Abstract
Introduction:
Erectile dysfunction (ED) is a significant health concern that greatly impacts quality of life, and is common in men as they age, impacting 52% of men between the ages of 40 and 70. A significant underlying cause of ED development is injury to the cavernous nerve (CN), a peripheral nerve that innervates the penis. CN injury also occurs in up to 82% of prostatectomy patients. We recently showed that Sonic hedgehog (SHH) protein delivered by peptide amphiphile (PA) nanofiber hydrogel to the CN and penis of a prostatectomy model of CN injury, is neuroprotective, accelerates CN regeneration, improves erectile function ~60%, preserves penile smooth muscle 56% and suppresses collagen deposition 30%. This regenerative potential is substantial in an adult prostatectomy model (P120). However prostatectomy patients are typically older (61.5 ± 9.6 years) and our models should mimic patient conditions more effectively when considering translation. In this study we examine regenerative potential in an aged prostatectomy model (P200–329).
Methods:
The caudal portion of the pelvic ganglia (MPG) and CN were dissected from adult (n=11), and aged (n=13) Sprague Dawley rats, and were grown in organ culture 3 days. Uninjured and 2 day CN crushed MPG/CN were exposed to Affi-Gel beads containing SHH protein, PBS (control), or 5e1 SHH inhibitor. Neurites were quantified by counting the number of growth cones normalized by tissue perimeter (mm) and immunohistochemistry for SHH, patched1 (PTCH1), smoothened (SMO), GLI1–3, and GAP43 were performed.
Results:
SHH treatment increased neurites 3.5-fold, in uninjured adult, and 5.7-fold in aged rats. Two days after CN crush, SHH treatment increased neurites 1.8-fold in adult rats and 2.5-fold in aged rats. SHH inhibition inhibited neurite formation in uninjured MPG/CN but not in 2 day CN crushed MPG/CN. PTCH1 and SMO (SHH receptors), and SHH transcriptional activators/repressors, GLI1–3, were abundant in aged MPG/CN with unaltered localization. ROCK1 was induced with SHH treatment.
Conclusions:
Reintroduction of SHH protein in an aged prostatectomy model is even more effective in promoting neurite formation/CN regeneration than in the adult. The first 48 hours after CN injury are a critical window when growth factors are released, that impact later neurite formation. These studies are significant because most prostatectomy patients are not young and healthy, as with adult rats, so the aged prostatectomy model will more accurately simulate ED patient response. Understanding how neurite formation changes with age is critical for clinical translation of SHH PA to prostatectomy patients.
Keywords: Neurite, aging, cavernous nerve regeneration, prostatectomy, Sonic hedgehog
Summary Sentence:
Reintroduction of sonic hedgehog protein in an aged prostatectomy model is even more effective in promoting neurite formation/cavernous nerve regeneration than in the adult. These studies are significant because most prostatectomy patients are not young and healthy, as with adult rats, so the aged prostatectomy model will more accurately simulate erectile dysfunction patient response. Understanding how neurite formation changes with age is critical for clinical translation of sonic hedgehog delivery to prostatectomy patients.
Introduction
Erectile dysfunction (ED) is a significant health concern that greatly impacts quality of life, and is common in men as they age, impacting 52% of men between the ages of 40 and 70 [1] and 22% of men under 40 [2]. Current treatments, including PDE5i, are ineffective in 61–69% of patients with peripheral neuropathy of the cavernous nerve (CN), which provides innervation to the penis [3, 4]. Injury to the CN and associated major pelvic ganglia (MPG) occurs during radical prostatectomy surgery to treat prostate cancer, in diabetic patients, and in aging patients. When the CN is injured (crush, tension, resection injury), this causes extensive down stream remodeling in the corpora cavernosa of the penis. With loss of innervation smooth muscle undergoes abundant and intensive apoptosis in the first week after injury in rodent models [5, 6] followed by collagen induction [7], making the tissue unable to respond to normal neurotransmitter (nitric oxide) signaling mechanisms, and ED is the end result. Parallel changes in corpora cavernosal remodeling have been documented in prostatectomy and diabetic patients with ED [8], although the remodeling process takes place over a longer time so there is a greater window of opportunity for intervention.
Regeneration of the CN is a primary goal in prostatectomy patients, with up to 85% of patients suffering ED after surgery [9, 10]. Some factors are known to be decreased in the MPG/CN with CN injury, such as nNOS, neurturin, glial cell line-derived neurotrophic factor alpha2, artemin, neurotrophin-4, and cilliary neurotrophic factor (7 days after CN resection) [11]. Several other factors have been implicated to play a role in neurite outgrowth, a key regulatory step in regeneration of peripheral nerves. BDNF and VEGF promote neurite outgrowth from cultured MPG neurons [12], as does SHH [13]. The SHH pathway is essential for embryonic development of many organs and its continuing role in adult tissues is less well defined. SHH is abundantly expressed in normal adult urogenital organs including the penis and MPG/CN tissue that provides innervation [14]. SHH protein is abundantly expressed in MPG neurons and glia [6, 15], and SHH protein is decreased within a day in the MPG and CN of adult rats with CN crush injury [6]. SHH treatment of cultured MPG/CN that were either uninjured or CN crushed, caused abundant neurite outgrowth of penile projecting neurons [Dobbs et al., 2018]. This finding is supported by our previous studies in which in vivo SHH treatment with peptide amphiphile nanofiber hydrogels after CN injury was neuroprotective, promoted CN regeneration, and improved erectile function ~60% 6 weeks after CN injury [6, 16].
