provisionally classified as Ochrobactrum sp. To our knowledge, this is the first study in which comparative genome analyses was performed for members of different Ochrobactrum species. Moreover, phenotype-to-genotype approach revealed the genetic background of the lack of motility in O. thiophenivorans DSM 7217T and showed the diversity of urease cluster organization in the studied group of Ochrobactrum spp. strains.
Materials and methods
Bacterial strains, media and growth conditions
Type strains of O. rhizosphaerae PR17T, O. thiophenivorans DSM 7216T, and O. pituitosum CCUG 50899T were purchased form Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Germany; strain O. pseudogrignonense CCUG 30717T was purchased from Culture Collection, University of Göteborg (CCUG), Sweden; strain O. anthropi ATCC 49188T was purchased form Belgian Co-ordinated Collections of Microorganisms (BCCM/LMG), Belgium; strain O. grignonense OgA9aT was a kind gift from prof. P. Kämpfer (Justus-Liebig-Universität Gießen, Germany). For routine propagation, all strains were cultured in Miller’s Lysogeny Broth (LB) or on LB solidified with agar (Novagen/Merck, Germany). Ochrobactrum spp. strains and P. parmentieri SCC3191 were grown at 28°C. Escherichia coli [pSB401] was grown at 37°C, with the addition of tetracycline (15 μg·mL-1). E. coli [pSB401] was used as a lux-based biosensor producing bioluminescence in the presence of AHLs [45]. The ability of Ochrobactrum spp. strains to grow at 7, 10, 20, 28, 37, and 42°C for a total of 7 days was assessed on LB agar plates, spot-inoculated with 2 μL of saline suspension of bacterial cells (turbidity adjusted to 0.3 McF). Additionally, the growth rate at 37°C was assessed in the LB medium for a total of 25 h, in 3 biological replicates, 4 technical replicates each. Salt tolerance was determined in LB base (10 g·L-1 peptone, 5 g·L-1 yeast extract) supplemented with NaCl concentrations ranging from 0 to 9%, in 3 replicates. All growth-related experiments in LB were performed in 96-well format, with orbital shaking (120 rpm) and 200 μl of medium inoculated with 4 μl of cell suspension (0.3 McF) per well. EnVision Multilabel Reader (PerkinElmer, USA) was used to measure OD600 of the cultures. The pH growth range of A44T was determined using the PM10 MicroPlate (Biolog, USA), in two replicates.
16S rRNA gene analysis and MLSA
Phylogenetic analyses were performed with the use of MEGA 7 software [58]. Dendrograms for the near complete 16S rRNA gene sequences (1335 bp) of A44T and the type strains of all 18 validly published Ochrobactrum spp. were obtained using the three clustering algorithms: maximum likelihood based on the Tamura 3-parameter model, neighbor joining, and maximum parsimony. All positions containing gaps and missing data were eliminated. There was a total of 1331 positions in the final dataset [59,60]. Multilocus Sequence Analysis (MLSA) [32] was performed for a concatenated set (3512 bp) of three genes: 16S rRNA gene (1335 bp), gyrB (1012 bp), and groEL (1165 bp).
Genome sequencing and genome-based phylogeny
Genome sequencing of Ochrobactrum sp. type strains, with the exception of O. pituitosum CCUG 50899T, was performed at BaseClear B.V., the Netherlands. Draft genome of O. pituitosum CCUG 50899T was obtained by a combined use of Illumina MiniSeq and Oxford Nanopore MinION platforms at IFB UG & MUG, Poland. Sequence of O. pituitosum CCUG 50899T was automatically annotated using the NCBI Prokaryotic Genome Annotation Pipeline [61]. All other sequences were annotated using the IGS Annotation Engine [33] (Institute for Genome Sciences, University of Maryland School of Medicine, USA). The whole-genome average nucleotide identity (ANIb) was calculated with the use of JSpecies software with default settings [62]. Digital DNA-DNA hybridization was carried out with Genome-to-Genome Distance Calculator 2.0 (GGDC) using the recommended BLAST+ alignment with formula 2 (identities/HSP length) (http://ggcd.dsmz.de/distcalc2.php) [63]. The single species thresholds recommended ANIb and GGDC are 95% [62] and 70% [63], respectively. Analysis of the functional annotation was done using the Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://www.genome.jp/kegg/) [38].
