Abstract
It is anticipated that bioactive fragments of the extracellular matrix (matrikines) can influence the development and progression of chronic diseases. The enzyme leukotriene A4 hydrolase (LTA4H) mediates opposing proinflammatory and anti-inflammatory activities, through the generation of leukotriene B4 (LTB4) and degradation of proneutrophilic matrikine Pro-Gly-Pro (PGP), respectively. We show that abrogation of LTB4 signaling ameliorated inflammation and airway hyperresponsiveness (AHR) in a murine asthma model, yet global loss of LTA4H exacerbated AHR, despite the absence of LTB4. This exacerbated AHR was attributable to a neutrophil-independent capacity of PGP to promote pathological airway epithelial remodeling. Thus, we demonstrate a disconnect between airway inflammation and AHR and the ability of a matrikine to promote an epithelial remodeling phenotype that negatively affects lung function. Subsequently, we show that substantial quantities of PGP are detectable in the sputum of moderate-severe asthmatics in two distinct cohorts of patients. These studies have implications for our understanding of remodeling phenotypes in asthma and may rationalize the failure of LTA4H inhibitors in the clinic.
INTRODUCTION
Tissue inflammation and remodeling are cardinal features of many chronic diseases, as exemplified within the lungs, whereby they are hallmarks of genetic disorders such as cystic fibrosis (CF), as well as common lung diseases such as asthma and chronic obstructive pulmonary disease (COPD), which have emerged as a leading cause of morbidity and mortality worldwide. It had traditionally been hypothesized that chronic inflammation was driving the airway remodeling, but it is increasingly accepted that these features of disease can develop in parallel and that remodeling can even develop independently of the inflammation (1). Consequently, the mediators and mechanisms that instigate airway remodeling are still incompletely understood but are critical in dictating future efforts to develop effective treatment strategies.
Leukotriene B4 (LTB4) is generated intracellularly by the enzyme leukotriene A4 hydrolase (LTA4H) (2) and upon release binds to leukotriene B4 receptor 1 (BLT1) (3). LTB4 functions as a potent chemotactic factor and activator for various inflammatory cells and instigates pathological inflammation observed in a multitude of chronic diseases (4–6). Consequently, there has been a concerted pharmaceutical effort to develop LTA4H inhibitors to ameliorate LTB4-mediated pathology, but these inhibitors have failed to demonstrate efficacy in the clinic (7). Most recently, the LTA4H inhibitor JNJ-40929837 was assessed in a human bronchial allergen challenge model of asthma, but despite demonstrating clear target engagement and reducing LTB4, this drug failed to show any clinical benefit over placebo (8).
The extracellular matrix (ECM) is the noncellular component of tissues that provides a scaffold for constituent cells and is critical in the provision of biological cues that dictate development, homeostasis, inflammation, and repair. Degradation of the ECM can liberate biologically active fragments, termed matrikines, which can dictate the progression of inflammation and injury seen in chronic lung diseases (9). One such matrikine is the tripeptide Pro-Gly-Pro (PGP) that is liberated from ECM collagen via the sequential enzymatic activity of matrix metalloproteinases (MMPs) and prolylendopeptidase (10). Once liberated, PGP can subsequently be chemically acetylated through the action of reactive aldehydes to a species that displays enhanced in vivo stability, AcPGP (N-acelytated PGP) (11). PGP and AcPGP function as neutrophil chemoattractants by mimicking key sequences found in glutamic acid, leucine, arginine–positive (ELR+) chemokines and binding to CXCR1/2 (10, 12). Previously, we demonstrated that LTA4H has a second anti-inflammatory activity, whereby it degrades PGP to facilitate the resolution of neutrophilic inflammation (11, 13, 14). Conversely, AcPGP is resistant to this LTA4H-mediated degradation (11). Accordingly, it seems that the LTA4H-PGP degradation pathway is perturbed in chronic lung diseases leading to the accumulation of PGP, which may subsequently be converted to AcPGP (11, 15), with both species subsequently driving inflammation and pathology (10, 15, 16). LTA4H therefore represents a highly unusual enzyme with opposing proinflammatory and anti-inflammatory activities that dictate the amplitude and persistence of inflammation, potentially accounting for the failure of LTA4H inhibitors in a clinical setting.
Here, we assessed this dual functionality of LTA4H in defining the pathogenesis of asthma. We demonstrate that global deletion of LTA4H abolished LTB4-driven inflammation but paradoxically exacerbated airway hyperresponsiveness (AHR) owing to PGP accumulation and a novel, neutrophil-independent activity for this peptide in promoting a profound pathological epithelial remodeling. Subsequently, we demonstrated that substantial quantities of AcPGP were present in the sputum of severe asthmatics from two separate patient cohorts, thus emphasizing the potential importance of this ECM-derived fragment in driving pathological airway remodeling in a clinical setting.
RESULTS
An absence of LTA4H leads to exacerbated AHR yet reduced airway inflammation in a house dust mite model of allergic airways disease
To dissect the significance of the dual roles of LTA4H in the context of allergic airways disease, we used a well-established murine house dust mite (HDM) model (Fig. 1A) (17). To define the specific role of LTB4 in the pathogenesis of disease, we used mice deficient in its receptor, BLT1 (fig. S1A). HDM-treated blt1−/− mice displayed a significant reduction in airway resistance relative to littermate controls (P < 0.05; fig. S1, B and C). Furthermore, analysis of hemotoxylin and eosin (H&E)–stained lung sections demonstrated a reduction in pulmonary inflammation in HDM-exposed blt1−/− mice relative to controls (fig. S1D). Thus, in our murine model, LTB4 contributes to the inflammatory and AHR changes during allergic airways disease.
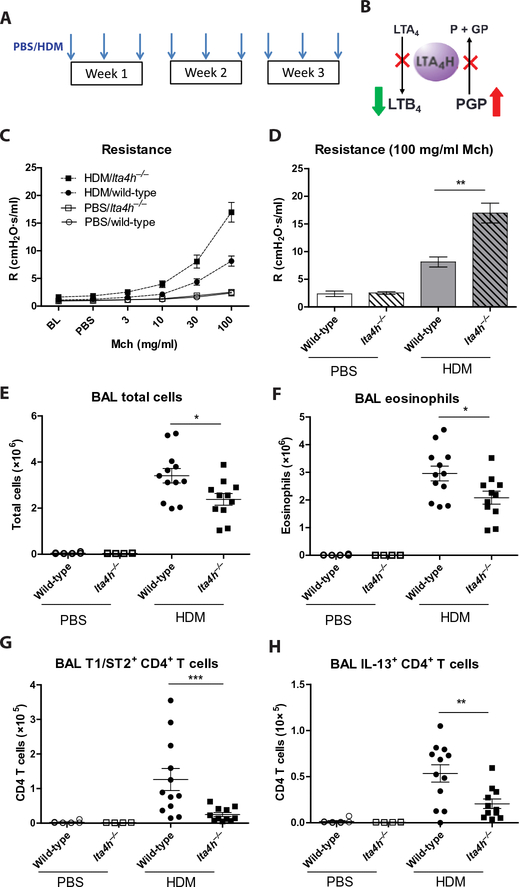
Fig. 1. Augmented airway resistance in HDM-treated lta4h−/− mice despite reduced airway inflammation.
(A) Wild-type and lta4h−/− mice were administered HDM or phosphate-buffered saline (PBS) intranasally three times per week for 3 weeks. (B) Schematic depicting the opposing proinflammatory (LTB4 generation) and anti-inflammatory (PGP degradation) activities of LTA4H. Abrogation of LTA4H activity (red crosses) will result in reduced LTB4 but increased PGP. (C) Airway resistance (R) to increasing doses of methacholine (Mch) was measured 24 hours after the final HDM/PBS exposure. (D) Airway resistance at 100 mg/ml methacholine. At 24 hours after final HDM/PBS exposure, total cell numbers in the BAL were assessed by trypan blue exclusion (E), and airway eosinophils (F), CD4+ T cells expressing T1/ST2 (G), and CD4+ T cells expressing IL-13 (H) were assessed by flow cytometry. Figures present combined data from two independent experiments with four to six mice per group in each experiment. Results depicted as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, using Mann-Whitney statistical test.
