Summary
Objective
To analyse gender differences in the clinical presentation and recovery of paediatric patients with Cushing’s disease (CD) after transsphenoidal surgery (TSS). Indeed, gender differences between paediatric patients with CD during presentation, after TSS and postoperative recovery have not been adequately studied.
Design
Data were obtained and retrospectively analysed from clinical reports and biochemical tests at the time of presentation, 5–9 days after TSS and at the 6 and 12 months postoperative follow-up visits to determine hypothalamic–pituitary–adrenal axis (HPAA) recovery.
Patients
Data from 102 paediatric patients (48 females, 54 males, mean age 12.9 ± 3.0) with CD who underwent TSS at the National Institute of Health (NIH) Clinical Center between 1997 and 2011.
Results
There was equal distribution of paediatric CD between males and females (53% vs 47%; n = 102, P = 0.484). Males were more likely than females to present with higher mean BMI Z-scores (2.2 ± 0.7 vs 1.9 ± 0.6, P = 0.0079), lower mean height Z-scores (−1.2 ± 1.3 vs −0.7 ± 1.1, P = 0.0467) and higher median plasma ACTH (12.2 vs 8.5 pmol/l; P = 0.0495). Females did not present more frequently with any single sign or symptom. No significant differences were found between males and females for CD cure rates 5–9 days after TSS (87.0% males vs 87.5% females, P = 1.0), long-term cure rates (86.5% vs 93.7%; n = 69; P = 0.4374) and HPAA recovery time (11.2 ± 2.5 vs 11.7 ± 2.5 months; n = 47; P = 0.1992).
Conclusions
Paediatric CD is found to have equal distribution between males and females, but male patients present with elevated BMI and potentially shorter height and higher plasma ACTH. There is no significant difference in the cure rate or HPAA recovery time after TSS between males and females.
Introduction
In adults, Cushing’s disease (CD) has been shown to have a female preponderance and present with more severe symptoms,1 but gender differences in paediatric patients with CD have yet to be studied. The incidence of CD in children is very rare compared to adults, but the potential consequences affecting normal growth and development can be long-term.2–5 We and others have reported that CD in children and adolescence leads to poor growth and development resulting in short stature, obesity, abnormal puberty and osteopenia.6–8 Gender differences between paediatric patients with CD during presentation, after TSS and postoperative recovery have not been adequately studied.
CD is the most common form of Cushing’s syndrome (CS) in childhood and adolescence, accounting for 75–80% of cases with most patients having the typical cushingoid appearance.2,5,8–10 There has been one study on a cohort of 50 paediatric patients with CD which described the relationship between gender and puberty: Storr et al.11 reported a higher incidence in prepubertal males but no increase in severity at presentation among either gender. More recently, a 2007 study reported that paediatric patients with CD had abnormal puberty due to excessive virilization caused by adrenal androgens.7
Here, we present a retrospective investigation of the most recent and largest cohort of paediatric patients with CD studying gender differences at three time points: at initial presentation, 3–9 days after TSS, and during recovery. Continuous and categorical data were collected and analysed from 102 paediatric patients with CD seen at the NIH within the last 15 years with the aim to describe gender differences in paediatric CD and to determine whether the differences in paediatric CD are consistent with those found in adult CD.
Methods
Data from 102 paediatric patients (48 female, 54 male; mean age 12.9 ± 3.0) seen at the NIH Clinical Center between 1997 and 2011 were retrospectively collected. Patient characteristics are summarized in Table 1. Of the 102 children with Cushing’s disease, the majority were Caucasian (65%), 8% were African American, 4% were Asian, and the remainder other/unknown race. Twenty-five percent of the children were of Hispanic/Latino ethnicity. Eighty-one of 102 children (80%) were from the United States or Canada, while 14 were from South America, 3 from the Middle East, 3 from Europe, and 1 from Oceania. Patients were included if they were diagnosed with CD and underwent TSS at the NIH before the age of 18; 6 of the patients in our cohort previously underwent TSS at a different institution, and their disease was not in remission prior to admission to the NIH. Data sets for patients were collected for the initial visit, after TSS and at approximately 6 (6.1 ± 1.5)-month and/or (12.3 ± 0.9)-month follow-up visits.