Comparative genomics
CDS (CoDing Sequences) count for O. pituitosum CCUG 50899T was obtained using Prokka [40]. Other CDS counts were derived from the respective GenBank files. Comparative genome analysis was performed using EDGAR plaftorm (http://edgar.computational.bio) [39]. The core genome and the singletons for a set of 6 related Ochrobactrum spp. strains were calculated for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio).
Determination of chemotaxonomic markers
Bacterial fatty acids were obtained from the whole bacterial cells grown overnight on the LB medium at 28°C. The fatty acids were extracted according to the method described by Bligh and Dyer, 1959 [42] and subjected to gas chromatography and mass spectrometry (Shimadzu QP 2010 SE system). Identification of FAMEs was performed with respect to standards (Bacterial Acid Methyl Esters, Sigma-Aldrich, USA). For the whole-cell MALDI-TOF mass spectrometry profiles, bacteria were grown overnight on LB agar plates at 28°C. The analysis was performed in ferulic acid (10 mg·mL-1) dissolved in 17% formic acid, 33% acetonitrile and 50% water as matrix. Protein mass fingerprints were obtained using the MALDI-TOF/TOF 5800 mass spectrometer (AB Sciex, Framingham, MA, USA), with detection in the linear middle mass (4000–20000 Da), positive ion mode for a total of 1000 laser shots by a 1 kHz OptiBeam laser (YAG, 349 nm). Registered spectra were analyzed with Data Explorer software (AB Sciex).
Biochemical and phenotypic assays
Biochemical traits and enzymatic activities were determined using GEN III Microplates (Biolog, USA) and API 20NE tests (bioMérieux, France) according to the manufacturer’s protocols, in two independent experiments. The urease activity of the tested strains was also verified, in two biological replicates, using the urease-indole diagnostic broth (BioMaxima, Poland). The results were collected after 24 and 48 hours of incubation at 28°C. The Columbia blood agar (BTL, Poland) was used to assess hemolytic activity. The MacConkey agar from BTL (Poland) was used to determine the growth on this medium. Catalase activity was determined by assessing the generation of oxygen bubbles in the presence of 3% (v/v) hydrogen peroxide. Oxidase activity was tested using plastic strips with a paper zone saturated with N,N-dimethyl-p-phenylenediamine oxolate and α-naphthol (Sigma-Aldrich, USA). LB agar and Tryptic Soy Agar TSA (Thermo Fisher Scientific, USA) were used for determination of colony morphology. Motility was assessed in glass tubes filled with 5 mL of the M9 minimal medium [64] containing 0.3% agar supplemented with 0.4% glycerol or 0.4% glucose, and with or without the addition of 0.5% casamino acids. Media in tubes were stab inoculated and incubated at 28°C for 4 days.
Cell micrography
Imaging of the A44T cells was performed with the use of atomic force microscope (AFM). Bacteria were grown in the M9 minimal medium supplemented with 0.4% glucose, overnight on a microscopy glass cover slide placed in a Petri dish, with gentle shaking (60 rpm) at 28°C. The slides were washed with water, samples were fixed with 2.5% glutaraldehyde (Sigma Aldrich) for 2 hours, washed again and dried in air. Cells were imaged in air using Bioscope Resolve (Bruker), in ScanAsyst (Peak Force Tapping) mode, with the application of ScanAsyst Air probe (f0 7.0 kHz, diameter <12 nm, k:0.4 N/m).
AHL inactivation assay
The ability of the tested Ochrobactrum sp. type strains to inactivate AHLs was determined in a modified assay described by Jafra and van der Wolf [44]. Briefly, bacterial cells from overnight cultures were harvested and re-suspended in the MOPS-buffered LB, pH 6.5. Turbidity was adjusted to 5 units in McFarland scale (McF) (~3.5×108 cfu·mL-1). 50 μl of cell suspension was added to 50 μl of 10 μM C6-HSL (Sigma-Aldrich, USA) in M63 0.12% agar in white/clear bottom 96-well plates [36]. The plates were incubated for 16 h at 28°C, without shaking. The remaining AHLs were detected using E. coli [pSB401] biosensor [45], emitting light in the presence of C6-HSL.
Plant protection assay on potato tuber slices
Potato tuber slices assay was performed as previously described [20]. Ware (table) potato tubers of cultivar Gala were obtained in local grocery stores (Gdansk, Poland).