We subsequently investigated the phenotype of HDM-exposed lta4h−/− mice, which cannot generate LTB4 but simultaneously cannot degrade PGP (Fig. 1B). Despite being unable to produce LTB4, HDM-administered lta4h−/− mice displayed a marked increase in airway resistance, both at baseline and to increasing doses of methacholine, relative to littermate controls (Fig. 1, C and D, and fig. S2, A and B) and a modest yet significant reduction in airway compliance (P < 0.01; fig. S2, C and D). Despite the exacerbated AHR, HDM- exposed lta4h−/− mice exhibited reduced total cellular infiltrate into their airways (Fig. 1E) and comparable cell numbers in their lung tissue relative to littermate controls (fig. S2E). A cardinal feature of allergic airways disease in this model is a robust eosinophilic infiltrate, but numbers of eosinophils in the airways of lta4h−/− mice were reduced relative to controls (Fig. 1F), and lung eosinophil numbers were comparable (fig. S2F). T helper 2 (TH2) cells expressing inter-leukin 1 receptor-like 1 (T1/ST2) (Fig. 1G) and IL-13+ (interleukin- 13–positive) CD4+ T cells (Fig. 1H) were also reduced in the airways of HDM-treated lta4h−/− mice relative to controls, although lung numbers were similar (fig. S2, G and H). Type 2 innate lymphoid cell (ILC2) numbers were equivalent between HDM-treated lta4h−/− mice and littermate controls (fig. S2, I and J). Moreover, numbers of CD11c+ airway macrophages were comparable between HDM- administered lta4h−/− mice and littermate controls (fig. S2K), and Ly6Chi monocytes were actually reduced in lta4h−/− mice (fig. S2L).
HDM-exposed lta4h−/− mice exhibit a reduction in classical drivers of AHR
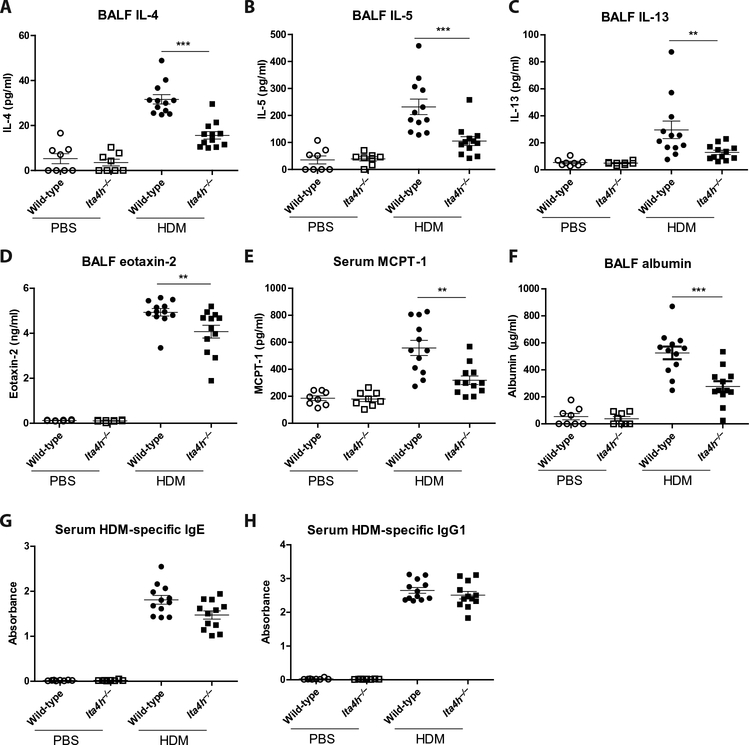
TH2 cytokines IL-4, IL-5, and IL-13 are implicated in driving the inflammation, remodeling, and ultimately AHR in our murine model of allergic airways disease (18). These TH2 cytokines were increased in bronchoalveolar lavage fluid (BALF; Fig. 2, A to C) and lung homogenate (fig. S3, A to C) of wild-type mice upon HDM exposure, but their concentrations were markedly lower in HDM-exposed lta4h−/− mice. Similarly, concentrations of the eosinophil chemoattractant eotaxin-2 were reduced in the BALF (Fig. 2D) and lung homogenate (fig. S3D) of HDM-treated lta4h−/− mice relative to littermate controls. Furthermore, lung homogenate concentrations of proinflammatory cytokines tumor necrosis factor–α (fig. S3E) and IL-6 (fig. S3F) were reduced in HDM-exposed lta4h−/− mice, whereas the concentration of IL-17 was comparable between wild-type and knockout mice (fig. S3G). Mast cells are a hallmark feature of allergic airway disease, but the concentration of mast cell protease-1 (MCPT-1) was lower in the serum of HDM-treated lta4h−/− mice relative to littermate controls (Fig. 2E). Edematous airways tend to be narrower and prone to airway hyperresponsive behavior (19); however, HDM-administered lta4h−/− mice demonstrated a marked reduction in BALF albumin relative to controls (Fig. 2F). Finally, total (fig. S3, H and I) and HDM-specific (Fig. 2, G and H) serum immunoglobulin E (IgE) and IgG1 were comparable between HDM-treated wild-type and lta4h−/− mice, highlighting comparable sensitization in allergen-exposed animals. Therefore, cellular and soluble mediators of inflammation commonly associated with allergic airways disease were generally reduced in lta4h−/− mice, most likely owing to the absence of LTB4, despite these animals presenting with an exacerbated AHR. Although loss of LTA4H function can lead to an accumulation of LTA4 and a shunt toward lipoxins or cysteinyl leukotrienes (20, 21), no lipoxins were detectable in BAL fluid (fig. S4A), and no significant differences were observed in cysteinyl leukotrienes (fig. S4, B and C).
Fig. 2. HDM-treated lta4h−/− mice exhibit reduced concentrations of TH2 inflammatory mediators relative to their littermate controls.
Wild-type and lta4h−/− mice were administered HDM or PBS intranasally three times per week for 3 weeks, and BALF and serum were collected 24 hours after the final HDM/PBS exposure. Concentrations of BALF IL-4 (A), BALF IL-5 (B), BALF IL-13 (C), BALF eotaxin-2 (D), serum MCPT-1 (E), BALF albumin (F), serum HDM-specific IgE antibodies (G), and serum HDM-specific IgG1 antibodies (H) were assessed by enzyme-linked immunosorbent assay (ELISA). Figures present combined data from two independent experiments with four to six mice per group in each experiment. Results depicted as means ± SEM. **P < 0.01, ***P < 0.001, using Mann-Whitney statistical test.
HDM-treated lta4h−/− mice exhibit PGP accumulation but comparable neutrophil numbers
To further investigate what may underlie the changes in lung function in these mice, we considered the secondary PGP- degrading action of LTA4H. Administration of HDM resulted in an elevation in BALF concentrations of PGP-generating enzymes MMP-9 and prolylendopeptidase (Fig. 3, A and B), but their concentrations were comparable between lta4h−/− mice and littermate controls. Consequently, both lta4h−/− mice and littermate controls have a comparable capacity to generate PGP from collagen. However, whereas no PGP or AcPGP was detectable in the BALF of wild-type mice, substantial concentrations of PGP (but not of AcPGP) were detectable in HDM-treated lta4h−/− mice (Fig. 3C), due to an inability of lta4h−/− animals to degrade PGP (Fig. 3, D and E).
Fig. 3. HDM-treated lta4h−/− mice exhibit PGP accumulation but comparable neutrophil infiltrate relative to littermate controls.
Wild-type and lta4h−/− mice were administered HDM or PBS intranasally three times per week for 3 weeks, and BALF and lung tissue were collected 24 hours after the final HDM/PBS exposure. Amounts of MMP-9 in BALF were assessed by ELISA (A) and prolylendopeptidase by Western blot (B). The concentration of PGP in BALF was determined by mass spectrometry (C). BALF was incubated with exogenous PGP and degradation assessed after 2 hours by mass spectrometry (D) or release of free proline (E). The number of neutrophils recruited into the lung tissue (F) and airways (G) was determined by flow cytometry. The concentration of MPO (H) in the BALF was determined by ELISA. Figures present combined data from two independent experiments with four to six mice per group in each experiment. Results depicted as means ± SEM. ***P < 0.001, using Mann-Whitney statistical test.