Factors that are upregulated or delivered in the first two days after CN injury have a profound effect on later sprouting potential [13]. Aging also greatly impacts the ability of the MPG neurons to support neurite outgrowth. Neurite growth from cultured MPGs derived from aged rats was not as robust as it was from MPGs from younger rats [L12], and neurite outgrowth in response to BDNF and VEGF was more robust in MPGs derived from young rats (6 months) than from aged rats (24 months) [12]. GFRα2 and nNOS mRNA expression levels in RT-PCR showed age-related decreases in 1–24 month old rats, and in situ hybridization showed that the number of GFRα2 positive neurons in MPG decreased with aging [17]. Morphology changes have also been observed in aged rat MPG including neuronal vacuolar degeneration with preserved nuclei [18]. In humans, pathological changes were identified after prostatectomy in pelvic plexus neurons including neuronophagia, neuron cell vacuolization, satellite cells vacuolization, cell pyknosis, and nageotte nodules. A number of these changes were increased with age [19]. In other organs such as skeletal muscle, SHH pathway signaling is impaired in aged mice with decreased upregulation of the pathway in response to injury [20–22]. We’ve shown that SHH protein decreases with age in the MPG/CN, with the precursor protein decreasing 37% and the active form 77% [6]. Since SHH signaling is important to maintain the architecture of the CN [16], increased age might affect not only cavernous tissue but also the neural plasticity of the CN related to erectile function [17].
The regenerative potential of SHH delivered by peptide amphiphile nanofiber hydrogel is promising and substantial in MPG/CN of an adult prostatectomy model (P120). However a P120 rat is comparable to a 20 year-old man, which is unlikely to develop ED. This model is state of the art in the ED field as most investigators study younger rats that have not finished penile development, and yet their findings are being considered equivalent to observations in ED patients. This occurs because of the higher cost and difficulty obtaining older rats, and no other investigators have performed penile postnatal development studies [14, 23, 17], so may not be aware of the model limitations. In our tissue bank, the average ED patient age ranges from 52–71 years with an average of 61.5 ± 9.6 years. This is consistent with average ED patient ages reported in the literature [1] and is equivalent to 1–2 rat years. It is important and innovative to accurately simulate ED patient conditions in our animal models to obtain clinically relevant information.
Thus we examine SHH pathway signing in an aged prostatectomy model that more accurately mimics ED patient conditions, and hypothesize that if the SHH receptors are intact in aged MPG/CN, that reintroduction of SHH protein to MPG/CN in an aged prostatectomy model would be more effective in promoting CN regeneration (neurite outgrowth) than in the adult. We will examine this in organ culture of uninjured, CN crushed, and SHH inhibited MPG/CN.
Materials and Methods
Animals:
Adult Sprague-Dawley rats postnatal day 115–120 (P115-P120, n=11) and aged Sprague Dawley rats (P200-P329, n=13) were obtained from Charles River (Wilmington, MA). The study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal care protocol was approved by the Office of Animal Care and Institutional Biosafety at the University of Illinois at Chicago and animals were cared for in accordance with institutional approval.
CN crush surgery:
MPG/CN were exposed and microforceps (size 0.02 × 0.06mm) were used to bilaterally crush the CN 30 seconds. This is a commonly used method of performing CN crush in rats [24, 25] and the extent and reproducibility of crush injury were previously validated in our laboratory [16], with a visible indent and change in color of the nerve apparent with crush injury. CN crushed rats were sacrificed after 2 days and were grown in organ culture.
Organ culture:
The MPG and attached CN were dissected from adult and aged Sprague Dawley rats. Only the caudal portion of the MPG, which innervates the penis was included in the culture. The pelvic, hypogastric and ancillary nerves, and the regions of the MPG that innervate the bladder, rectum and prostate, were excluded from culture. MPG/CN were placed in sterile culture plates containing 150 μl of reduced growth factor Matrigel (Corning Life Sciences 356231). Reduced growth factor Matrigel was used for this study so that the Martigel did not influence neurite formation. Affi-Gel beads (100–200 mesh, Bio Rad) were incubated overnight at 4°C with 1XPBS, SHH protein (25μl of a 1μg/μl solution, R&D Systems), or 5E1 SHH inhibitor (388μg/mL, Hybridoma Bank), and were placed on top of the Matrigel near the MPG/CN. Matrigel was gelled at 37°C for 5 minutes prior to adding RPMI media (Sigma) and an antibiotic cocktail containing Penicillin-Streptomycin-Glutamine (100X, Thermo Fisher Scientific). Culture plates were placed in an atmosphere controlled incubator (5% CO2) at 37°C for three days. Groups were uninjured adult and aged MPG/CN treated with PBS (control, n=6), SHH protein (n=6) and 5E1 SHH inhibitor (n=2), and CN crushed MPG/CN from adult and aged rats treated with PBS (n=9), SHH protein (n=6), and 5E1 SHH inhibitor (n=2). Additional aged rat MPG/CN including the hypogastric and pelvic nerves, underwent CN crush and after 2 days in vivo, were grown in organ culture with PBS (n=2) or SHH protein (n=2) as outlined above.
Quantification of neurites:
Neurites were quantified by counting the total number of growth cones visible for each tissue grown in organ culture by a blinded observer at 160X magnification, normalized by tissue perimeter length (mm). Photos of the MPG/CN were magnified on the computer screen until growth cones were visible, and growth cones in the entire tissue were counted and the perimeter was measured. Three to five photos for each tissue were counted and averaged. Images were obtained using an Olympus SZ-CTV dissecting microscope and Axiocam R1.2 Carl Zeiss digital camera. Representative photos for each group are presented in the figures.
