Abstract
Patients with chronic kidney diseases are at risk for further loss of kidney function and death, which occur despite reasonable blood pressure treatment. To determine whether arterial stiffness influences chronic kidney disease progression and death, independently of blood pressure we conducted a prospective cohort study of chronic kidney disease patients enrolled in the Chronic Renal Insufficiency Cohort Study. Using carotid-femoral pulse wave velocity we examined the relationship between pulse wave velocity and end stage kidney disease (ESRD), ESRD or halving of estimated glomerular filtration rate, or death from any cause. The 2795 participants we enrolled had a mean age of 60 years, 56.4% were men, 47.3% had diabetes, and the average estimated glomerular filtration rate at entry was 44.4 mL/min/1.73m2. During follow-up there were 504 ESRD events, 628 ESRD or halving of estimated glomerular filtration rate events, and 394 deaths. Patients with the highest tertile of pulse wave velocity (>10.3 meters/second) were at higher risk for ESRD (Hazard ratio [95% CI]; 1.37 [1.05–1.80]), ESRD or 50% decline in estimated glomerular filtration rate (1.25 [0.98–1.58]) or death (1.72 [1.24–2.38]). Pulse wave velocity is a significant predictor of chronic kidney disease progression and death in people with impaired kidney function. Incorporation of pulse wave velocity measurements may help define better the risks for these important health outcomes in patients with chronic kidney diseases. Interventions that reduce aortic stiffness deserve study in people with chronic kidney disease.
Keywords: chronic kidney disease, progression, end stage renal disease, arterial stiffness
Introduction
The kidneys are exposed to an extraordinary volume of blood flow across under a wide range of hemodynamic conditions. The relatively low vascular resistance that enables such a high flow rate also renders the kidneys vulnerable to barotrauma since they are susceptible to the pulsatile aspects of blood pressure and blood flow. In particular, excessive pulsatility in blood pressure damages the glomerulus resulting in proteinuria, and a loss of kidney function1. The low resistance in the kidney allows the pressure wave of each heartbeat to penetrate deeply into the microvasculature in animal models2. Prospective studies in humans show that elevated blood pressure plays a substantial role in initiating kidney damage, and participates in the progressive loss of kidney function that frequently ensues once kidney function impairment is detected3;4.
Recent observations show that arterial stiffness contributes independently of brachial blood pressure to death and cardiovascular outcomes in end stage renal disease (ESRD)5–7, and to incident heart failure events in non-dialyzed chronic kidney disease (CKD) patients8. Arterial stiffness is estimated from the pulse wave velocity (PWV) traveling along a defined arterial segment, such as aorta, in which the carotid and femoral arteries are typical sites of pulse wave measurement9. Less is known about the role of arterial stiffness in the progressive loss of kidney function in patients with established CKD, although the vasodilated state of the kidney suggests it would be an important influence2. Some studies of CKD patients support an independent role for arterial stiffness in kidney function decline in established CKD10;11, while others do not12–14.
To examine the role of arterial stiffness in CKD patients with impaired kidney function but not on dialysis, we evaluated the relationship between aortic PWV as an independent predictor of CKD progression, and death from any cause, among men and women enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study.
Methods
The demographic data on this CRIC cohort is available at the NIDDK website repository by request (https://repository.niddk.nih.gov/studies/cric/?query=None accessed February 13 2018). The PWV data was not publicly available at the time of this manuscript submission. This is an ancillary study to the CRIC Study. The CRIC Study enrolled 3,939 men and women with CKD between May 2003 and August 2008 at seven U.S. clinical centers (Ann Arbor, MI; Baltimore, MD; Chicago, IL; Cleveland, OH; New Orleans, LA; Oakland, CA and Philadelphia, PA). They were aged 21–74 years with an estimated glomerular filtration rate (eGFR) of 20–70 ml/min/1.73 m2. The design of the study and the baseline characteristics of the participants were described previously15;16. Persons with prior dialysis for more than one month, New York Heart Association Class III/IV heart failure, polycystic kidney disease, or other primary renal diseases requiring active immunosuppression, human immunodeficiency virus infection and pregnancy were not enrolled. Each year participants underwent an in-person study visit and an interim (6 month) telephone contact. Measurements of PWV were begun in July 2005. Most participants (72%) had their first PWV measurement on, or before, the second year in-center follow-up visit. The study was approved by the Institutional Review Board of each site and written informed consent was obtained from all study participants.