This model of allergic airways disease is predominantly eosinophilic with a minimal neutrophilic infiltrate (fig. S5, A and B). Given the capacity of PGP to function as a neutrophil chemoattractant, we questioned whether PGP accumulation in the HDM-treated lta4h−/− mice promoted a more neutrophilic asthma—a phenotype associated with more severe forms of the disease in humans (22–24). Surprisingly, PGP accumulation in this setting did not lead to enhanced neutro-phil numbers in the lung tissue (Fig. 3F) or airways (Fig. 3G) of HDM-treated lta4h−/− mice at 3 weeks or earlier time points (fig. S5, A and B). Although the neutro-phil chemoattractant PGP is elevated in HDM-treated lta4h−/− mice, these animals cannot generate neutrophil chemoattractant LTB4 (fig. S5C); thus, the two mediators may differentially compensate. The concentration of classical mouse neutrophil chemokines, keratinocyte chemoattractant (KC) and macrophage inflammatory protein-2 (MIP-2), was modestly elevated in HDM-exposed animals, but no differences were apparent between wild-type and lta4h−/− mice (fig. S5, D and E). Finally, we considered whether neutrophil activation may be altered because of the presence of PGP, leading to augmented release of potentially pathological products. However, the concentration of both primary granule- derived myeloperoxidase (MPO; Fig. 3H and fig. S5F) and tertiary granule- derived MMP-9 (Fig. 3A and fig. S5G) was comparable between HDM- administered lta4h−/− mice and littermate controls.
PGP drives airway epithelial remodeling and AHR in the context of allergic airways disease
It was postulated that airway structural changes may account for the exacerbated airway resistance observed in the lta4h−/− mice administered HDM. Accordingly, observation of H&E-stained lung sections revealed that airway epithelial cell height was significantly augmented in the HDM-treated lta4h−/− mice relative to littermate controls, with epithelial cells projecting out to occlude the lumen of the airways (P < 0.001; Fig. 4, A and B, and fig. S6). Furthermore, some of the airways of lta4h−/− mice administered HDM were filled with mucus (Fig. 4A and fig. S6), and periodic acid–Schiff (PAS) staining demonstrated enhanced mucus production by the airway epithelia of HDM-treated lta4h−/− mice relative to littermate controls (Fig. 4, C and D). Subsequently, we questioned whether other aspects of airway remodeling were also altered in the lta4h−/− mice, but no differences were apparent in airway smooth muscle or collagen deposition (fig. S7).
Fig. 4. HDM-treated lta4h−/− mice exhibit augmented airway epithelial remodeling relative to littermate controls.
Wild-type and lta4h−/− mice were administered HDM or PBS intranasally three times per week for 3 weeks, and 24 hours after the final HDM/PBS exposure, lungs were removed for histological examination. (A) Representative H&E-stained lung sections from wild-type and lta4h−/− mice administered PBS or HDM. (B) Epithelial cell height around medium-sized conducting airways was assessed from H&E-stained lung sections. Data presented are an average per mouse. (C) Representative PAS-stained lung sections from wild-type and lta4h−/− mice administered PBS or HDM. (D) Goblet cells were scored from PAS-stained sections. The sum of airway scores from each lung was divided by the number of airways examined and expressed as mucus cell score in arbitrary units. Figures present combined data from two independent experiments with four to six mice per group in each experiment. Results depicted as means ± SEM. *P < 0.05, ***P < 0.001, using Mann-Whitney statistical test. Scale bars, 20 μm.
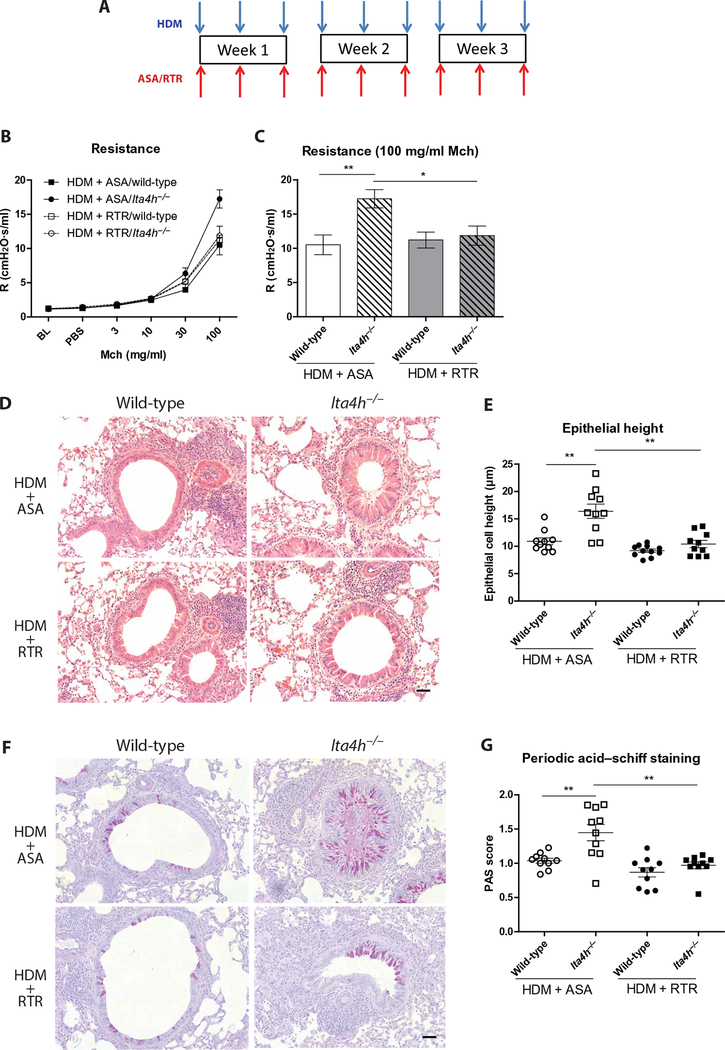
The tripeptide arginine-threonine-arginine (RTR) functions to bind and neutralize PGP both in vitro and in vivo (25). HDM-exposed wild-type and lta4h−/− mice were therefore administered RTR or control peptide, alanine-serine-alanine (ASA), to directly probe the role of PGP in driving the remodeling and lung function changes (Fig. 5A). Administration of RTR to HDM-exposed lta4h−/− mice markedly reduced airway resistance (Fig. 5, B and C), epithelial height (Fig. 5, D and E), and epithelial mucus production (Fig. 5, F and G), demonstrating that all these changes were mediated through the action of PGP. Thus, PGP accumulation in HDM-treated lta4h−/− animals can drive pathological epithelial remodeling and ensuing AHR even in the absence of the important pathological mediator LTB4.
Fig. 5. PGP neutralization attenuates the heightened airway resistance and epithelial remodeling observed in HDM-exposed lta4h−/− mice.
(A) Wild-type and lta4h−/− mice were administered HDM intranasally three times per week for 3 weeks. Mice were concomitantly administered PGP antagonist, RTR, or control peptide, ASA. (B) Airway resistance to increasing doses of methacholine was measured 24 hours after the final HDM exposure. (C) Airway resistance at 100 mg/ml methacholine. (D) Representative H&E-stained lung sections from wild-type and lta4h−/− mice administered HDM with either RTR or ASA. (E) Epithelial cell height around medium-sized conducting airways was assessed from H&E-stained lung sections. Data presented are an average per mouse. (F) Representative PAS-stained lung sections from wild-type and lta4h−/− mice administered HDM with either RTR or ASA. (G) Goblet cells were scored from PAS-stained sections. The sum of airway scores from each lung was divided by the number of airways examined and expressed as mucus cell score in arbitrary units. Figures present combined data from two independent experiments with five mice per group in each experiment. Results depicted as means ± SEM. *P < 0.05, **P < 0.01, using Mann-Whitney statistical test. Scale bars, 20 μm.
