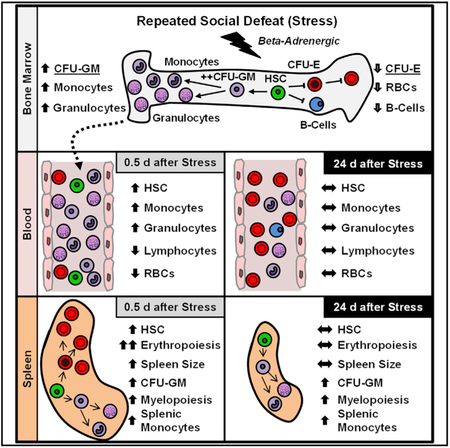
SUMMARY
Psychosocial stress accelerates myelopoietic production of monocytes and neutrophils that contributes to a variety of health complications ranging from atherosclerosis to anxiety. Here, we show that social stress in mice mobilizes hematopoietic stem progenitor cells (HSPCs) from the bone marrow that enter circulation, engraft into the spleen, and establish a persistent extramedullary hematopoietic depot. These splenic progenitors actively proliferate and differentiate into multiple cell types, including monocytes, neutrophils, and erythrocytes. Splenic erythropoiesis partially abrogates stress-induced anemia. Repeated injection with isoprenaline induces progenitor mobilization to the spleen, identifying a key role for β-adrenergic signaling. Moreover, protracted splenic production of CD11b+ cells persists for at least 24 days after the cessation of social stress. Thus, chronic stress establishes a persistent extramedullary hematopoietic depot that can modify a wide range of chronic disease processes and alter homeostasis of the bi-directional regulatory axis between the nervous and immune systems.
Graphical Abstract
In Brief
McKim et al. show that social stress enhances innate immune cell production in the bone marrow and mobilizes blood cell progenitors to the spleen, where they establish protracted ectopic production of innate immune cells. This represents a mechanism by which stress causes protracted changes in immunity and inflammation.
INTRODUCTION
Immunological adaptations contribute to the negative health outcomes associated with chronic psychological stress. Stress regulates immune cell inflammatory biology via descending neuroendocrine signaling pathways, including the hypothalamic pituitary adrenal (HPA) axis and the sympathetic nervous system (SNS). The effects of stress on the immune system are complex and depend on the nature, duration, and identity of the leukocytes in question (Wohleb et al., 2015; Reader et al., 2015). Nonetheless, some consensus has recently emerged from the human clinical, epidemiologic, and social genomics literature. Chronic social adversity in humans is associated with increased monocyte and neutrophil production (Heidt et al., 2014; Powell et al., 2013), exaggerated cytokine responses to leukocyte stimulation (Rohleder et al., 2009), insensitivity to the anti-inflammatory effects of glucocorticoids (Cohen et al., 2012; Rohleder, 2012), and impaired expression of genes involved in humoral immunity (Kiecolt-Glaser et al., 1996). This profile is consistent with the “conserved transcriptional response to adversity” (CTRA) that has been observed in leukocyte profiles of individuals experiencing chronic stress (Cole et al., 2011, 2015a; Powell et al., 2013; Miller et al., 2014). CTRA and stress-induced myelopoiesis are major predictors of disease outcomes, including atherosclerosis (Tawakol et al., 2017; Heidt et al., 2014), cancer (Antoni et al., 2016), and anxiety disorders (Kohrt et al., 2016; Wohleb et al., 2015). These observations demonstrate that immune adaptations to chronic stress are important mediators of the social determinants of health. For instance, Tawakol et al. (2017) reported that enhanced hematopoiesis in the bone marrow and spleen accounted for the majority of increased cardiovascular incidents in high-stress patients. Similarly, Heidt et al. (2014) showed that chronic variable stress in mice increased monocyte and neutrophil production in bone marrow that accelerated atherosclerotic plaque production. Immune adaptations to stress are also predictive of mental health outcomes. For instance, post-traumatic stress disorder (PTSD) symptom severity and progression are predicted by CTRA profiles (Kohrt et al., 2016).
