Abstract
To test the association between impaired olfaction and other prodromal features of PD in the Parkinson At-Risk Syndrome Study. The onset of olfactory dysfunction in PD typically precedes motor features, suggesting that olfactory testing could be used as a screening test. A combined strategy that uses other prodromal nonmotor features, along with olfactory testing, may be more efficient than hyposmia alone for detecting the risk of PD. Individuals with no neurological diagnosis completed a mail survey, including the 40-item University of Pennsylvania Smell Identification Test, and questions on prodromal features of PD. The frequency of reported nonmotor features was compared across individuals with and without hyposmia. A total of 4,999 subjects completed and returned the survey and smell test. Of these, 669 were at or below the 15th percentile based on age and gender, indicating hyposmia. Hyposmics were significantly more likely to endorse nonmotor features, including anxiety and depression, constipation, and rapid eye movement sleep behavior disorder symptoms, and to report changes in motor function. Twenty-six percent of subjects with combinations of four or more nonmotor features were hyposmic, compared to 12% for those reporting three or fewer nonmotor features (P < 0.0001). Hyposmia is associated with other nonmotor features of PD in undiagnosed individuals. Further assessment of hyposmic subjects using more specific markers for degeneration, such as dopamine transporter imaging, will evaluate whether combining hyposmia and other nonmotor features is useful in assessing the risk of future neurodegeneration.
Keywords: Parkinson’s disease, olfaction, early detection, nonmotor features
PD has a prodromal phase during which physiological changes as well as nonmotor clinical features are present.1–3 Clinical aspects of the premotor phase of PD include constipation, daytime sleepiness, and rapid eye movement sleep behavior disorder (RBD), affective symptoms, and impaired sense of smell.4
Of these prodromal features, olfactory loss may be particularly useful for early diagnosis or even premotor identification of PD. Olfactory deficits are common in PD, affecting up to 85% of PD patients.5 Hyposmia develops early in the disease course6,7 and is largely independent of motor and cognitive status and medication use.8–10 Several studies have found olfactory deficits in asymptomatic relatives of PD patients,11,12 some of whom have subsequently developed clinically manifest PD. 13 In a population-based study of World War II veterans, olfactory deficits predicted risk of subsequent PD. 14 Lesions in the olfactory system are also some of the earliest pathological manifestations in PD.15,16
The utility of olfactory testing could potentially be improved by combining it with other prodromal PD features. Hyposmic individuals who also have other prodromal signs may be at particularly high risk for PD and could be the best candidates for screening with a more definitive test, such as dopamine transporter (DAT) imaging. In this analysis, we hypothesized that prodromal nonmotor features would be more common among hyposmic individuals and that such an association could be used to identify individuals at higher risk for hyposmia, and thereby improve the efficiency of screening for individuals for PD.
Patients and Methods
Study Design
This study was conducted as part of the Parkinson At-Risk Syndrome (PARS) Study, a multicenter study coordinated at the Institute for Neurodegenerative Disorders (New Haven, CT) and conducted by 20 clinical centers in the United States (see Appendix). The purpose of the PARS study is to test a two-stage screening strategy for PD consisting of olfactory testing followed by DAT imaging. All activities were approved by the Western Institutional Review Board (IRB), the Department of Defense Research Review Board, and the relevant IRBs at participating centers.
Research Participants
Study participants included individuals without a diagnosis of PD or other neurodegenerative disorder. One group of participants was comprised of first-degree relatives of patients with PD. The second group of subjects did not have a family history of PD. These subjects were identified through purchased mailing lists using the following criteria: nurses in Connecticut; veterans from Conncecticut, Boston, Philadelphia, and Rhode Island; and home owners in Connecticut. Other participants were identified through postings on the PARS website or other media (e.g., the National Parkinson Foundation website, PatientsLikeMe; blog.patientslikeme.com/tag/national-parkinsons-foundation/). To be eligible for the study, participants had to be over 50 years of age (or within 10 years of the age of onset of their affected relative for individuals with an affected family member), not diagnosed with PD or other neurodegenerative condition, and free of other conditions that could affect olfactory function (e.g., history of nasal trauma, sinusitis, or other local olfactory problems).
Assessments
Potential subjects who returned interest forms received a study packet by mail. The packet contained the following: (1) a written informed consent document; (2) a 40-item University of Pennsylvania Smell Identification Test (UPSIT); and (3) a questionnaire with items covering demographics, standard measures of nonmotor features of PD, risk factors for PD, and screening questions for parkinsonian symptoms.