As would be anticipated, no PGP was detectable in the BALF of HDM-exposed blt1−/− mice that have functional LTA4H (fig. S8A). Furthermore, epithelial height was actually reduced in HDM-exposed blt1−/− mice relative to wild-type controls (fig. S8B) in keeping with the general reduction in inflammation, remodeling, and ameliorated AHR in these knockout mice that presents as a consequence of the loss of LTB4. This further validates the concept that the exacerbated AHR observed in HDM-exposed lta4h−/− mice is attributable to the accumulated PGP-driven epithelial pathology.
Direct application of AcPGP induces epithelial remodeling and mucus production
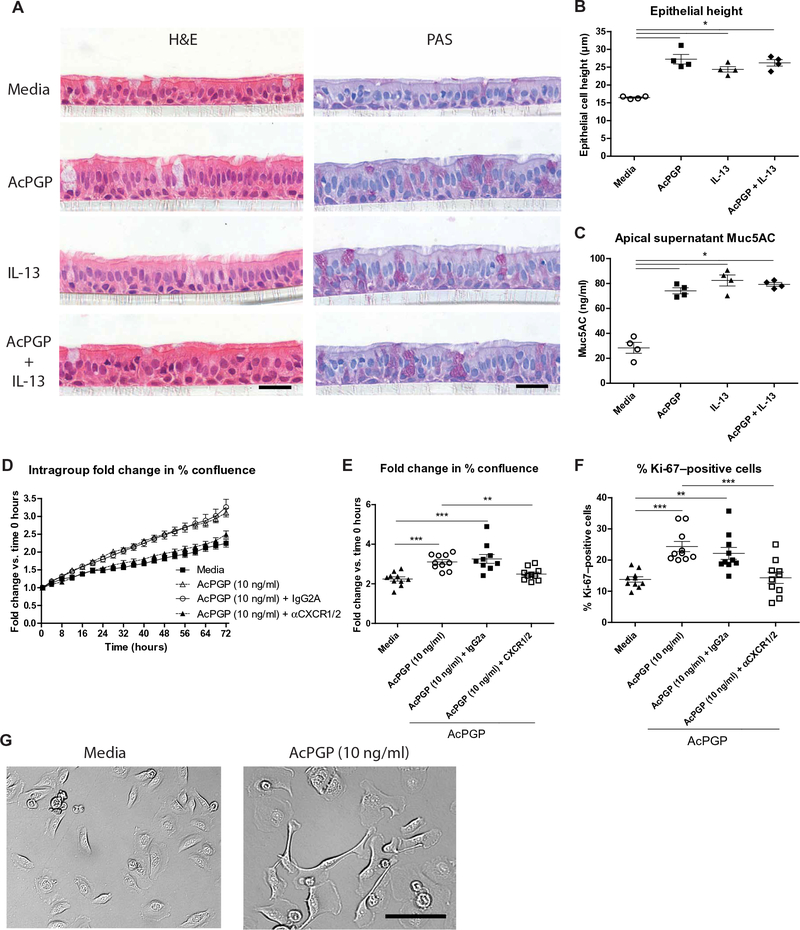
We subsequently assessed whether this epithelial remodeling effect of PGP was a consequence of a direct, sustained interaction of the peptide with epithelial cells. Normal human bronchial epithelial cells at air-liquid interface (ALI) were therefore cultured in media or media supplemented with AcPGP for 7 days. AcPGP as opposed to PGP was used in these studies because it is functionally comparable to PGP but is resistant to any endogenous LTA4H that may be released from the bronchial epithelial cells. Analysis of H&E-stained sections of these cells after 7 days demonstrated that AcPGP treatment resulted in an increase in epithelial height (Fig. 6, A and B). In addition, AcPGP supplementation enhanced mucus production in apical supernatants collected at day 7 of culture (Fig. 6, A and C). These changes elicited by AcPGP were of a comparable magnitude to those observed after sustained stimulation of normal human bronchial epithelial cells at ALI with IL-13, a potent driver of goblet cell hyperplasia and mucus production (Fig. 6, A to C).
Fig. 6. AcPGP induces remodeling of human bronchial epithelial cells.
Normal human bronchial epithelial cells were cultured at ALI. Respective wells were treated with media or media supplemented with AcPGP (10 μg/ml) and/or IL-13 (10 ng/ml) for 7 days. Apical supernatants were collected, and cells were fixed for histological analysis. (A) Representative H&E- and PAS-stained sections of ALI culture epithelium after 7 days of treatment. (B) Epithelial cell height was assessed from H&E-stained sections, with data presented as an average per well. (C) Apical supernatant Muc5AC was assessed by ELISA at day 7 after treatment. Undifferentiated normal human bronchial epithelial cells were cultured in media or media supplemented with AcPGP (10 ng/ml) and visualized over a period of 72 hours using a JuLI Stage automated cell imaging system. In some groups, AcPGP-treated cells were preincubated with either anti-CXCR1/2 antibodies or IgG2a isotype control antibody. (D) Intragroup fold change in percent cell confluence of cells at 72 hours after each treatment relative to 0 hours. (E) Fold change in cell confluence over 72-hour period depicted for individual wells in each treatment group. (F) After 72 hours, the cells were fixed and stained for Ki-67, with Ki-67–positive cells expressed as a percentage of 4′,6-diamidino-2-phenylindole (DAPI)–positive cells. (G) Bright-field image of epithelial cells treated with media or AcPGP (10 ng/ml) after 72 hours. Figures (A) to (C) present combined data from two independent experiments with two wells per group in each experiment. Figures (D) to (F) represent data combined from two independent experiments with five wells per group in each experiment. Results depicted as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, using Mann-Whitney statistical test (B and C) or analysis of variance (ANOVA) with Bonferroni correction (E and F). Scale bars, 20 μm.
To further define the effect of PGP on epithelia, normal undifferentiated primary bronchial epithelial cells were cultured with different concentrations of AcPGP and visualized over a period of 72 hours. AcPGP caused an increase in epithelial cell confluence at all concentrations of the peptide tested (fig. S9, A to D), with this phenotype prominent at AcPGP concentrations as low as 10 ng/ml (Fig. 6, D and E). The increase in confluence was, in part, attributable to an increase in cellular proliferation in response to AcPGP, with a significant increase in the proportion Ki-67–positive cells after 72 hours of peptide stimulation (P < 0.001; Fig. 6F and fig. S9, E and F). Biological effects of PGP on other cells have been demonstrated to be CXCR1/2- dependent (12, 26, 27). Anti-CXCR1/2 blocking antibodies significantly reduced the AcPGP-driven changes in epithelial confluence (P < 0.01; Fig. 6, D and E) and proliferation (P < 0.001; Fig. 6F), demonstrating that these effects were also imparted via CXCR1/2 signaling. In addition, AcPGP-treated epithelial cells displayed enhanced radial spreading that would also contribute to the augmented confluency observed (movie S1). At later time points, in particular, AcPGP-treated cells also displayed marked morphological changes with prominent lamellipods, indicative of enhanced cellular motility, and some evidence of a loss of contact inhibition of locomotion (Fig. 6G and movie S1). Thus, AcPGP directly elicits functional and morphological changes in bronchial epithelial cells that could account for the epithelial phenotype observed in HDM-treated lta4h−/− animals. These studies highlight the capacity of a matrikine, generated during inflammation, to subsequently drive pathological airway remodeling, which, in turn, accounts for the marked disconnect between airway inflammation and lung function.
AcPGP is elevated in the sputum of severe asthmatic patients
Whereas no PGP or AcPGP was detectable in HDM-exposed wild-type mice, human severe asthma is a heterogeneous disease with variable inflammatory, remodeling, and clinical phenotypes, and PGP/AcPGP may naturally be present and contributing to pathology in a subset of patients. The concentrations of PGP and AcPGP were subsequently assessed in the sputum of a cohort of 42 patients with moderate-severe asthma and 15 healthy controls (table S1). Although AcPGP was undetectable in the sputum of any of the healthy controls, all of the asthma patients presented with substantial and remarkably similar quantities of the peptide (Fig. 7A). To corroborate these findings, we assessed the concentration of AcPGP in the sputum of a secondary, independently processed and analyzed, cohort of eight severe asthmatics and nine nonsevere asthmatics (table S2). Once again, all severe asthmatics had substantial concentrations of AcPGP in their sputum, which were significantly higher than those observed in nonsevere asthmatics (P < 0.01; Fig. 7B). Specifically, in this secondary cohort, no AcPGP was detectable in most of the patients (six of nine patients) with nonsevere asthma, with the remainder presenting with a relatively low amount of peptide compared to the severe asthmatics (Fig. 7B).