Immunohistochemical analysis (IHC):
IHC was performed on frozen MPG/CN which were cut to 12μm thickness and were post fixed in acetone for 15 minutes at 4°C. OCT was removed with 1X PBS washes prior to blocking with 3% milk in PBS at 4°C for one hour. Sections were incubated overnight at 4°C with 1/100 goat polyclonal antibody against patched (PTCH1) and GLI1 (Santa Cruz), rabbit polyclonal antibody against smoothened (SMO, LSBio), GLI2 and GLI3 (Rockland), and mouse monoclonal antibodies against neuronal nitric oxide synthase (nNOS, Transduction Laboratories), β–III tublin (Abcam), growth associated protein 43 (GAP43, Chemicon), Rho-activated serine/threonine kinase (ROCK1, Transduction) and ROCK2 (Santa Cruz). Fluorescent secondary antibodies were chicken anti-goat 488, chicken anti-rabbit 594, and donkey anti-mouse 594 (Molecular Probes, 1/150). HRP secondary antibodies were 1/150 donkey anti-goat (Sigma-Aldrich), and mouse anti-rabbit (Santa Cruz). Control slides in which the primary antibody was omitted, were performed for all secondary antibodies, to ensure artifact staining was not present from the secondary antibodies. Sections were mounted using DPX Mounting media (Electron Microscopy Sciences, Hatfield, PA). Microscopy was performed using a Leica DM2500 microscope.
Statistical Analysis:
Statistical analysis was performed by ANOVA with a Scheffe’s posthoc test using the SPSS program. The results were reported ± the standard error of the mean. Results were significantly different if p≤0.05.
Results
Anatomy of the pelvic plexus:
Photos were taken of the pelvic plexus from adult (P115–120) and aged (P200–329) Sprague Dawley rats (Figure 1A). The pelvic plexus was identified. The CN appears flat and thin in the adult rat with a small, clearly defined MPG (Figure 1A). There is extensive vascular supply accompanying the neural tissue. In the aged rat, the CN is thicker and rounder in appearance (Figure 1A). The MPG is larger and less well defined. The vascular supply does not appear diminished with age.
Figure 1:

(A) Photos of the pelvic plexus from adult (P115–120) and aged (P200–329) Sprague Dawley rats. The CN is flat and thin in the adult rat with a small, clearly defined MPG. In the aged rat, the CN is thicker and rounder in appearance, with a larger, less well-defined MPG. The vascular supply does not appear diminished with age. (B) Magnified view of MPG treated with SHH protein, shows growing neurites with clearly visible growth cones at the tips and elongating fibers. (C) Neurites were confirmed with GAP43 (growth cones marker). 100–800X magnification.
Neurite formation in uninjured MPG/CN:
Organ culture was performed on MPG/CN taken from uninjured Sprague Dawley rats and tissues were treated with SHH. A highly magnified view of the MPG shows growing neurites with clearly visible growth cones at the tips and elongating fibers (Figure 1B, arrows indicate visible growth cones). In order to confirm the presence of neurites, we performed GAP43 (growth cones marker) immunohistochemical analysis. GAP43 was identified in elongating neurites of the CN (Figure 1C).
Comparison of neurite formation in uninjured/normal adult and aged rat MPG:
Uninjured/normal adult and aged MPG were grown in organ culture for 3 days with PBS (control) or SHH protein and neurites were quantified by counting the number of growth cones/mm of tissue. The number of neurites/mm increased 3.5 fold with SHH treatment in adult rats (249%, p=0.0001, Figure 2A and B). The number of neurites/mm increased 5.7-fold with SHH treatment in aged rats (468%, p=0.013, Figure 2A and B). There was no difference in neurite formation in adult and aged rats treated with PBS (control, p=0.085, Figure 2A and B). SHH treatment was 2.7-fold less in aged rats in comparison to SHH treated adult rats (63%, p=0.0.001, Figure 2A and B).
Figure 2:

Uninjured adult and aged MPG were grown in organ culture for 3 days with PBS (control) or SHH protein and neurites were quantified (A and B). The number of neurites/mm increased 3.5 fold with SHH treatment in adult rats (249%, p=0.0001). The number of neurites/mm increased 5.7-fold with SHH treatment in aged rats (468%, p=0.013). There was no difference in neurite formation in adult and aged rats treated with PBS (control, p=0.085). SHH treatment was 2.7-fold less in aged rats in comparison to SHH treated adult rats (63%, p=0.001). 800X magnification.
Comparison of neurite formation in 2 day CN crushed adult and aged rat MPG:
Adult and aged MPG that underwent CN crush were isolated after two days and were grown in organ culture for 3 days with PBS (control) or SHH protein and neurites were quantified. The number of neurites/mm increased 1.8 fold with SHH treatment in adult rats (82%, p=0.044, Figure 3A and B). The number of neurites/mm increased 2.5-fold with SHH treatment in aged rats (150%, p=0.030, Figure 3A and B). There was no difference in neurite formation in adult and aged rats treated with PBS (control, p=0.298) or SHH (p=0.0.197, Figure 3A and B).
Figure 3:

Adult and aged MPG that underwent CN crush and were isolated after two days were grown in organ culture for 3 days with PBS (control) or SHH protein, and neurites were quantified (A and B). The number of neurites/mm increased 1.8 fold with SHH treatment in adult rats (82%, p=0.044). The number of neurites/mm increased 2.5-fold with SHH treatment in aged rats (150%, p=0.030). There was no difference in neurite formation in adult and aged rats treated with PBS (control, p=0.298) or with SHH protein (p=0.197). Red line indicates enlarged region. 800X magnification.
SHH inhibition suppressed neurite formation:
Uninjured aged MPG/CN were grown in organ culture for three days with Affi-Gel beads delivering 5E1 SHH inhibitor or PBS control. SHH inhibition of uninjured MPG/CN had neurite formation in only one small portion away from the Affi-Gel beads containing SHH inhibitor (Figure 4A). In MPG that underwent CN crush and then were cultured after 2 days, SHH inhibition did not decrease neurite formation (Figure 4B), indicating that the first 48 hours after CN injury are a critical window when growth factors, including SHH, are released, that impact later neurite formation.
