Abstract
There is now overwhelming experimental and clinical evidence that atherosclerosis is a chronic inflammatory disease. Lessons from genome-wide association studies, advanced in vivo imaging techniques, transgenic lineage tracing mice, and clinical interventional studies have shown that both innate and adaptive immune mechanisms can accelerate or curb atherosclerosis. Here, we summarize and discuss the pathogenesis of atherosclerosis with a focus on adaptive immunity. We discuss some limitations of animal models and the need for models that are tailored to better translate to human atherosclerosis and ultimately progress in prevention and treatment.
Keywords: Atherosclerosis, immunology, inflammation, T-cells, B-cells, myeloid cells, vaccination, LDL
Subject Terms: Atherosclerosis, inflammation
Introduction
Atherosclerosis is the most common underlying pathology of coronary artery disease (CAD), peripheral artery disease (PAD), and cerebrovascular disease1, 2. The chronic build-up of vessel-occluding plaques in the subendothelial intimal layer of large and medium sized arteries eventually results in significant stenosis that restricts blood flow and causes critical tissue hypoxia3. The most common complications, myocardial infarction (MI) and stroke, are caused by spontaneous thrombotic vessel occlusion and represent the most common cause of death worldwide4, 5. Current clinical guidelines focus on the treatment of these complications6. Clinically used therapies that efficiently prevent or curb the progression of atherosclerosis are limited to drugs that lower low-density lipoprotein (LDL) cholesterol. Traditionally, atherosclerosis was regarded as a cholesterol storage disease caused by the retention of lipoproteins including LDL in the intimal space of arteries. Retained LDL is modified and taken up by scavenger receptor-mediated phagocytosis. This process results in the continuous growth of fatty infiltrates rich in inflammatory leukocytes that macroscopically appear as plaques. Levels of plasma cholesterol, LDL cholesterol, and apolipoproteins, including Apolipoprotein B (ApoB), are highly correlated with clinical atherosclerosis7, 8. Mice along with other animal models suggest causality: Elevating plasma cholesterol levels, as achieved by genetic knock-outs of LDL-receptor (LDLR) or Apolipoprotein E (ApoE) in mice, causes atherosclerosis in C57BL/6 mice that otherwise do not develop spontaneous disease9, 10. Genome-wide association studies (GWAS) have correlated many single nucleotide polymorphisms (SNPs) in or near the genes encoding lipid-associated proteins. Examples include LDLR, APOB and proprotein convertase subtilisin/kexin type 9 (PCSK9), which modulate LDL cholesterol levels, as risk factors in atherosclerosis and MI11, 12. In addition, atherosclerosis is accompanied by a chronic, low-grade inflammatory response that attracts cells of the innate and adaptive immune systems into the atherosclerotic plaque3, some of them recognizing ApoB, the core protein of LDL particles. Thus, atherosclerosis is a chronic inflammatory disease with an autoimmune component13. This autoimmune response is clinically best documented by antibodies against LDL and other atherosclerosis antigens, which are found in all patients and animal models. In many studies, low-affinity ‘natural’ antibodies against oxidation epitopes in LDL were found to be negatively correlated with atherosclerosis, while high-affinity antibodies secreted by IgG-producing plasma cells were positively correlated14. Here, we will summarize and discuss the adaptive autoimmune mechanisms that accompany and modify atherosclerotic disease.
LDL accumulation initiates vascular inflammation
The atherogenic process starts with the accumulation of several plasma lipoproteins in the subendothelial space at sites of flow perturbation and endothelial dysfunction. This is best documented for LDL, whose accumulation correlates with classical risk factors, such as smoking, hypertension, and metabolic dysregulation in obesity and diabetes15. In the intima, LDL undergoes oxidative modifications by reactive-oxygen species (ROS), which promote the uptake of oxLDL into macrophages16. In addition, oxidized phospholipids per se trigger inflammation of the arterial wall17 by binding to Toll-like receptors (TLRs), a group of widely expressed pattern-recognition receptors (PRRs) that cause pro-inflammatory signaling18. Clinically, oxLDL is a marker of plaque inflammation19. Native LDL can also be taken up by macrophages by micropinocytosis, or in its aggregated form as cholesterol complexes or -crystals by phagocytosis. The sustained influx of cholesterol eventually exceeds the phagocytes’ metabolic capacity and intracellular lipid droplets form. Microscopically, cholesterol-laden macrophages are ‘foam cells’. Cholesterol loading is thought to cause a myeloid cell response with pro-inflammatory cytokine secretion, in situ macrophage proliferation, and further recruitment of myeloid cells (summarized in20). A clinically important consequence of cholesterol loading is the formation of intracellular cholesterol microcrystals that activate the inflammasome, a molecular machinery comprising molecules of the cytosolic-nucleotide binding domain and leucine-rich repeat gene family (NLRP3) that cleaves pro-IL-1β into its biologically active form21. IL-1β serves as an inflammatory master cytokine that enhances the expression of many pro-inflammatory cytokines, as well as of CRP22. Notably, attenuating cholesterol storage and enhancing cholesterol efflux pathways may favor the resolution of plaque inflammation end even promote plaque regression (summarized in23). The myeloid response is accompanied by the infiltration of cells of the adaptive immune system, B and T cells24, 25. Notably, the plaque’s growing content of myeloid cells and lymphocytes correlates with clinical complications and may predispose for future thromboembolic events caused by large cellular infiltrates and a thin fibrous cap (‘unstable plaque’)26, 27.
Evidence for an autoimmune response in atherosclerosis
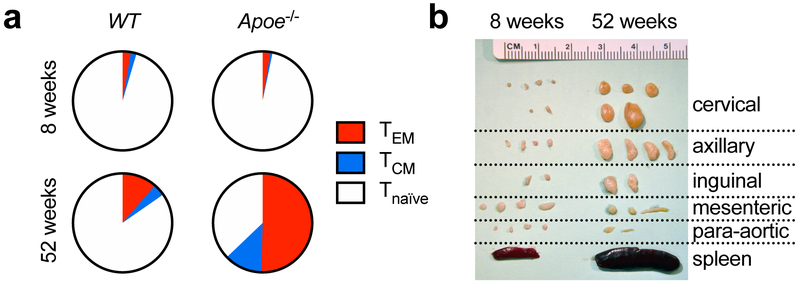
The presence of T and B cells in the plaque28 sparked the hypothesis that atherosclerosis includes an autoimmune response. Adaptive immunity in infection and autoimmunity proceeds by a humoral arm that comprises specific antibodies against the antigen secreted by plasma cells, and a cellular arm with T cells that either activate B cells during co-stimulation or differentiate into effector T cells with pro- or anti-inflammatory cytokine production29. CD8+ and CD4+ T cells only initiate immune responses to peptides presented MHC-I on all nucleated cells or MHC-II on antigen-presenting cells (APCs), respectively. Such responses are MHC-restricted, i.e. they only occur in individuals expressing a specific MHC-allele with the ability to bind the relevant peptide epitope. Binding of a specific T cell receptor (TCR) concomitant with co-stimulatory events provided by APCs activates T cells and causes their clonal proliferation30. In mouse atherosclerosis, 2-photon microscopy has revealed an increased rate of APC-CD4+ T-helper cell interactions in the plaque specifically in the setting of hypercholesterolemia that resulted in pro-inflammatory cytokine secretion31. In addition, T-helper cells show an increasing maturation into antigen-experienced effector/memory (TEM) and central-memory (TCM) T cells in the lymph nodes (Figure 1a) that is also observed in atherosclerotic plaques28, 31. Sequencing of the TCR revealed an oligoclonal origin of lesional T cells32, 33 suggesting that some (antigen-specific) T cell clones actively expand in the plaque. The enhanced activation of T cells is accompanied by an expansion of lymph nodes draining the atherosclerotic aorta in aged atherosclerotic Apoe−/− mice (Figure 1b) and a local and systemic pro-inflammatory response that is further enhanced by a hypercholesterolemia-inducing diet34-36. These findings support the concept that specific antigens drive an immune-response in the aorta and lymph nodes during atherosclerosis.
Figure 1: Activation of T cells is a hallmark of atherosclerosis.
(a) During feeding with a Western Diet (WD), CD4+ T-helper cells from atherosclerosis-prone Apoe−/− build-up a significant immune memory with more than one half of T cells express markers of CD62L− CD44+ T-effector memory cells (TEM) and CD62L+ CD44+ central-memory cells (TCM) when compared to atherosclerosis-free wildtype (WT) mice. (b) Along with enhanced T cell activation, lymph nodes draining the aorta and supra-aortic arteries (cervical, axillary lymph nodes) massively increase in size. Courtesy of D. Wolf and K. Ley
LDL – an autoantigen within the plaque
Of all candidates that may serve as B- and T cell activating antigens, plasma levels of LDL and its core protein ApoB show the strongest clinical and causal link with atherosclerosis in humans37. ApoB-containing triglyceride-rich remnant particles also show a strong association with CVD, inflammation, and immune pathways7. Indeed, LDL as (auto-) antigen was first suggested by Gero et al in 1959: immunization with LDL protected against atherosclerosis in rabbits38, suggesting that autoimmune response against LDL can be atheroprotective39. Many CD4+ T cells in human plaques recognize oxLDL40 by binding to MHC-presented peptide epitopes from ApoB-10041, 42. A tetramer of recombinant MHC-molecules loaded with an ApoB-derived peptide – a tool to detect antigen-specific T-helper cells in vivo43 – found a naturally occurring population of CD4+ T cells in the blood that recognizes the human peptide ApoB3036-305042. Furthermore, atherosclerosis is accompanied by IgG-antibodies against LDL, oxLDL, and ApoB44. Collectively, these findings strongly suggest LDL as a relevant self-antigen that drives an autoimmune response against self-proteins in the atherosclerotic plaque. Besides LDL/ApoB, heat shock proteins (HSPs)45-47 and some foreign peptides from pathogens such as Cytomegalovirus (CMV), Hepatitis C Virus (HCV), Human Immunodeficiency Virus (HIV), Human Papilloma Virus (HPV), and others48-50 have been proposed as atherosclerosis-relevant antigens.