Fig. 7. The matrikine AcPGP is elevated in the sputum of severe asthmatics.
(A) The concentration of AcPGP peptide in the sputum of patient cohort 1 (UK cohort), consisting of healthy controls (n = 15) and moderate-severe asthmatics (mod-sev asthma; n = 42), as determined by mass spectrometry. (B) The concentration of AcPGP peptide in the sputum of patient cohort 2 (US cohort), consisting of severe (n = 8) and nonsevere asthmatics (n = 9), as determined by mass spectrometry. The horizontal bar depicts the median of each group. Results depicted as means ± SEM. **P < 0.01, ***P < 0.001, using Mann-Whitney statistical test.
To determine why AcPGP accumulated in severe asthmatics, we assessed concentrations of PGP-generating MMP-9 and PGP-degrading extracellular LTA4H in the sputum of the primary patient cohort. Although MMP-9 concentrations were significantly elevated in the sputum of severe asthmatics relative to healthy controls (fig. S10A), extracellular LTA4H concentrations were comparable (fig. S10B), resulting in a marked imbalance in levels of PGP-generating to PGP-degrading enzymes that would facilitate PGP accumulation (fig. S10C). The subsequent cause of PGP acetylation in severe asthmatics remains to be determined.
DISCUSSION
Here, we dissected the dual roles of LTA4H in driving the inflammatory and remodeling aspects of allergic airways disease. Selective abrogation of LTB4 signaling ameliorated inflammation and AHR in our murine HDM model, supportive of previous assertions that LTB4 is an important pathological mediator of asthma (20, 28–31). Conversely, global loss of LTA4H resulted in an exacerbated AHR—seemingly paradoxical given the absence of LTB4 and the ensuing reduction in inflammatory cells and mediators. We subsequently revealed that this exacerbated AHR was attributable to accumulation of PGP and a neutrophil-independent capacity of this peptide to elicit a thickening of the airway epithelial layer and elevated mucus production, leading to occlusion of the airways and limitation of airflow. Thus, we demonstrate a clear potential to disconnect airway inflammation from the remodeling and clinical aspects of allergic airways disease and highlight the critical importance of specific remodeling changes in driving lung function. We went on to demonstrate that AcPGP is elevated in two separate cohorts of asthmatic patients, highlighting the potential physiological relevance of this matrikine in defining clinical disease.
Whereas various cytokines and growth factors are recognized as instigators of inflammation and remodeling, the ECM is well-placed to sense tissue injury and initiate an appropriate localized response. Thus, the release of biologically active matrikines from the ECM is not only effectively able to function as damage- associated molecular patterns to promote inflammation but also appropriately placed to dictate and influence the course of wound repair. Consequently, a measured and controlled matrikine system could function to ensure an appropriate response at the specific site of injury. However, excessive matrikine production or persistence, as seen in many chronic lung diseases, would result in pathological sequelae. PGP/AcPGP is classically viewed as a neutrophil chemoattractant, but it is increasingly apparent that this peptide can also modulate the behavior of other cells. For example, recent studies have demonstrated that PGP/AcPGP acts via CXCR2 on endothelial cells to promote vascular permeability (26) and on endothelial progenitor cells to promote migration, proliferation, and tube-forming activity (27). Airway epithelial cells, such as their endothelial counterparts, have been shown to express CXCR2 (32), and PGP liberated from the proximally located basement membrane is positioned to modulate epithelial behavior. We demonstrate that direct application of AcPGP to primary human bronchial epithelial cells promoted CXCR1/2-dependent proliferation and radial spreading and recapitulated the in vivo phenotype when the cells were cultured at ALI. Furthermore, AcPGP induced morphological changes in epithelial cells with prominent lamellipods indicative of enhanced cellular motility and some evidence of a loss of contact inhibition of locomotion. It is feasible that PGP elicits an epithelial response that is conducive to localized wound repair; however, if PGP persists as a result of aberrant LTA4H activity or is converted to LTA4H-resistant AcPGP, then this response becomes pathological. Thus, although it is recognized that fragments generated from the ECM can modulate the inflammatory response, we now demonstrate that the matrikine PGP/AcPGP also exhibits a remarkable and potent capacity to directly drive airway remodeling.
Previously, we have shown that a failure of LTA4H-mediated PGP degradation in acute, self-resolving pulmonary neutrophilic models results in an exacerbated neutrophilia (14). However, in the context of our TH2-dominant allergic airways disease model, it seems that PGP accumulation does not promote an augmented neutrophilic recruitment or activation. Within this model, HDM administration to wild-type mice results in a hypereosinophilic inflammation with minimal neutrophil recruitment, and it may be that this polarized TH2 environment is less conducive to neutrophil recruitment or persistence. Furthermore, although PGP accumulates in lta4h−/− mice, LTB4 is absent and may thus counteract the increase in PGP in the context of allergic airways disease. Given that the BALF concentrations of PGP described in this study are lower than those reported previously in neutrophilic inflammatory models or diseases, it is also feasible that the concentration of the peptide generated is insufficient to promote a robust neutrophil infiltrate. Conversely, we demonstrate that PGP can drive the observed epithelial changes even at very low concentrations.
Whereas no PGP or AcPGP was detectable in HDM-exposed wild-type mice, we demonstrated that AcPGP was present in the sputum of severe asthmatics, rationalizing the physiological relevance of our findings to a clinical setting. Although AcPGP was absent in all healthy controls, it was detectable in all severe asthmatics across two distinct cohorts of patients. In addition, the concentration of AcPGP was greater in severe asthmatics compared to those patients with non-severe disease in the second cohort, highlighting the potential usefulness of AcPGP as an indicator and instigator of severe pathological inflammation and remodeling. Given the function of PGP/AcPGP, it is pertinent that severe asthma phenotypes that exhibit pronounced neutrophilic inflammation (22–24) and greater airway epithelial thickness and proliferation that contribute to progressive decline in lung function have been described (33). Although concentrations of AcPGP detectable in the sputum of severe asthmatics were relatively similar across the two cohorts, it should be noted that differences in sputum processing limit the ability to make direct intercohort comparisons. In addition, it should be stressed that, although we have demonstrated that PGP has a marked capacity to elicit both murine and human epithelial remodeling, the association with AHR has only been demonstrated in mice. Previous studies have highlighted the significance of specific pathways in driving AHR in mice but have not been recapitulated in human disease. Thus, the significance of PGP in defining AHR in patients in severe asthma is yet to be validated and remains a limitation of this study. No correlation was detected within the moderate-severe asthma cohort between any lung function parameters and levels of AcPGP. However, a limitation of this patient group is that spirometry was performed at the time of patient enrollment, which was often many months before sputum acquisition. AcPGP is a dynamic, changeable readout that has previously been seen to fluctuate with exacerbations in chronic lung disease, and thus, it is perhaps unsurprising that there is no correlation.
PGP and AcPGP are elevated in other chronic lung diseases (10, 12) as a consequence of aberrant LTA4H-mediated PGP degradation and subsequent acetylation of the PGP. The observed disconnect between PGP-generating MMP-9 and PGP-degrading LTA4H in our cohorts of severe asthmatics could rationalize the accumulation of PGP, but it must then be acetylated because only AcPGP was detectable in these patients. We have previously demonstrated the capacity of reactive aldehydes in tobacco smoke to acetylate PGP to LTA4H-resistant AcPGP (11, 34), but smoking status seemingly had little impact on AcPGP concentrations within our cohorts of severe asthmatics. Reactive aldehydes can also be generated physiologically during episodes of severe inflammation, with acrolein, for example, being a product of lipid peroxidation and the MPO-catalyzed oxidation of threonine (35, 36). The source of reactive aldehydes, be it intrinsic or environmentally derived, in these cohorts of severe asthmatics is yet to be unequivocally defined.