Brachial BP measurement:
At the annual clinic visit, three seated BP measurements were obtained using a Tyco Classic aneroid sphygmomanometer following a standardized protocol17. The average seated brachial BP measurement was used as the BP value. Brachial mean arterial pressure (MAP) was calculated as the diastolic blood pressure plus one-third of the [systolic – [minus] diastolic] blood pressure. Brachial pulse pressure was calculated by subtracting the diastolic blood pressure from the systolic blood pressure value.
PWV measurement:
Methods for assessment of aortic PWV in the CRIC Study have been previously described18. Briefly, carotid-to-femoral PWV measurements were performed in the supine position after at least 5 minutes of rest. Three electrocardiographic (ECG) leads were attached: one to the right arm, one to the left arm and one to the left lower abdomen or leg providing a standard limb lead II ECG tracing. The head was turned between 45 – 60° away from the examiner and the right carotid pulse was palpated. The distance from the suprasternal notch to the point of the palpable carotid pulse was measured in millimeters. The right femoral pulse was palpated and the distance to the umbilicus from the suprasternal notch, and then from the umbilicus to the point of femoral palpation was also measured. The travel distance was the notch-to-femoral distance minus the notch-to-carotid distance. A Millar tonometer attached to an electronic module interface was placed perpendicular to the carotid pulse and repositioned in small increments until a stable wave form was observed9. Pulse waveforms from right carotid and right femoral arteries were captured with the Sphygmocor PVx System (AtCor Medical, Sydney, Australia)18. The operator captured 10 seconds of stable carotid waveform and repeated the sequence using the femoral artery. After second waveform capture, the computer generated a PWV value with a standard deviation. If the standard deviation was more than 15% of the PWV value the study was repeated. Because of the large waist size in many CRIC participants, which artificially increases the sternal notch to umbilicus distance, we applied a correction to this distance using a formula incorporating waist size, gender and height18.
Outcomes:
At each yearly visit, and each 6 month intermittent phone contact, participants were queried regarding interval medical history including hospitalizations. Serum creatinine and cystatin C were measured yearly to estimate glomerular filtration rates (eGFR)16;19. Outcomes were ESRD, a 50% reduction in eGFR and/or ESRD, or death from any cause occurring after the first PWV measurement. ESRD, defined as receiving dialysis or a kidney transplant, was determined by participant self-report/local clinical center ascertainment and supplemented by cross-linkage with the United States Renal Data System. Deaths from all causes were determined through contact with surviving family members and adjudicated by two CRIC study physicians, supplemented by cross-linkage to the Social Security Death Master File15. Participant follow-up in this study was censored either at the time of death, withdrawal, lost to follow-up, or the end of the follow-up period (March 2013).
Data and Analyses:
Data are expressed as mean ± standard deviation (S.D.), or proportions (%) where appropriate. For unadjusted tests of differences between groups, analysis of variance was used for continuous variables and chi-square tests were used for categorical variables.
To compare whether subjects who had successful PWV measurement differed from those who did not, we stratified on whether PWV was obtained and compared summary statistics on a wide range of variables. We used the baseline period for entry into the CRIC Study to compare demographic and clinical characteristics of persons who did with those who did not have PWV measured. This was because the time of PWV measurement occurred at different points in follow-up.
For time-to-event analyses, the index visit or time 0, was the visit at which each subject first had a PWV measurement. Covariate information that was obtained at or prior to that visit were used in multivariable analyses. Cox proportional hazards models were for the three time-to-event outcomes. We used splines to test for departures from linearity in our predictors of interest: MAP and PWV. If there was evidence of non-linearity, then tertiles would be used. The reason for using tertiles rather than some other model that does categorize the exposures (such as splines), is that non-linear relationships are more difficult to interpret and we do not expect any loss of power to be a major concern from a study of this size.
For each outcome, MAP and PWV were included in the Cox model, along with established risk factors: age, sex, race, proteinuria, eGFR, and study site. Because the relationship between PWV and the outcomes might vary with MAP, we also fitted models that included interactions between PWV and MAP. We formally tested for significance of both the main effects and interactions.
Kaplan-Meier curves, stratified by tertiles of PWV, were used to graphically summarize the (unadjusted) relationship between PWV and the time-to-event outcomes.