Odor identification was assessed using the UPSIT. 17 The UPSIT is a standardized, four-alternative, forced-choice test comprised of four booklets containing 10 odorants apiece, with one odorant per page. The UPSIT is packaged in envelopes and comes with easy-to-follow instructions, making it suitable for home administration. The specific stimuli, basis for their selection, and reliability and sensitivity of this test have been described in detail previously.17,18 Age- and gender-specific norms were calculated for this study based on the entire baseline cohort. Overall, these norms were similar to those previously published for the UPSIT.17 For this study, hyposmia was defined as age- and gender-normed UPSIT scores at or below the 15th percentile.
Constipation was assessed by a single self-report item on bowel movement frequency adapted from the Honolulu Asian Aging Study (HAAS).19 Constipation was defined as one bowel movement every other day or less. RBD was assessed using four separate items from a published RBD questionnaire.20 The RBD questions probed (1) acting out dreams, (2) excessive movement during sleep, and (3) violent movements during sleep, including thrashing and hitting. There was also a single question that asked whether the subject had been diagnosed with RBD.
Anxiety was measured using the State-Trait Anxiety Inventory (STAI), which contains items on state (form A) and trait (form B) anxiety.21 The STAI has been used extensively in PD research. A score of greater than 39 on either scale represents significant anxiety.22 Depression was assessed by the Center for Epidemiological Studies Depression Scale (CES-D).23 The CES-D consists of 20 items. A score of 16 or greater indicates significant depression.24 Self-reported change in motor symptoms was captured using a nine-item parkinsonism questionnaire.25,26–28
Epidemiological risk factors previously associated with PD were assessed with single-item questions. Caffeine consumption was defined as intake of all types of caffeine-containing beverages (e.g., tea, coffee, cola, and Mellow Yellow). Smoking status was categorized as current, former, and never smoked. In addition to these items, exposure to pesticides, heavy metals, chemical solvents, and whether the subject had suffered a head injury were assayed with single questions. Subjects were asked if they exercised regularly; women were asked if they had ovaries removed. Finally, there were questions on exposure to medications linked to PD risk, including nonsteroidal anti-inflammatory drug (NSAID) medications, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (i.e., statins), hormone replacement therapy, and laxatives.
Statistical Analysis
Descriptive statistics, including means, medians, percentages, and standard deviations (SDs), when appropriate, were calculated for the UPSIT, demographic variables, risk factors, and questions on PD symptoms. To confirm the validity of parametric analyses, sample distributions were visually inspected using histograms or scatter plots, and outlying values were checked for accuracy.
Subjects completing and not completing the UPSIT were compared using chi-square tests or t tests, as appropriate. Univariate associations between hyposmia and other prodromal features of PD were tested using chi-square tests for dichotomous and categorical variables. Cochran-Armitage tests for linear trend were used for ordered categorical variables (e.g., bowel movement frequency). Furthermore, t tests were used to test differences in mean scores on continuous scales (e.g., the CES-D). Logistic regression was used to calculate odds ratios (ORs) for association between hyposmia and other prodromal features. Multiple logistic regression models were constructed to test for independent associations between hyposmia and prodromal features.
All P values reported are based on two-tailed statistical tests. All analyses were performed using SAS software (version 9.2; SAS Institute, Inc., Cary, NC).