Table 1.
Paediatric CD patient characteristics and data at initial presentation
| Patient characteristics (N = 102) | Mean ± SD |
|---|---|
| Males/Females (n) | 54/48 |
| Age (years) | 12·9 ± 3·0 |
| BMI Z-score | 2·1 ± 0·7 |
| Height Z-score | −1·0 ± 1·2 |
| Median (Range) | |
| Midnight Cortisol (nmol/l) (normal <121·4 nmol/l) | 419·5 (27·6–3753·6) |
| Plasma ACTH (normal <10·1 pmol/l) | 10 (0·77–69·4) |
| Mean UFC/BSA (nmol/day/m2) | 375·5 (15·9–23702·8) |
| Time since onset of symptoms (months) | 32·05 (1·71–175·79) |
| Mean time to full recovery (months) (n = 47) | 12 (4·8–15·2) |
| (by peak cortisol of >496·8 nmol/l) |
| Patient characteristics | Male (N = 54) Mean ± SD |
Female (N = 48) Mean ± SD |
P-value |
|---|---|---|---|
| Age at Onset (years) (n = 83) | 9·0 ± 2·9 | 10·2 ± 3·3 | 0·0787 |
| BMI Z-score | 2·2 ± 0·7 | 1·9 ± 0·6 | 0·0079 |
| Height Z-Score | −1·2 ± 1·3 | −0·7 ± 1·1 | 0·0467 |
| Male (N = 54) Median (Range) |
Female (N = 48) Median (Range) |
P-value | |
| Adenoma size (mm) (n = 48) | 5 (3–20) | 6 (3–32) | 0·1260 |
| Midnight cortisol (nmol/l) (n = 101) | 456·8 (27·6–959·1) | 400·2 (56·6–3753·6) | 0·3972 |
| Plasma ACTH (pmol/l) | 12·2 (1·1–69·4) | 8·5 (0·8–35·2) | 0·0495 |
| UFC/BSA (nmol/day/m2) | 400·9 (18·6·4594) | 300·2 (15·9–23702·8) | 0·1746 |
| Fasting glucose (mmol/l) | 5·1 (3·7–14·9) | 5·0 (3·0–14·2) | 0·7852 |
| Fasting insulin (mg/dl) (n = 61) | 27·9 (9·0–226·0) | 27·6 (2·7–96·2) | 0·5786 |
| HOMA (mg/dl) (n = 61) | 6·5 (1·9–46·3) | 6·6 (0·6–20·7) | 0·8566 |
| Total cholesterol (mmol/l) (n = 71) | 4·7 (2·4–7·5) | 4·7 (3·1–11·9) | 0·2369 |
| HDL (mmol/l) (n = 70) | 1·4 (0·8–2·4) | 1·3 (0·7–2·1) | 0·0969 |
| LDL (mmol/l) (n = 71) | 2·7 (0·6–6·7) | 3·2 (1·8–10·3) | 0·0968 |
| Triglyceride (mmol/l) (n = 71) | 0·9 (0·3–4·0) | 1·1 (0·4–6·2) | 0·1233 |
The initial visit data included the age, height, weight, BMI Z-score, 24-h UFC, midnight serum cortisol levels, plasma total cholesterol, HDL, LDL, fasting insulin, fasting glucose, calculated homoeostasis model assessment–insulin resistance (HOMA–IR), bone age Z-scores (BAZ), physical symptoms (webbed neck, facial plethora, acne, striae) and duration since onset of symptoms. Physical symptom data were obtained from patient notes and surveys.