Sensitivity analyses were performed using multiple imputation for any missing covariates, which increased the sample size due to the distribution of missing data, but did not meaningfully influence the results.
Results
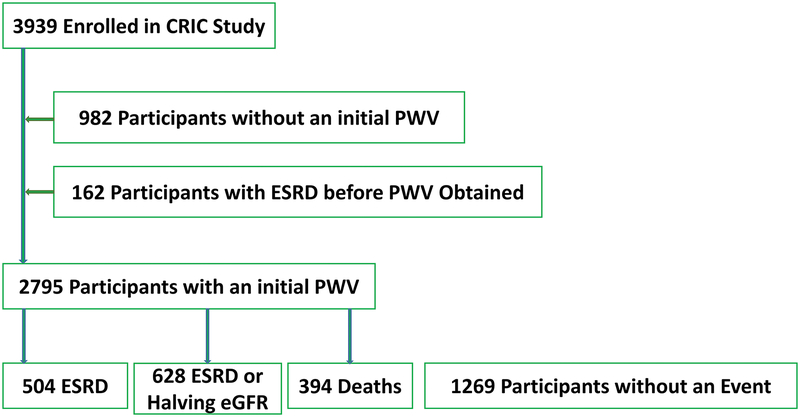
Among the 3939 enrolled CRIC participants, 2795 had a successful carotid-femoral PWV measurement, typically in their second year of follow up. Figure 1 shows the participant flow in this study.
Figure 1.
Enrollment and Outcomes flow chart.
Table 1 presents the baseline characteristics of the CRIC participants in whom carotid-femoral PWV was compared with those in whom it was not successfully obtained. As we reported previously, those in whom a carotid-femoral PWV could not be obtained had higher BMI, lower eGFR, and lower Hemoglobin levels18.
Table 1:
Characteristics of CRIC participants without and with a successful aortic PWV measurement
| Characteristic | All Eligible | PWV Available | P Value | |
|---|---|---|---|---|
| n=3939 | No n=1144 | Yes n=2795 | ||
| Age (years) | 59.08 (11.08) | 56.99 (11.51) | 59.93 (10.78) | <.0001 |
| Gender (% Male) | 2161 (54.9%) | 585 (51.1%) | 1576 (56.4%) | 0.0026 |
| Race / ethnicity | . | |||
| Hispanic | 497 (12.6%) | 170 (14.9%) | 327 (11.7%) | <.0001 |
| Non-Hispanic Black | 1650 (41.9%) | 562 (49.1%) | 1088 (38.9%) | . |
| Non-Hispanic White | 1638 (41.6%) | 372 (32.5%) | 1266 (45.3%) | . |
| Other | 154 (3.91%) | 40 (3.5%) | 114 (4.1%) | . |
| Diabetes | 1996 (50.7%) | 673 (58.8%) | 1323 (47.3%) | <.0001 |
| eGFR (mL/min/1.73m2) (19) | 42.54 (17.54) | 38.10 (14.90) | 44.38 (18.21) | <.0001 |
| Weight (kg) | 91.35 (23.53) | 95.60 (28.31) | 89.60 (21.02) | <.0001 |
| Systolic BP (mmHg) | 128.75 (22.69) | 133.52 (24.14) | 126.79 (21.77) | <.0001 |
| Diastolic BP (mmHg) | 70.52 (12.98) | 72.20 (13.63) | 69.83 (12.64) | <.0001 |
| Seated Pulse measure (beats/min) | 68.04 (11.46) | 69.25 (11.79) | 67.54 (11.28) | <.0001 |
| Hemoglobin (g/dL) | 12.60 (1.80) | 12.21 (1.84) | 12.76 (1.76) | <.0001 |
| Serum Creatinine (mg/dL) | 1.98 (0.88) | 2.07 (0.75) | 1.94 (0.92) | <.0001 |
| Triglycerides | 156.28 (116.50) | 171.45 (131.71) | 148.88 (107.56) | <.0001 |