Genome mining for urease and flagella-related genes
BLAST search tools [65] available from NCBI were used to determine the presence of urease and flagella-related genes in the genomes of A44T and the related Ochrobactrum spp. (GenBank accession numbers for genomes given in Table 1). For O. pituitosum, annotation of clusters A (extracted from PYSY02000003.1) and B (extracted from PYSY02000002.1) was performed with RAST: (http://rast.nmpdr.org/). The CLC Main Workbench 7 (QIAGEN Bioinformatics) and PowerPoint 2016 (Microsoft) were applied to visualize the urease clusters.
Supporting information
(A) The phylogenetic tree obtained using the neighbor-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. (B) Dendrogram obtained using the maximum parsimony method. Tree #1 out of 2 most parsimonious trees (length = 392) is shown. The consistency index is (0.489051), the retention index is (0.585799), and the composite index is 0.376585 (0.286486) for all sites and parsimony-informative sites (in parentheses). The MP tree was obtained using the Subtree-Pruning-Regrafting (SPR) algorithm with search level 1 in which the initial trees were obtained by the random addition of sequences (10 replicates). For both (A) and (B), the analysis involved 20 nucleotide sequences. All positions containing gaps and missing data were eliminated. There was a total of 1331 positions in the final dataset. The analyses were conducted in MEGA7.
(TIF)
The analysis was performed in ferulic acid (10 mg·mL-1) dissolved in 17% formic acid, 33% acetonitrile, and 50% water as matrix. Protein mass fingerprints were obtained using the MALDI-TOF/TOF 5800 mass spectrometer (AB Sciex, Framingham, MA, USA), with detection in the linear middle mass (4000–20000 Da), positive ion mode for a total of 1000 laser shots by a 1 kHz OptiBeam laser (YAG, 349 nm). Registered spectra were analyzed with Data Explorer software (AB Sciex). All MALDI-TOF MS spectra analyses used in this study were averages of at least four replicated measurements per analyzed strain.
(TIF)
(TIF)
The experiment was performed in a 96-well format, with the use of EnVision automatic plate reader. Each point represents an average value of 12 measurements, taken in 3 biological replicates, 4 technical replicates each.
(TIF)
Cells were imaged in air using Bioscope Resolve (Bruker), in ScanAsyst (Peak Force Tapping) mode, with the application of ScanAsyst Air probe (f0 7.0 kHz, diameter <12 nm, k: 0.4 N/m).
(TIF)
(XLSX)
(XLSX)
Analysis was performed using JSpeciesWS. Shown in bold are sequences obtained in this study.
(XLSX)
(XLSX)
The core genome was calculated for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
(XLSX)
(XLSX)
Data was collected following 19 h of incubation at 28°C.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors thank Prof. P. Kämpfer (Justus-Liebig-Universität Gießen, Germany) for providing the strain O. grignonense OgA9aT, S. Nadendla from the Institute for Genome Sciences Annotation Engine (University of Maryland School of Medicine, USA) for providing annotations of bacterial genomes, Dr Jochen Blom (Justus-Liebig-Universität Gießen, Germany) for the opportunity to use EDGAR and A. Lewandowska (University of Gdansk, Poland) for professional English language correction.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the National Science Centre, Poland (Narodowe Centrum Nauki, Polska) research grant 2014/13/B/NZ9/02136 to SJ. DK was supported by a personal scholarship START 2017 (START 40.2017) granted by the Foundation for Polish Science (FNP).