The murine repeated social defeat stress model increases myelopoiesis and produces a CTRA profile similar to that observed in humans with low socioeconomic status (Powell et al., 2013). Using this model, we have found that stress-induced monocyte production contributes to prolonged and recurring anxiety-like responses in mice. For example, SNS activation during repeated social defeat stress increases monocyte production in the bone marrow, release into circulation, and accumulation in the brain (Wohleb et al., 2011; Hanke et al., 2012). Congruent with this, Heidt et al. (2014) found that sympathetic activation during chronic variable stress in mice also increased myeloid cell production and accumulation in atherosclerotic lesions. In our work, prevention of monocyte trafficking to the brain with sympathetic inhibition or CCR2 deficiency prohibited the induction of anxiety-like behavior and reduced neuroinflammatory signaling (Wohleb et al., 2011,2013; McKim et al., 2018). As with PTSD in humans (American Psychiatric Association, 2000), the impact of stress on immune function and inflammatory biology are protracted following social stress in mice. For example, immune and behavioral sensitization following repeated social defeat stress persisted for at least 24 days (Wohleb et al., 2014; McKim et al., 2016b). We found that this persistent vulnerability was mediated by a long-lasting pool of releasable monocytes in the spleen. This extended immunologic impact could not be explained by any extended change in bone marrow myelopoiesis (Wohleb et al., 2014), nor could this be explained by the persistence of a stress-induced bolus of long-lived monocytes (Yona et al., 2013; Patel et al., 2017). We hypothesized that the protracted oversupply of splenic monocytes may be mediated by the induction of extramedullary myelopoiesis in the spleen following stress. This hypothesis was supported by the observation that the sympathetic activation has been linked to the release of hematopoietic stem cells from their niches in the bone marrow (Méndez-Ferrer et al., 2008). In the present study, we sought to test this hypothesis and identify the biological mechanism of chronic stress-induced augmentation of myelopoiesis. Unlike existing reports on stress and bone marrow hematopoiesis (Heidt et al., 2014; Powell et al., 2013), results here show that social stress mobilized hematopoietic progenitor cells to the spleen, which established a persistent depot of erythromyeloid production. This discovery provides a mechanism by which transient stress exposure leads to a protracted influence on hematopoiesis.
RESULTS
Social Stress Enhances Myelopoiesis at the Expense of Lymphopoiesis and Erythropoiesis
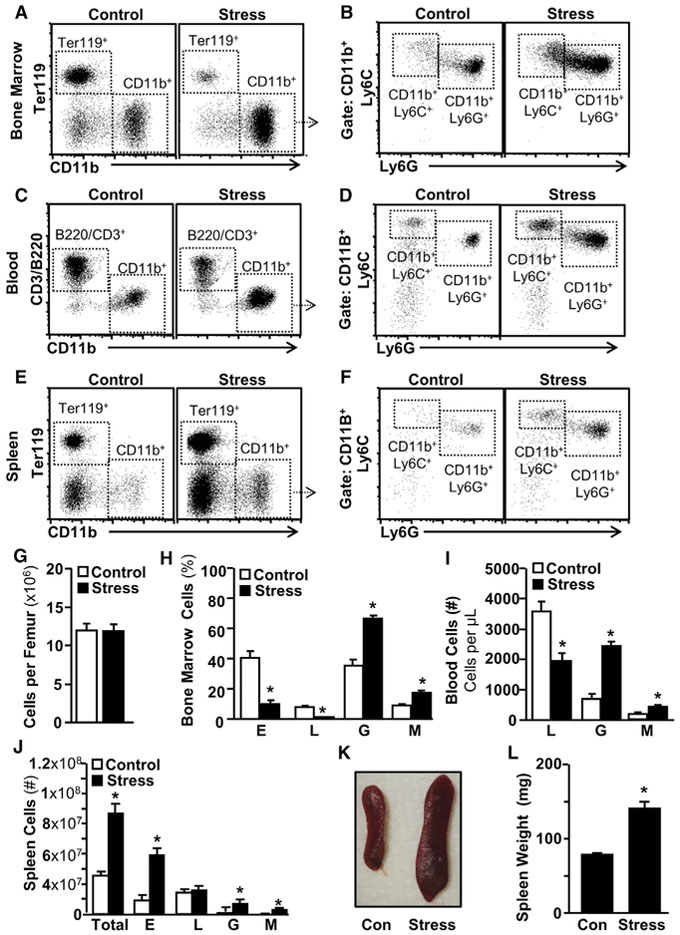