Our findings also have direct implications for the assessment of LTA4H inhibitors, which have entered the clinic and failed to demonstrate efficacy (7) and which we have recently shown to abrogate PGP-degrading activity of LTA4H (37). The recent failure of JNJ-40929837 to demonstrate efficacy in a human bronchial allergen challenge model of asthma, despite clearly reducing the concentration of LTB4 (8), could be attributable to a confounding increase in PGP. Finally, we have previously demonstrated that PGP is elevated in chronic neutrophilic lung diseases such as COPD and CF (10, 12, 38). Aberrant epithelial remodeling with excessive mucus production is hallmark of these diseases that could in part be attributable to the previously unappreciated role for PGP now identified in this study.
In conclusion, we have identified a role for the matrikine PGP in driving pathological airway epithelial remodeling. Accordingly, we demonstrate that a failure of lta4h−/− mice to degrade PGP in a physiologically relevant murine model of allergic airways disease leads to severe adverse effects and exacerbated AHR, despite the absence of LTB4. These studies have implications for future assessment of LTA4H inhibitors in the clinic, as well as, more broadly, our understanding of inflammatory and remodeling changes in severe asthma, COPD, and CF.
MATERIALS AND METHODS
Study design
The primary objective of this study was to define the importance of the proinflammatory (LTB4 generation) and anti-inflammatory (PGP degradation) activities of LTA4H in defining the inflammatory and remodeling features of allergic airways disease. All mouse experiments were performed in accordance with the recommendations in the Guide for the Use of Laboratory Animals of Imperial College London, with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. All animal procedures and care conformed strictly to the UK Home Office Guidelines under the Animals (Scientific Procedures) Act 1986, and the protocols were approved by the Home Office of Great Britain. In all experiments, littermate controls were used to all knockout strains, and mice were reared under the same environmental conditions and were age-matched. Adult female mice were randomly placed in either the control group or the experimental group. Authors were blinded for cell counts and histology analysis. The number of mice in each group was determined by power calculations based on extensive previous experience with the model system and is defined in the respective figure legends. The number of independent replicates for each experiment is defined within the respective figure legends. No samples or animals were excluded from data analyses.
The human component of the study was designed to define the presence and levels of PGP and/or AcPGP in sputum derived from asthmatic patients and healthy controls. PGP/AcPGP levels were assessed by mass spectrometry and relevant proteases by ELISA, as described elsewhere in Materials and Methods. There were no previous data to inform power calculations in asthmatic patients, and thus, sample size was opportunistic and used historical sample sets. PGP/AcPGP levels were assessed in two independently acquired, processed, and analyzed patient cohorts. Authors were blinded for analysis of PGP/AcPGP levels. All research ethics and patient consent were in place and are detailed in the “Patient populations and sample collection” section, along with patient inclusion criteria and the number of patients within each cohort. Primary data are in table S4.
Patient populations and sample collection
For the primary patient cohort, sputum samples (spontaneous or induced by using hypertonic saline) were collected from 15 healthy individuals and 42 patients with moderate-severe asthma fulfilling the British Thoracic Society criteria (disease steps 3 to 5). Sputum samples were processed as previously described (39), and supernatants were stored at −80°C. Clinical characteristics of patients are presented in table S1. All samples were collected at the Manchester University NHS Foundation Trust/North West Lung Centre under the framework of the Manchester Allergy, Respiratory and Thoracic Surgery Biobank (study nos. M2014–17 and M2015–32) with ethical approval of the National Research Ethics Service (Research Ethics Committee reference 10/H1010/7). All patients provided written informed consent before participation in the study. Spirometry was performed at the time of patient recruitment and thus may not be reflective of lung function at the time of sputum acquisition.
The secondary cohort was derived through the asthma clinic at the University of Alabama at Birmingham (UAB). The asthma clinic maintains a database, which includes demographics, spirometric values, and laboratory data. For those who consent to participate, blood and induced sputum samples are stored in a clinical specimen biorepository (UAB institutional review board number F150812003). Sputum was induced in patients using the Hargreaves protocol (40). Sputum samples were processed as previously described (41), and supernatants were stored at −80°C. Clinical characteristics of patients are presented in table S2. The asthmatic populations are defined as severe versus nonsevere based on the need for high-dose inhaled corticosteroids (ICS) plus a second controller agent to maintain control of their asthma or remained uncontrolled despite use of these medications (42) at the time of sputum collection. High-dose corticosteroids are defined according to the Global Initiative for Asthma steroid equivalency table (www.ginasthma.org) or as the highest-dose ICS combined with long-acting beta agonist in the U.S. Food and Drug Administration–approved medication available in the United States.
Allergic airway disease model
Respective wild-type and lta4h−/− mice, on a 129/S6 background, were administered 25 μg of HDM extract (Greer Laboratories) in 50 μl of sterile PBS intranasally three times a week for 3 weeks. Respective wild-type and blt1−/− mice, on a C57BL/6 background, were administered 25 μg of HDM extract in 50 μl of sterile PBS intranasally five times a week for 3 weeks. These differing dosing protocols used in 129/S6 and C57BL/6 mice have been optimized to elicit comparable inflammatory, remodeling, and AHR in these distinct inbred strains. Control mice were administered 50 μl of sterile PBS intranasally at analogous time points. All mice were culled 24 hours after the final dose of HDM or PBS.
For the PGP neutralization studies, respective wild-type and lta4h−/− mice were administered HDM and either 50 μg of RTR or control peptide, ASA (AnaSpec peptides) intranasally three times a week for 3 weeks. All mice were culled 24 hours after the final dose of HDM.
Lung function
Measurements of dynamic resistance, elastance, and compliance were performed in anesthetized and tracheotomized mice using a flexiVent system (SCIREQ) in response to increasing concentrations (0, 3, 10, 30, 100, and 300 mg/ml) of methacholine (Sigma-Aldrich), as described previously (43).
Tandem mass spectrometry for PGP/AcPGP detection
For peptide quantification in BALF, PGP/AcPGP was measured using a MDS Sciex (Applied Biosystems) API-4000 spectrometer equipped with a Shimadzu high-performance liquid chromatography (HPLC). For peptide quantification from degradation experiments, PGP was measured using a Thermo Accela Pump and Autosampler coupled to a Thermo TSQ Quantum Access. HPLC was performed using a 2.0 mm × 150 mm Jupiter 4u Proteo column (Phenomenex) with A (0.1% HCOOH) and B (MeCN + 0.1% HCOOH): 0 to 0.5 min 5% buffer B/95% buffer A, then increased over 0.5 to 2.5 min to 100% buffer B/0% buffer A. Background was removed by flushing with 100% isopropanol/0.1% formic acid. Positive electrospray mass transitions were at 270/70, 270/116, and 270/173 for PGP and 312/112 and 312/140 for AcPGP. Peak area was measured, and peptide concentrations were calculated relative to heavy-labeled PGP and AcPGP internal standards using a relative standard curve method, as previously described (12).
Histological assessment of lung sections
The inferior lobe of the right lung was inflated with and stored in 10% neutral buffered formalin for 24 hours before paraffin wax embedding. The paraffin-embedded sections (4 μm thick) were subsequently stained for H&E to evaluate inflammation and general morphology. Epithelial cell height around medium-sized conducting airways (150 and 250 μm in diameter) was assessed, with 10 measurements made per airway and a minimum of 10 airways measured per section. Data presented are an average per mouse.
Goblet cells were visualized on PAS-stained sections and scored double-blind using a numerical scoring system (0, <5% goblet cells; 1, 5 to 25%; 2, 25 to 50%; 3, 50 to 75%; 4, >75%). The sum of airway scores from each lung was divided by the number of airways examined (20 to 50 airways per mouse) and expressed as mucus cell score in arbitrary units, as previously described (17).
Collagen deposition was assessed on Sirius red–stained sections. Image analysis was performed using Scion Image (Scion Corporation).