References
- 1.Velasco J, Romero C, López-Goñi I, Leiva J, Díaz R, Moriyón I. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int J Syst Evol Microbiol 1998; 48: 759–768.2. [DOI] [PubMed] [Google Scholar]
- 2.Scholz HC, Al Dahouk S, Tomaso H, Neubauer H, Witte A, Schloter M, et al. Genetic diversity and phylogenetic relationships of bacteria belonging to the Ochrobactrum–Brucella group by recA and 16S rRNA gene-based comparative sequence analysis. Syst Appl Microbiol 2008; 31: 1–16. 10.1016/j.syapm.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 3.Bohlin J, Snipen L, Cloeckaert A, Lagesen K, Ussery D, Kristoffersen AB, et al. Genomic comparisons of Brucella spp. and closely related bacteria using base compositional and proteome based methods. BMC Evol Biol. 2010;10: 249 10.1186/1471-2148-10-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes B, Popoff M, Kiredjian M, Kersters K. Ochrobactrum anthropi gen. nov., sp. nov. from human clinical specimens and previously known as group Vd. Int J Syst Bacteriol. 1989; 38: 406–416. [Google Scholar]
- 5.Lebuhn M, Achouak W, Schloter M, Berge O, Meier H, Barakat M, et al. Taxonomic characterization of Ochrobactrum sp. isolates from soil samples and wheat roots, and description of Ochrobactrum tritici sp. nov. and Ochrobactrum grignonense sp. nov. Int J Syst Evol Microbiol 2000;50: 2207–2223. 10.1099/00207713-50-6-2207 [DOI] [PubMed] [Google Scholar]
- 6.Kämpfer P, Sessitsch A, Schloter M, Huber B, Busse HJ, Scholz HC. Ochrobactrum rhizosphaerae sp. nov. and Ochrobactrum thiophenivorans sp. nov., isolated from the environment. Int J Syst Evol Microbiol. 2008;58: 1426–1431. 10.1099/ijs.0.65407-0 [DOI] [PubMed] [Google Scholar]
- 7.Trujillo ME, Willems A, Abril A, Planchuelo A-M, Rivas R, Ludeña D, et al. Nodulation of Lupinus albus by strains of Ochrobactrum lupini sp. nov. Appl Environ Microbiol 2005;71: 1318–27. 10.1128/AEM.71.3.1318-1327.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dirksen P, Marsh SA, Braker I, Heitland N, Wagner S, Nakad R, et al. The native microbiome of the nematode Caenorhabditis elegans: gateway to a new host-microbiome model. BMC Biology. 2016;14: 38 10.1186/s12915-016-0258-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S, Sheng P, Zhang H. Isolation and Identification of Cellulolytic Bacteria from the Gut of Holotrichia parallela larvae (Coleoptera: Scarabaeidae). Int J Mol Sci 2012;13: 2563–2577. 10.3390/ijms13032563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu XH, Bai WQ, Zhong QZ, Li M, He FQ, Li BT. Isolation and characterization of a bacterial strain of the genus Ochrobactrum with methyl parathion mineralizing activity. J Appl Microbiol 2006;101: 986–994. 10.1111/j.1365-2672.2006.03016.x [DOI] [PubMed] [Google Scholar]
- 11.Woźniak-Karczewska M, Čvančarová M, Chrzanowski Ł, Kolvenbach B, Corvini PFX, Cichocka D. Isolation of two Ochrobactrum sp. strains capable of degrading the nootropic drug—Piracetam. New Biotechnol 2017. 10.1016/j.nbt.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Sanjeev Kumar S, Kumar MS, Siddavattam D, Karegoudar TB. Generation of continuous packed bed reactor with PVA–alginate blend immobilized Ochrobactrum sp. DGVK1 cells for effective removal of N,N-dimethylformamide from industrial effluents. J Hazard Mater 2012; 199–200: 58–63. 10.1016/j.jhazmat.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 13.Sipahutar MK, Vangnai AS. Role of plant growth-promoting Ochrobactrum sp. MC22 on triclocarban degradation and toxicity mitigation to legume plants. J Hazard Mater 2017;329: 38–48. 10.1016/j.jhazmat.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 14.Pozo C, Rodelas B, De La ES, Gonzalez-Lopez J. d,l-Hydantoinase activity of an Ochrobactrum anthropi strain. J Appl Microbiol 2002;92: 1028–1034. [DOI] [PubMed] [Google Scholar]
- 15.Sonke T, Ernste S, Tandler RF, Kaptein B, Peeters WP, van Assema FB, et al. L-selective amidase with extremely broad substrate specificity from Ochrobactrum anthropi NCIMB 40321. Appl Environ Microbiol 2005;71: 7961–7973. 10.1128/AEM.71.12.7961-7973.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumayo M, Hahm M-S, Ghim S-Y. Determinants of plant growth-promoting Ochrobactrum lupini KUDC1013 involved in induction of systemic resistance against Pectobacterium carotovorum subsp. carotovorum in tobacco leaves. Plant Pathol J 2013;29: 174–181. 10.5423/PPJ.SI.09.2012.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulucci NS, Gallarato LA, Reguera YB, Vicario JC, Cesari AB, García de Lema MB, et al. Arachis hypogaea PGPR isolated from Argentine soil modifies its lipids components in response to temperature and salinity. Microbiol Res. 2015;173: 1–9. 10.1016/j.micres.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 18.Zurdo-Pineiro JL, Rivas R, Trujillo ME, Vizcaino N, Carrasco JA, Chamber M, et al. Ochrobactrum cytisi sp. nov., isolated from nodules of Cytisus scoparius in Spain. Int J Syst Evol Microbiol 2007;57: 784–788. 10.1099/ijs.0.64613-0 [DOI] [PubMed] [Google Scholar]