Results
Characteristics of Research Participants
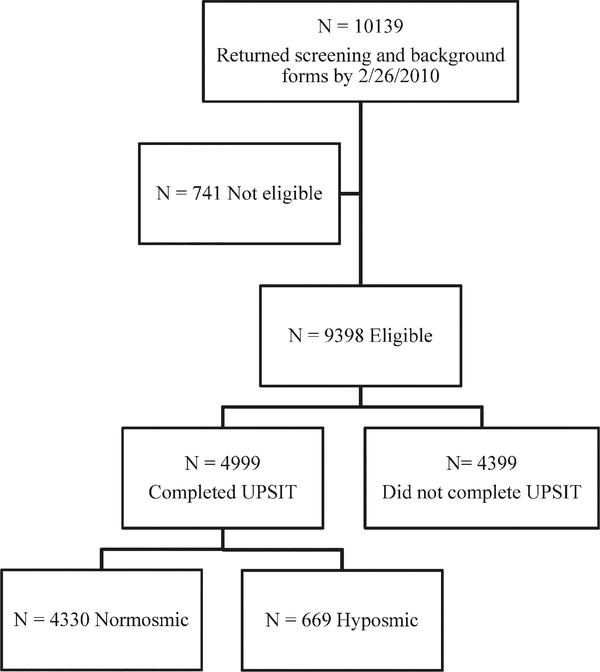
A total of 10,139 subjects returned valid screening and background forms by the closing date of February 26, 2010 (Fig. 1). Of these responses, 741 individuals were not eligible. The reasons for ineligibility included age under 50 (n = 319), diagnosis of parkinsonism (n = 208), other neurological condition (n = 125), history of significant sinus condition (n = 72), and other (n = 17). Of the eligible subjects, 4,999 of 9,398 (53%) completed and returned the UPSIT. Younger subjects were more likely than older subjects to complete the UPSIT (mean age, 64 versus 68; P < 0.0001). Females were more likely than males (60% versus 48%; P < 0.0001) and Caucasians more likely than non-Caucasians (64% versus 33%; P < 0.0001) to complete the UPSIT. Sixty-five percent of subjects having a family member with PD returned the UPSIT, compared with 46% of those who did not (P < 0.0001). Subjects who reported that they thought they had a decreased sense of smell were less likely to return the UPSIT than those who did not so report (51% versus 54%; P = 0.04). Demographic characteristics of subjects completing the UPSIT are shown in Table 1, based on hyposmia status. There are no significant differences in age or gender, because UPSIT percentile scores are standardized for age and gender. However, older age (r2 = −0.33; P < 0.0001) and male gender (male = 31.3 versus female = 33.8; P < 0.0001) were significantly associated with lower unstandardized UPSIT scores.
FIG. 1.
Flow diagram of ascertainment of study cohort. Reasons for ineligibility are given in the text.
TABLE 1.
Demographic Characteristics of the Study Cohort Comparing Hyposmic and Normosmic Subjects Who Completed the UPSIT
| Normosmics( N = 4,330) | Hyposmics (N = 669) | P Value | |
|---|---|---|---|
| Age (Mean, SD) | 63.9 (9.6) | 64.5 (9.3) | 0.13 |
| Male gender (N, %) | 2,200 (51) | 355 (53) | 0.28 |
| White/Caucasian (N, %) | 3,820 (98) | 583 (96) | 0.0138 |
| PD in family (N, %) | 0.55 | ||
| None | 2,393 (55) | 378 (57) | |
| 1 | 1,760 (41) | 265 (40) | |
| >1 | 177 (4) | 26 (4) | |
| Current/former smoker (N, %) | 2,272 (53) | 359 (55) | 0.57 |
Overall UPSIT Performance
Among those who completed the UPSIT, 669 subjects were judged to be hyposmic based on the norms developed for the study. The average raw UPSIT score was 34.1 (SD, 3.2) for normosmics and 22.6 (SD, 6.4) for hyposmics.
Relationship Between Prodromal Features of PD and UPSIT Performance
There were significant associations between hyposmia and prodromal features of PD, including constipation, RBD symptoms, depression/anxiety, and self-reported change in motor function (Table 2). All RBD items were endorsed significantly more frequently by hyposmics than normosmics. In addition, significantly more hyposmics reported having at least one RBD symptom at least one time per month (34% versus 26%; P = 0.0007). Not surprisingly, self-reported loss of sense of smell was strongly associated with hyposmia, but only 38% of hyposmics were aware of their deficit. All items on the screening questionnaire of motor symptoms were endorsed more frequently by hyposmics, compared to normosmics (Table 3). There was also a significant difference in the number of hyposmic subjects that endorsed at least two motor symptoms, compared to normosmics (25% versus 17%; P < 0.0001).
TABLE 2.