| Calcium (mg/dL) | 9.26 (0.53) | 9.12 (0.56) | 9.31 (0.50) | <.0001 |
| Phosphate (mg/dL) | 3.82 (0.72) | 3.91 (0.73) | 3.56 (0.64) | <.0001 |
| Total Parathyroid Hormone (pg/ml) | 84.13 (85.25) | 93.96 (86.40) | 68.15 (80.89) | <.0001 |
| 24H Urine Protein (g/24H) | 1.16 (2.45) | 1.82 (3.25) | 0.86 (1.90) | <.0001 |
| Hemoglobin A1C (%) | 6.91 (1.62) | 6.98 (1.75) | 6.86 (1.52) | 0.0746 |
| Uric Acid (mg/dL) | 7.60 (1.98) | 7.76 (1.97) | 7.17 (1.94) | <.0001 |
| % Participants on ACE-inhibitor or ARB | 2714 (69.4%) | 786 (69.3%) | 1928 (69.4%) | 0.9434 |
| % Participants on Calcium antagonist | 1621 (41.4%) | 521 (45.9%) | 1100 (39.6%) | 0.0003 |
| % Participants on Beta blockers | 2009 (51.4%) | 658 (58%) | 1351 (48.6%) | <.0001 |
| % Participants on Diuretics | 2341 (59.9%) | 791 (69.8%) | 1550 (55.8%) | <.0001 |
| # Anti-HT Drug Classes | 2.52 (1.28) | 2.76 (1.19) | 2.42 (1.30) | <.0001 |
| Age (years) | 59.08 (11.08) | 56.99 (11.51) | 59.93 (10.78) | <.0001 |
| Gender (% Male) | 2161 (54.9%) | 585 (51.1%) | 1576 (56.4%) | 0.0026 |
The mean age of our cohort was 60 years, 56.4% were men, 47.3% had diabetes, and the average estimated glomerular filtration rate at entry into this ancillary study was 44.4 mL/min/1.73m2. The mean follow-up time for the ESRD outcome was 4.9±2.1 years. The mean follow-up time for the ESRD or halving of eGFR was 4.1±2.3 years. The mean follow-up time (+S.D.) for the outcome of death was 5.4±1.8 years. During follow-up there were 504 ESRD events, 628 ESRD or halving of eGFR events, and 394 deaths.
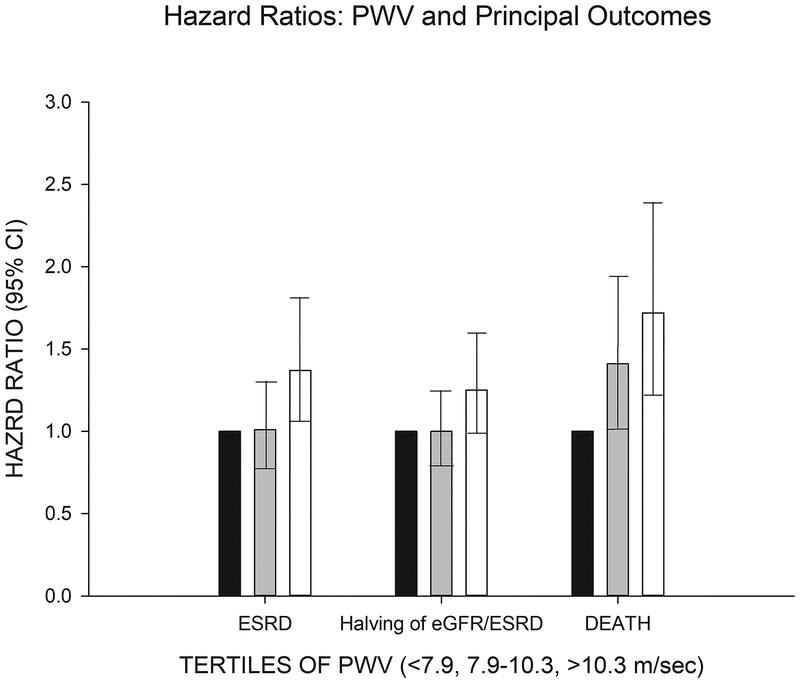
The analysis of PWV as a continuous variable in the Cox Proportional Hazards survival analyses demonstrated a non-linear relationship of PWV to ESRD, halving of eGFR/ESRD, and death outcomes, thus, tertiles of PWV were used instead as we previously published8. Table 2 shows the complete multivariable regression model components for the three outcomes adjusting for MAP in the models as recommended by the recent AHA scientific statement20. Patients with the highest tertile of pulse wave velocity (>10.3 m/sec) were at higher risk for ESRD (Hazard ratio [95% CI]; 1.37 [1.05–1.80]), ESRD or 50% decline in eGFR (1.25 [0.98–1.58]) or death (1.72 [1.24–2.38]).