- 19.Li L, Li Y-Q, Jiang Z, Gao R, Nimaichand S, Duan Y-Q, et al. Ochrobactrum endophyticum sp. nov., isolated from roots of Glycyrrhiza uralensis. Arch Microbiol 2016;198: 171–179. 10.1007/s00203-015-1170-8 [DOI] [PubMed] [Google Scholar]
- 20.Jafra S, Przysowa J, Czajkowski R, Michta A, Garbeva P, Van der Wolf J. Detection and characterization of bacteria from the potato rhizosphere degrading N-acyl-homoserine lactone. Can J Microbiol 2006;52: 1006–1015. 10.1139/w06-062 [DOI] [PubMed] [Google Scholar]
- 21.Czajkowski R, Krzyżanowska D, Karczewska J, Atkinson S, Przysowa J, Lojkowska E, et al. Inactivation of AHLs by Ochrobactrum sp. A44 depends on the activity of a novel class of AHL acylase. Environ Microbiol Rep 2011;3: 59–68. 10.1111/j.1758-2229.2010.00188.x [DOI] [PubMed] [Google Scholar]
- 22.Fuqua WC, Winans SC. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol 1994;176: 2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swift S, Rowe MC, Kamath M. Quorum Sensing In: El-Sharoud W, editor. Bacterial Phisiology-A Molecular Approach. Berlin, Heidelberg: Springer Berlin Heidelberg; 2008. pp. 179–232. [Google Scholar]
- 24.Grandclément C, Tannières M, Moréra S, Dessaux Y, Faure D. Quorum quenching: role in nature and applied developments. FEMS Microbiol Rev 2016;40: 86–116. 10.1093/femsre/fuv038 [DOI] [PubMed] [Google Scholar]
- 25.Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nature Rev Microbiol 2009;7: 836 10.1038/nrmicro2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirhonen M, Flego D, Heikinheimo R, Palva ET. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J 1993;12: 2467–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee A, Cui Y, Lui Y, Dumenyo CK, Chatterjee AK. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density-sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl Environ Microbiol 1995;61: 1959–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aujoulat F, Romano-Bertrand S, Masnou A, Marchandin H, Jumas-Bilak E. Niches, population structure and genome reduction in Ochrobactrum intermedium: Clues to technology-driven emergence of pathogens. PLoS ONE. 2014; 9: e83376 10.1371/journal.pone.0083376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni G, Gohil K, Misra V, Kakrani AL, Misra SP, Patole M, et al. Multilocus sequence typing of Ochrobactrum spp. isolated from gastric niche. J Infect Public Health. 2017; 10: 201–210. 10.1016/j.jiph.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Kämpfer P, Scholz HC, Huber B, Falsen E, Busse HJ. Ochrobactrum haematophilum sp. nov. and Ochrobactrum pseudogrignonense sp. nov., isolated from human clinical specimens. Int J Syst Evol Microbiol 2007; 57: 2513–2518. 10.1099/ijs.0.65066-0 [DOI] [PubMed] [Google Scholar]
- 31.Huber B, Scholz HC, Kämpfer P, Falsen E, Langer S, Busse H-J. Ochrobactrum pituitosum sp. nov., isolated from an industrial environment. Int J Syst Evol Microbiol 2010;6 0: 321–326. 10.1099/ijs.0.011668-0 [DOI] [PubMed] [Google Scholar]
- 32.Ramette A, Frapolli M, Fischer-Le Saux M, Gruffaz C, Meyer J-M, Défago G, et al. Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2,4-diacetylphloroglucinol and pyoluteorin. Syst Appl Microbiol 2011; 34: 180–188. 10.1016/j.syapm.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 33.Galens K, Orvis J, Daugherty S, Creasy HH, Angiuoli S, White O, et al. The IGS standard operating procedure for automated prokaryotic annotation. Stand Genomic Sci 2011;4: 244–251. 10.4056/sigs.1223234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison PW, Lower RPJ, Kim NKD, Young JPW. Introducing the bacterial ‘chromid’: not a chromosome, not a plasmid. Trends Microbiol 2010;18: 141–148. 10.1016/j.tim.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 35.Dziewit L, Bartosik D. Comparative analyses of extrachromosomal bacterial replicons, identification of chromids, and experimental evaluation of their indispensability In: Mengoni A, Galardini M, Fondi M, editors. Bacterial Pangenomics: Methods and Protocols. New York, NY: Springer New York; 2015. pp. 15–29. [DOI] [PubMed] [Google Scholar]