To further evaluate the clinically significant differences in BMI Z-scores between genders at initial presentation, we analysed other components of the metabolic syndrome based on accepted normal paediatric values. Cholesterol, HDL, LDL and triglyce-rides were defined as high risk if serum levels were greater than 5.18 mmol/l, less than 0.91 mmol/l, greater than 2.85 mmol/l and greater than 1.70 mmol/l, respectively. Patients with high fasting glucose levels above 6.99 mmol/l were determined to be diabetic, and patients with a HOMA–IR level above 2.6 mg/dl were considered insulin resistant.
For the identification of metabolic syndrome, Weber et al.12 showed that a BMI Z-score of 0 890 provides good discriminatory ability, which we used for our patients. In addition, according to the WHO clinical criteria for metabolic syndrome,13 insulin resistance has to be first identified, followed by any two criteria amongst hypertension, elevated plasma triglycerides, low HDL cholesterol levels, high BMI, elevated urine albumin excretion rate or albumin/creatinine ratio.
Data collected at the time of surgery included adenoma size if visualized on MRI, histopathology of resected tumour, postoperative 24-h urinary free cortisol (UFC) and plasma cortisol. Adenoma size is reported as the size based on the largest reported diameter as reported by a neuroradiologist. The plasma cortisol and 24-h UFC measured on postoperative days 5–9 were used to determine if the patient was cured by the surgery; a UFC less than 27.6 nmol/day and/or plasma cortisol less than 55.2 nmol/l are defined as cured, as previously described14,15. Patients were administered hydrocortisone replacement postoperatively as previously described.16 Patients with continuous remission of signs and symptoms at a most recent follow-up date (>17 months) were considered cured long-term.
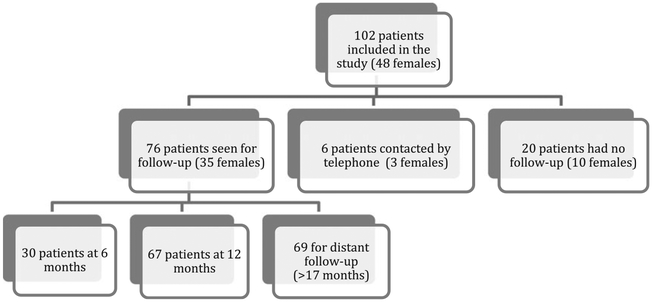
Data collected from follow-up visits included peak cortisol levels from ACTH (250 μg) stimulation test and 24-h UFC. Of the 102 patients (Figure 1), 76 (74.5%) returned for a follow-up visit; 30 (29.4%) patients came for a follow-up visit at approximately 6 months postoperative, 67 (65.7%) patients had a follow-up visit at approximately 12 months postoperative; 21 (20.6%) patients had both a 6- and 12-month visit; and 26 (25.5%) did not follow up at all. HPAA recovery was defined as ACTH stimulation test resulting in a peak cortisol level of ≥496–8 nmol/l as previously described.16
Fig. 1.
Flow diagram regarding patients’ follow-up.
Data were described using simple descriptive statistics or frequency distributions and are presented as range and median or percents, and mean ± standard deviation (SD) for the description of age, BMI and height Z-scores. Continuous data were log-transformed if necessary and compared between the genders using t-tests, or Wilcoxon rank-sum test, as appropriate. Categorical data were compared by Fisher’s exact test, and the proportions of gender distributions were compared by the Z-test for proportions. SAS system software v. 9.2 (SAS Institute, Inc, Cary, NC, USA) and PEPI v. 4.0 (Gahlinger & Abramson, Salt Lake City, UT, USA) were used to analyse the data. A two-sided P-value of less than 0–05 was considered statistically significant.