Based on our previous research documenting stress-mediated induction of a long-lasting pool of splenic myeloid cells (McKim et al., 2016b; Wohleb et al., 2014), the present study sought to delineate the effect of stress on hematopoiesis and investigate how myeloid cells home to and persist in the spleen long after stress cessation. To address this question, mice were subjected to the repeated social defeat model of social stress, and the percentage of erythrocytes (Es), lymphocytes (Ls), monocytes (Mos), and granulocytes (Gs) were determined in the bone marrow (BM), blood, and spleen 0.5 days later. This time point (0.5 days after the final cycle of stress) was chosen to avoid the detection of transient leukocyte dynamics that occur immediately following stress-induced neuroendocrine activation (Dhabhar et al., 2012). Figures 1A–1F show representative examples of the effect of social stress on Es (Ter119+), Mos (CD11b+/ Ly6C+), Gs (CD11b+/Ly6G+), and Ls (B220+ or CD3+) in BM (Figures 1A and 1B), blood (Figures 1C and 1D), and spleen (Figures 1E and 1F). Because stress does not alter total BM cellularity (Figure 1G), cells in BM are expressed as a percentage (Figure 1H). Stress increased MOs and Gs in the BM with a reciprocal reduction in Es (Ter119+) and Ls (p < 0.01 for each, Figure 1H). Similar effects were also observed in the blood (Figures 1C and 1D), which showed stress-dependent increases in Mos and Gs (p < 0.01) and a decrease in Ls (p < 0.01, Figure 1 I). Figures 1E and 1F show the effect of stress on spleen Es (Ter119+), Mos (CD11b+/Ly6C+), and Gs (CD11b+/Ly6C+). The total number of cells within the spleen was increased by ~2.6-fold after stress (Figure 1J), reflecting increased numbers of Es, Gs, and Mos (p <0.01, for each, Figure 1J). This increased spleen cellularity was associated with an ~2-fold increase in spleen size and mass (Figures 1K and 1L).
Figure 1. Social Stress Enhanced Myelopoiesis at the Expense of Lymphopoiesis and Erythropoiesis.
(A–F) Male C57BL/6 mice were exposed to 6 repeated cycles of social defeat stress (Stress) or left undisturbed as controls. BM, blood, and spleen samples were collected 0.5 days after the final cycle of stress. Representative bivariate dot plots show the labeling and gating strategies for (A and B) BM, (C and D) blood, and (E and F) spleen.
(G–J) Ter119+ erythrocytes (Es), B220+ or CD3+ lymphocytes (Ls), CD11b+/Ly6G+ granulocytes (Gs), and CD11b+/Ly6Chi monocytes (Mos) in (G and H) BM, (I) blood, and (J) spleen.
(K) Representative picture of spleen size.
(L) Spleen weight.
Data from (A)–(F) and (H)–(L) derived from 1 experiment (n = 6). Data in (G) derived from 2 experiments (N = 12). Bars represent means ± SEMs. Means with asterisks are significantly different from control (p < 0.05).