Frequency of Any Prodromal Features of PD in Normosmic and Hyposmic Participants
| Normosmics (N = 4,330) | Hyposmics (N = 669) | P Value | |
|---|---|---|---|
| Self-reported decreased sense of smell (N, %) | <0.0001 | ||
| No | 3,473 (80) | 330 (49) | |
| Yes | 412 (10) | 256 (38) | |
| Don’t know | 430 (10) | 82 (12) | |
| Bowel movement frequency (%) | 0.0065 | ||
| <1 per day | 696 (16) | 136 (21) | |
| 1 per day | 2,222 (52) | 335 (51) | |
| >1 per day | 1,372 (32) | 189 (29) | |
| Anxiety (STAI score >39 on part A, state) (%) | 619 (14) | 127 (19) | 0.0017 |
| Anxiety (STAI score >39 on part B, trait) (%) | 744 (17) | 153 (23) | 0.0004 |
| Depression (CES-D score >15) (%) | 463 (11) | 124 (19) | <0.0001 |
| RBD questions | N = 3,148 | N = 465 | |
| Limb/body movements (%) | 0.0001 | ||
| Never | 1,316 (47) | 167 (41) | |
| <1 per month | 866 (31) | 123 (30) | |
| 1 to 3 times per month | 325 (12) | 53 (13) | |
| 1 per week | 101 (4) | 17 (4) | |
| >1 per week | 201 (7) | 51 (12) | |
| Violent movements (%) | <0.0001 | ||
| Never | 2,595 (85) | 340 (75) | |
| <1 per month | 342 (11) | 69 (15) | |
| 1 to 3 times per month | 56 (2) | 25 (6) | |
| 1 per week | 30 (1) | 6 (1) | |
| >1 per week | 33 (1) | 13 (3) | |
| Reported diagnosed by doctor with RBD (%) | 63 (2) | 18 (4) | 0.0114 |
TABLE 3.
Frequency of Self-Reported Change in Motor Function for Normosmics and Hyposmics
| Normosmics (%)(N = 4,330) | Hyposmics (%) (N = 669) | P Value | |
|---|---|---|---|
| Trouble rising from chair | 845 (20) | 154 (23) | 0.0372 |
| Handwriting smaller | 386 (9) | 100 (15) | <0.0001 |
| Voice softer | 256 (6) | 66 (10) | 0.0001 |
| Balance poor | 538 (13) | 111 (17) | 0.0024 |
| Feet freeze | 29 (1) | 12 (2) | 0.0027 |
| Face less expressive | 175 (4) | 51 (8) | <0.0001 |
| Arms/legs shake | 155 (4) | 47 (7) | <0.0001 |
| Trouble with buttons | 316 (7) | 72 (11) | 0.0019 |
| Shuffle/take tiny steps | 112 (3) | 44 (7) | <0.0001 |
Relationship Between PD Risk Factors and UPSIT Performance
There was no significant association between any of the reported risk factors for PD and hyposmia in our cohort, with the exception of a history of oophorectormy among women (29% versus 22%; P = 0.009) and laxative use (7% verus 5%; P = 0.032; Supporting Table). Negative findings included lack of association with cigarette smoking, for which there have been strong and consistent inverse associations reported for PD.29 Previously, we had reported an inverse association between hyposmia and caffeine consumption in first-degree relatives in a smaller, separate cohort.30 When a similar analysis, with the total UPSIT score as the outcome measure, was performed in the PARS cohort, results were again significant (P = 0.04), particularly among males (P = 0.02). However, the difference in mean adjusted UPSIT score was small.
Association Between Hyposmia and Combinations of Prodromal Features
Clinical features that were significant in univariate analyses (e.g., constipation, depression, anxiety, and self-reported motor symptoms) were entered into a multiple logistic regression model (Table 4). Of these factors, depression had the highest odds of being associated with hyposmia. Although there were significant associations between these prodromal features and hyposmia, the overall predictive accuracy of the model containing all of these features was low (c-statistic = 0.574). Adding a single question on whether the subject was aware of olfactory loss increased the predictive power of the model substantially (c-statistic = 0.688); individuals aware of their olfactory loss were also more likely to report other prodromal features.
TABLE 4.
Odds of Hyposmia for Significantly Associated Variables
| Unadjusted* |
Adjusted**
|
|||||
|---|---|---|---|---|---|---|
| OR | 95% | CI | OR | 95% | CI | |
| Constipation | 1.37 | 1.11 | 1.68 | 1.31 | 1.06 | 1.62 |
| Depression | 1.93 | 1.55 | 2.41 | 1.95 | 1.44 | 2.65 |
| Anxiety | 1.38 | 1.14 | 1.67 | 0.85 | 0.66 | 1.11 |
| Motor symptoms | 1.66 | 1.36 | 2.02 | 1.38 | 1.12 | 1.72 |
ORs and CIs are shown for factors significantly associated with hyposmia in univariate analysis. Constipation is defined as less than one bowel movement per day. Depression is defined as a CES-D score of >15; anxiety is defined as a STAI A or B score of >39. Self-reported change in motor symptoms is defined as two or more positive responses on the screening questionnaire.