T-helper cell dependent immunity in atherosclerosis
Early evidence from immunohistochemistry studies28, 51, more recent scRNAseq24, 52, and CyTOF approaches24, 53 have estimated that ~ 25-38 % of all leukocytes in mouse aortic and human atherosclerotic plaques are CD3+ T cells, with CD3+CD4+ T-helper cells accounting for ~ 10 %. T cells predominantly populate atherosclerotic lesions with an enrichment in the fibrous cap28, 51, but are also found in the adventitia of older lesions24, 54. Their recruitment to the plaque occurs via chemokine receptors C-C chemokine receptor type 5 (CCR5), -X-C Motif Chemokine Receptor 6 (CXCR6), and others55, 56. CD4+ T cells are critical regulators of the adaptive immune response with the ability to differentiate into distinct T-helper subtypes that can either be immune-dampening or activating to other T cells, exert direct anti- or pro-inflammatory effects on tissue resident cells, provide B cell help to induce the production of high-affinity IgG antibodies, or exhibit cytolytic activity29 (Figure 2). Thus, the function of T-helper cells in atherosclerosis is multi-faceted and depends on specific transcriptional programs and patterns of cytokine secretion that can either fuel or attenuate atherosclerosis. Early evidence from Rag-1 deficient mice, which cannot produce mature T- and B cells, suggested a pathogenic role for T and B lymphocytes only in early atherosclerosis with moderately enhanced lipid levels, but not in severely hypercholesteremic Apoe−/− mice57, 58. Genetic absence of T cells in athymic nu/nu mice or a depletion of CD4+ T cells by anti-CD4 antibodies protected from lesion development59. After antigen presentation by APCs, lesional T cells differentiate into functionally distinct T-helper subtype (TH) −1, −2, −17, T-regulatory cells (Treg), T-follicular helper cells (TFH) and Type 1 regulatory (TR1) cells60. Atherosclerosis is a known TH1-disease. Many CD4+ T cells in the plaque express the pro-inflammatory, TH1-associated cytokines IFN-γ, IL-2, IL-3, TNF, and lymphotoxin (LT), which can activate macrophages, T cells, and other components of the plaque, and thereby aggravate the inflammatory response61. T cells that express the plaque-homing chemokine receptor CCR5 in lymph nodes, and T cells from atherosclerotic lesions secrete IFN-γ and express T-bet, the TH1-lineage defining transcription factor55, 62. Knocking out IFN-γ, its receptor, or T-bet protects mice from atherosclerosis63-65. IFN-γ may directly reduce plaque stability by inhibiting smooth muscle cell proliferation66, affecting macrophage polarization, and modulating cardiovascular risk factors67. On the other hand, regulatory CD4+ T cells (Tregs) that express the transcription factor FoxP3 and the high affinity IL-2 receptor CD25 protect mice from atherosclerosis68, 69. Tregs exert their atheroprotective properties by secreting the anti-inflammatory cytokine IL-1070, plaque-stabilizing TGF-β71, and by suppressing the proliferation of pro-inflammatory T-effector cells72. Atheroprotective effects of in-vivo treatment with IL-2 complexes73 and anti-CD3 treatment74, 75 have been attributed to a relative increase of Tregs. In addition, TR1 cells that lack FoxP3 expression but express CD49b and Lag-3 secrete IL-10 and are atheroprotective76, 77. In the atherosclerotic plaque, a substantial proportion of T cells moreover express transcripts for the TH2 cytokines IL-4, IL-5, and IL-1324. In contrast to abdominal aortic aneurysm formation, which is a clear TH2-dependent disease78 and the negative correlation of IL-4 with clinical atherosclerosis79, the relevance of TH-2 immunity in atherosclerosis remains unclear. The TH2 cytokine IL-4 antagonizes TH1 responses and diminished lesion formation in one study80, while depletion of IL-4 has also been reported to be atheroprotective81. Likewise, the role of TH17 cells in atherosclerosis is controversial: Deletion or neutralization of the master cytokine IL-17 protected from atherosclerosis82-84, while other studies reported that TH17 immunity protected from atherosclerosis and induced a stable plaque phenotype85-87. T-follicular helper cells (TFH), which are required to co-stimulate B cells and to induce an Ig-class switch, have also been proposed to be pro-atherogenic88 or to protect from atherosclerosis by secreting LDL-lowering/neutralizing anti-LDL/ApoB secreting antibodies89. The different findings in these studies may reflect different but unknown antigen specificities of the T cells studied.
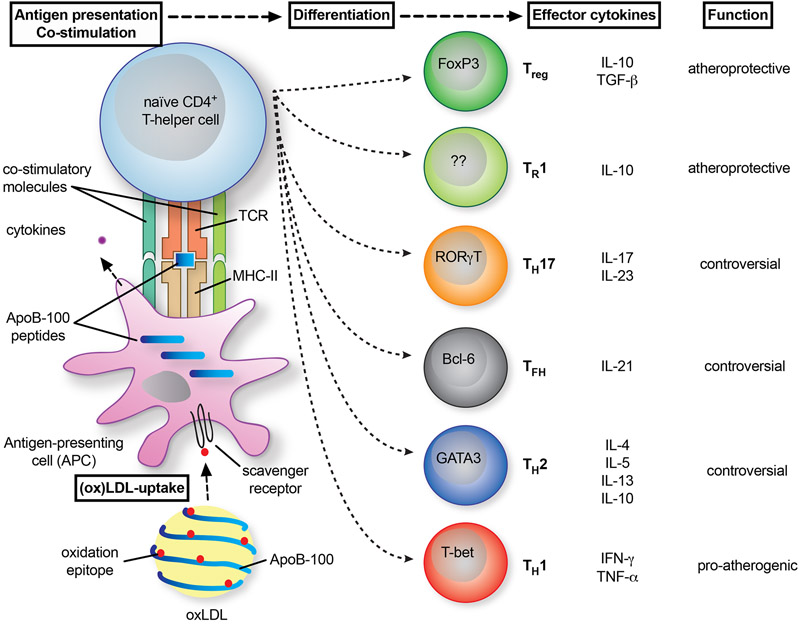
Figure 2: T cell polarization in atherosclerosis.
Naïve T helper cells (TH) acquire the complete phenotype of an effector T cell in the plaque after presentation of antigenic peptides from ApoB by antigen-presenting cells (APCs). Therefore, an APC takes up (oxidized) LDL-cholesterol particles, processes, and presents peptides from ApoB on MHC-II. The T cell recognized this complex by a specific T cell receptor (TCR). This process is guided by the binding of co-stimulatory ligands to their corresponding receptors on T cells. As a result of co-stimulatory signals and cytokines secreted by the APC, T cells express transcription factors (denoted in the cells) that favor the differentiation into distinct TH-types. These express specific cytokines that can either act in an atheroprotection or pro-atherogenic manner. The relevance for atherosclerosis remains controversial for some TH-phenotypes.
It is noteworthy that antigen presentation initiates and modulates the CD4+ T-helper cell response atherosclerosis. T cell activation in an antigen-specific manner is an exclusive consequence of antigen-presenting cells (APCs) that present antigenic peptides displayed on MHC molecules29. Blocking MHC-II during co-stimulation or on APCs abrogates the downstream CD4+ T cell response31, 90. T cell immune responses are typically initiated by antigen-loaded dendritic cells (DCs) migrating to lymph nodes. Several cells in the atherosclerotic plaque act as APCs for recall responses of antigen-experienced effector and memory T cells, including macrophages in the plaque, B cells in the adventitia, along with conventional DCs and plasmacytoid dendritic cells (pDCs). Depending on co-stimulatory signals and cytokines provided by these APCs, the immune response can be skewed into a tolerogenic (immune-suppressive) or an immunogenic response (summarized in91, 92).
The role of other T cell subsets remains less well defined. It has been suggested that MHC-I dependent cytotoxic CD8+ T cells contribute to plaque inflammation and the build-up of the necrotic core93, 94, but antigen specificity has not been considered95. Natural killer (NK) cells regulate antigen-specific T cell immunity besides the killing of infected and tumor cells. They are detected at low frequencies in the plaque and may therefore modulate atherosclerosis96. In contrast to earlier studies, a recent report, however, suggested that NK cells do not affect atherogenesis97-100. In addition, CD1d-restricted NK-cells can recognize glycolipid antigens. Some NKT cell subsets were reported to aggravate atherosclerosis, but the atherosclerosis-relevant glycolipids detected by these NKT-cells remain unknown101-103.