Figure 4:
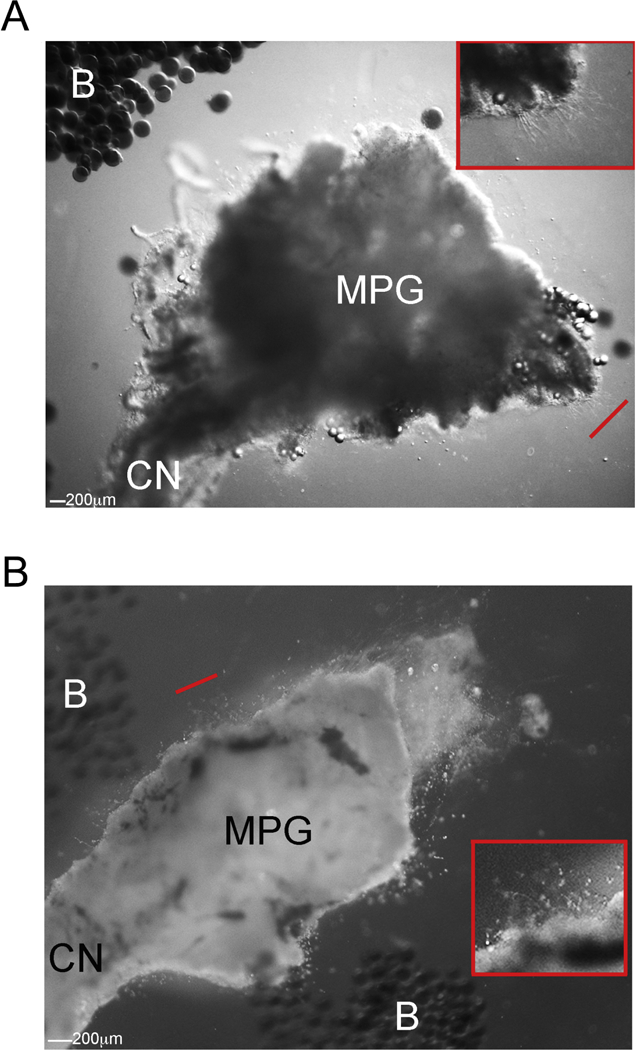
Uninjured (A) and 2 day CN crushed (B) aged MPG/CN were grown in organ culture for three days with Affi-Gel beads delivering 5E1 SHH inhibitor. SHH inhibition of uninjured MPG/CN had neurite formation in only one small region away from the Affi-Gel beads containing SHH inhibitor (A). In MPG that underwent CN crush and then were cultured after 2 days, SHH inhibition did not decrease neurite formation (B). 800X magnification.
Neurite formation occurs in all nerves of the pelvic plexus:
MPG from aged Sprague Dawley rats that underwent CN crush/MPG tension injury were dissected after two days and grown in organ culture for three days with PBS or SHH protein. Aged rat MPG responded to CN injury with neurite formation in the CN, and also in the pelvic nerve and hypogastric nerve, which innervate the bladder (Figure 5A). Aged pelvic plexus were responsive to SHH treatment (Figure 5B).
Figure 5:

MPG from aged Sprague Dawley rats that underwent CN crush/MPG tension injury were dissected after two days and grown in organ culture for three days with PBS (A) or SHH protein (B). Aged rat MPG responded to CN injury with neurite formation in the CN, and also in the pelvic nerve and hypogastric nerve, which innervate the bladder (A). Aged pelvic plexus were responsive to SHH treatment (B). 800X magnification.
Immunohistochemical analysis of MPG neurons:
IHC analysis was performed on uninjured adult and aged rat MPG that was grown in organ culture for 3 days. In aged rats, nNOS was less abundant by visual observation in MPG neurons that innervate the penis (Figure 6A). This is in keeping with previous observations in the literature. The SHH receptors, PTCH1 and SMO, are abundant in adult and aged rat MPG neurons that innervate the penis, with no apparent difference with age (Figure 6A). PTCH1, the binding part of the SHHH receptor, was identified in growing neurites (Figure 6B). This was confirmed with dual staining for β–III tublin, which is present in elongating neurites (Figure 6B) [26]. GLI, the transcriptional activator of the SHH pathway, has three isoforms which were all identified in glial cells surrounding MPG neurons of adult and aged rats (Figure 7), indicating that the SHH pathway is active in cultured MPG/CN.