Separate models, each adjusted for age, gender, and family member with PD.
Combined model, adjusted for age, gender, and family member with PD.
Because the sample of individuals with bed partners that responded to the RBD questionnaire was a subset of the entire cohort (n = 3,613), a separate model was constructed to assess the association between reported RBD symptoms (defined as having one of the following at least once a month: moving while sleeping, acting out dreams, or thrashing violently while sleeping) and hyposmia. This model was adjusted only for age, gender, and family history of PD. The OR for hyposmia for subjects with reported RBD was 1.46 (95% confidence interval [CI] = 1.17, 1.83; P = 0.001). After additional adjustment for depression, anxiety, constipation, and reported motor symptoms, there was still a significant association between RBD symptoms and hyposmia (OR = 1.33, 95% CI = 1.05, 1.68; P = 0.019).
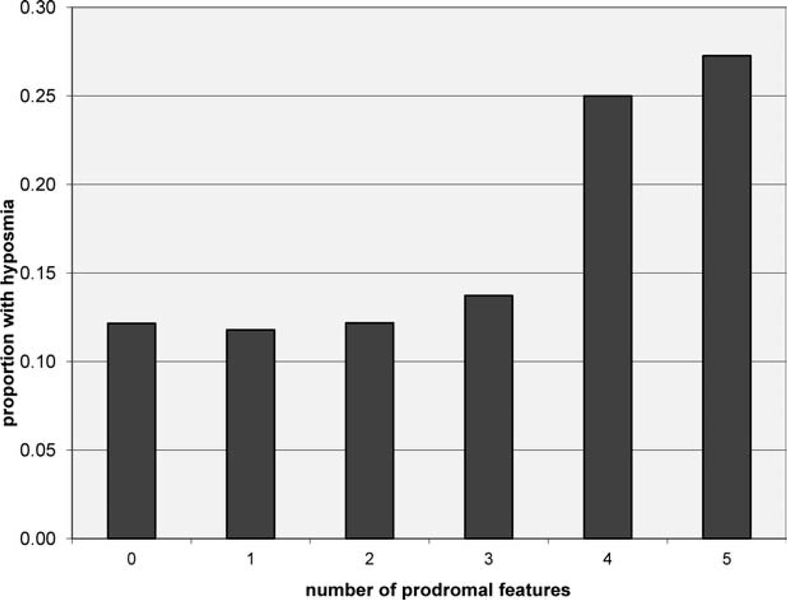
The proportion of subjects with combinations of prodromal features (e.g., depression, anxiety, constipation, RBD symptoms, or motor symptoms) who also had hyposmia was evaluated (Fig. 2). Subjects with increasing numbers of prodromal features were substantially more likely to be hyposmic (P < 0.0001), particularly those with four or five features. There were relatively small numbers of individuals in these categories (183 of 3,107 = 5.9%), and a small proportion of all hyposmics come from these groups (47 of 401 = 11.7%).
FIG. 2.
Proportion of individuals with hyposmia in subjects with combinations of prodromal features. Prodromal features included the following: (1) having any RBD symptom at least once a month; (2) endorsing two or more items on the PD symptom self-report questionnaire; (3) having a CES-D score of greater than 15; (4) having a STAI-A or -B score of >39; or (5) having bowel movement frequency of <1 per day. Individuals with higher numbers of prodromal features, particularly four or five, are more likely to be hyposmic (P <0.0001).
Discussion
In this report, we detail the range of prodromal features of PD, including hyposmia, RBD symptoms, anxiety and depression, constipation, and self-reported change in motor function in approximately 5,000 individuals without a diagnosis of PD participating in the PARS study. There were significant relationships between hyposmia and the number of prodromal features of PD. In general, the magnitude of the associations was modest, but detectable, given the size of the PARS cohort.
Although prodromal PD features were more common in hyposmic individuals, they were also observed in normosmic individuals. More than half of hyposmics exhibited only one or no prodromal PD features. Overall, these results suggest that hyposmic subjects are more likely to manifest other nonmotor features of PD, but these features are not sufficient to either guarantee or exclude the possibility that an individual may have significant olfactory impairment. Individuals who had more than one prodromal feature were more likely to be hyposmic, but a small proportion of the overall cohort had multiple prodromal features.