The function of ApoB-specific, auto-reactive T-helper cells
It has been challenging to determine the phenotype of ApoB-specific CD4+ T cells, i.e. the fraction of T cells with a TCR recognizing ApoB-peptides presented on MHC-II, within the pool of all lesional T cells. In animal models, ApoB-specific T cells have been expanded in-vitro or by vaccination against LDL or ApoB-peptides in-vivo. A direct transfer of vaccination-induced T cells in one study aggravated atherosclerotic disease104. T cells re-stimulated with oxLDL ex-vivo promoted atherosclerotic disease after adoptive transfer in a model of immunodeficient scid/Apoe−/− mice105. Neutralization of T cells that responded to oxLDL stimulation by a monoclonal antibody directed against the TCRBV31 chain protected from atherosclerosis106, suggestive of a pro-atherogenic function of ApoB-reactive T cells. However, more recent technologies to specifically detect ApoB-specific T cells in-vivo suggest the opposite: Tracking of ApoB-reactive T cells in mice and humans suggests that a majority of antigen-specific T cells are immunosuppressive Tregs42. This is consistent with recent work from Gistera et al. who transferred ApoB-reactive T cells from a mouse with a transgenic TCR directed against oxLDL/ApoB, which protected from atherosclerosis89. These mixed results obscure the exact function of antigen-specific T cells. Likely, their phenotype and function depend on presented peptides, the microenvironment, and cytokine milieu, which potentially affects T cell polarization. Some pro-atherogenic antigen-specific T cells104, 106 were isolated and cloned for in vitro assays by a procedure known to pre-dispose and select pathogenic TH1 and to neglect Treg clones. It is possible that the population of antigen-specific T cells may be multi-potent to give rise to several TH-lineages in-vivo – an idea consistent with the recent observation that MHC-II multimer selected ApoB-reactive T cells can express several TH-defining transcription factors simultaneously42.
The Treg-switch hypothesis – how protective immunity turns into a pathogenic response
The notion that atherosclerosis has an autoimmune component raised the question whether atherosclerosis is prevented in an antigen-specific manner by ApoB-reactive Tregs39 in healthy individuals. Tregs prevent the onset on autoimmune disease107. Naturally occurring Tregs are generated in the thymus (nTregs) and peripheral are induced Tregs from naïve T cells (iTregs). Despite the proven atheroprotective role of bulk Tregs68, 69 it has been unclear whether Tregs reactive to ApoB exist and how these may contribute to disease. Interestingly, ample clinical data suggest a strong inverse relationship between Tregs and atherosclerosis: Numbers of Tregs and IL-10 expression are lower in patients with myocardial infarction108, 109. Low Treg numbers predict cardiovascular events110. Blood Treg numbers in established murine atherosclerosis decline in later disease, while effector T cells increase36 (Figure 3a). However, in subclinical human atherosclerosis, Treg numbers correlate positively with LDL111. Likewise, in mice hypercholesterolemia initially favors the differentiation of Tregs112, an effect that may be a counter-regulatory response to enhanced inflammation36, intracellular lipid accumulation113, or an antigen-specific response. The latter hypothesis was supported by enhanced T cell receptor (TCR) downstream signaling events in hypercholesterolemic mice114, suggesting that a sub-population of T cells responds to antigens associated with increased LDL levels or to components of LDL particles itself. Thus, these data indirectly suggest the existence of LDL/ApoB-reactive Tregs that bear a TCR specifically responding to ApoB auto-peptides. These cells respond when the corresponding peptides are presented by MHC-II molecules by various APCs. Indeed, we directly demonstrated the existence of such ApoB-reactive T cells by MHC-II tetramers loaded with the human and mouse auto-peptide ApoB3036-3050. Using this tool, we showed that among all ApoB3036-3050-reactive CD4+ T-cells in patients free of cardiovascular disease, two thirds exclusively expressed FoxP3, indicative of a large population of ApoB-reactive Tregs. In patients with subclinical atherosclerosis, the percentage of exclusively FoxP3-positive T cells declined to ~ 30%, while a substantial proportion of the remaining FoxP3+ T cells acquired simultaneous expression of ROR-γT or T-bet, the TH17 and TH1-defining transcription factors, respectively42. These observations, along with the diminished pool of Tregs in later mouse and human disease, support the idea that the immunosuppressive phenotype of Tregs disappears as atherosclerosis progresses. Consist with this hypothesis, Tregs in late atherosclerosis in mice simultaneously express T-bet, lose their ability to regulate and to protect from atherosclerosis, while retaining some phenotypic similarity with Tregs, such as some residual expression of FoxP336, 55, 62. Gaddis et al. recently proposed that FoxP3 expression may be lost in favor of the transcription factor Bcl-6, the defining transcription factor for follicular-helper T cells88. Adoptively transferred ApoB+ T-helper cells turned into TFH cells after adoptive transfer89. In other autoimmune conditions, such as experimental autoimmune encephalitis (EAE) and arthritis, an instability of FoxP3 expression triggers the formation of antigen-specific, but dysfunctional, partially non-protective former Tregs (exTregs)115-117. The instability of FoxP3 may be caused by increased methylation of the FoxP3 locus, which is observed in patients with severe cardiovascular disease118 and that may be prevented by modifications of lipid metabolism or anti-cytokine interventions88, 115. In addition, the function of FoxP3 may be regulated by alternative splicing favoring pathogenic transcriptional programs119. These data suggest that the initial protective immune response by Tregs switches into a pathogenic response as atherosclerosis progresses39 (Figure 3b,c).
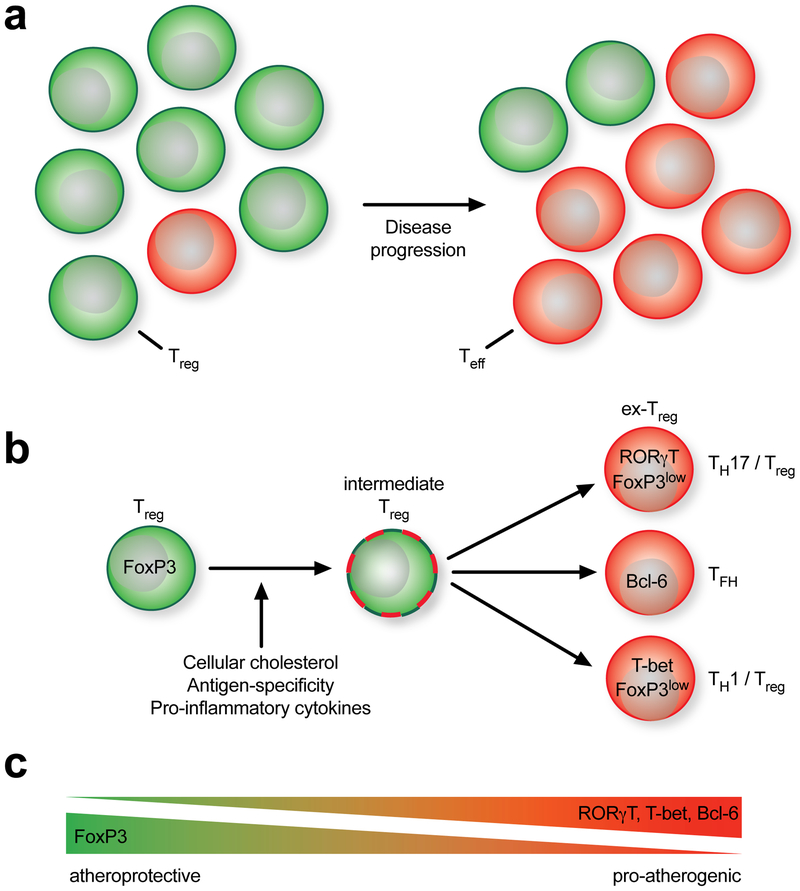
Figure 3: Decline of protective T-regulatory cells (Treg) in the course of atherosclerosis.
(a) As disease progresses, the pool of Treg-dominated antigen-specific T cells is overwhelmed by effector T cells (Teff) with a presumably pro-atherogenic function. (b) Over time, Tregs expressing their defining transcription factor FoxP3 start to express alternative TH-transcription factors, such as RORγ-T (TH17), Bcl-6 (TFH), or T-bet (TH1). FoxP3 either remains co-expressed or disappears. Likely, this switch into FoxP3-low expressed or -negative exTregs may be caused by antigen-specificity of the T cell, the cytokine milieu in the atherosclerotic plaque, or the loading of the T cell with intracellular cholesterol. (c) These observations have built the concept of an increasing replacement of (athero-) protective immunity with a pro-atherogenic response.