- 36.Chain PSG, Lang DM, Comerci DJ, Malfatti SA, Vergez LM, Shin M, et al. Genome of Ochrobactrum anthropi ATCC 49188 T, a versatile opportunistic pathogen and symbiont of several eukaryotic hosts. J Bacteriol. 2011;193: 4274–4275. 10.1128/JB.05335-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu B, Paulson JN, Zheng X, Kolter R. Simplified and representative bacterial community of maize roots. Proc Natl Acad Sci U S A 2017; 114: E2450–E2459. 10.1073/pnas.1616148114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 2000;28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blom J, Albaum SP, Doppmeier D, Pühler A, Vorhölter F-J, Zakrzewski M, et al. EDGAR: a software framework for the comparative analysis of prokaryotic genomes. BMC Bioinformatics. 2009; 10: 154 10.1186/1471-2105-10-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014; 30: 2068–2069. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 41.Guimarães LC, Florczak-Wyspianska J, de Jesus LB, Viana MVC, Silva A, Ramos RTJ, et al. Inside the Pan-genome—Methods and Software Overview. Curr Genomics. 2015; 16: 245–252. 10.2174/1389202916666150423002311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37: 911–917. 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- 43.Teyssier C, Marchandin H, Jean-Pierre H, Diego I, Darbas H, Jeannot J-L, et al. Molecular and phenotypic features for identification of the opportunistic pathogens Ochrobactrum spp. J Med Microbiol 2005; 54: 945–953. 10.1099/jmm.0.46116-0 [DOI] [PubMed] [Google Scholar]
- 44.Jafra S, van der Wolf JM. Fast screening method for detection of acyl-HSL-degrading soil isolates. J Microbiol Methods 2004; 57: 415–420. 10.1016/j.mimet.2004.01.015 [DOI] [PubMed] [Google Scholar]
- 45.Winson MK, Swift S, Fish L, Throup JP, Jorgensen F, Chhabra SR, et al. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett. 1998; 163: 185–92. 10.1111/j.1574-6968.1998.tb13044.x [DOI] [PubMed] [Google Scholar]
- 46.McMillan DJ, Mau M, Walker MJ. Characterisation of the urease gene cluster in Bordetella bronchiseptica. Gene. 1998; 208: 243–251. 10.1016/S0378-1119(97)00651-3. [DOI] [PubMed] [Google Scholar]
- 47.Kulkarni G, Dhotre D, Dharne M, Shetty S, Chowdhury S, Misra V, et al. Draft genome of Ochrobactrum intermedium strain M86 isolated from non-ulcer dyspeptic individual from India. Gut Pathog. 2013; 5: 7 10.1186/1757-4749-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sangari FJ, Seoane A, Rodríguez MC, Agüero J, García Lobo JM. Characterization of the urease operon of Brucella abortus and assessment of its role in virulence of the bacterium. Infect Immun. 2007; 75: 774–780. 10.1128/IAI.01244-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gohil KN, Neurgaonkar PS, Paranjpe A, Dastager SG, Dharne MS. Peeping into genomic architecture by re-sequencing of Ochrobactrum intermedium M86 strain during laboratory adapted conditions. Genomics Data. 2016; 8: 72–6. 10.1016/j.gdata.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pohlner M, Marshall I, Schreiber L, Cypionka H, Engelen B. Draft genome sequence of Pseudoruegeria sp. SK021, a representative of the marine Roseobacter group, isolated from North Sea sediment. Genome Announc 2017; 5 pii: e00541-17. 10.1128/genomeA.00541-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mobley HL, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiological Reviews. 1995; 59:451–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koning-Ward TF, Robins-Browne RM. A novel mechanism of urease regulation in Yersinia enterocolitica. FEMS Microbiol Lett 1997; 147: 221–226. 10.1111/j.1574-6968.1997.tb10245.x [DOI] [PubMed] [Google Scholar]
- 53.Kulkarni GJ, Shetty S, Dharne MS, Shouche YS. Genome sequencing analysis reveals virulence-related gene content of Ochrobactrum intermedium strain 229E, a urease-positive strain isolated from the human gastric niche. FEMS Microbiol Lett 2014; 359: 12–15. 10.1111/1574-6968.12549 [DOI] [PubMed] [Google Scholar]
- 54.Toffanin A, Cadahia E, Imperial J, Ruiz-Argüeso T, Palacios J. Characterization of the urease gene cluster from Rhizobium leguminosarum bv. viciae. Arch Microbiol 2002; 177: 290–298. 10.1007/s00203-001-0392-0 [DOI] [PubMed] [Google Scholar]
- 55.De Reuse H, Skouloubris S. Nitrogen metabolism. Helicobacter pylori: American Society of Microbiology; 2001.