Results
Among our 102 patients, there was equal distribution between males and females (53% vs 47% P = 0.484). There also was an equal gender distribution of CD in prepubertal and pubertal patients; six prepubertal and 31 pubertal males compared to nine prepubertal and 28 pubertal females (P = 0 385). Mean breast Tanner stage for females was 3 (IQR 3–5),-while mean testicular volume by Prader orchidometer in males was 8 ml (IQR 5–10). Regarding the female group’s menstrual cycle, we have data for 39 of the total 48 patients who were initially evaluated. Their age ranged from 6.53 to 18.19 years with a mean of 12.87 ± 3 years. Sixteen patients were premenarchal, four presented with primary amenorrhoea, 5 with secondary amenorrhoea and 8 with irregular menstruation (73.9% of the postmenarchal patients had either amenorrhoea -or irregular menstruation). Paediatric female patients did not present more frequently with any single sign or symptom, and preoperative MRI showed similar tumour sizes based on the largest measured diameter.
All patients had high midnight cortisol, plasma ACTH and 24-h UFC levels at initial presentation, and there were no statistically significant differences between males and females except for a borderline difference in plasma ACTH (Table 1). The med ian midnight cortisol for the group was 419.5 nmol/l (males 456 8 vs females 400.2 nmol/l; P = 0 3972; normal <110 4 nmol/median plasma ACTH was 10.0 pmol/l (males 12.2 vs females 8.5 pmol/l; P = 0.0495; normal <10.1 pmol/l), and the median 24-h UFC/BSA was 375–5 nmol/day/m2 (males 400.9 vs females 300.2 nmol/day/m2; P = 0 1746).
Initial visit characteristics of the 102 patients are summarized in Table 1. Analysis at initial presentation showed males presenting with CD with higher mean BMI Z-scores (2.2 ± 0.7 vs 1.9 ± 0.6, P = 0.0079), and borderline lower mean height Z-scores (−1.2 ± 1.3 vs −0.7 ± 1.1, P = 0.0467) Table 2.
Table 2.
Percent of paediatric CD males (N = 54) and females (N = 48) with sign/symptom at initial clinical presentation
| Sign/Symptom | Male (%) | Female (%) | P-value |
|---|---|---|---|
| Obesity | 74·1 | 60·4 | 0·2030 |
| Acne | 73·5 | 86·7 | 0·2275 |
| Facial Plethora | 75·0 | 60·0 | 0·2659 |
| Striae | 71·4 | 81·3 | 0·4004 |
| Buffalo Hump | 51·9 | 50·0 | 1·000 |
| Diabetes | 7·4 | 14·6 | 0·3405 |
| Myopathy | 44·0 | 35·3 | 0·7504 |
Statistical analysis of metabolic syndrome revealed that females had an increased risk based on LDL (68 8% females vs 43.6% males P = 0.0208) (Table 3). There were no other significant findings when comparing the remaining metabolic significant findings when syndrome parameters in males and females. Of note, a high proportion of the cohort was found to be at risk for metabolic syndrome. Of the patients who had cholesterol readings, 23 of 71 (32–4%) individuals were considered high risk; 11 of 39 (28.2%) males and 12 of 32 (37.5%) females. LDL levels were high or borderline and at risk in 54.9% of patients, while HDL levels were low and considered at risk in only 7.1% of patients. Triglyceride levels were considered at risk in 12 of 71 patients (16.9%); five of 39 (12.8%) males and seven of 32 (21.9%) females. There were 11 of 102 (10.8%; four males vs seven females) with inappropriately elevated fasting glucose levels and defined as patients with diabetes. Fasting insulin levels were considered at risk in 49 of 61 (80.3%); 25 of 31 (80.7%) males and 24 of 30 (80.0%) females. The HOMA–IR ratio showed 54 of 61 patients (88.5%) with insulin resistance; 27 of 31 (87.1%) males and 27 of 30 (90.0%) females.
Table 3.