Pro- and anti-atherogenic B cell responses in atherosclerosis
Classically, two types of B cells can be distinguished: B1 cells that are part of the innate immune system and secrete germ-line encoded IgM antibodies in a T cell independent manner, and B2 cells that need to be activated by T follicular helper cells (TFH) to differentiate into plasma cells that secrete IgG antibodies. In infection and vaccination against pathogens, B cell-derived plasma cells secrete IgG antibodies that neutralize or opsonize bacteria and viruses29. In addition, B cells can secrete numerous cytokines that distinctly affect inflammation. Examples include IRA-B cells, which are pro-atherogenic and secrete GM-CSF to drive myeloid cell activation and to induce pro-atherogenic TH1 immunity120. B-regulatory cells (Breg) secrete the anti-inflammatory cytokine IL-10 and induce protective T-regulatory cells or directly act anti-inflammatory121, although the relevance for atherosclerosis is controversial122. The role of other cytokine producing B-effector (Be) cells is unclear. Only a few B cells are found in the atherosclerotic plaque24; the majority of B cells reside in the adventitia, in particular in aged atherosclerotic animals, where arterial tertiary lymphoid organs (ATLOs) form123. B cells in the spleen respond to a high cholesterol diet124, suggesting local and systemic B cell responses in atherosclerosis. Global gain and loss of function experiments have suggested an overall protective role of B cells125, 126. In general, innate B1 response seem to be atheroprotective and adaptive B2 responses pro-atherogenic (Figure 4):
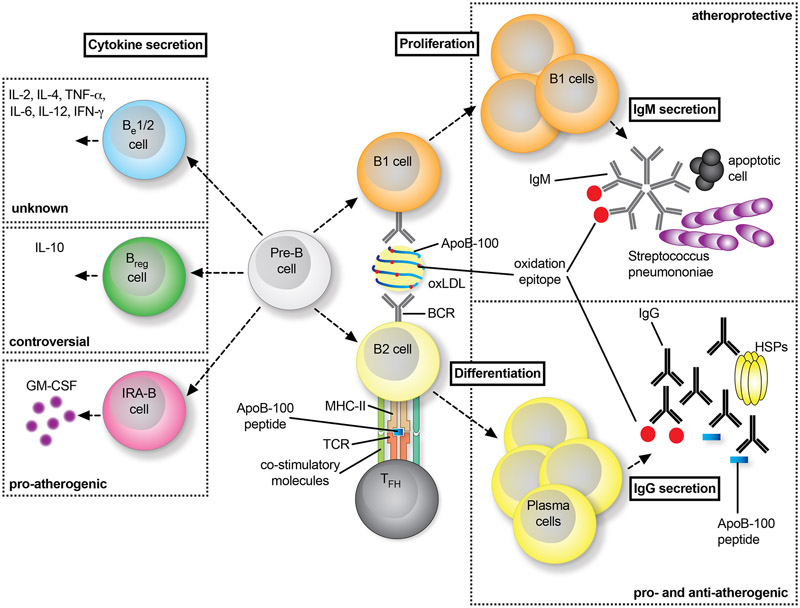
Figure 4: Distinct role of B cells in atherosclerosis.
B cells on a developmental stage (Pre-B) turn into innate-like B1 cells or adaptive, conventional B2 cells (right panel). B1 cells recognize epitopes on LDL and oxLDL particles, which leads to their activation and expression of low-affinity IgM antibodies by proliferation. Often, these IgM show cross-reactivity with epitopes on bacteria such as Streptococcus pneumoniae or on apoptotic cells. Interfering with B1 functionality aggravates atherosclerosis. B2 cells require co-stimulation by TFH cells by MHC-II:peptide:TCR interactions and co-stimulatory signaling events to fully differentiate into plasma cells that express high-affinity IgG antibodies against atherogenic antigens, such as ApoB, oxLDL, or heat-shock proteins (HSP). Neutralizing B2 cells is atheroprotective, while the role of IgG-antibodies remains controversial with reported pro- and anti-atherogenic functions. Independent of the classification of B1/2 cells, distinct B cell subsets have been shown to express non-exclusive sets of cytokines, which allows the definition of cytokine-secreting B-effector (Be) −1 and −2 cells, regulatory B cells (Bregs), and innate-response activator (IRA) B cells (left panel).
B1 cells:
B 1 cells represent a first-line, innate defense against common pathogens. In mice, they are characterized as CD11b+CD43+CD23−B220lowCD19+ cells and may be sub-divided into B1a and B1b cells depending on their location and surface markers127. Typically, most B1 cells reside in the peritoneal cavity. In the atherosclerotic plaque, a few CD11b+ B220negCD19+ B1-like cells are found that further decrease in more advanced disease24. B1 cells secrete germ-line encoded IgM. Typically, B1-derived IgM recognize phosphocholine (PC) head groups of polysaccharides in the wall of bacteria, such as S. pneumoniae. The same IgMs also bind oxidation-specific neo-epitopes on LDL and epitopes on apoptotic cells128-130. Oxidative neo-epitopes also seem to be generated in the spleen during sterile inflammation131. In cardiovascular disease, IgM recognizing epitopes on LDL or ApoB are inversely correlated with atherosclerosis, complications, and outcome132-138. It has been shown that IgMs directed against oxLDL inhibit its uptake by macrophages and prevent myeloid-cell inflammation139, 140. Consistently, several studies with gain- and loss-of-function experiments have established an atheroprotective role for B1 cells141-145.
B2 cells:
IgG antibodies originate from plasma cells that have undergone B cell maturation with the help of TFH cells in germinal centers, which causes a switch from low-affinity IgM to high-affinity IgG146. IgG antibody titers to native and oxidized LDL or ApoB are positively correlated with atherosclerotic disease in mice and humans133, 147-149. Inhibiting B2 cells is reportedly atheroprotective150-152, while specifically interfering with plasma cell functioning seems to be proatherogenic153. The role of IgG antibodies in atherosclerosis is controversial: It was suggested that IgGs against ApoB aggravate154 or protect from atherosclerosis155, 156. A clinical phase II study (Goal of Oxidized LDL and Activated Macrophage Inhibition by Exposure to a Recombinant Antibody, ‘GLACIER’) using a monoclonal IgG antibody against a human ApoB-peptide failed to show its expected atheroprotective effect157. The design of the study with the use of 8F-fluorodeoxyglucose (FDG) PET-imaging as surrogate for plaque inflammation instead of cardiovascular endpoints, the short observation period of 85 days, and the small study population may have contributed to its lack of efficacy.
Vaccination against atherosclerosis – a translatable strategy?
The discovery of the autoimmune component of atherosclerosis has sparked the idea of immunizing with LDL or peptides from ApoB to prevent atherosclerosis by inducing or maintaining the traits of protective immunity against ApoB. Almost 60 years ago, it was shown that rabbits develop smaller atherosclerotic lesions after subcutaneous injection of LDL38. That vaccination with LDL can be atheroprotective was confirmed in a variety of species, LDL preparations, routes, and adjuvants158-160. At least seven MHC-II-restricted peptides from ApoB, which contains the immunodominant epitopes of LDL, are protecting from atherosclerosis when used in vaccines: p3, p6, p101, p102, p103, p18, p21042, 161-163. An ongoing challenge is to decipher the mechanism of action, which is critically required to define vaccination protocols translatable to humans. It has been proposed that either Tregs42, 164-167, IL-1042, 161, 167, 168, or vaccination-induced IgG-antibodies may confer atheroprotection, depending on doses, routes, and adjuvants used44. Recent studies, however, suggest that atheroprotection does not require IgG-antibodies169 and primarily proceeds by IL-10+ ApoB-specific Tregs42.
Whether vaccination strategies can be translated to humans remains unclear. A first step towards a translatable approach was the identification of human ApoB-peptide epitopes accessible to immunomodulation in two recent studies42, 170. In mice, ApoB-peptides have been delivered in the non-translatable classical adjuvants Complete Freund’s Adjuvant (CFA), an emulsion of mineral oil supplemented with inactivated mycobacteria, or Incomplete Freund’s Adjuvant (IFA), which lacks the mycobacteria component of CFA. Subcutaneous or intraperitoneal injections of both, CFA and IFA, were shown to elicit non-specific inflammation171, 172. This limitation was recently overcome by the discovery that a squalene oil, a class of adjuvants already used in clinical practice, can be used as an adjuvant for ApoB-peptides169. In addition, it remains unclear whether vaccination is effective in established atherosclerosis as most studies tested the prevention of de-novo atherosclerosis in rodents.
Limitations of animal models
The principles of the cellular and humoral adaptive immune response in experimental murine atherosclerosis have been established. The efficacy of anti-inflammatory therapy in human atherosclerosis has been validated in the CANTOS trial recently. However, significant challenges remain for the translation of animal studies to humans. First, mice, which represent the most widely employed atherosclerosis model, neither develop spontaneous atherosclerosis, nor do atherosclerotic knockout mice develop coronary artery disease. In addition, spontaneous atherothrombotic events resembling heart attacks and strokes do not occur in atherosclerotic mice. Also, blood lipoprotein profiles in mice are unlike those in humans, even in the genetic atherosclerosis models. Second, most atherosclerosis studies are conducted in a single mouse strain (C57BL/6) that cannot capture the genetic diversity seen in humans. Genetic diversity is known to modulate the response against antigens and atherosclerosis-relevant stimuli within a spectrum from pro- to anti-inflammatory173,174. Third, some cytokines and immune receptors are not conserved between mice and humans, because the immune systems of both species are under intense evolutionary pressure. Fourth, mice represent a simplified model system for antigen presentation and recognition. Unlike humans, mice are housed in specific-pathogen free (SPF) facilities, which neglects the likely pathogen-driven activation, antigenic repertoire, and differentiation of immune cells175. While C57BL/6 mice bear just one MHC-II allele/molecule (I-Ab), humans express several alleles of a large pool of different MHC-II variants that are termed human leukocyte antigens (HLA) with over 10,000 different HLA allelic forms. This extreme variability renders the direction and amplitude of autoimmunity in humans difficult to predict.