Table 2:
Hazards of ESRD, ESRD or halving of eGFR, and Death by tertile of PWV*
| ESRD | ESRD or 50% decline in eGFR | DEATH | |
|---|---|---|---|
| Variable | HR(95%CI) | HR(95%CI) | HR(95%CI) |
| PWV Tert 7.9-<=10.3 | 1.01(0.78–1.32) | 1(0.79–1.25) | 1.41(1.02–1.95) |
| PWV Tert >10.3 | 1.37(1.05–1.8) | 1.25(0.98–1.58) | 1.72(1.24–2.38) |
| MAP Tert 82.2-<=93.3 | 1.34(1.03–1.74) | 1.25(0.99–1.57) | 0.88(0.68–1.14) |
| MAP Tert >93.3 | 1.37(1.06–1.77) | 1.37(1.1–1.72) | 0.93(0.71–1.21) |
| Age (years) | 0.98(0.97–0.99) | 0.98(0.98–0.99) | 1.04(1.03–1.05) |
| Female | 0.73(0.6–0.9) | 0.85(0.71–1.02) | 0.64(0.51–0.81) |
| Diabetes | 1.28(1.04–1.57) | 1.48(1.23–1.77) | 1.42(1.14–1.77) |
| Hispanic | 1.09(0.7–1.69) | 1.27(0.86–1.89) | 1.37(0.81–2.31) |
| Non-Hispanic Black | 1.45(1.14–1.84) | 1.52(1.24–1.88) | 1.1(0.87–1.4) |
| Other | 1.65(1.04–2.62) | 1.85(1.23–2.78) | 1.27(0.75–2.15) |
| Proteinuria: 0.10 - <0.50 g/24hr | 2.65(1.67–4.18) | 2.5(1.73–3.6) | 1.21(0.91–1.6) |
| Proteinuria: 0.50 - <1.50 g/24hr | 5.89(3.75–9.27) | 6.01(4.16–8.67) | 1.21(0.86–1.7) |
| Proteinuria: 1.50+ g/24hr | 11.04(7.02–17.37) | 11.51(7.96–16.64) | 1.39(0.98–1.95) |
| eGFR (mL/min/1.73m2) | 0.89(0.88–0.9) | 0.92(0.92–0.93) | 0.97(0.96–0.97) |
Using a time to event analysis anchored to first PWV measurement
Figure 2 depicts the adjusted hazards of each outcome with the lowest tertile of PWV as the referent group showing the independent relationship of PWV in the highest tertile for each outcome. Our basic model in Table 2 was enlarged further to include BMI, triglyceride concentrations, heart rate, smoking and usage of ACE or ARB therapy (Supplemental Table S1). The addition of these covariates did not alter the statistical significance of HRs associated with the highest PWV tertile on the three outcomes.
Figure 2.
Depicts the adjusted hazard ratios of ESRD, ESRD or halving of eGFR, or death events on the Y axis [with 95% CI in the error bars] by tertiles of pulse wave velocity. The solid black bar is the referent population (PWV < 7.9 m/sec). The grey bar is the second tertile of PWV (7.9–10.3 m/sec). The open bar is the highest tertile of PWV (>10.3 m/sec).
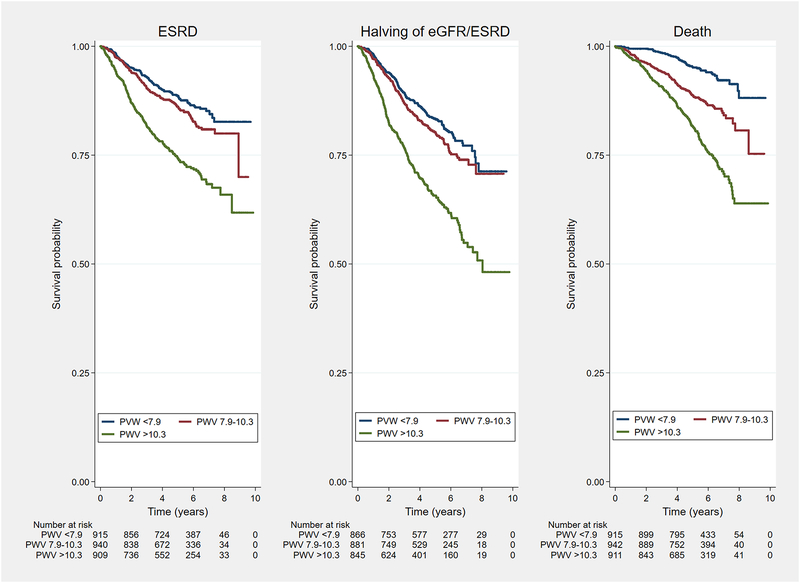
The Figure 3 shows a Kaplan-Meier plot of the unadjusted relationship of PWV tertiles to the three outcomes. In Supplemental Table S2 an analysis of the three outcomes stratified by eGFR <30 mL/min/1.73m2 versus > 30 mL/min/1.73m2 showed that the hazard for death was higher in the subgroup with more preserved kidney function, and that the hazard for the kidney outcomes was higher in those with the lower values of kidney function.