Smooth muscle actin staining was performed using a rabbit anti-mouse α–smooth muscle actin (Thermo Fisher Scientific) antibody diluted 1:1500 using an avidin/biotin staining strategy. Tissue sections were visualized using a Leica DM2500 microscope and QWin software (Leica Microsystems Ltd.).
Assessment of human bronchial epithelial cell proliferation
Primary human normal bronchial epithelial cells (Lonza) were cultured for expansion in a 75-cm3 flask (Corning), coated with type 1 collagen (30 μg/ml; Sigma-Aldrich), in Lonza bronchial epithelial growth medium (BEGM). Once more than 90% confluence was achieved, the cells were removed with trypsin (TrypLE Express Enzyme, Gibco) and plated onto a 96-well plate at a density of 5000 cells per well. The cells were supplemented with BEGM or with BEGM containing AcPGP (10 μg/ml, 1 μg/ml, 100 ng/ml, or 10 ng/ml). For assessment of a CXCR1/2-dependent mechanism, epithelial cells were incubated for 1 hour at 37°C with anti-CXCR1 and anti-CXCR2 antibodies (1 μg/ml; R&D Systems) or IgG2a isotype control antibodies (2 μg/ml; R&D Systems) before addition of AcPGP (10 ng/ml). The cells were placed in an incubator for 4 hours for acclimatization and to avoid potential condensation on the lid. Snapshots of each well were recorded at 30-min intervals over 72 hours using the JuLI Stage Real-Time Cell History Recorder (NanoEnTek Inc.). Percentage cell confluence was automatically recorded for every snapshot of each respective well. At the end of the experiment, the cells were fixed in 10% neutral buffered formalin (Sigma-Aldrich) for subsequent analysis. Ki-67 staining was performed using a rat anti-mouse Ki-67 primary antibody diluted 1:500 (eBioscience). A Cy3-conjugated goat anti-rat antibody (Jackson ImmunoResearch) diluted 1:500 was subsequently added to enumerate Ki-67 positively stained cells. The cells were then counterstained with a DAPI dye (Sigma-Aldrich) diluted 1:1000. The number of DAPI-positive cells and the number of DAPI and Ki-67 double-positive cells were counted to determine the percentage Ki-67–positive cells per well.
ALI culture studies
Polarized human bronchial epithelial cell cultures (MucilAir) were provided by Epithelix (Sàrl Epithelix). The primary cells were generated from a single healthy donor (71-year-old female with no smoking history). The MucilAir cell culture medium was changed every 2 days until experimentation. Respective wells were treated with MucilAir cell culture medium containing AcPGP (10 μg/ml; AnaSpec peptides), human recombinant IL-13 (10 ng/ml; Peprotech), or a combination of AcPGP (10 μg/ml) and human recombinant IL-13 (10 ng/ml). MucilAir cell culture medium was used for media control wells. The respective wells were treated apically overnight and then continuously fed basolaterally every 2 days with medium containing respective treatments. Apical supernatant was collected by washing the apical side of the Transwell with 200 μl of PBS on day 7 and stored at −80°C for subsequent analysis. At the end of the experiment, inserts were fixed in 4% paraformaldehyde and processed for histological analysis.
Statistical analysis
Statistical significance was calculated with a nonparametric Mann-Whitney statistical test (two-sided) and GraphPad Prism software (GraphPad Software Inc.). ANOVA with Bonferroni correction was used for multiple comparisons. Results are depicted as means ± SEM unless stated otherwise. All P values of <0.05, <0.01, and <0.001 were considered significant and are referred to as such in the text.
Supplementary Material
Acknowledgments:
We thank A. Caldwell and the Centre of Excellence for Mass Spectrometry at King’s College London for the use of equipment and technical assistance. We thank L. Lawrence for histological sectioning and staining. This report includes independent research supported by the National Institute for Health Research (NIHR) South Manchester Respiratory and Allergy Clinical Research Facility at Manchester University NHS Foundation Trust (Wythenshawe) and by the NIHR Manchester Biomedical Research Centre. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Funding: R.J.S. is a Wellcome Trust Senior Research Fellow in Basic Biomedical Sciences (209458/Z/17/Z), and parts of this work were also funded through an Asthma UK Innovations award (AUK-IG-2014–286). C.M.L. is a Wellcome Trust Senior Fellow in Basic Biomedical Sciences (107059/Z/15/Z). T.P. is supported by a Marie Curie Intra European Fellowship within the Seventh European Community Framework Programme (FP7-PEOPLE-2013-IEF no. 627374). The National Heart, Lung and Blood Institute funds J.E.B. (HL077783, HL110950, HL114439, and HL126596), C.S. (HL122426 and HL136211), and A.G. (HL102371). A.G. is also funded through the Veterans Administration (1I01BX001756). The UAB Lung Health Center Pulmonary Proteomics Laboratory is funded through the UAB Health Service Foundation General Endowment Fund. A. Simpson is supported by the Manchester Biomedical Research Centre. Parts of this research were supported by a precompetitive, open innovation award to the Manchester Collaborative Centre for Inflammation Research by AstraZeneca and GlaxoSmithKline.
Footnotes
SUPPLEMENTARY MATERIALS
http://www.sciencetranslationalmedicine.org/cgi/content/full/10/455/eaaq0693/DC1
Materials and Methods
Fig. S1. HDM-treated blt1−/− mice show ameliorated AHR and airway inflammation relative to wild-type controls.
Fig. S2. HDM-treated lta4h−/− mice have comparable lung tissue inflammation to littermate controls despite exhibiting an exacerbated AHR.
Fig. S3. HDM-treated lta4h−/− mice exhibit reduced concentrations of TH2 inflammatory mediators in their lung tissue relative to their littermate controls.
Fig. S4. Lipoxin A4 and cysteinyl leukotrienes are not elevated in HDM-treated lta4h−/− mice.
Fig. S5. Comparable neutrophilic inflammation and mediators in HDM-exposed wild-type and lta4h−/− mice.
Fig. S6. HDM-treated lta4h−/− mice exhibit augmented airway epithelial remodeling relative to littermate controls.
Fig. S7. Comparable smooth muscle and collagen deposition in HDM-treated wild-type and lta4h−/− mice.
Fig. S8. HDM-treated blt1−/− mice do not show PGP accumulation or increased epithelial height compared to wild-type mice.
Fig. S9. Direct application of AcPGP to bronchial epithelial cells increases cell proliferation and confluence.
Fig. S10. A protease imbalance could facilitate PGP accumulation in severe asthmatics.
Fig. S11. Flow cytometry gating strategy used to define myeloid cell populations.
Fig. S12. Flow cytometry gating strategy used to define CD4+ T cell population and associated markers.
Fig. S13. Flow cytometry gating strategy used to define ILC2 population and associated markers.
Table S1. Clinical features of patients with severe asthma and healthy subjects from the primary cohort (UK) included in the study.
Table S2. Clinical features of patients with severe and nonsevere asthma from the secondary cohort (United States) included in the study.
Table S3. Differential cell types were defined by specific cell surface markers using flow cytometry.
Table S4. Primary data.