Description of metabolic syndrome risk at time of presentation in paediatric patients with CD
| Plasma component | %Total at risk (N = 102) |
%Males at risk (N = 54) |
%Females at risk (N = 48) |
P-value |
|---|---|---|---|---|
| Total cholesterol (n = 71) | 32·4 | 28·2 | 37·5 | 0·1428 |
| LDL (n = 71) | 54·9 | 43·6 | 68·8 | 0·0208 |
| HDL (n = 70) | 7·1 | 7·9 | 6·3 | 1·000 |
| Triglycerides (n = 71) | 16·9 | 12·8 | 21·9 | 0·1514 |
| Fasting glucose (n = 102) | 10·8 | 7·4 | 14·6 | 0·1307 |
| Fasting insulin (n = 61) | 80·3 | 80·7 | 80·0 | 1·000 |
| HOMA-IR (n = 61) | 88·5 | 87·1 | 90·0 | 1·000 |
Pre- and post-TSS BMI Z-scores were available in 27 females, and pre-TSS, 24 had a BMI Z-score higher than 0 89 (88 9%), whereas post-TSS, 16 met the criteria for metabolic- syndrome12 (59.3%). In our male group, we had data for BMI Z-scores pre- and postoperatively in 30 patients. Pre-TSS, 28 had a BMI Z-score higher than 0.89 (93.3%), and post-TSS, only 19 males (63.3%). These data show that there was a decrease in the prevalence of metabolic syndrome post-TSS in females and males of 29.6% and 30%, respectively, taking into consideration only the patients’ BMI status. According to the WHO clinical criteria for metabolic syndrome,13 of the 21 female patients, eight met the criteria pre-operatively (38.1%) and two postoperatively for metabolic syndrome (9.5%). Concerning the male patients, initially, 10 met the criteria (50%) compared to only one of them after the resection (5%). There was, therefore, a decrease of 28.6% and 45% postoperatively in the prevalence of metabolic syndrome in the female and male group, respectively.
The bone age Z-score (BAZ) was calculated before and after TSS. In the female group, 24 had a mean BAZ of 0.5 ± 1.7 pre-TSS and −0.2 ± 1.3 post-TSS after 1.2 ± 0.2 years. We had data from 30 males who had a mean BAZ of 1 ± 1.3 pre-operatively and 0.5 ± 1.5 postoperatively after a period of 1.2 ± 0.3 years. For patients who were Tanner stages 1–4 at the time of TSS, and in whom we had at least 1 year of follow-up growth data, the mean annual growth velocity following TSS was 7.35 ± 4.06 cm, as compared to noted growth deceleration/plateau prior to surgery.17
No significant gender differences were found on the immediate and long-term cure rates, or HPAA recovery. Of the 102 patients, 89 individuals (87.2%) were found to be cured 5–9 days after TSS (87.0% males vs 87.5% females, P = 1.0). For the patients who returned for a follow-up at a later time (>17 months), 62 of 69 (89.9%) patients remained cured (86.5% males vs 93.7% females; P = 0.4374). Of the 76 patients who returned for either a 6 or 12 month follow-up visit, 47 recovered HPAA function (21 males vs 26 females; P = 0.0578) with an average recovery time of 11.5 ± 2.5 months (11.2 ± 2.5 months for males vs 11.7 ± 2.5 months for females; n = 47; P = 0.1992). Due to the fact that many patients had difficulties coming for follow-up at the NIH, there was an attempt to contact them by telephone so that we could be informed about their current situation. Of the 26 patients who were not seen for follow-up, we managed to communicate with six patients, five of whom were cured, and their length of follow-up in months ranged from 4.6 to 64.2 with a median of 33.3 months (Fig. 1).
Of the 76 patients who were evaluated for postoperative complications, 16 (five female) were found to have hypopituitarism. In the female group, diabetes insipidus (DI) occurred in four and hypothyroidism in two of them. In the male group, eight presented with hypothyroidism, five with DI, four with hypogonadism and one with growth hormone deficiency. All patients were administered the appropriate hormonal replacement medication.