Figure 3:
Shown are Kaplan-Meier survival curves depicting the unadjusted relationship between tertiles of PWV and the time-to-event for the CRIC outcomes of ESRD (Left), halving of estimated GFR or ESRD (Center), and Death (Right). Text at bottom shows number of participants at risk at the timepoints.
Discussion
Among persons with primarily stage 3 and 4 CKD, including substantial proportions of African Americans and persons with diabetes, we observed that aortic PWV independently predicted both measures of CKD progression, and all-cause death, when evaluated in models that adjusted for mean arterial pressure. In all these analyses, the models incorporated important covariates that predict death or kidney function loss, including blood pressure, eGFR, proteinuria, diabetes, age, gender and race. These observations add to our initial investigations of the importance of aortic PWV in CKD18;21, and are consistent with some prior investigations10;11, but not others13;14. The positive and independent effects of arterial stiffness on death and worsening of kidney function we observed in our study are likely related to the large size of our cohort, the reasonably long follow up of our participants, and the high degree of retention of our cohort.
PWV studies primarily focus on the velocity of pulse wave travel in the aorta, since abundant literature attests to the value of studying this particular vascular bed7;22. The recent Scientific Statement from the American Heart Association recommends using the aorta as the primary vessel in pursuing research into the relationship between arterial stiffness and outcomes20. Pursuing the role of arterial stiffness in CKD progression and outcomes such as death is complicated by effects of blood pressure itself on PWV, and vice-versa. When using the carotid-femoral PWV as a factor involved in outcome, or when PWV is the target of an intervention, it should be adjusted by the MAP at the time it is measured, as recommended by the recent AHA statement.
Blood pressure influences the course of kidney disease progression, and prior work from the CRIC Study confirms the importance of blood pressure measurements in CKD progression4. Our data incorporating PWV into models predicting kidney disease progression are consistent with the observation of the vulnerability of the kidney to hemodynamic trauma given the remarkable vasodilation of the kidney, the “torrential” blood flow in the kidneys, and the penetration of the pulse wave deep into the microvasculature of the kidney2.
Our findings are consistent with some studies of CKD progression, where arterial stiffness provides independent predictive potential10;11, and are at odds with other studies which did not find an independent association of arterial stiffness with CKD progression13;14. In particular, the study of Michener and colleagues did not find a relationship between PWV and eGFR in an older population, however, they felt that elderly age may have overshadowed any relationship. The large size of the CRIC Study, the range of ages, ethnic diversity among the participants, the large proportion of diabetics, and the long follow-up in CRIC make it difficult to render comparisons to other CKD cohorts which tend to be smaller and more ethnically homogenous.
The aortic PWV has shown an independent relationship to cardiovascular outcomes, including death, when factored into models that also include blood pressure in longitudinal studies, including ESRD cohorts7. Although the PWV in an individual is influenced by the MAP23, other factors besides blood pressure also contribute to arterial stiffness18. Moreover, antihypertensive treatment does not always improve arterial stiffness despite effective reduction in blood pressure5. Thus, measurements of PWV add complementary value to standard blood pressure values and common demographic factors in predicting outcomes.
Aortic stiffening has a strong influence on left ventricular hypertrophy and coronary ischemia which may explain the increase in cardiovascular mortality noted in longitudinal studies24–28. In studies incorporating aortic PWV prospectively, up to half the deaths occur from non-cardiovascular causes7. Although mechanisms linking measures of arterial stiffness to all cause death remain to be determined, it is probable that common pathogenic processes such as inflammation, aging, and oxidant stress contribute both to arterial stiffness as well as to death from non-cardiovascular causes7. Guidelines encourage performance of arterial stiffness measurements, arguing that PWV represents a valid intermediate surrogate for prediction of all-cause mortality29. Our observation in CRIC that PWV was a significant predictor of death, is consistent with similar findings in ESRD24. A small study in Germany also observed an association of PWV with death in CKD stages 2–46.