Clinical considerations
Decreasing LDL levels and attenuating the inflammatory response represent the two fundamental therapeutic strategies against atherosclerosis available today. The most successful causal medication as measured by event-free person years are inhibitors of endogenous cholesterol synthesis by the HMG-CoA reductase (statins)176, 177, which lower LDL-cholesterol and have pleiotropic anti-inflammatory effects beyond what can be expected from the reduction of LDL178. Statins can prevent, reduce, and even reverse atherosclerotic plaque burden179. Monoclonal antibodies to Proprotein convertase subtilisin/kexin type 9 (PCSK9) lower LDL-cholesterol even more dramatically by blocking LDL degradation180, 181 without apparent impact on levels of CRP levels182, a biomarker of systemic inflammation. However, even after LDL-lowering with statins and PCSK9-inhibition, a substantial residual inflammatory risk remains183. These observations have established the distinct, but overlapping, roles of inflammation- and lipid associated risk. Low-dose treatment with the anti-proliferative and anti-inflammatory drug colchicine prevented cardiovascular events in a small prospective clinical trial184. In addition, the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) showed that inhibition of inflammation by the Interleukin 1-β (IL1-β) antibody canakinumab reduced cardiovascular end-points in patients with established atherosclerosis by 15%185. Strikingly, these observations have proven the inflammatory hypothesis on a conceptual basis, yet it is unclear, which patients may benefit from novel anti-inflammatory therapies: First, inhibition of IL1-β impaired host defense, which was reflected by an increased incidence of lethal infections185. Second, the recent Cardiovascular Inflammation Reduction Trial (CIRT) that tested low-dose anti-inflammatory methotrexate in patients with coronary heart disease did not reach its endpoints186. This lack of efficacy was partially explained by the inclusion of patients at low inflammatory risk and calls for a future personalized risk stratification (inflammatory versus lipid risk) and treatment once anti-inflammatory therapy is available in clinical practice. Whether the autoimmune component of atherosclerosis may already be addressable by unspecific anti-inflammatory therapy is currently unknown. However, vaccination and immunomodulation may provide a future antigen-specific therapy that is unlikely to impair host defense. The first validation of MHC-II tetramers to quantify the ApoB-reactive T cell responses42 and the measurement of auto-antibodies187 in humans may provide feasible risk stratification tools in the challenge to define patients at a high immune risk for atherosclerosis in future.
Conclusion
Atherosclerosis is a chronic inflammatory disease of the vessel wall that is largely driven by an innate immune response through myeloid cells as monocytes and macrophages. Autoimmunity against ApoB and other antigens involve CD4+ T-helper cells that instruct myeloid cells and antigen-specific antibodies that may directly modify the pathogenicity of these antigens. This autoimmune response is detectable in humans and animal models with atherosclerosis. While the classical perception is that autoimmunity is pathogenic per se, recent evidence suggests that ApoB-specific CD4+ T-helper cells are already detectable in subjects without clinical atherosclerosis, where many of them show atheroprotective features. As atherosclerosis progresses, the protective auto-immune response converts into a pathogenic one. It is unknown whether this switch in functionality represents a cause or a consequence of atherosclerosis and inflammation. It is clear that the adaptive immune system in atherosclerosis can be pro- or anti-inflammatory and thus pro- or anti-atherogenic. Manipulating the adaptive immune system by immunomodulatory strategies or vaccination is an attractive concept. Limitations in the predictive power of animal models and a lack of a full understanding of the role of auto-antibodies, B- and T cells present formidable hurdles to clinical translation.
Acknowledgments
Sources of fundind
This work was supported by grants to D. Wolf from the Deutsche Forschungsgemeinschaft (DFG WO1994/1). K. Ley was supported by grants HL115232, HL88093, and HL121697 from the National Heart, Lung, and Blood Institute.
Non-Standard Abbreviations and Acronyms
- scRNAseq
Single-cell RNA sequencing
- CyTOF
Cytometry by Time of Flight (mass cytometry)
- TH
T-helper cell
- Treg
T-regulatory cell
- ATLO
Artery tertiary lymphoid organ
- FACS
Fluorescence-activated cell sorting
- LDL
Low-density lipoprotein
- LDLR
Low-density lipoprotein receptor
- ApoE
Apolipoprotein E
- PCSK9
Proprotein convertase subtilisin/kexin type 9
- GWAS
Genome-wide association studies
- CRP
C-reactive protein
- MHC
Major Histocompatibility complex
- HDL
High-density lipoprotein
Footnotes
Disclosures
K. Ley has received research funding for developing an atherosclerosis vaccine formulation from UBI.
References
- 1.Gallino A, Aboyans V, Diehm C, Cosentino F, Stricker H, Falk E, et al. Non-coronary atherosclerosis. European heart journal. 2014;35:1112–1119 [DOI] [PubMed] [Google Scholar]
- 2.Ross R Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340:115–126 [DOI] [PubMed] [Google Scholar]
- 3.Libby P Inflammation in atherosclerosis. Nature. 2002;420:868–874 [DOI] [PubMed] [Google Scholar]
- 4.Kruk ME, Gage AD, Joseph NT, Danaei G, Garcia-Saiso S, Salomon JA. Mortality due to low-quality health systems in the universal health coverage era: A systematic analysis of amenable deaths in 137 countries. Lancet. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–546 [DOI] [PubMed] [Google Scholar]
- 6.Braunwald E The treatment of acute myocardial infarction: The past, the present, and the future. Eur Heart J Acute Cardiovasc Care. 2012;1:9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547–563 [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Harker L. Hyperlipidemia and atherosclerosis. Science. 1976;193:1094–1100 [DOI] [PubMed] [Google Scholar]
- 9.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein e. Science. 1992;258:468–471 [DOI] [PubMed] [Google Scholar]
- 11.Do R, Stitziel NO, Won HH, Jorgensen AB, Duga S, Angelica Merlini P, et al. Exome sequencing identifies rare ldlr and apoa5 alleles conferring risk for myocardial infarction. Nature. 2015;518:102–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McPherson R, Tybjaerg-Hansen A. Genetics of coronary artery disease. Circ Res. 2016;118:564–578 [DOI] [PubMed] [Google Scholar]
- 13.Kobiyama K, Ley K. Atherosclerosis. Circ Res. 2018;123:1118–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B cells and humoral immunity in atherosclerosis. Circ Res. 2014;114:1743–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimbrone MA Jr., Garcia-Cardena G Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134:611–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtiss LK, Tobias PS. Emerging role of toll-like receptors in atherosclerosis. J Lipid Res. 2009;50 Suppl:S340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senders ML, Que X, Cho YS, Yeang C, Groenen H, Fay F, et al. Pet/mr imaging of malondialdehyde-acetaldehyde epitopes with a human antibody detects clinically relevant atherothrombosis. Journal of the American College of Cardiology. 2018;71:321–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nahrendorf M Myeloid cell contributions to cardiovascular health and disease. Nat Med. 2018;24:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. Nlrp3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libby P Interleukin-1 beta as a target for atherosclerosis therapy: Biological basis of cantos and beyond. Journal of the American College of Cardiology. 2017;70:2278–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell rna-sequencing and mass cytometry. Circ Res. 2018;122:1675–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially l-selectin dependent. J Exp Med. 2006;203:1273–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292 [DOI] [PubMed] [Google Scholar]
- 27.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. The New England journal of medicine. 2011;364:226–235 [DOI] [PubMed] [Google Scholar]
- 28.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of t cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138 [DOI] [PubMed] [Google Scholar]
- 29.Adler R Janeway’s immunobiology. Choice: Current Reviews for Academic Libraries. 2008;45:1793–1794 [Google Scholar]
- 30.Steinman RM. Decisions about dendritic cells: Past, present, and future. Annu Rev Immunol. 2012;30:1–22 [DOI] [PubMed] [Google Scholar]
- 31.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, et al. Dynamic t cell-apc interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulsson G, Zhou X, Tornquist E, Hansson GK. Oligoclonal t cell expansions in atherosclerotic lesions of apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:10–17 [DOI] [PubMed] [Google Scholar]
- 33.Lin Z, Qian S, Gong Y, Ren J, Zhao L, Wang D, et al. Deep sequencing of the t cell receptor beta repertoire reveals signature patterns and clonal drift in atherosclerotic plaques and patients. Oncotarget. 2017;8:99312–99322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centa M, Prokopec KE, Garimella MG, Habir K, Hofste L, Stark JM, et al. Acute loss of apolipoprotein e triggers an autoimmune response that accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caligiuri G, Nicoletti A, Zhou X, Tornberg I, Hansson GK. Effects of sex and age on atherosclerosis and autoimmunity in apoe-deficient mice. Atherosclerosis. 1999;145:301–308 [DOI] [PubMed] [Google Scholar]
- 36.Maganto-Garcia E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic changes in regulatory t cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124:185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colantonio LD, Bittner V, Reynolds K, Levitan EB, Rosenson RS, Banach M, et al. Association of serum lipids and coronary heart disease in contemporary observational studies. Circulation. 2016;133:256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gero S, Gergely J, Jakab L, Szekely J, Virag S, Farkas K, et al. Inhibition of cholesterol atherosclerosis by immunisation with beta-lipoprotein. Lancet. 1959;2:6–7 [DOI] [PubMed] [Google Scholar]