There were no associations between the frequency of hyposmia and environmental factors that have been linked to PD. Previously, a number of environmental and lifestyle factors have been associated with PD, including an inverse relationship with caffeine consumption31 and cigarette smoking.29 We did replicate our previous finding of a difference in mean adjusted UPSIT score for heavy, compared to low, caffeine drinkers. However, the effect was small, and caffeine consumption was not useful in differentiating hyposmics from normosmics based on the 15th percentile cutoff to define hyposmia.
Associations have also been reported with use of certain prescription drugs, such as NSAID medications, cholesterol-lowering medications and estrogens,32–34 and exposure to pesticides.35 These exposures were investigated by questionnaire and no relationships with hyposmia were identified, except for laxative use. Likewise, there was no association between hyposmia and vigorous exercise. It is not surprising that these associations were not observed, because the vast majority of hyposmia results from causes other than incipient PD.36 In spite of the large sample size of this cohort, only very strong signals coming from the subset of hyposmics who are about to develop PD could be detected. It may be somewhat surprising that hyposmia was not more common in individuals with a family history of PD. However, most participants who were relatives of PD patients had only one affected family member, and the risk of PD in this group may not have been substantial enough to be detectable using hyposmia as a marker for PD.
Previous studies have examined the association between hyposmia and subsequent PD risk. Patients with idiopathic RBD often have olfactory deficits.37 These same individuals have approximately a 4% per year risk of developing PD.38 In the HAAS study of World War II veterans, hyposmic individuals were at substantially higher risk of PD within 4 years after identification of the olfactory deficit.14 In another study, hyposmic relatives of PD patients were at high risk for deficits on DAT imaging.39 In the same study, individuals who were hyposmic and had abnormal imaging had very substantial risk of developing PD within 2 years.13 Normosmic individuals had a low risk of DAT deficit or clinical evidence of neurodegeneration.
In the context of these previous studies, the current results have important clinical implications. As has been demonstrated, olfactory testing could be used as the first part of a two-step screening strategy to detect very early or premotor PD. The current results confirm the relationship between hyposmia and other nonmotor features of PD, adding to the evidence of a prodromal constellation of nonmotor features, including hyposmia, that precede the diagnosis of PD. However, the results of this study demonstrate that most hyposmics do not have nonmotor features of PD. Hyposmia is most often the result of causes other than PD.36 Moreover, many people with normal olfactory function endorse symptoms such as anxiety or depression. Still, prescreening for nonmotor features of PD could cut down on the number of individuals who would need to be smell tested for the purpose of identifying a research cohort of at-risk individuals. However, it is neither sensitive nor specific enough to be used in any clinical screening or early diagnosis program.
Several limitations to this study should be noted. All assessments in this segment of the PARS study were conducted by mail, and none of the subjects were examined. The study relied on self-report for responses to the survey items and on in-home completion of the olfactory testing. Because of this methodology, it is likely that there was some misclassification of subjects, both in terms of risk factors and hyposmic status. It is less likely that there was systematic bias, but rather that the study might have detected larger effects if more precise measures of the various prodromal features had been undertaken. However, the aim of the PARS Study is to test a large-scale screening strategy for PD. It would not be possible or even desirable to directly examine or assess each of 5,000 PARS participants, given the need to establish the feasibility of the PARS sequential biomarker hypothesis. A subset of PARS subjects will have both DAT imaging and longitudinal clinical follow-up. In the course of this follow-up, it may be possible to determine whether the combination of hyposmia and other nonmotor signs helps predict which subjects are at risk for abnormal imaging and clinical PD.
Conclusion
The PARS Study demonstrates the feasibility of large-scale olfactory testing of a community population with and without family members with PD. In the future, modalities such as heart rate variability assessment40 or transcranial ultrasound41 could be incorporated into prescreening paradigms for PD to reduce the number of individuals who would require DAT imaging. Future PD screening studies will likely rely on consortia to develop the resources required to efficiently conduct these large studies. Despite these caveats and challenges, the opportunity to potentially identify those at risk for PD prior to motor symptoms raises the potential for the eventual prevention of PD and provides a rationale for studies to optimize PD-screening paradigms.
Supplementary Material
Acknowledgments
Relevant conflicts of interest/financial disclosures: Nothing to report. Full financial disclosures and author roles may be found in the online version of this article.