Arterial stiffness is a contributing factor, independent of blood pressure to incident CKD. In the Rotterdam Study each 1 SD in PWV was associated with a 13% increase in the likelihood of incident CKD, and with greater rate of progression of kidney function loss30. Similarly, a Japanese workplace study also observed a 36% higher likelihood of incident CKD with each 1 meters/second increase in arterial stiffness31.
Several limitations are important to note. First, although PWV was offered to all CRIC participants, the first measurements were, by protocol, usually not obtained until the beginning of the third year of participation, thus some patients died, or developed CKD endpoints before their first measurement could be undertaken. Additionally, the difficulties obtaining the femoral waveform have been described in our prior report18. In about 20% of the eligible CRIC participants we were unable to obtain adequate waveforms. These subjects weighed, on average, 11 kg more than participants with successful femoral waveform captured.
In summary we observed that aortic PWV was an independent predictor of death and CKD progression in the CRIC Study. Our observations contribute to this area of investigation because of the large size, extensive phenotype, ethnic diversity, large number of diabetics, and long term follow up of this CKD cohort. Our results also suggest that efforts to de-stiffen the aorta could be of benefit in this population with a high risk for death and CKD progression.
Perspectives
Chronic kidney diseases (CKD) cause substantial morbidity and mortality. Although elevated blood pressure contributes to further kidney function loss and death, patients with CKD continue to lose function and to die despite reasonable blood pressure control. Arterial stiffness may be an important contributor to this.
Supplementary Material
Novelty and Significance.
What is new: Our study builds on the current literature regarding arterial stiffness and outcomes in chronic kidney diseases by showing in a large, ethnically diverse population that pulse wave velocity is a predictor of further kidney function loss and death.
What is relevant: The relevance in our study is the recognition that this simply-measured blood vessel quality, arterial stiffness, shows values higher than the general population in people with chronic kidney disease, and it identifies patients at higher risk for death who already have impaired kidney function.
Summary: stiff arteries predict more rapid kidney function loss, and death, in patients who already have impaired kidney function.
Acknowledgements
*CRIC Study Investigators:
Lawrence J. Appel, MD, MPH
Harold I. Feldman, MD, MSCE
Alan S. Go, MD
Jiang He, MD, PhD
John W. Kusek, PhD
James P. Lash, MD
Akinlolu Ojo, MD, PhD
Mahboob Rahman, MD
Raymond R. Townsend, MD
Funding Sources
Funding for the CRIC Study was supported by cooperative agreements from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: K01DK092353, the University of Pennsylvania CTRC CTSA UL1 RR-024134, Johns Hopkins University UL1 RR-025005, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1RR024986, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser NIH/NCRR UCSF-CTSI UL1 RR-024131.