- 39.Ley K 2015 russell ross memorial lecture in vascular biology: Protective autoimmunity in atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tse K GA, Sidney J, Ouyang H, Witztum J, Sette A, Tse H, Ley K. Atheroprotective vaccination with mhc-ii restricted peptides from aopb-100. Frontiers in Immunology. 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura T, Kobiyama K, Winkels H, Tse K, Miller J, Vassallo M, et al. Regulatory cd4(+) t cells recognize mhc-ii-restricted peptide epitopes of apolipoprotein b. Circulation. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, et al. Naive cd4(+) t cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura T, Tse K, Sette A, Ley K. Vaccination to modulate atherosclerosis. Autoimmunity. 2015;48:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wick G, Jakic B, Buszko M, Wick MC, Grundtman C. The role of heat shock proteins in atherosclerosis. Nature reviews. Cardiology. 2014;11:516–529 [DOI] [PubMed] [Google Scholar]
- 46.Zhu J, Quyyumi AA, Rott D, Csako G, Wu H, Halcox J, et al. Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: Evidence for an autoimmune component of atherogenesis. Circulation. 2001;103:1071–1075 [DOI] [PubMed] [Google Scholar]
- 47.George J, Afek A, Gilburd B, Shoenfeld Y, Harats D. Cellular and humoral immune responses to heat shock protein 65 are both involved in promoting fatty-streak formation in ldl-receptor deficient mice. Journal of the American College of Cardiology. 2001;38:900–905 [DOI] [PubMed] [Google Scholar]
- 48.Lawson JS, Glenn WK, Tran DD, Ngan CC, Duflou JA, Whitaker NJ. Identification of human papilloma viruses in atheromatous coronary artery disease. Front Cardiovasc Med. 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: Update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost. 2011;106:858–867 [DOI] [PubMed] [Google Scholar]
- 50.Pothineni NVK, Subramany S, Kuriakose K, Shirazi LF, Romeo F, Shah PK, et al. Infections, atherosclerosis, and coronary heart disease. European heart journal. 2017;38:3195–3201 [DOI] [PubMed] [Google Scholar]
- 51.Hansson GK, Jonasson L, Lojsthed B, Stemme S, Kocher O, Gabbiani G. Localization of t lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis. 1988;72:135–141 [DOI] [PubMed] [Google Scholar]
- 52.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, et al. Single-cell rna-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122:1661–1674 [DOI] [PubMed] [Google Scholar]
- 53.Cole JE, Park I, Ahern DJ, Kassiteridi C, Danso Abeam D, Goddard ME, et al. Immune cell census in murine atherosclerosis: Cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovascular research. 2018;114:1360–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged apoe−/− mice. J Exp Med. 2009;206:233–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, McArdle S, Gholami A, Kimura T, Wolf D, Gerhardt T, et al. Ccr5+t-bet+foxp3+ effector cd4 t cells drive atherosclerosis. Circ Res. 2016;118:1540–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galkina E, Harry BL, Ludwig A, Liehn EA, Sanders JM, Bruce A, et al. Cxcr6 promotes atherosclerosis by supporting t-cell homing, interferon-gamma production, and macrophage accumulation in the aortic wall. Circulation. 2007;116:1801–1811 [DOI] [PubMed] [Google Scholar]
- 57.Dansky HM, Charlton SA, Harper MM, Smith JD. T and b lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein e-deficient mouse. Proc Natl Acad Sci U S A. 1997;94:4642–4646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emeson EE, Shen ML, Bell CG, Qureshi A. Inhibition of atherosclerosis in cd4 t-cell-ablated and nude (nu/nu) c57bl/6 hyperlipidemic mice. Am J Pathol. 1996;149:675–685 [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf D, Zirlik A, Ley K. Beyond vascular inflammation--recent advances in understanding atherosclerosis. Cell Mol Life Sci. 2015;72:3853–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robertson AK, Hansson GK. T cells in atherogenesis: For better or for worse? Arteriosclerosis, thrombosis, and vascular biology. 2006;26:2421–2432 [DOI] [PubMed] [Google Scholar]
- 62.Butcher MJ, Filipowicz AR, Waseem TC, McGary C, Crow KJ, Magilnick N, et al. Atherosclerosis-driven treg plasticity results in formation of a dysfunctional subset of plastic ifngamma+ th1/tregs. Circ Res. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the ldlr-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460 [DOI] [PubMed] [Google Scholar]
- 65.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. Ifn-gamma potentiates atherosclerosis in apoe knock-out mice. J Clin Invest. 1997;99:2752–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1991;11:1223–1230 [DOI] [PubMed] [Google Scholar]
- 67.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, et al. Interferon-gamma, a th1 cytokine, regulates fat inflammation: A role for adaptive immunity in obesity. Circ Res. 2008;103:467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, et al. Natural regulatory t cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180 [DOI] [PubMed] [Google Scholar]
- 69.Klingenberg R, Gerdes N, Badeau RM, Gistera A, Strodthoff D, Ketelhuth DF, et al. Depletion of foxp3+ regulatory t cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA, et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–2853 [DOI] [PubMed] [Google Scholar]
- 71.Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of tgf-beta signaling in t cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foks AC, Lichtman AH, Kuiper J. Treating atherosclerosis with regulatory t cells. Arterioscler Thromb Vasc Biol. 2015;35:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, Toh BH, et al. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands cd4+cd25+foxp3+ regulatory t cells and attenuates development and progression of atherosclerosis. Circulation. 2012;126:1256–1266 [DOI] [PubMed] [Google Scholar]
- 74.Kita T, Yamashita T, Sasaki N, Kasahara K, Sasaki Y, Yodoi K, et al. Regression of atherosclerosis with anti-cd3 antibody via augmenting a regulatory t-cell response in mice. Cardiovascular research. 2014;102:107–117 [DOI] [PubMed] [Google Scholar]
- 75.Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein e knockout mice. Mol Med. 2003;9:10–17 [PMC free article] [PubMed] [Google Scholar]
- 76.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, et al. Coexpression of cd49b and lag-3 identifies human and mouse t regulatory type 1 cells. Nat Med. 2013;19:739–746 [DOI] [PubMed] [Google Scholar]
- 77.Mallat Z, Gojova A, Brun V, Esposito B, Fournier N, Cottrez F, et al. Induction of a regulatory t cell type 1 response reduces the development of atherosclerosis in apolipoprotein e-knockout mice. Circulation. 2003;108:1232–1237 [DOI] [PubMed] [Google Scholar]
- 78.Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of ifn-gamma signaling induce aneurysms in allografted aortas. J Clin Invest. 2004;114:300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engelbertsen D, Andersson L, Ljungcrantz I, Wigren M, Hedblad B, Nilsson J, et al. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler Thromb Vasc Biol. 2013;33:637–644 [DOI] [PubMed] [Google Scholar]
- 80.Mallat Z, Taleb S, Ait-Oufella H, Tedgui A. The role of adaptive t cell immunity in atherosclerosis. J Lipid Res. 2009;50 Suppl:S364–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female ldl receptor−/− mice. Arteriosclerosis, thrombosis, and vascular biology. 2002;22:456–461 [DOI] [PubMed] [Google Scholar]
- 82.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, et al. Blockade of interleukin-17a results in reduced atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2010;121:1746–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, et al. A critical function of th17 proinflammatory cells in the development of atherosclerotic plaque in mice. Journal of immunology. 2010;185:5820–5827 [DOI] [PubMed] [Google Scholar]
- 84.Nordlohne J, Helmke A, Ge S, Rong S, Chen R, Waisman A, et al. Aggravated atherosclerosis and vascular inflammation with reduced kidney function depend on interleukin-17 receptor a and are normalized by inhibition of interleukin-17a. JACC Basic Transl Sci. 2018;3:54–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, et al. Interleukin-17a deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein e-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:273–280 [DOI] [PubMed] [Google Scholar]
- 86.Gistera A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, et al. Transforming growth factor-beta signaling in t cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013;5:196ra100. [DOI] [PubMed] [Google Scholar]
- 87.Brauner S, Jiang X, Thorlacius GE, Lundberg AM, Ostberg T, Yan ZQ, et al. Augmented th17 differentiation in trim21 deficiency promotes a stable phenotype of atherosclerotic plaques with high collagen content. Cardiovascular research. 2018;114:158–167 [DOI] [PubMed] [Google Scholar]
- 88.Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, et al. Apolipoprotein ai prevents regulatory to follicular helper t cell switching during atherosclerosis. Nat Commun. 2018;9:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gistera A, Klement ML, Polyzos KA, Mailer RK, Duhlin A, Karlsson MCI, et al. Ldl-reactive t cells regulate plasma cholesterol levels and development of atherosclerosis in humanized hypercholesterolemic mice. Circulation. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sage AP, Murphy D, Maffia P, Masters LM, Sabir SR, Baker LL, et al. Mhc class ii-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic t cell immunity. Circulation. 2014;130:1363–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zernecke A Dendritic cells in atherosclerosis: Evidence in mice and humans. Arterioscler Thromb Vasc Biol. 2015 [DOI] [PubMed] [Google Scholar]
- 92.Koltsova EK, Ley K. How dendritic cells shape atherosclerosis. Trends Immunol. 2011;32:540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A, et al. Cytotoxic and proinflammatory cd8+ t lymphocytes promote development of vulnerable atherosclerotic plaques in apoe-deficient mice. Circulation. 2013;127:1028–1039 [DOI] [PubMed] [Google Scholar]