Social Stress Mobilizes Hematopoietic Progenitors to the Blood and Spleen
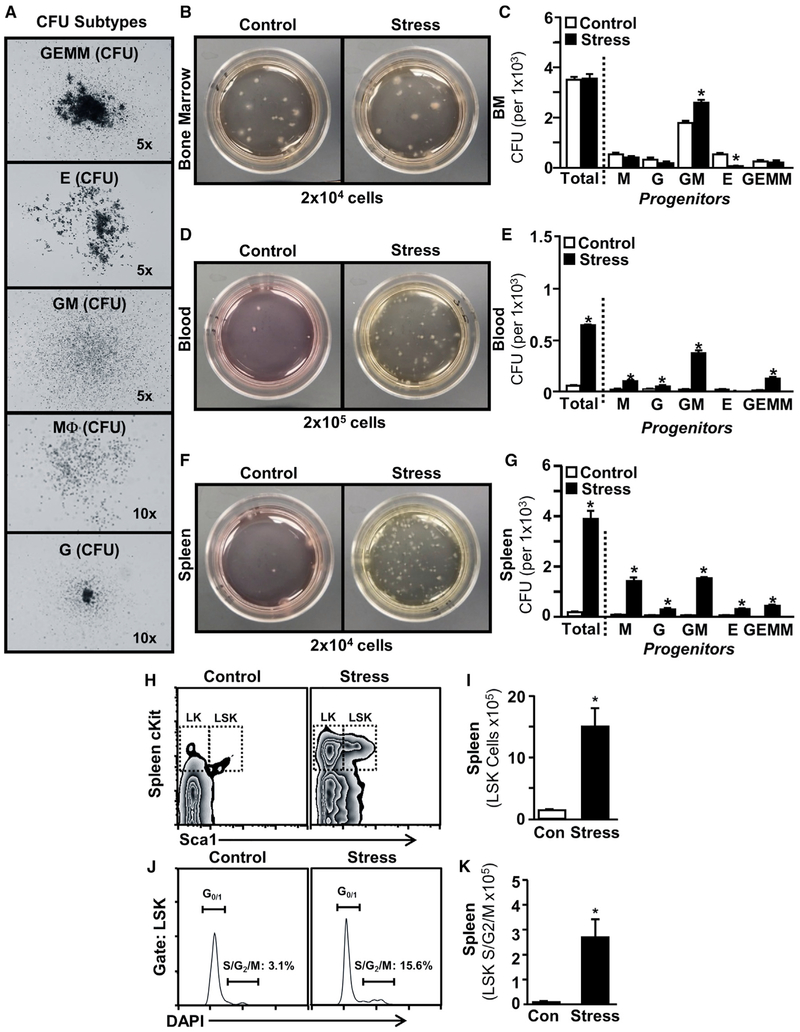
To determine whether stress-induced myelopoiesis stemmed from increased mobilization of hematopoietic progenitor cells, colony-forming units (CFUs) of hematopoietic progenitors were determined in the BM, blood, and spleen 0.5 days after stress (Figure 2). In this assay, non-lineage-restricted hematopoietic progenitors expanded into large multipotent granulocyte-erythrocyte-macrophage-megakaryocyte (GEMM) colonies, and lineage-restricted progenitors expanded into granulocyte-macrophage (GM), macrophage (M), G, and E colonies (Figure 2A). In the BM, the total number of CFUs was unchanged (Figures 2B and 2C), but GM-CFUs were increased at the expense of E-CFUs (p < 0.05 for each, Figure 2C). This is consistent with the shift toward Mo and G production in the BM after stress (Figure 1). In contrast, both blood and spleen showed a significant increase in the total number of hematopoietic progenitor cells following stress. M-, G-, GM-, and GEMM-CFU were robustly increased in both blood and spleen (p < 0.05 for each, Figures 2C–2F). Notably, there were few CFUs in the blood or spleen of control mice. In addition, social stress induced an ~5-fold increase in the number of splenic Lin−/cKit+/Sca1 + (LSK) cells (p < 0.05, Figures 2H and 2I) and a >5-fold increase in the proportion of splenic LSK cells that were proliferating (p < 0.05, Figures 2J and 2K).
Figure 2. Social Stress Mobilized Hematopoietic Progenitors to the Blood and Spleen.
Male C57BL/6 mice were exposed to 6 repeated cycles of social defeat stress (Stress) or left undisturbed as controls. BM, blood, and spleen samples were collected 0.5 days after the final cycle of stress.
(A–G) Representative images of (A) phenotyped colony forming units (CFUs) of cell lineages: GEMM, E, GM, Ms, and G. Representative pictures of CFUs collected and quantification of phenotyped CFUs are shown from the (B and C) BM, (D and E) blood, and (F and G) spleen of control and stress mice. In a separate experiment, male C57BL/6 mice were exposed to 6 repeated cycles of social defeat stress (Stress) or left undisturbed as controls. Spleen samples were collected 0.5 days after the final cycle of stress.
(H) Representative bivariate density plots show the labeling and gating strategy for detection of Lin−/cKit+/Sca1+ (LSK) cells in the spleen.
(I) Number of LSK cells in the spleen.
(J) Representative histogram of DAPIhi S-G2-M phase LSK cells.