Figure 6:
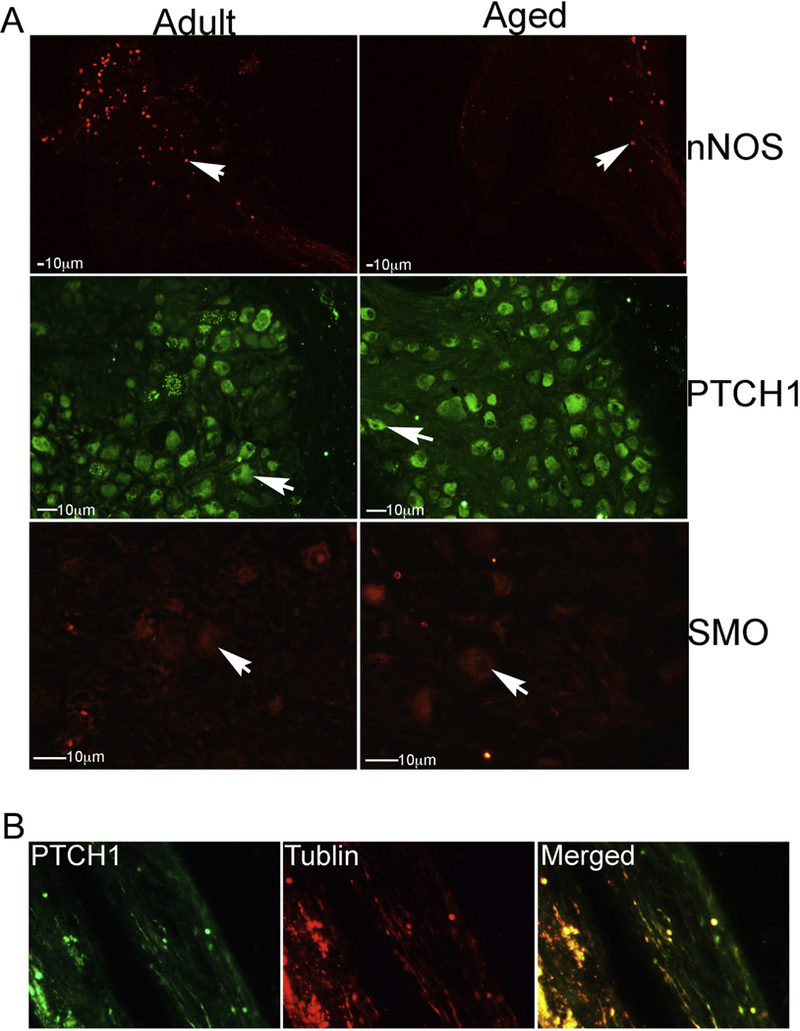
IHC analysis was performed in uninjured adult and aged rat MPG/CN that was grown in organ culture for 3 days. (A) In aged rats, nNOS was less abundant by visual observation in MPG neurons that innervate the penis. The SHH receptors, PTCH1 and SMO, were identified in adult and aged rat MPG neurons that innervate the penis, with no apparent difference with age. (B) PTCH1, the binding part of the SHHH receptor, was identified in growing neurites, as confirmed with dual staining for β–III tublin. Arrows indicate protein. 100–1000X magnification.
Figure 7:

IHC analysis was performed on uninjured adult and aged rat MPG/CN that was grown in organ culture for 3 days and were assayed for GLI1–3. All three GLI isoforms were identified in glial cells surrounding MPG neurons of adult and aged rats. Arrows indicate GLI1–3 proteins. 1000X magnification.
G-coupled proteins have been implicated as targets of SHH signaling in other organs. ROCK2 was abundant in uninjured MPG/CN treated with PBS and SHH (Figure 8A). ROCK1 was not identified in uninjured MPG/CN (Figure 8A), however ROCK1 was induced in response to SHH treatment (Figure 8A). ROCK2 was localized in MPG neurons, while ROCK1 was present in associated glial cells (Figure 8B).
Figure 8:
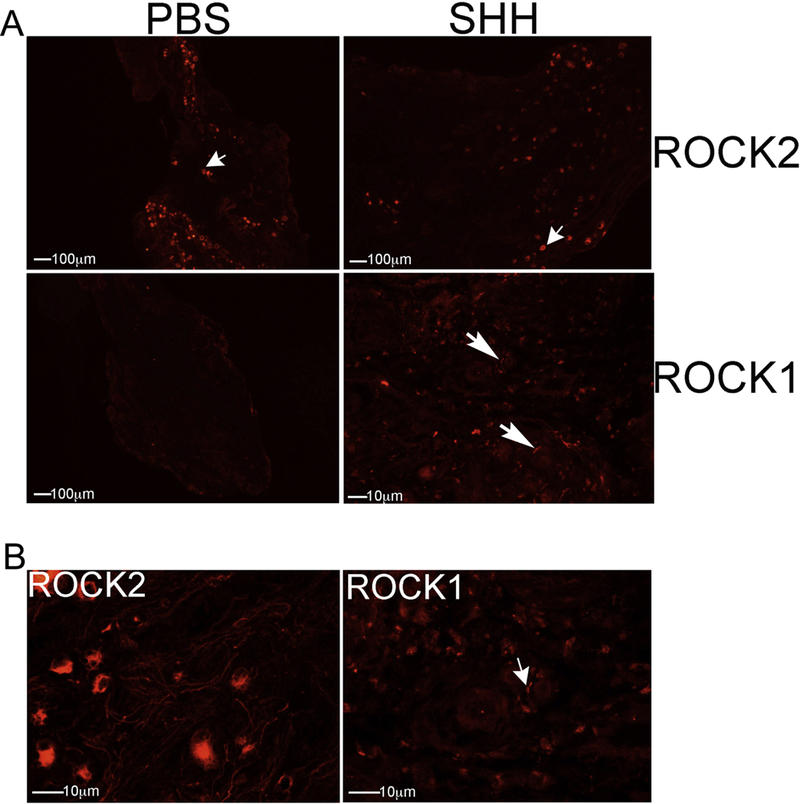
IHC analysis was performed on uninjured MPG/CN from aged rats that were treated with PBS or SHH protein. ROCK2 was abundant in uninjured MPG/CN treated with PBS and SHH (A). ROCK1 was not identified in uninjured MPG/CN (A), however ROCK1 was induced in response to SHH treatment (A). ROCK2 was localized in MPG neurons, while ROCK1 was present in associated glial cells (B). 50–400X magnification.
Discussion
Little is known about the role of SHH signaling in the development and adult maintenance of peripheral nerves, such as the CN. MPG neurons more than doubled between birth and adulthood [27] and the adult number of neurons was achieved in the MPG/CN by P7 for sympathetic neurons, and P21 for parasympathetic neurons. Much more is known about the role of SHH signaling in the central nervous system than the peripheral nerves. During early development, Shh induces the differentiation of dopaminergic neurons [28], and Shh, and its receptors, PTCH1 and SMO, are expressed in postnatal and adult hippocampal neurons [29]. Shh signaling regulates the structure and functional properties of presynaptic terminals of hippocampal neurons [29]. Shh and its receptors were expressed in adult dorsal root ganglia neurons, axons and glia and trended toward higher levels following axotomy injury [30]. In our studies of the MPG/CN, we have shown that SHH is abundantly expressed in penile projecting neurons and associated glia of the MPG/CN, SHH inhibition causes axonal degradation of CN fibers, SHH protein is upregulated in Schwann cells that surround the injury site in the CN to facilitate regeneration [31], there is decreased precursor and active SHH protein in the MPG/CN with CN injury [32], and decreased SHH protein with age in the MPG/CN (precursor protein decreasing 37% and the active form 77%) [16].