APPENDIX
The PARS Study investigators and coordinators are: David Russell, MD, Abby Fiocco, Candace Cotto, R-N, The Institute for Neurodegenerative Disorders, New Haven, CT; Kapil Sethi, MD, Paula Jackson, Medical College of Georgia, Augusta, GA; Samuel Frank, MD, Anna Hohler, MD, Cathi A. Thomas, MS, RN, Raymond C. James, Boston University Medical Center, Boston, MA; Tanya Simuni, MD, Emily Borushko, MPH, Northwestern University, Chicago, IL; Matt Stern, MD, Jacqueline Rick, PhD, University of Pennsylvania PDMDC, Philadelphia, PA; Robert Hauser, MD, Leyla Khavarian, Theresa McClain, ARNP, University of South Florida, Tampa, FL; Irene Richard, MD, Cheryl Deely, University of Rochester, Rochester, NY; Grace S. Liang, MD, Liza Reys, The Parkinson’s Institute, Sunnyvale, CA; Charles H. Adler, MD, PhD, Amy K. Duffy, Mayo Clinic Arizona, Scottsdale, AZ; Rachel Saunders-Pullman, MD, MPH, Beth Israel Medical Center, New York, NY; Marian L. Evatt, MD, Linda McGinn, RN, Emory University, Atlanta, GA; Eugene Lai, MD, Shawna Johnson, RN, BSN, Farah Atassi, MD, MPH, Michael E. DeBakey Department of Veteran’s Affairs Medical Center, Houston, TX; Indu Subramanian, MD, Angelina Gratiano, UCLA Medical Center, Los Angeles, CA; Kathryn Chung, MD, Brenna Lobb, Susan O’Conner, Portland VA Medical Center, Portland, OR.
PARS Study project management, data management, and imaging processing: Maria R. Albelo, Carolyn Cioffi, MBA, Allison Gadoury, Brian Howard, Valerie Iannucci, Shirley Lasch, Susan Mendick, MPH, Donna Miles, Katrina Miles, Emily Virden, Gary Wisniewski.
References
- 1.Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry 1998;64:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varrone A, Marek KL, Jennings D, Innis RB, Seibyl JP. [(123)I]beta-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson’s disease and multiple system atrophy. Mov Disord 2001;16:1023–1032. [DOI] [PubMed] [Google Scholar]
- 3.Gonera EG, Van’t Hof M, Berger HJC, Van Weel C, Horstink WIM. Symptoms and duration of the prodromal phase in Parkinson’s disease. Mov Disord 1997;12:871–876. [DOI] [PubMed] [Google Scholar]
- 4.Stern MB. The preclinical detection of Parkinson’s disease: ready for prime time? Ann Neurol 2004;56:169–171. [DOI] [PubMed] [Google Scholar]
- 5.Stern MB, Doty RL, Dotti M, et al. Olfactory function in Parkinson’s disease subtypes. Neurology 1994;44:266–268. [DOI] [PubMed] [Google Scholar]
- 6.Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 1988;38:1237–1244. [DOI] [PubMed] [Google Scholar]
- 7.Siderowf A, Newberg AB, Chou KL, et al. TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson’s disease. Neurology 2005;64:1716–1720. [DOI] [PubMed] [Google Scholar]
- 8.Doty RL.Odor perception in neurodegenerative diseases and schizophrenia In:Doty RL, ed. Handbook of Olfaction and Gustation. New York: Marcel Dekker; 2003:479–502. [Google Scholar]
- 9.Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1992; 55:138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tissingh G, Berendse HW, Bergmans P, et al. Loss of olfaction in de novo and treated Parkinson’s disease: possible implications for early diagnosis. Mov Disord 2001;16:41–46. [DOI] [PubMed] [Google Scholar]
- 11.Markopoulou K, Larsen KW, Wszolek EK, et al. Olfactory dysfunction in familial parkinsonism. Neurology 1997;49:1262–1267. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery EBJ, Baker KB, Lyons K, Koller WC. Abnormal performance on the PD test battery by asymptomatic first-degree relatives. Neurology 1999;52:757–762. [DOI] [PubMed] [Google Scholar]
- 13.Ponsen MM, Stoffers D, Booij J, van Eck-Smit BLF, Wolters EC, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol 2004;56:173–181. [DOI] [PubMed] [Google Scholar]
- 14.Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol 2008;63:167–173. [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol 2002;249(Suppl 3):1–5. [DOI] [PubMed] [Google Scholar]
- 16.Beach TG, Adler CH, Lue LF, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment, and motor dysfunction. Acta Neuropathol 2009;117:613–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984;32:489–502. [DOI] [PubMed] [Google Scholar]