- 56.Fujii T, Kato T, Hiraoka KD, Miyata T, Minamino T, Chevance FFV, et al. Identical folds used for distinct mechanical functions of the bacterial flagellar rod and hook. Nature Commun 2017;8:14276 10.1038/ncomms14276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosselló-Móra R, Trujillo ME, Sutcliffe IC. Introducing a digital protologue: a timely move towards a database-driven systematics of archaea and bacteria. Antonie van Leeuwenhoek. 2017; 110: 455–456. 10.1007/s10482-017-0841-7 [DOI] [PubMed] [Google Scholar]
- 58.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol Biol Evol 2016; 33: 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol Biol Evol 1992; 9: 678–687. 10.1093/oxfordjournals.molbev.a040752 [DOI] [PubMed] [Google Scholar]
- 60.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 1980; 16: 111–120. 10.1007/bf01731581 [DOI] [PubMed] [Google Scholar]
- 61.Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 2016;44: 6614–6624. 10.1093/nar/gkw569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A 2009; 106: 19126–19131. 10.1073/pnas.0906412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013; 14: 60 10.1186/1471-2105-14-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual,). Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2001. pp3. 17–3.32.
- 65.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997;25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The phylogenetic tree obtained using the neighbor-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. (B) Dendrogram obtained using the maximum parsimony method. Tree #1 out of 2 most parsimonious trees (length = 392) is shown. The consistency index is (0.489051), the retention index is (0.585799), and the composite index is 0.376585 (0.286486) for all sites and parsimony-informative sites (in parentheses). The MP tree was obtained using the Subtree-Pruning-Regrafting (SPR) algorithm with search level 1 in which the initial trees were obtained by the random addition of sequences (10 replicates). For both (A) and (B), the analysis involved 20 nucleotide sequences. All positions containing gaps and missing data were eliminated. There was a total of 1331 positions in the final dataset. The analyses were conducted in MEGA7.
(TIF)
The analysis was performed in ferulic acid (10 mg·mL-1) dissolved in 17% formic acid, 33% acetonitrile, and 50% water as matrix. Protein mass fingerprints were obtained using the MALDI-TOF/TOF 5800 mass spectrometer (AB Sciex, Framingham, MA, USA), with detection in the linear middle mass (4000–20000 Da), positive ion mode for a total of 1000 laser shots by a 1 kHz OptiBeam laser (YAG, 349 nm). Registered spectra were analyzed with Data Explorer software (AB Sciex). All MALDI-TOF MS spectra analyses used in this study were averages of at least four replicated measurements per analyzed strain.
(TIF)
(TIF)
The experiment was performed in a 96-well format, with the use of EnVision automatic plate reader. Each point represents an average value of 12 measurements, taken in 3 biological replicates, 4 technical replicates each.
(TIF)
Cells were imaged in air using Bioscope Resolve (Bruker), in ScanAsyst (Peak Force Tapping) mode, with the application of ScanAsyst Air probe (f0 7.0 kHz, diameter <12 nm, k: 0.4 N/m).
(TIF)
(XLSX)
(XLSX)
Analysis was performed using JSpeciesWS. Shown in bold are sequences obtained in this study.
(XLSX)
(XLSX)
The core genome was calculated for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
The analysis was performed for Prokka-annotated genomes using EDGAR (http://edgar.computational.bio). Genome of O. quorumnocens was used as a reference.
(XLSX)
(XLSX)
(XLSX)
Data was collected following 19 h of incubation at 28°C.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.