While the importance of Hh signaling in embryonic development is undisputed, the requirement for pathway activity in the adult organism is less well defined [22]. Hh signaling is activated in situations of tissue repair, a process considered less effective in older individuals [22]. In other tissues such as skeletal muscle, the SHH pathway is postnatally recapitulated after injury and during regeneration to regulate angiogenesis and myogenesis [20]. Hh signaling and the subsequent improvement in angiogenesis and myogenesis are impaired in aged mice and can be improved by introduction of exogenous Hh ligands [22]. Total Hh activity or its inducibility upon damage was significantly dampened in older animals, contributing to an overall phenotype with less regenerative capability [22]. In organ culture of both uninjured and CN crushed MPG/CN, we observed decreased neurite formation with age (Figure 2 and 3). CN injury increased neurite formation in both young and aged rat MPG/CN. Surprisingly, we identified that SHH treatment was more effective in promoting neurite formation in both uninjured and CN crushed MPG/CN of aged rats (1.6 and 1.4-fold increased with age) in comparison to adults (Figures 2 and 3). This is likely because the signaling machinery (PTCH1, SMO and GLI) are intact in aged rats, it is just the overall level of SHH protein that is decreased in MPG/CN [6]. In ischemic muscle repair in old mice, delay in repair was associated with an impaired upregulation of Gli1 [21], and SMO expression was significantly down regulated [21]. While a detailed quantitative analysis of the MPG/CN is not possible because of the minute tissue size (<100μ), we did not observe a difference in localization or apparent abundance of PTCH1 and SMO on visual observation of sections of the MPG/CN cultures, despite nNOS being clearly less abundant in the aged rat MPG/CN (Figure 6). The increased effectiveness of SHH treatment in promoting neurite formation in aged MPG/CN suggests that the nanofiber hydrogel delivery of SHH that we have previously developed for adult CN regeneration [16], will likely be even more effective in our aged prostatectomy model.
We previously showed that SHH, PTCH1 and SMO proteins are abundantly expressed in both MPG neurons and associated glial cells, with SHH mRNA only expressed in neurons by in situ hybridization [6]. Whereas GLI1–3, the transcriptional activators and repressors of the SHH pathway, are exclusively expressed in satellite glial cells surrounding penile projecting neurons of the MPG. This suggests that the glial cells are the target of SHH signaling from the associated neurons. Glial cells play a significant role in maintenance of MPG neurons and during regeneration. Glial cells are active partners in neuronal communication [33]. Bidirectional signaling selectively occurs between specific subpopulations of glia, neurons, and synapses [34] and is important for neuronal function. Local conditions influence how initial regenerative axon sprouts emerge from parent axons [32], while CN injury triggers a cascade of events in MPG neurons, including changes in expression of neurotransmitters, neurotrophic factors, cytokine production, and SHH pathway signaling [9, 5, 6]. Since glial cells undergo apoptosis prior to neuronal apoptosis following CN injury [35], they appear to be more sensitive to change in the MPG microenvironment.
The processes that are set into motion after CN injury are complex with a defined time frame for Wallerian degeneration. Separation of proximal and distal ends of the injury site occurs within 30 minutes [36] and degeneration of the distal segment begins within 24–36 hours [37]. Neurites initiate from the proximal end of damaged axons within 4 days [38, 39]. In uninjured MPG/CN cultured with SHH inhibitor, neurite outgrowth was inhibited (Figure 4). However if the CN was crushed and the tissue left in vivo for two days, later SHH inhibition did not affect neurite outgrowth (Figure 4). This finding suggests that the first 48 hours are an important window after CN injury in which growth factors are released that later impact neurite outgrowth. Ideally a therapy to regenerate the CN should be applied immediately in this early window when it has the opportunity to significantly impact later neurite outgrowth. Two days after CN injury, the process of neurite formation has already been set in motion and inhibiting SHH at this time cannot reverse the already primed neurite sprouting process.
ROCK1 and ROCK2 do not always have the same functions, and their activation can be isoform-specific [40]. We show that ROCK2 is normally expressed in MPG/CN, however ROCK1 is not normally expressed in cultured MPG/CN, but was up-regulated in response to SHH protein treatment (Figure 8). Depending on their subcellular localization, activation, and other environmental factors, ROCK signaling can have different effects on cellular function [40]. We observed different localization for ROCK1 and ROCK2 with ROCK2 present in MPG neurons and ROCK1 identified in associated glial cells. This is consistent with glial cells being the target of SHH produced in MPG neurons since GLI1–3, the transcriptional activators and repressor of the pathway, are expressed in glial cells (Figure 7). Non-canonical signaling of SHH involving Rho/ROCK has been suggested in other systems, including angiogenesis of endothelial cells [41], in astrocytes [42], and in microparticles carrying SHH [43]. The function of ROCK1 in this system requires further future investigation.
Conclusions
Reintroduction of SHH protein in an aged prostatectomy model is even more effective in promoting neurite formation/CN regeneration than in the adult. The first 48 hours after CN injury are a critical window when growth factors are released, that impact later neurite formation. These studies are significant because most prostatectomy patients are not young and healthy, as with adult rats, so the aged prostatectomy model will more accurately simulate erectile dysfunction patient response. Understanding how neurite formation changes with age is critical for clinical translation of SHH to prostatectomy patients.