Discussion
This is the first comprehensive study of paediatric patients with CD treated at the NIH Clinical Center since 1993, with the exception of a recent manuscript focusing on surgical outcomes.18 We investigated a new and larger group of 102 patients seen over the last 15 years. Our study shows an equal distribution of CD in paediatric males and females. We have also found significant differences showing that males present with a higher BMI and possibly shorter stature. As the pubertal growth spurt in males occurs later than in females, the timing of puberty and its relationship to stature is inherently different between males and females; however, comparing Z-scores which are corrected for gender should normalize this difference in gender-specific ages of maturation. Physical signs of CD manifested equally in both genders with potentially higher biochemical levels of ACTH in the males. Pre-operative pituitary MRI showed tumours of similar size in both genders. Interestingly, a study in adult patients with CD that had shown the expected female preponderance also showed males presenting at a younger age with more severe clinical symptoms, and increased UFC and ACTH.1
As our statistical analysis revealed higher BMI Z-levels in males, we further investigated lipid profiles and other risks associated with metabolic syndrome in our cohort. It is important to point out that following cure, elements of metabolic syndrome improved in the majority of the patients. Interestingly, despite the increased BMI Z in males, we found females were at significantly higher risk based on LDL than that of males. No other significant differences were identified in the other parameters for metabolic syndrome in males and females. Nonetheless, it is noteworthy to report the high prevalence of risk factors contributing to metabolic syndrome. Assessing the risk for metabolic syndrome in paediatric patients with CD would further define the long-term implications of the disease. The incidence of metabolic syndrome in paediatric patients with normal BMI has been reported to be 3–4%,19 but Cruz and Goran show an increased incidence of 30% in children who are overweight (BMI >95 percentile) and with those who are insulin resistant.20
Our data suggest that paediatric patients with CD are at a higher risk of metabolic syndrome showing 68% of patients are considered obese, while 89% had HOMA–IR values indicating insulin resistance at initial presentation. Keil et al.21 previously described a similar pattern of increase in fasting insulin, LDL, HOMA, glucose, cholesterol and triglycerides in a smaller subset of our patients.
TSS is the treatment of choice for CD22–24; long-term cure after TSS has been shown to reverse hypercortisolism, increase growth velocity, and improve bone mineral density.25,26 The most recent visit of each patient was at an average of 2.6 ± 2.4 years after TSS. It has been reported that long-term surveillance is required for all patients who underwent TSS to detect disease recurrence.25,26 Analysis of the data did not reveal any gender differences of paediatric CD cure rates or recovery of HPAA after TSS, although a longer period of follow-up would be ideal. The reported average remission rate for CD after TSS is 72% with an average time of relapse of 4.2 years.27,28
Why males present with higher BMI Z, shorter stature and higher plasma ACTH is unclear. In other paediatric pituitary tumours, such as prolactinomas boys tend to have more aggressive disease but this is also accompanied by larger tumour size.29 We may speculate that overall paediatric pituitary adenomas are more aggressive in boys; in prolactinomas, however, hormonal secretion does not lead to any symptoms in prepubertal boys (other than when the tumour produces mass effects).30 ACTH-producing adenomas have a hormonal phenotype which makes them detectable earlier. The molecular signatures of paediatric pituitary tumours are not known, and it is possible that paediatric pituitary adenomas are different from those in adults.
The retrospective design of our study has a number of limitations. For example, while importing data to analyse the risks of metabolic disorder in our cohort, many patients had missing values for total cholesterol, HDL, LDL, fasting insulin and fasting glucose. This is because these parameters of study were not added to our protocol until 2004. There were also a significant number of patients (20) who were neither seen for follow-up nor able to be contacted by telephone. This lack of data limits the conclusions we can draw. In addition, due to the descriptive nature of the study, there were numerous comparisons with a few borderline statistically significant differences that need to be considered with caution.