This work was supported by R01-DK067390 from the NIDDK
Footnotes
VA Statement
Views expressed are those of the authors and not necessarily those of the Department of Veterans Affairs
Disclosures and Conflicts of Interest
Raymond R. Townsend, MD: NIH grants, Consultant to Medtronic and Janssen
Amanda Hyre Anderson, PhD
Julio A. Chirinos MD, PhD
Harold I. Feldman, MD NIH Grants, Kyowa Kirin (Speaker), Glaxo SmithKline (Speaker)
Juan Grunwald, MD
Lisa Nessel
Jason Roy, PhD
Boyang Chai, PhD
Matthew Weir, MD NIH Grants, Janssen, Astra Zeneca, MSD, Akebia, Boston Scientific, Relypsa, Boeheinger-Ingelheim, Bayer
Mahboob Rahman, MD
Jackson T Wright, Jr, MD NIH Grants
Nisha Bansal, MD
Chi-yuan Hsu, MD, MSc
John Kusek, PhD
Reference List
- 1.Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension 2011; 58:839–846. [DOI] [PubMed] [Google Scholar]
- 2.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005; 46:200–204. [DOI] [PubMed] [Google Scholar]
- 3.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. Clinical Nephrology 1996; 334:13–18. [DOI] [PubMed] [Google Scholar]
- 4.Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, Charleston J, He J, Kallem R, Lash JP, Miller ER III, Rahman M, Steigerwalt S, Weir M, Wright JT Jr., Feldman HI. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med 2015; 162:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 2001; 103:987–992. [DOI] [PubMed] [Google Scholar]
- 6.Baumann M, Wassertheurer S, Suttmann Y, Burkhardt K, Heemann U. Aortic pulse wave velocity predicts mortality in chronic kidney disease stages 2–4. J Hypertens 2014; 32:899–903. [DOI] [PubMed] [Google Scholar]
- 7.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 8.Chirinos JA, Khan AM, Bansal N, Dries DL, Feldman HI, Ford V, Anderson AH, Kallem R, Lash JP, Ojo A, Schreiber M, Sheridan A, Strelsin J, Teal V, Go AS, Townsend RR. Arterial Stiffness, Central Pressures and Incident Hospitalized Heart Failure in the Chronic Renal Insufficiency Cohort (CRIC) Study. Circ Heart Fail 2014; 7:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deloach SS, Townsend RR. Vascular stiffness: Its measurement and significance for epidemiologic and outcome studies. CJASN 2008; 3:184–192. [DOI] [PubMed] [Google Scholar]
- 10.Weber T, Ammer M, Gunduz D, Bruckenberger P, Eber B, Wallner M. Association of increased arterial wave reflections with decline in renal function in chronic kidney disease stages 3 and 4. Am J Hypertens 2011; 24:762–769. [DOI] [PubMed] [Google Scholar]
- 11.Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension 2010; 55:1110–1115. [DOI] [PubMed] [Google Scholar]
- 12.Michener KH, Mitchell GF, Noubary F, Huang N, Harris T, Andresdottir MB, Palsson R, Gudnason V, Levey AS. Aortic stiffness and kidney disease in an elderly population. Am J Nephrol 2015; 41:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P. Arterial remodeling associates with CKD progression. J Am Soc Nephrol 2011; 22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra P, Sands RL, Gillespie BW, Levin NW, Kotanko P, Kiser M, Finkelstein F, Hinderliter A, Rajagopalan S, Sengstock D, Saran R. Relationship between heart rate variability and pulse wave velocity and their association with patient outcomes in chronic kidney disease. Clin Nephrol 2014; 81:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER III, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol 2003; 14(7 Supple 2):S148–S153. [DOI] [PubMed] [Google Scholar]
- 16.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 2009; 4:1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O’Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend RR, Weir M, Wright JT. Hypertension Awareness, Treatment, and Control in Adults with Chronic Kidney Disease: Results from the Chronic Renal Insufficiency Cohort (CRIC) Study. American Journal of Kidney Diseases 2009; 55:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townsend RR, Wimmer NJ, Chirinos JA, Parsa A, Weir M, Perumal K, Lash JP, Chen J, Steigerwalt SP, Flack J, Go AS, Rafey M, Rahman M, Sheridan A, Gadegbeku CA, Robinson NA, Joffe M. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens 2010; 23:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2012; 60:250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 2015; 66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safar ME, Nawar T, Plante GE. Large arteries and the kidney. J Am Soc Hypertens 2007; 1:169–177. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergel DH. The static elastic properties of the arterial wall. J Physiol 1961; 156:445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999; 99:2434–2439. [DOI] [PubMed] [Google Scholar]
- 25.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 26.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005; 111:3384–3390. [DOI] [PubMed] [Google Scholar]
- 27.Kitahara T, Ono K, Tsuchida A, Kawai H, Shinohara M, Ishii Y, Koyanagi H, Noguchi T, Matsumoto T, Sekihara T, Watanabe Y, Kanai H, Ishida H, Nojima Y. Impact of brachial-ankle pulse wave velocity and ankle-brachial blood pressure index on mortality in hemodialysis patients. Am J Kidney Dis 2005; 46:688–696. [DOI] [PubMed] [Google Scholar]
- 28.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 29.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De BG, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013. ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 30.Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA Franco OH, Dehghan A. Arterial Stiffness and Decline in Kidney Function. Clin J Am Soc Nephrol 2015;10(12):2190–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomiyama H, Tanaka H, Hashimoto H, Matsumoto C, Odaira M, Yamada J, Yoshida M, Shiina K, Nagata M, Yamashina A. Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis 2010;212(1):345–350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.