Highlights.
Reintroduction of SHH protein in an aged prostatectomy model is even more effective in promoting neurite formation/CN regeneration than in the adult.
The first 48 hours after CN injury are a critical window when growth factors are released, that impact later neurite formation.
SHH pathway signaling machinery, including PTCH1, SMO and GLI proteins, remain intact in aged MPG/CN, and are able to respond to exogenous SHH protein.
These studies are significant because most prostatectomy patients are not young and healthy, as with adult rats, so the aged prostatectomy model will more accurately simulate ED patient response.
Understanding how neurite formation changes with age is critical for clinical translation of SHH PA to prostatectomy patients.
Acknowledgments
This project was supported by NIH/NIDDK Award number R01DK101536. Cell culture equipment was provided by the laboratory of Gail Prins, Ph.D.
Grant Sponsor:
NIH/NIDDK DK101536
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest:
Declarations of interest include iEDISON 0577703-18-0007, -0008, -0009
References
- 1.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994; 151: 54–61. [DOI] [PubMed] [Google Scholar]
- 2.Heruti R, Shochat T, Tekes-Manova D, Ashkenazi I, Justo D (2004) Prevalence of erectile dysfunction among young adults: results of a large-scale survey. J Sex Med 2004; 1: 284–291. [DOI] [PubMed] [Google Scholar]
- 3.Pace G, Del Rosso A, Vicentini C. Penile rehabilitation therapy following radical prostatectomy. Disabil Rehabil 2010; 32: 1204–1208. [DOI] [PubMed] [Google Scholar]
- 4.Perimenis P, Markou S, Gyftopoulos K, Athanasopoulos A, Giannitsas K, Barbalias G. Switching from long-term treatment with self-injections to oral sildenafil in diabetic patients with severe erectile dysfunction. European Urology 2002; 41: 387–391. [DOI] [PubMed] [Google Scholar]
- 5.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol 2003; 169: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 6.Angeloni N, Bond CW, Harrington D, Stupp S, Podlasek CA. Sonic hedgehog is neuroprotective in the cavernous nerve with rush injury. J Sex Med 2013; 10: 1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choe S, Veliceasa D, Bond CW, Harrington DA, Stupp SI, McVary KT, Podlasek CA. Sonic hedgehog delivery from self-assembled nanofiber hydrogels reduces the fibrotic response in models of erectile dysfunction. Acta Biomateralia 2016; 32: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angeloni NL, Bond CW, McVary KT, Podlasek CA. Sonic hedgehog protein is decreased and penile morphology is altered in prostatectomy and diabetic patients. PLOS ONE 2013; 8: e70985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendirci M, Hellstrom WJ. Current concepts in the management of erectile dysfunction in men with prostate cancer. Clin Prostate Cancer 2004; 3: 87–92. [DOI] [PubMed] [Google Scholar]
- 10.Emanu JC, Avildsen IK, Nelson CJ. Erectile dysfunction after radical prostatectomy: prevalence, medical treatments, and psychosocial interventions. Curr Opin Support Palliat Care 2016; 10: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hlaing SM, Garcia LA, Kovanecz I, Martinez RA, Shah S, Artaza JN, Ferrini MG. Sildenafil promotes neuroprotection of the pelvic ganglia neurons after bilateral cavernosal nerve resection in the rat. BJU Int 2013; 111: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin G, Shindel AW, Fandel TM, Bella AJ, Lin CS, Lue TF. Neurotrophic effects of brain-derived neurotrophic factor and vascular endothelial growth factor in major pelvic ganglia of young and aged rats. BJU Int 2010; 105: 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobbs R, Choe S, Kalmanek E, Harrington DA, Stupp SI, McVary KT, Podlasek CA. Peptide amphiphile delivery of sonic hedgehog protein promotes neurite formation in penile projecting neurons. Nanomedicine: Nanotechnology, Biology and Medicine 2018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Podlasek CA, Zelner DJ, Jiang HB, Tang Y, Houston J, McKenna KE, McVary KT. Sonic hedgehog cascade is required for penile postnatal morphogenesis, differentiation, and adult homeostasis. Biol Reprod 2003; 68: 423–438. [DOI] [PubMed] [Google Scholar]
- 15.Choe S, Bond CW, Harrington DA, Stupp SI, McVary KT, Podlasek CA. Peptide amphiphile nanofiber hydrogel delivery of sonic hedgehog protein to the cavernous nerve to promote regeneration and prevent erectile dysfunction. Nanomedicine: Nanotechnology, Biology and Medicine 2017; 13: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angeloni NL, Bond CW, Tang Y, Harrington DA, Zhang S, Stupp SI, McKenna KE, Podlasek CA. Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials 2011; 32: 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hisasue S, Kato R, Suetomi T, Kato K, Suzuki K, Kobayashi K, Itoh N, Kiyama H, Tsukamoto T. Age-related alteration of neurturin receptor GFRa2 and nNOS in pelvic ganglia. Neurobiol Aging 2006; 27: 124–1530. [DOI] [PubMed] [Google Scholar]
- 18.Golomb E, Scolnik M, Koren R, Servadio C, Sandbank U, Abramovici A. Effects of senescence and citral on neuronal vacuolar degeneration in rat pelvic ganglia. NeuroToxicology 2001; 22: 73–77. [DOI] [PubMed] [Google Scholar]