- 18.Doty RL, Frye RE, Agrawal U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys 1989;45:381–384. [DOI] [PubMed] [Google Scholar]
- 19.Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 2001;57:456–462. [DOI] [PubMed] [Google Scholar]
- 20.Comella CL, Nardine TM, Diederich NJ, Stebbins GT. Sleeprelated violence, injury, and REM sleep behavior disorder in Parkinson’s disease. Neurology 1998;51:526–529. [DOI] [PubMed] [Google Scholar]
- 21.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press, Inc.; 1983. [Google Scholar]
- 22.Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression Scale. Br J Clin Psychol 1983;22: 245–249. [DOI] [PubMed] [Google Scholar]
- 23.Radloff L The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385–401. [Google Scholar]
- 24.Zich JM, Atkinsson CC, Greenfield TK. Screening for depression in primary care clinics: the CES-D and the BDI. Int J Psychiatry Med 1990;20:259–277. [DOI] [PubMed] [Google Scholar]
- 25.Tanner CM, Ellenberg J, Mayeux R, Ottman R, Langston JW. A sensitive and specific screening method for Parkinson’s disease. Neurology 1994;32:397–398. [Google Scholar]
- 26.Höglinger GU, Rissling I, Metz A, et al. Enhancing recognition of early Parkinsonism in the community. Mov Disord 2004;19: 505–512. [DOI] [PubMed] [Google Scholar]
- 27.Pramstaller PP, Falk M, Schoenhuber R, Poewe W. Validation of a mail questionnaire for parkinsonism in two languages (German and Italian). J Neurol 1999;246:79–86. [DOI] [PubMed] [Google Scholar]
- 28.Duarte J, Claveria LE, de Pedro CJ, Sempere AP, Coria F, Calne DB. Screening Parkinson’s disease: a validated questionnaire of high specificity and sensitivity. Mov Disord 1995;10:643–649. [DOI] [PubMed] [Google Scholar]
- 29.Morens DM, Grandinetti A, Reed D, White LR, Ross GW. Cigarette smoking and protection from Parkinson’s disease: false association or etiologic clue. Neurology 1995;45:1041–1051. [DOI] [PubMed] [Google Scholar]
- 30.Siderowf A, Jennings D, Connolly J, Doty RL, Marek K, Stern MB. Risk factors for Parkinson’s disease and impaired olfaction in relatives of patients with Parkinson’s disease. Mov Disord 2007; 22:2249–2255. [DOI] [PubMed] [Google Scholar]
- 31.Ross GW, Abbott RD, Petrovitch H, et al. Association of coffee and caffeine intake with risk of Parkinson’s disease. JAMA 2000; 283:2674–2679. [DOI] [PubMed] [Google Scholar]
- 32.Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology 2007;69:1836–1842. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Jacobs E, Schwarzschild MA, et al. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann Neurol 2005;58:963–967. [DOI] [PubMed] [Google Scholar]
- 34.Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S. L-type calcium channel blockers and Parkinson disease in Denmark. Ann Neurol 2010;67:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ascherio A, Chen H, Weisskopf MG, et al. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol 2006;60:187–203. [DOI] [PubMed] [Google Scholar]
- 36.London B, Nabet B, Fisher AR, White B, Sammel MD, Doty RL. Predictors of prognosis in patients with olfactory disturbance. Ann Neurol 2008;63:159–166. [DOI] [PubMed] [Google Scholar]
- 37.Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology 2006;66:845–851. [DOI] [PubMed] [Google Scholar]
- 38.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009;72:1296–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berendse HW, Booij J, Francot CM, et al. Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann Neurol 2001;50: 34–41. [DOI] [PubMed] [Google Scholar]
- 40.Haapaniemi TH, Pursiainen V, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllyla VV. Ambulatory ECG and analysis of heart rate variability in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2001;70:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berg D, Siefker C, Becker G. Echogenicity of the substantia nigra in Parkinson’s disease and its relation to clinical findings. J Neurol 2001;248:684–689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.