- 94.Kolbus D, Ramos OH, Berg KE, Persson J, Wigren M, Bjorkbacka H, et al. Cd8+ t cell activation predominate early immune responses to hypercholesterolemia in apoe(−)(/)(−) mice. BMC immunology. 2010;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cochain C, Zernecke A. Protective and pathogenic roles of cd8(+) t cells in atherosclerosis. Basic Res Cardiol. 2016;111:71. [DOI] [PubMed] [Google Scholar]
- 96.Winkels H, Ley K. Natural killer cells at ease: Atherosclerosis is not affected by genetic depletion or hyperactivation of natural killer cells. Circ Res. 2018;122:6–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schiller NK, Boisvert WA, Curtiss LK. Inflammation in atherosclerosis: Lesion formation in ldl receptor-deficient mice with perforin and lyst(beige) mutations. Arterioscler Thromb Vasc Biol. 2002;22:1341–1346 [DOI] [PubMed] [Google Scholar]
- 98.Whitman SC, Rateri DL, Szilvassy SJ, Yokoyama W, Daugherty A. Depletion of natural killer cell function decreases atherosclerosis in low-density lipoprotein receptor null mice. Arterioscler Thromb Vasc Biol. 2004;24:1049–1054 [DOI] [PubMed] [Google Scholar]
- 99.Selathurai A, Deswaerte V, Kanellakis P, Tipping P, Toh BH, Bobik A, et al. Natural killer (nk) cells augment atherosclerosis by cytotoxic-dependent mechanisms. Cardiovascular research. 2014;102:128–137 [DOI] [PubMed] [Google Scholar]
- 100.Nour-Eldine W, Joffre J, Zibara K, Esposito B, Giraud A, Zeboudj L, et al. Genetic depletion or hyperresponsiveness of natural killer cells do not affect atherosclerosis development. Circ Res. 2018;122:47–57 [DOI] [PubMed] [Google Scholar]
- 101.Aslanian AM, Chapman HA, Charo IF. Transient role for cd1d-restricted natural killer t cells in the formation of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2005;25:628–632 [DOI] [PubMed] [Google Scholar]
- 102.Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, et al. Cd1d-dependent activation of nkt cells aggravates atherosclerosis. J Exp Med. 2004;199:417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Kanellakis P, Hosseini H, Cao A, Deswaerte V, Tipping P, et al. A cd1d-dependent lipid antagonist to nkt cells ameliorates atherosclerosis in apoe−/− mice by reducing lesion necrosis and inflammation. Cardiovascular research. 2016;109:305–317 [DOI] [PubMed] [Google Scholar]
- 104.Shaw MK, Tse KY, Zhao X, Welch K, Eitzman DT, Thipparthi RR, et al. T-cells specific for a self-peptide of apob-100 exacerbate aortic atheroma in murine atherosclerosis. Front Immunol. 2017;8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou X, Robertson AK, Hjerpe C, Hansson GK. Adoptive transfer of cd4+ t cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:864–870 [DOI] [PubMed] [Google Scholar]
- 106.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, et al. Inhibition of t cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sakaguchi S Naturally arising cd4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562 [DOI] [PubMed] [Google Scholar]
- 108.Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of cd4(+)cd25(+) regulatory t cells in patients with acute coronary syndromes. European heart journal. 2006;27:2530–2537 [DOI] [PubMed] [Google Scholar]
- 109.George J, Schwartzenberg S, Medvedovsky D, Jonas M, Charach G, Afek A, et al. Regulatory t cells and il-10 levels are reduced in patients with vulnerable coronary plaques. Atherosclerosis. 2012;222:519–523 [DOI] [PubMed] [Google Scholar]
- 110.Wigren M, Bjorkbacka H, Andersson L, Ljungcrantz I, Fredrikson GN, Persson M, et al. Low levels of circulating cd4+foxp3+ t cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler Thromb Vasc Biol. 2012;32:2000–2004 [DOI] [PubMed] [Google Scholar]
- 111.Guasti L, Maresca AM, Schembri L, Rasini E, Dentali F, Squizzato A, et al. Relationship between regulatory t cells subsets and lipid profile in dyslipidemic patients: A longitudinal study during atorvastatin treatment. BMC Cardiovasc Disord. 2016;16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mailer RKW, Gistera A, Polyzos KA, Ketelhuth DFJ, Hansson GK. Hypercholesterolemia induces differentiation of regulatory t cells in the liver. Circ Res. 2017;120:1740–1753 [DOI] [PubMed] [Google Scholar]
- 113.Cheng HY, Gaddis DE, Wu R, McSkimming C, Haynes LD, Taylor AM, et al. Loss of abcg1 influences regulatory t cell differentiation and atherosclerosis. J Clin Invest. 2016;126:3236–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mailer RKW, Gistera A, Polyzos KA, Ketelhuth DFJ, Hansson GK. Hypercholesterolemia enhances t cell receptor signaling and increases the regulatory t cell population. Sci Rep. 2017;7:15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, et al. Self-antigen-driven activation induces instability of regulatory t cells during an inflammatory autoimmune response. Immunity. 2013;39:949–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of foxp3+ t cells into th17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68 [DOI] [PubMed] [Google Scholar]
- 117.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory t cells accumulate in the cns but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jia L, Zhu L, Wang JZ, Wang XJ, Chen JZ, Song L, et al. Methylation of foxp3 in regulatory t cells is related to the severity of coronary artery disease. Atherosclerosis. 2013;228:346–352 [DOI] [PubMed] [Google Scholar]
- 119.Joly AL, Seitz C, Liu S, Kuznetsov NV, Gertow K, Westerberg LS, et al. Alternative splicing of foxp3 controls regulatory t cell effector functions and is associated with human atherosclerotic plaque stability. Circ Res. 2018;122:1385–1394 [DOI] [PubMed] [Google Scholar]
- 120.Hilgendorf I, Theurl I, Gerhardt LM, Robbins CS, Weber GF, Gonen A, et al. Innate response activator b cells aggravate atherosclerosis by stimulating t helper-1 adaptive immunity. Circulation. 2014;129:1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sage AP, Nus M, Baker LL, Finigan AJ, Masters LM, Mallat Z. Regulatory b cell-specific interleukin-10 is dispensable for atherosclerosis development in mice. Arterioscler Thromb Vasc Biol. 2015;35:1770–1773 [DOI] [PubMed] [Google Scholar]
- 122.Strom AC, Cross AJ, Cole JE, Blair PA, Leib C, Goddard ME, et al. B regulatory cells are increased in hypercholesterolaemic mice and protect from lesion development via il-10. Thromb Haemost. 2015;114:835–847 [DOI] [PubMed] [Google Scholar]
- 123.Srikakulapu P, Hu D, Yin C, Mohanta SK, Bontha SV, Peng L, et al. Artery tertiary lymphoid organs control multilayered territorialized atherosclerosis b-cell responses in aged apoe−/− mice. Arterioscler Thromb Vasc Biol. 2016;36:1174–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nus M, Sage AP, Lu Y, Masters L, Lam BYH, Newland S, et al. Marginal zone b cells control the response of follicular helper t cells to a high-cholesterol diet. Nat Med. 2017;23:601–610 [DOI] [PubMed] [Google Scholar]
- 125.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by b cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in ldl receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898 [DOI] [PubMed] [Google Scholar]
- 127.Srikakulapu P, McNamara CA. B cells and atherosclerosis. American journal of physiology. Heart and circulatory physiology. 2017;312:H1060–H1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hosseini H, Li Y, Kanellakis P, Tay C, Cao A, Tipping P, et al. Phosphatidylserine liposomes mimic apoptotic cells to attenuate atherosclerosis by expanding polyreactive igm producing b1a lymphocytes. Cardiovascular research. 2015 [DOI] [PubMed] [Google Scholar]
- 129.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: Molecular mimicry between streptococcus pneumoniae and oxidized ldl. Nat Med. 2003;9:736–743 [DOI] [PubMed] [Google Scholar]
- 131.Grasset EK, Duhlin A, Agardh HE, Ovchinnikova O, Hagglof T, Forsell MN, et al. Sterile inflammation in the spleen during atherosclerosis provides oxidation-specific epitopes that induce a protective b-cell response. Proc Natl Acad Sci U S A. 2015;112:E2030–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin m type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112 [DOI] [PubMed] [Google Scholar]
- 133.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, et al. Relationship of igg and igm autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–433 [DOI] [PubMed] [Google Scholar]
- 134.Hulthe J, Bokemark L, Fagerberg B. Antibodies to oxidized ldl in relation to intima-media thickness in carotid and femoral arteries in 58-year-old subjectively clinically healthy men. Arterioscler Thromb Vasc Biol. 2001;21:101–107 [DOI] [PubMed] [Google Scholar]
- 135.Dotevall A, Hulthe J, Rosengren A, Wiklund O, Wilhelmsen L. Autoantibodies against oxidized low-density lipoprotein and c-reactive protein are associated with diabetes and myocardial infarction in women. Clin Sci (Lond). 2001;101:523–531 [PubMed] [Google Scholar]
- 136.Ravandi A, Boekholdt SM, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, et al. Relationship of igg and igm autoantibodies and immune complexes to oxidized ldl with markers of oxidation and inflammation and cardiovascular events: Results from the epic-norfolk study. J Lipid Res. 2011;52:1829–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tsimikas S, Miyanohara A, Hartvigsen K, Merki E, Shaw PX, Chou MY, et al. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. Journal of the American College of Cardiology. 2011;58:1715–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sjogren P, Fredrikson GN, Samnegard A, Ericsson CG, Ohrvik J, Fisher RM, et al. High plasma concentrations of autoantibodies against native peptide 210 of apob-100 are related to less coronary atherosclerosis and lower risk of myocardial infarction. European heart journal. 2008;29:2218–2226 [DOI] [PubMed] [Google Scholar]
- 139.Gillotte-Taylor K, Boullier A, Witztum JL, Steinberg D, Quehenberger O. Scavenger receptor class b type i as a receptor for oxidized low density lipoprotein. J Lipid Res. 2001;42:1474–1482 [PubMed] [Google Scholar]