- 19.Rath-Wolfson L, Shvero A, Bubis G, Buzaverov G, Zeidman A, Ran E, Koren R. Morphological changes in peri-prostatic sympathetic ganglion cells in aging males. Molecular and Clinical Oncology 2017; 6: 713–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccioni A, Gaetani E, Neri V, Gatto I, Palladino M, Silver M, Smith RC, Giarretta I, Pola E. Hkatky L, Pola R Sonic hedgehog therapy in a mouse model of age-associated impairment of skeletal muscle regeneration. J Genontol A Biol Sci Med Sci 2014; 69: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renault MA, Robbesyn F, CHapouly C, Yao Q, Vandierdonck S, Reynaud A, Belloc I, Traiffort E, Ruat M, Desgranges C, Gadeau AP. Hedgehog-dependent regulation of angiogenesis and myogenesis is impaired in aged mice. Arterioscler Thromb Vasc Biol 2013; 33: 2858–2866. [DOI] [PubMed] [Google Scholar]
- 22.Lauth M Sonic the Hedgehog: A game about aging? Emerging evidence for anti-geriatric effects of Hedgehog signaling. Bioessays 2014; 36: 1128. [DOI] [PubMed] [Google Scholar]
- 23.Bond CW, Angeloni NL, Podlasek CA. Analysis of testosterone effects on sonic hedgehog signaling in juvenile, adolescent and adult Sprague dawley rat penis. J Sex Med 2010; 7: 1116–1125. [DOI] [PubMed] [Google Scholar]
- 24.Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP. Functional sequelae of cavernous nerve injury in the rat: is there model dependency. J Sex Med 2006; 3: 77–83. [DOI] [PubMed] [Google Scholar]
- 25.Nangle MR, Keast JR. Reduced efficacy of nitrergic neurotransmission exacerbates erectile dysfunction after penile nerve injury despite axonal regeneration. Exp Neurol 2007; 207: 30–41. [DOI] [PubMed] [Google Scholar]
- 26.Paden CM, Zhou X, Watt JA, Burton R, Pickett J, Oblinger MM. Coordinated upregulation of alpha 1- and beta II-tubulin mRNAS during collateral axonal sprouting of central peptidergic neurons. J Neurosci Res 1995; 42: 402–412. [DOI] [PubMed] [Google Scholar]
- 27.Yan H, Keast JR. Neurturin regulates postnatal differentiation of parasympathetic pelvic ganglion neurons, initial axonal projections and maintenance of terminal fields in male urogenital organs. J Comp Neurol 2008; 507: 1169–1183. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Pace J, Filichia E, Davis B, Hoffer B, Selman W, Luo Y. Effect of the sonic hedgehog receptor smoothened on the survival and function of dopaminergic neuros. Exp Neurol 2016; 283: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell N, Petralia RS, Currier DG, Wang YX, Kim A, Mattson MP, Yal PJ. Sonic hedgehog regulates presynaptic terminal size, ultrastructure and function in hippocampal neurons. J Cell Sci 2012; 125: 4207–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez JA, Kobayashi M, Krishnan A, Webber C, Christie K, Guo G, Singh V, Zochodne DW. Intrinsic facilitation of adult peripheral nerve regeneration by the sonic hedgehog morphogen. Experimental Neurology 2015; 271: 493–505. [DOI] [PubMed] [Google Scholar]
- 31.Bond C, Tang Y, Podlasek CA. Neural influences on sonic hedgehog and apoptosis in the rat penis. Biol Reprod 2008; 78: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Podlasek CA, Meroz CL, Tang Y, McKenna KE, McVary KT. Regulation of cavernous nerve injury-induced apoptosis by sonic hedgehog. Biol Reprod 2007; 76: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallina A, Ferrari M, Suardi N, Capitanio U, Abdollah F, Tutolo M, Bianchi M, Sacca A, Salonia A, Rigatti P, Montorsi F, Briganti A. erectile function outcome after bilateral nerve sparing radical prostatectomy: Which patients may be left untreated? J Sex Med 2012; 9: 903–8. [DOI] [PubMed] [Google Scholar]
- 34.Pace G, Del Rosso A, Vicentini C. Penile rehabilitation therapy following radical prostatectomy. Disabil Rehabil 2010; 32: 1204–8. [DOI] [PubMed] [Google Scholar]
- 35.Hehemann M, Choe S, Kalmanek E, Harrington D, Stupp SI, McVary KT, Podlasek CA. Pelvic and hypogastric nerves are injured in a rat prostatectomymodel, contributing to the development of stress urinary incontinence. Submitted Experimental Neurology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nature Medicine 2005; 11: 572–577. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Sanford MT, Xin Z, Lin G, Lue TF. Role of Schwann cells in the regeneration of penile and peripheral nerves. Asian Journal of Andrology 2015; 17: 776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sunderland S Nerves and nerve injuries. Edinburgh: Churchill Livingstone, 1978. [Google Scholar]
- 39.Lundborg G, Dahlin L, Danielsen N, Zhao Q. Trophism, trophism, and specificity in nerve regeneration. J Reconstr Microsurg 1994; 10: 345–354. [DOI] [PubMed] [Google Scholar]
- 40.Hartmann S, Ridley A, Lutz S. The function of Rho-Associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease. Front Pharmacol 2015; 6: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renault MA, Roncalli J, Tongers J, Thorne T, Klyachko E, Misener S, Volpert OV, Mehta S, Burg A, Luedemann C, Qin G, Kishore R, Losordo DW. Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells. J Mol Cell Cardiol 2010; 49: 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He QW, Xia XP, Chen SC, Wang Y, Huang M, Huang Y, Li JY, Li YN, Gao Y, Mao L, Mei YW, Hu B. Astrocyte-derived sonic hedgehog contributes to angiogenesis in brain microvascular endothelial cells via RhoA/ROCK pathway after oxygen-glucose deprivation. Mol Neurobiol 2013; 47: 976–987. [DOI] [PubMed] [Google Scholar]
- 43.Soleti R, Benameur T, Porro C, Panaro MA, Andriansitohaina R, Martinez MC. Microparticles harboring sonic hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis 2009; 30: 580–588. [DOI] [PubMed] [Google Scholar]