In conclusion, paediatric CD is found to have unexpectedly and contrary to the situation in adults, an equal distribution between males and females. There are no significant differences in the cure rate or HPAA recovery time after TSS. However, male patients presented with higher BMI, possibly shorter height and increased plasma ACTH, suggesting a more aggressive form of CD in boys, as is the case in paediatric prolactinomas. Further studies are needed to elucidate the molecular and patho-physiological basis for this gender-dependent biologic behaviour of paediatric pituitary adenomas.
Acknowledgements
Nothing to declare.
References
- 1.Pecori Giraldi F, Moro M & Cavagnini F (2003) Gender-related differences in the presentation and course of Cushing’s disease. Journal of Clinical Endocrinology and Metabolism, 88, 1554–1558. [DOI] [PubMed] [Google Scholar]
- 2.Magiakou MA, Mastorakos G, Oldfield EH et al. (1994) Cushing’s syndrome in children and adolescents. Presentation, diagnosis, and therapy. New England Journal of Medicine, 331, 629–636. [DOI] [PubMed] [Google Scholar]
- 3.Streeten DH, Faas FH, Elders MJ et al. (1975) Hypercortisolism in childhood: shortcomings of conventional diagnostic criteria. Pediatrics, 56, 797–803. [PubMed] [Google Scholar]
- 4.Strickland AL, Underwood LE, Voina SJ et al. (1972) Growth retardation in Cushing’s syndrome. American Journal of Diseases of Children, 123, 207–213. [DOI] [PubMed] [Google Scholar]
- 5.Weber A, Trainer PJ, Grossman AB et al. (1995) Investigation, management and therapeutic outcome in 12 cases of childhood and adolescent Cushing’s syndrome. Clinical Endocrinology (Oxford), 43, 19–28. [DOI] [PubMed] [Google Scholar]
- 6.Lodish MB, Hsiao HP, Serbis A et al. (2010) Effects of Cushing disease on bone mineral density in a pediatric population. Journal of Pediatrics, 156, 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupuis CC, Storr HL, Perry LA et al. (2007) Abnormal puberty in paediatric Cushing’s disease: relationship with adrenal androgen, sex hormone binding globulin and gonadotrophin concentrations. Clinical Endocrinology (Oxford), 66, 838–843. [DOI] [PubMed] [Google Scholar]
- 8.Storr HL, Chan LF, Grossman AB et al. (2007) Paediatric Cushing’s syndrome: epidemiology, investigation and therapeutic advances. Trends in Endocrinology and Metabolism, 18, 167–174. [DOI] [PubMed] [Google Scholar]
- 9.Magiakou MA & Chrousos GP (2002) Cushing’s syndrome in children and adolescents: current diagnostic and therapeutic strategies. Journal of Endocrinological Investigation, 25, 181–194. [DOI] [PubMed] [Google Scholar]
- 10.McArthur RG, Cloutier MD, Hayles AB et al. (1972) Cushing’s disease in children. Findings in 13 cases. Mayo Clinic Proceedings, 47, 318–326. [PubMed] [Google Scholar]
- 11.Storr HL, Isidori AM, Monson JP et al. (2004) Prepubertal Cushing’s disease is more common in males, but there is no increase in severity at diagnosis. Journal of Clinical Endocrinology and Metabolism, 89, 3818–3820. [DOI] [PubMed] [Google Scholar]
- 12.Weber DR, Leonard MB, Shults J et al. (2014) A Comparison of Fat and Lean Body Mass Index to BMI for the Identification of Metabolic Syndrome in Children and Adolescents. Journal of Clinical Endocrinology and Metabolism, 99, 3208–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy SM, Brewer HB Jr, Cleeman JI et al. (2004) Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation, 109, 433–438. [DOI] [PubMed] [Google Scholar]