(K) Number of proliferating S-G2-M phase LSK cells in the spleen.
Bars represent means ± SEMs. Data from (A)–(G) derived from 1 experiment, with n = 4, and data from (H)–(K) derived from 1 experiment, with n = 6. Means with asterisks are significantly different from control (p < 0.05).
Social Stress Increases the Recruitment of Hematopoietic Stem and Progenitor Cells to the Spleen
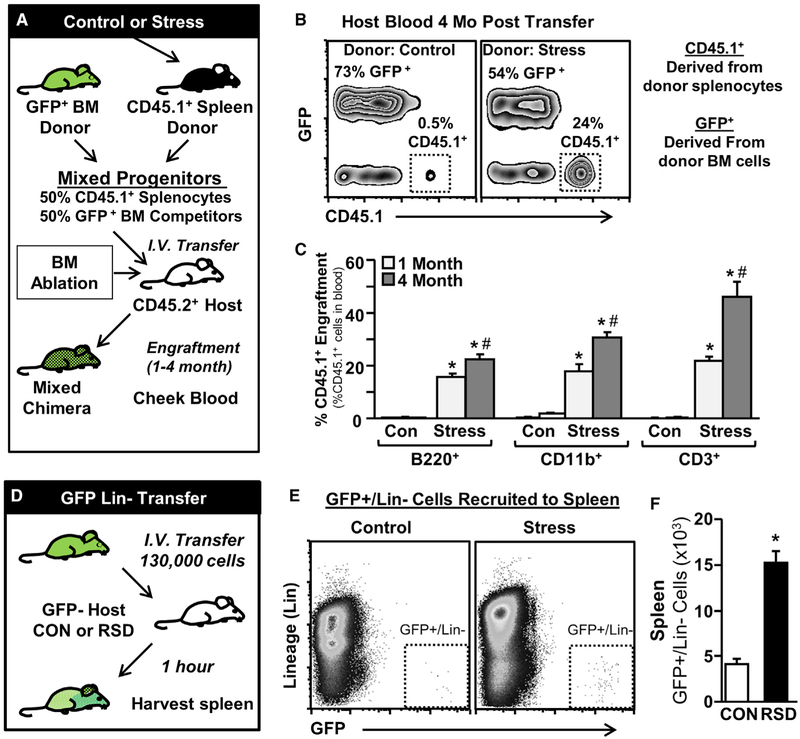
To determine whether the hematopoietic progenitors in the spleen following stress reflect bona fide multipotent and self-renewing hematopoietic stem and progenitor cells (HSPCs), a competitive repopulation assay was completed. To do this, GFP+ BM competitor cells were mixed with an equal number of CD45.1 + splenocytes from either control or stressed mice, and this mixture was intravenously (i.v.) transferred into mye-loablated CD45.2 host mice (Figure 3A). Splenocytes from stressed mice but not control mice were significantly enriched for HSPCs and were able to competitively engraft into host mice (Figure 3B). Engrafted HSPCs from stress spleens produced multi-lineage progeny that were detectable in the blood at 1 and 4 months post-transfer (Figure 3C), including B cells (B220+; F(1,23) = 162.3, p < 0.0001), Mos and neutrophils (CD11b+; F(1,23) = 110.9, p < 0.0001), and T cells (CD3+; F(1,23) = 77.1, p < 0.0001). Engraftment of HSPCs from stressed spleens progressively increased from 1 to 4 months post-transfer (stress × time interaction: B220 F(1,23) = 5.1, p < 0.05, CD11b F(1,23) = 6.9, p < 0.05, and CD3 F(1,23) = 9.9, p < 0.01). To test whether HSPCs were being recruited to the spleen from the blood, 130,000 GFP+ lineage-depleted cells were i.v. transferred into control and stress mice (Figure 3D), and recruitment of GFP+/Lin− cells to the spleen was assessed 1 hr later by flow cytometry (Figure 3E). Stress significantly increased the recruitment of GFP+/Lin− cells to the spleen (p <0.05, Figure 3F). Collectively, stress mobilized HSPCs into circulation and were actively recruited into the spleen.
Figure 3. Social Stress Increased Self-Renewing and Long-Lived HSPCs in the Spleen.