- 140.Horkko S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin m is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cesena FH, Dimayuga PC, Yano J, Zhao X, Kirzner J, Zhou J, et al. Immune-modulation by polyclonal igm treatment reduces atherosclerosis in hypercholesterolemic apoe−/− mice. Atherosclerosis. 2012;220:59–65 [DOI] [PubMed] [Google Scholar]
- 143.Que X, Hung MY, Yeang C, Gonen A, Prohaska TA, Sun X, et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature. 2018;558:301–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, et al. B1a b lymphocytes are atheroprotective by secreting natural igm that increases igm deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109:830–840 [DOI] [PubMed] [Google Scholar]
- 145.Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, et al. B-1b cells secrete atheroprotective igm and attenuate atherosclerosis. Circ Res. 2015;117:e28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ait-Oufella H, Sage AP, Mallat Z, Tedgui A. Adaptive (t and b cells) immunity and control by dendritic cells in atherosclerosis. Circ Res. 2014;114:1640–1660 [DOI] [PubMed] [Google Scholar]
- 147.Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized ldl correlate with arterial accumulation and depletion of oxidized ldl in ldl receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:95–100 [DOI] [PubMed] [Google Scholar]
- 148.Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain igg that recognizes epitopes of oxidized ldl. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1994;14:32–40 [DOI] [PubMed] [Google Scholar]
- 149.Bjorkbacka H, Alm R, Persson M, Hedblad B, Nilsson J, Fredrikson GN. Low levels of apolipoprotein b-100 autoantibodies are associated with increased risk of coronary events. Arterioscler Thromb Vasc Biol. 2016;36:765–771 [DOI] [PubMed] [Google Scholar]
- 150.Kyaw T, Tay C, Hosseini H, Kanellakis P, Gadowski T, MacKay F, et al. Depletion of b2 but not b1a b cells in baff receptor-deficient apoe mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS One. 2012;7:e29371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, et al. Conventional b2 b cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185:4410–4419 [DOI] [PubMed] [Google Scholar]
- 152.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sage AP, Nus M, Bagchi Chakraborty J, Tsiantoulas D, Newland SA, Finigan AJ, et al. X-box binding protein-1 dependent plasma cell responses limit the development of atherosclerosis. Circ Res. 2017;121:270–281 [DOI] [PubMed] [Google Scholar]
- 154.Tay C, Liu YH, Kanellakis P, Kallies A, Li Y, Cao A, et al. Follicular b cells promote atherosclerosis via t cell-mediated differentiation into plasma cells and secreting pathogenic immunoglobulin g. Arterioscler Thromb Vasc Biol. 2018;38:e71–e84 [DOI] [PubMed] [Google Scholar]
- 155.Schiopu A, Bengtsson J, Soderberg I, Janciauskiene S, Lindgren S, Ares MP, et al. Recombinant human antibodies against aldehyde-modified apolipoprotein b-100 peptide sequences inhibit atherosclerosis. Circulation. 2004;110:2047–2052 [DOI] [PubMed] [Google Scholar]
- 156.Schiopu A, Frendeus B, Jansson B, Soderberg I, Ljungcrantz I, Araya Z, et al. Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in apobec-1(−/−)/low-density lipoprotein receptor(−/−) mice. Journal of the American College of Cardiology. 2007;50:2313–2318 [DOI] [PubMed] [Google Scholar]
- 157.Lehrer-Graiwer J, Singh P, Abdelbaky A, Vucic E, Korsgren M, Baruch A, et al. Fdg-pet imaging for oxidized ldl in stable atherosclerotic disease: A phase ii study of safety, tolerability, and anti-inflammatory activity. JACC Cardiovasc Imaging. 2015;8:493–494 [DOI] [PubMed] [Google Scholar]
- 158.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (ldl) receptor-deficient rabbits with homologous malondialdehyde-modified ldl reduces atherogenesis. Proc Natl Acad Sci U S A. 1995;92:821–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Freigang S, Horkko S, Miller E, Witztum JL, Palinski W. Immunization of ldl receptor-deficient mice with homologous malondialdehyde-modified and native ldl reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol. 1998;18:1972–1982 [DOI] [PubMed] [Google Scholar]
- 160.Zhu L, He Z, Wu F, Ding R, Jiang Q, Zhang J, et al. Immunization with advanced glycation end products modified low density lipoprotein inhibits atherosclerosis progression in diabetic apoe and ldlr null mice. Cardiovasc Diabetol. 2014;13:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kimura T, Tse K, McArdle S, Gerhardt T, Miller J, Mikulski Z, et al. Atheroprotective vaccination with mhc-ii-restricted apob peptides induces peritoneal il-10-producing cd4 t cells. American journal of physiology. Heart and circulatory physiology. 2017;312:H781–H790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fredrikson GN, Soderberg I, Lindholm M, Dimayuga P, Chyu KY, Shah PK, et al. Inhibition of atherosclerosis in apoe-null mice by immunization with apob-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003;23:879–884 [DOI] [PubMed] [Google Scholar]
- 163.Honjo T, Chyu KY, Dimayuga PC, Yano J, Lio WM, Trinidad P, et al. Apob-100-related peptide vaccine protects against angiotensin ii-induced aortic aneurysm formation and rupture. Journal of the American College of Cardiology. 2015;65:546–556 [DOI] [PubMed] [Google Scholar]
- 164.Wigren M, Kolbus D, Duner P, Ljungcrantz I, Soderberg I, Bjorkbacka H, et al. Evidence for a role of regulatory t cells in mediating the atheroprotective effect of apolipoprotein b peptide vaccine. Journal of internal medicine. 2011;269:546–556 [DOI] [PubMed] [Google Scholar]
- 165.Herbin O, Ait-Oufella H, Yu W, Fredrikson GN, Aubier B, Perez N, et al. Regulatory t-cell response to apolipoprotein b100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:605–612 [DOI] [PubMed] [Google Scholar]
- 166.Klingenberg R, Lebens M, Hermansson A, Fredrikson GN, Strodthoff D, Rudling M, et al. Intranasal immunization with an apolipoprotein b-100 fusion protein induces antigen-specific regulatory t cells and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:946–952 [DOI] [PubMed] [Google Scholar]
- 167.Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein b-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123:1083–1091 [DOI] [PubMed] [Google Scholar]
- 168.Tse K, Gonen A, Sidney J, Ouyang H, Witztum JL, Sette A, et al. Atheroprotective vaccination with mhc-ii restricted peptides from apob-100. Front Immunol. 2013;4:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kobiyama K, Vassallo M, Mitzi J, Winkels H, Pei H, Kimura T, et al. A clinically applicable adjuvant for an atherosclerosis vaccine in mice. Eur J Immunol. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gistera A, Hermansson A, Strodthoff D, Klement ML, Hedin U, Fredrikson GN, et al. Vaccination against t-cell epitopes of native apob100 reduces vascular inflammation and disease in a humanized mouse model of atherosclerosis. Journal of internal medicine. 2017;281:383–397 [DOI] [PubMed] [Google Scholar]
- 171.Wigren M, Bengtsson D, Duner P, Olofsson K, Bjorkbacka H, Bengtsson E, et al. Atheroprotective effects of alum are associated with capture of oxidized ldl antigens and activation of regulatory t cells. Circ Res. 2009;104:e62–70 [DOI] [PubMed] [Google Scholar]
- 172.Khallou-Laschet J, Tupin E, Caligiuri G, Poirier B, Thieblemont N, Gaston AT, et al. Atheroprotective effect of adjuvants in apolipoprotein e knockout mice. Atherosclerosis. 2006;184:330–341 [DOI] [PubMed] [Google Scholar]
- 173.Buscher K, Ehinger E, Gupta P, Pramod AB, Wolf D, Tweet G, et al. Natural variation of macrophage activation as disease-relevant phenotype predictive of inflammation and cancer survival. Nat Commun. 2017;8:16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bennett BJ, Davis RC, Civelek M, Orozco L, Wu J, Qi H, et al. Correction: Genetic architecture of atherosclerosis in mice: A systems genetics analysis of common inbred strains. PLoS genetics. 2016;12:e1005913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. The effects of lowering ldl cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM Jr., Kastelein JJP, et al. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. 2008;359:2195–2207 [DOI] [PubMed] [Google Scholar]
- 178.Schonbeck U, Libby P. Inflammation, immunity, and hmg-coa reductase inhibitors: Statins as antiinflammatory agents? Circulation. 2004;109:II18–26 [DOI] [PubMed] [Google Scholar]
- 179.Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, et al. Effect of two intensive statin regimens on progression of coronary disease. The New England journal of medicine. 2011;365:2078–2087 [DOI] [PubMed] [Google Scholar]
- 180.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. The New England journal of medicine. 2015;372:1489–1499 [DOI] [PubMed] [Google Scholar]
- 181.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. The New England journal of medicine. 2017;376:1713–1722 [DOI] [PubMed] [Google Scholar]
- 182.Sahebkar A, Di Giosia P, Stamerra CA, Grassi D, Pedone C, Ferretti G, et al. Effect of monoclonal antibodies to pcsk9 on high-sensitivity c-reactive protein levels: A meta-analysis of 16 randomized controlled treatment arms. Br J Clin Pharmacol. 2016;81:1175–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bohula EA, Giugliano RP, Leiter LA, Verma S, Park JG, Sever PS, et al. Inflammatory and cholesterol risk in the fourier trial. Circulation. 2018;138:131–140 [DOI] [PubMed] [Google Scholar]
- 184.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. Journal of the American College of Cardiology. 2013;61:404–410 [DOI] [PubMed] [Google Scholar]
- 185.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. The New England journal of medicine. 2017 [DOI] [PubMed] [Google Scholar]
- 186.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-dose methotrexate for the prevention of atherosclerotic events. The New England journal of medicine. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Taleb A, Tsimikas S. Lipoprotein oxidation biomarkers for cardiovascular risk: What does the future hold? Expert Rev Cardiovasc Ther. 2012;10:399–402 [DOI] [PubMed] [Google Scholar]