- 14.Batista DL, Oldfield EH, Keil MF et al. (2009) Postoperative testing to predict recurrent Cushing disease in children. Journal of Clinical Endocrinology and Metabolism, 94, 2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rollin GA, Ferreira NP, Junges M et al. (2004) Dynamics of serum cortisol levels after transsphenoidal surgery in a cohort of patients with Cushing’s disease. Journal of Clinical Endocrinology and Metabolism, 89, 1131–1139. [DOI] [PubMed] [Google Scholar]
- 16.Lodish M, Dunn SV, Sinaii N et al. (2012) Recovery of the hypothalamic-pituitary-adrenal axis in children and adolescents after surgical cure of Cushing’s disease. Journal of Clinical Endocrinology and Metabolism, 97, 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodish MB, Gourgari E, Sinaii N et al. (2014) Skeletal maturation in children with Cushing syndrome is not consistently delayed: the role of corticotropin, obesity, and steroid hormones, and the effect of surgical cure. Journal of Pediatrics, 164, 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lonser RR, Wind JJ, Nieman LK et al. (2013) Outcome of surgical treatment of 200 children with Cushing’s disease. Journal of Clinical Endocrinology and Metabolism, 98, 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook S, Weitzman M, Auinger P et al. (2003) Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Archives of Pediatrics and Adolescent Medicine, 157, 821–827. [DOI] [PubMed] [Google Scholar]
- 20.Cruz ML & Goran MI (2004) The metabolic syndrome in children and adolescents. Current Diabetes Reports, 4, 53–62. [DOI] [PubMed] [Google Scholar]
- 21.Keil MF, Graf J, Gokarn N et al. (2012) Anthropometric measures and fasting insulin levels in children before and after cure of Cushing syndrome. Clinical Nutrition, 31, 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ammini AC, Bhattacharya S, Sahoo JP et al. (2011) Cushing’s disease: results of treatment and factors affecting outcome. Hormones (Athens), 10, 222–229. [DOI] [PubMed] [Google Scholar]
- 23.Mampalam TJ, Tyrrell JB & Wilson CB (1988) Transsphenoidal microsurgery for Cushing disease. A report of 216 cases. Annals of Internal Medicine, 109, 487–493. [DOI] [PubMed] [Google Scholar]
- 24.Styne DM, Grumbach MM, Kaplan SL et al. (1984) Treatment of Cushing’s disease in childhood and adolescence by transsphenoidal microadenomectomy. New England Journal of Medicine, 310, 889–893. [DOI] [PubMed] [Google Scholar]
- 25.Devoe DJ, Miller WL, Conte FA et al. (1997) Long-term outcome in children and adolescents after transsphenoidal surgery for Cushing’s disease. Journal of Clinical Endocrinology and Metabolism, 82, 3196–3202. [DOI] [PubMed] [Google Scholar]
- 26.Leinung MC, Kane LA, Scheithauer BW et al. (1995) Long term follow-up of transsphenoidal surgery for the treatment of Cushing’s disease in childhood. Journal of Clinical Endocrinology and Metabolism, 80, 2475–2479. [DOI] [PubMed] [Google Scholar]
- 27.Guilhaume B, Bertagna X, Thomsen M et al. (1988) Transsphenoidal pituitary surgery for the treatment of Cushing’s disease: results in 64 patients and long term follow-up studies. Journal of Clinical Endocrinology and Metabolism 66, 1056–1064. [DOI] [PubMed] [Google Scholar]
- 28.Devoe DJ, Miller WL, Conte FA et al. (1997) Long-term outcome in children and adolescents after transsphenoidal surgery for Cushing’s disease. Journal of Clinical Endocrinology and Metabolism 82, 3196–3202. [DOI] [PubMed] [Google Scholar]
- 29.Fideleff HL, Boquete HR, Suarez MG et al. (2009) Prolactinoma in children and adolescents. Hormone Research, 72, 197–205. [DOI] [PubMed] [Google Scholar]
- 30.Catli G, Abaci A, Altincik A et al. (2012) Hyperprolactinemia in children: clinical features and long-term results. Journal of Pediatric Endocrinology and Metabolism, 25, 1123–1128. [DOI] [PubMed] [Google Scholar]