(A) Schematic representation of experimental design. CD45.1+ splenocytes from control or stress mice were mixed 1:1 with GFP+ BM competitor cells and were i.v. injected into CD45.2 myoablated host mice. Engraftment of donor cells was assessed in cheek blood 1 and 4 months after i.v. transfer.
(B) Representative bivariate density plots of GFP+ and CD45.1+ cells collected from cheek blood 1 month after i.v. transfer.
(C) The ratioofCD45.1 + cells to GFP+ cells (%)for each cell type (B220, CD11b, CD3) detected in the cheek blood from CD45.2 host mice at 1 or 4 months after i.v. transfer.
(D) Schematic representation of experimental design. Magnetic-activated cell sorting (MACS)-isolated GFP+Lin− BM cells were i.v. transferred into control or stressed mice 0.5 days after stress. Recruitment of transferred GFP+/Lin− cells to the spleen was assessed 1 hr later.
(E) Bivariate density plots of GFP+Lin− cells in the spleen.
(F) Number of GFP+Lin− cells that were recovered in spleen. RSD, repeated social defeat.
Data from (A)–(C) are derived from 1 experiment (n = 6–7), and data from (D)–(F) are derived from 2 experiments (N = 6). Bars represent means ± SEMs. Means with asterisks are significantly different from control (p < 0.05).
β-Adrenergic Signaling Enhances Myelopoiesis and Increases HSPCs in the Blood and Spleen
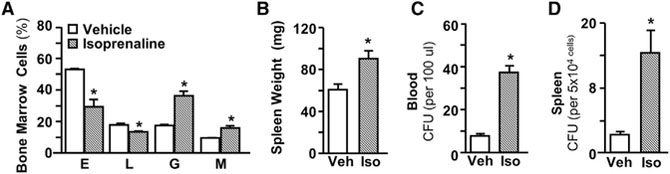
We have reported that stress-induced Mo accumulation is mediated by the activation of β-adrenergic receptors and is blocked by pharmacologic β-adrenergic antagonists (Hanke et al., 2012; Powell et al., 2013; Wohleb et al., 2011). To determine whether β-adrenergic signaling alone is sufficient to induce HSPC and myelopoietic activation, mice were treated with the pharmacologic β-agonist, isoprenaline (Iso; injected 2× daily at 10 mg/kg for 6 days). Iso increased BM production of Mos (M) and Gs, with a corresponding decrease in Ls and Es (Figure 4A, p < 0.05 for each). Iso also induced splenomegaly (Figure 4, p < 0.05) and increased CFUs in blood and spleen (Figures 4C and 4D, p < 0.05 for each).
Figure 4. β-Adrenergic Signaling Enhanced Myelopoiesis and Increased HSPCs in the Blood and Spleen.
Male C57BL/6 mice were injected i.p. twice daily with vehicle (Veh) or 10 mg/kg/day isoprenaline (Iso). BM, blood, and spleen samples were collected 0.5 days after the final injection.
(A) Percentage of TER119+ E, B220+ or CD3+ L, CD11b+/Ly6G+ Gs, and CD11b+/Ly6Chi Mos (M) in BM.
(B–D) Spleen weight is shown (B). Quantification of CFU in (C) blood and (D) spleen are shown.
Data from (A)–(D) derived from 2 experiments (N = 6). Bars represent means ± SEMs. Means with asterisks are significantly different from control (p < 0.05).