Movie S1. Real-time imaging of AcPGP-induced changes in epithelial cell confluence and morphology.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Saglani S, Lloyd CM, Novel concepts in airway inflammation and remodelling in asthma. Eur. Respir. J 46, 1796–1804 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Haeggström JZ, Tholander F, Wetterholm A, Structure and catalytic mechanisms of leukotriene A4 hydrolase. Prostaglandins Other Lipid Mediat. 83, 198–202 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Tager AM, Luster AD, BLT1 and BLT2: The leukotriene B4 receptors. Prostaglandins Leukot. Essent. Fatty Acids 69, 123–134 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Di Gennaro A, Haeggström JZ, The leukotrienes: Immune-modulating lipid mediators of disease. Adv. Immunol 116, 51–92 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Di Gennaro A, Haeggström JZ, Targeting leukotriene B4 in inflammation. Expert Opin. Ther. Targets 18, 79–93 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Ohnishi H, Miyahara N, Gelfand EW, The role of leukotriene B4 in allergic diseases. Allergol. Int 57, 291–298 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Fourie AM, Modulation of inflammatory disease by inhibitors of leukotriene A4 hydrolase. Curr. Opin. Investig. Drugs 10, 1173–1182 (2009). [PubMed] [Google Scholar]
- 8.Barchuk W, Lambert J, Fuhr R, Jiang JZ, Bertelsen K, Fourie A, Liu X, Silkoff PE, Barnathan ES, Thurmond R, Effects of JNJ-40929837, a leukotriene A4 hydrolase inhibitor, in a bronchial allergen challenge model of asthma. Pulm. Pharmacol. Ther 29, 15–23 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Gaggar A, Weathington N, Bioactive extracellular matrix fragments in lung health and disease, J. Clin. Invest 126, 3176–3184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE, A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J. Immunol 180, 5662–5669 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, Shastry S, Rowe SM, Shim YM, Hussell T, Blalock JE, A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science 330, 90–94 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE, A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med 12, 317–323 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Snelgrove RJ, Leukotriene A4 hydrolase: An anti-inflammatory role for a proinflammatory enzyme. Thorax 66, 550–551 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Akthar S, Patel DF, Beale RC, Peiró T, Xu X, Gaggar A, Jackson PL, Blalock JE, Lloyd CM, Snelgrove RJ, Matrikines are key regulators in modulating the amplitude of lung inflammation in acute pulmonary infection. Nat. Commun 6, 8423 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells JM, O’Reilly PJ, Szul T, Sullivan DI, Handley G, Garrett C, McNicholas CM, Roda MA, Miller BE, Tal-Singer R, Gaggar A, Rennard SI, Jackson PL, Blalock JE, An aberrant leukotriene A4 hydrolase-proline-glycine-proline pathway in the pathogenesis of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 190, 51–61 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardison MT, Galin FS, Calderon CE, Djekic UV, Parker SB, Wille KM, Jackson PL, Oster RA, Young KR, Blalock JE, Gaggar A, The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J. Immunol 182, 4423–4431 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory LG, Mathie SA, Walker SA, Pegorier S, Jones CP, Lloyd CM, Overexpression of Smad2 drives house dust mite-mediated airway remodeling and airway hyperresponsiveness via activin and IL-25. Am. J. Respir. Crit. Care Med 182, 143–154 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory LG, Lloyd CM, Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 32, 402–411 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung KF, Rogers DF, Barnes PJ, Evans TW, The role of increased airway microvascular permeability and plasma exudation in asthma. Eur. Respir. J 3, 329–337 (1990). [PubMed] [Google Scholar]
- 20.Rao NL, Dunford PJ, Xue X, Jiang X, Lundeen KA, Coles F, Riley JP, Williams KN, Grice CA, Edwards JP, Karlsson L, Fourie AM, Anti-inflammatory activity of a potent, selective leukotriene A4 hydrolase inhibitor in comparison with the 5-lipoxygenase inhibitor zileuton. J. Pharmacol. Exp. Ther 321, 1154–60 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Rao NL, Riley JP, Banie H, Xue X, Sun B, Crawford S, Lundeen KA, Yu F, Karlsson L, Fourie AM, Dunford PJ, Leukotriene A4 hydrolase inhibition attenuates allergic airway inflammation and hyperresponsiveness. Am. J. Respir. Crit. Care Med 181, 899–907 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ, Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am. J. Respir. Crit. Care Med 156, 737–743 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Fahy JV, Kim KW, Liu J, Boushey HA, Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J. Allergy Clin. Immunol 95, 843–852 (1995). [DOI] [PubMed] [Google Scholar]
- 24.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, Wenzel SE, Peters SP, Meyers DA, Bleecker ER; National Heart, Lung, and Blood Institue’s Severe Asthma Research Program, Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J. Allergy Clin. Immunol 133, 1557–1563.e5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Houwelingen AH, Weathington NM, Verweij V, Blalock JE, Nijkamp FP, Folkerts G, Induction of lung emphysema is prevented by l-arginine-threonine-arginine. FASEB J. 22, 3403–3408 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn CS, Scott DW, Xu X, Roda MA, Payne GA, Wells JM, Viera L, Winstead CJ, Bratcher P, Sparidans RW, Redegeld FA, Jackson PL, Folkerts G, Blalock JE, Patel RP, Gaggar A, The matrikine N-α-PGP couples extracellular matrix fragmentation to endothelial permeability. Sci. Adv 1, e1500175 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon YW, Heo SC, Lee TW, Park GT, Yoon JW, Jang IH, Kim S-C, Ko H-C, Ryu Y, Kang H, Ha CM, Lee SC, Kim JH, N-acetylated proline-glycine-proline accelerates cutaneous wound healing and neovascularization by human endothelial progenitor cells. Sci. Rep 7, 43057 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waseda K, Miyahara N, Kanehiro A, Ikeda G, Koga H, Fuchimoto Y, Kurimoto E, Tanimoto Y, Kataoka M, Tanimoto M, Gelfand EW, Blocking the leukotriene B4 receptor 1 inhibits late-phase airway responses in established disease. Am. J. Respir. Cell Mol. Biol 45, 851–817 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taube C, Miyahara N, Ott V, Swanson B, Takeda K, Loader J, Shultz LD, Tager AM, Luster AD, Dakhama A, Gelfand EW, The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness. J. Immunol 176, 3157–3164 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Terawaki K, Yokomizo T, Nagase T, Toda A, Taniguchi M, Hashizume K, Yagi T, Shimizu T, Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J. Immunol 175, 4217–4225 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Turner CR, Breslow R, Conklyn MJ, Andresen CJ, Patterson DK, Lopez-Anaya A, Owens B, Lee P, Watson JW, Showell HJ, In vitro and in vivo effects of leukotriene B4 antagonism in a primate model of asthma. J. Clin. Invest 97, 381–387 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Boer WI, Cytokines and therapy in COPD: A promising combination? Chest 121, 209S–218S (2002). [DOI] [PubMed] [Google Scholar]
- 33.Cohen L, Tarsi XE,J, Ramkumar T, Horiuchi TK, Cochran R, DeMartino S, Schechtman KB, Hussain I, Holtzman MJ, Castro M; NHLBI Severe Asthma Research Program (SARP), Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am. J. Respir. Crit. Care Med 176, 138–145 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardison MT, Brown MD, Snelgrove RJ, Blalock JE, Jackson P, Cigarette smoke enhances chemotaxis via acetylation of proline-glycine-proline. Front. Biosci. (Elite Ed) 4, 2402–2409 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E, Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J. Biol. Chem 273, 16058–1066 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Anderson MM, Hazen SL, Hsu FF, Heinecke JW, Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated. J. Clin. Invest 99, 424–432 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Low CM, Akthar S, Patel DF, Löser S, Wong C-T, Jackson PL, Blalock JE, Hare SA, Lloyd CM, Snelgrove RJ, The development of novel LTA4H modulators to selectively target LTB4 generation. Sci. Rep 7, 44449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, Blalock JE, N-α-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir. Res 10, 38 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pizzichini E, Pizzichini MM, Efthimiadis A, Hargreave FE, Dolovich J, Measurement of inflammatory indices in induced sputum: Effects of selection of sputum to minimize salivary contamination. Eur. Respir. J 9, 1174–1180 (1996). [DOI] [PubMed] [Google Scholar]
- 40.Pin I, Gibson PG, Kolendowicz R, Girgis-Gabardo A, Denburg JA, Hargreave FE, Dolovich J, Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax 47, 25–29 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells JM, Jackson PL, Viera L, Bhatt SP, Gautney J, Handley G, King RW, Xu X, Gaggar A, Bailey WC, Dransfield MT, Blalock JE, A randomized, placebo-controlled trial of roflumilast. Effect on proline-glycine-proline and neutrophilic inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 192, 934–942 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet L-P, Brightling C, Chanez P, Dahlen S-E, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG, International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J 43, 343–373 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Löser S, Gregory LG, Zhang Y, Schaefer K, Walker SA, Buckley J, Denney L, Dean CH, Cookson WOC, Moffatt MF, Lloyd CM, Pulmonary ORMDL3 is critical for induction of Alternaria-induced allergic airways disease. J. Allergy Clin. Immunol 139, 1496–1507.e3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.