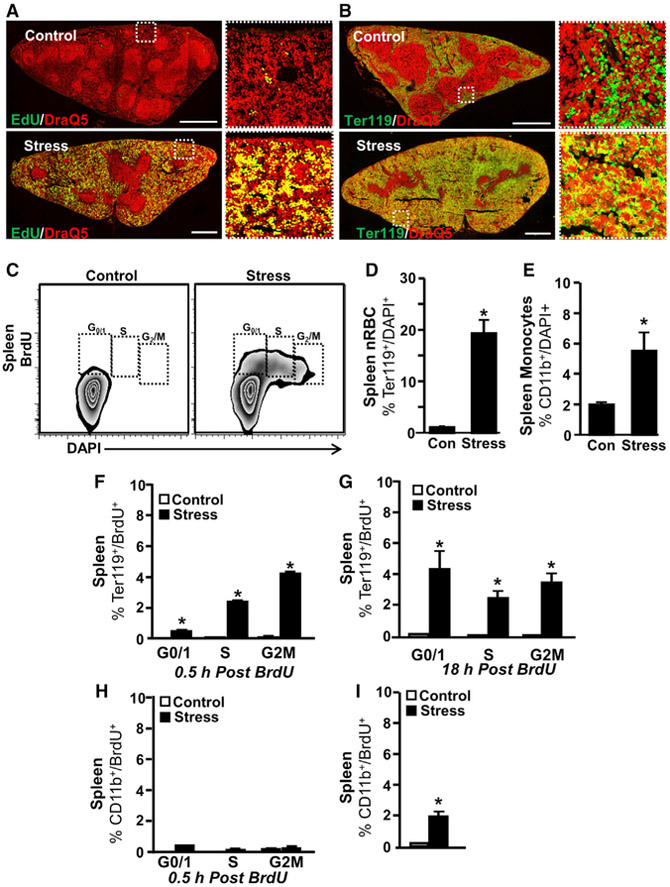
Social Stress Enhances Erythromyeloid Progenitor Proliferation in the Spleen
To identify the specific lineage differentiation mechanisms underlying the observed splenic myelopoiesis, mice were pulsed with ethynyldeoxyuridine (EdU) 0.5 hr before spleens were collected for sectioning and analysis. Notably, spleens from control mice had few EdU+ proliferating cells (Figure 5A, top and inset). The spleen from stressed mice, however, had large numbers of EdU+/DraQ5+ nucleated proliferating cells throughout the red pulp (Figure 5A, bottom). The size and distribution of these proliferating cells suggested that many of them were E progenitors. These cells, unlike mature Es, are nucleated. While control spleens contained a large number of DraQ5− non-nucleated Ter119+ mature Es (Figure 5B, top inset), spleens from stressed mice had a large number of DraQ5+/Ter119+ nucleated E progenitors throughout the red pulp (Figure 5B, bottom). DraQ5+/Ter119+ E progenitors in stressed spleens were located along the outside edge of dense clusters of nucleated cells, which had varying levels of Ter119 expression toward the center of the clusters (Figure 5B, inset). Ter119lo/− cells in the center of these clusters may be proliferating common myeloid-erythroid progenitors that have not yet become fully restricted to the E lineage.
Figure 5. Social Stress Enhanced Erythromyeloid Progenitor Proliferation in the Spleen.
Male C57BL/6 mice were exposed to 6 repeated cycles of social defeat (stress) or left undisturbed as controls. EdU (50 mg/kg) was injected intraperitoneally (i.p.) 0.5 days after stress and the spleen was collected 30 min later.
(A) Representative collages of spleen cross-sections for EdU (green) and DraQ5 nuclear (red) labeling.
(B) Representative collages of spleen cross-sections for Ter119 (green) and DraQ nuclear (red) labeling. White boxes highlight magnified regions, which are shown at right. White scale bars, 1 mm. In a separate experiment, mice were subjected to stress, as above. The spleen was collected 0.5 or 18 hr after injection with BrdU (50 mg/kg).
(C) Representative bivariate density plots of BrdU and DAPI labeling for cell-cycle analysis.
(D) Percentage of nucleated Es (Ter119/ DAPI+) in the spleen.
(E) Percentage of nucleated Mos (CD14/ DAPI+) in the spleen. Percentage of proliferating DAPI+ nucleated Es in the spleen (F) 0.5 hr and (G) 18 hr post-BrdU injection. Percentage of CD11b+/ BrdU+ cells in the spleen (H) 0.5 hr and (I) 18 hr post-BrdU injection.
Data derived from 2 experiments (N = 7–8). Bars represent means ± SEMs. Means with asterisks are significantly different from control (p < 0.05).
In bromodeoxyuridine (BrdU) pulse analysis of cell-cycle phase, social stress substantially increased the number of S-G2-M phase cells within the spleen (Figure 5C). Consistent with the above data, there was a significant increase in both Ter119+/DAPI+ nucleated red blood cells (nRBCs; Figure 5D, p < 0.05) and CD11b+ cells (Figure 5E, p < 0.05). Cell-cycle analysis shows that nucleated E progenitors in the spleen after stress were rapidly proliferating; > 50% of Ter119+/DAPI+ were BrdU+ at both 0.5 and 18 hr post-injection (Figures 5F and 5G). Stress increased the number of proliferated G0 BrdU+/CD11b+ cells in the spleen at 18 hr but not 0.5 hr post-injection (Figures 5H and 5I). These data are consistent with findings that Mos and neutrophils originate from asymmetric mitosis of CD11b− myeloid progenitors, and there is a lag before daughter cells upregulate mature myeloid cell markers such as CD11b (Hettinger et al., 2013). These data demonstrate that stress increased the extramedullary production of CD11b+ and Ter119+ cells in the spleen.
Splenic Erythropoiesis Partially Mitigates Anemia during Social Stress
To determine whether secondary erythropoiesis represents a physiological compensation to reduce stress-induced anemia, the effect of splenectomy on stress-induced changes in complete blood counts was assessed (Table 1). Social stress significantly reduced red blood cell count (F(1,11) = 109.9, p <0.0001), hemoglobin (F(1,11) = 159.7, p < 0.0001), and hematocrit levels (F(1,11) = 197.0, p < 0.0001) compared to controls. Moreover, each of these indicators of anemia was further exacerbated in splenectomized stress mice (red blood cell count [F(1,11) = 3.7, p = 0.08], hemoglobin [F(1,11) = 8.5, p < 0.05], and hematocrit [F(1,11) = 11.6, p < 0.01]) compared to controls.
Table 1. Splenic Erythropoiesis Partially Mitigated Anemia during Social Stress.
| Control |
Stress |
|||
|---|---|---|---|---|
| Blood | Sham | SPLX | Sham | SPLX |
| RBC (K/μL) | 9.8 ± 0.1 | 9.6 ± 0.3 | 7.2 ± 0.2* | 5.7 ± 0.3*,# |
| Hemoglobin (g/dL) | 14.9 ± 0.1 | 14.9 ± 0.4 | 11 ± 0.4* | 8.7 ± 0.3*,# |
| Hematocrit (%) | 45.7 ± 0.9 | 45.8 ± 0.9 | 34.3 ± 0.9* | 27 ± 0.9*,# |
| MCV (fl) | 46.6 ± 0.3 | 47.9 ± 0.7 | 47.9 ± 0.5 | 47.5 ± 0.9 |
| MC HGB (pg) | 15.1 ± 0.1 | 15.5 ± 0.2 | 15.4 ± 0.1 | 15.2 ± 0.2 |
| RBC distribution | 17.7 ± 0 | 18.6 ± 1.1 | 18.3 ± 0.5 | 19.9 ± 1 |
| Platelet (K/μL) | 1,300 ± 78 | 1,505.3 ± 153 | 1,279 ± 39 | 1,432.8 ± 112 |
| MPV (fl) | 5.3 ± 0.2 | 5.1 ± 0.1 | 5.1 ± 0.1 | 5.2 ± 0.1 |
| Retic % | 2.2 ± 0.1 | 2.7 ± 0.2 | 3.4 ± 0.8 | 5.3 ± 1.3 |
Male C57BL/6 mice were splenectomized (SPLX) or given sham surgery. Then, 14 days later, mice were exposed to 6 repeated cycles of social defeat (stress) or left undisturbed as controls. Blood samples were collected for automated complete blood counts 0.5 days after the final cycle of stress. Main effects of stress: red blood cell (RBC) count (F(1,11) = 109.9, p < 0.0001), hemoglobin (F(1,11) = 159.7, p < 0.0001), and hematocrit (F(1,11)= 197.0, p <0.0001). Stress × SPLX interaction effects: RBC (F(1,11) = 3.7, p = 0.08), hemoglobin (F(1,11) = 8.5, p < 0.05), and hematocrit (F(1,11) = 11.6, p<0.01). MCV, mean cell volume; MCHGB, mean cell hemoglobin; MPV, mean platelet volume. Numbers represent means ± SEMs. Data derived from 2 experiments (N = 6). Means with asterisks are significantly different from sham control (p < 0.05) and means with hash mark were significantly different (p < 0.05) from sham stress.
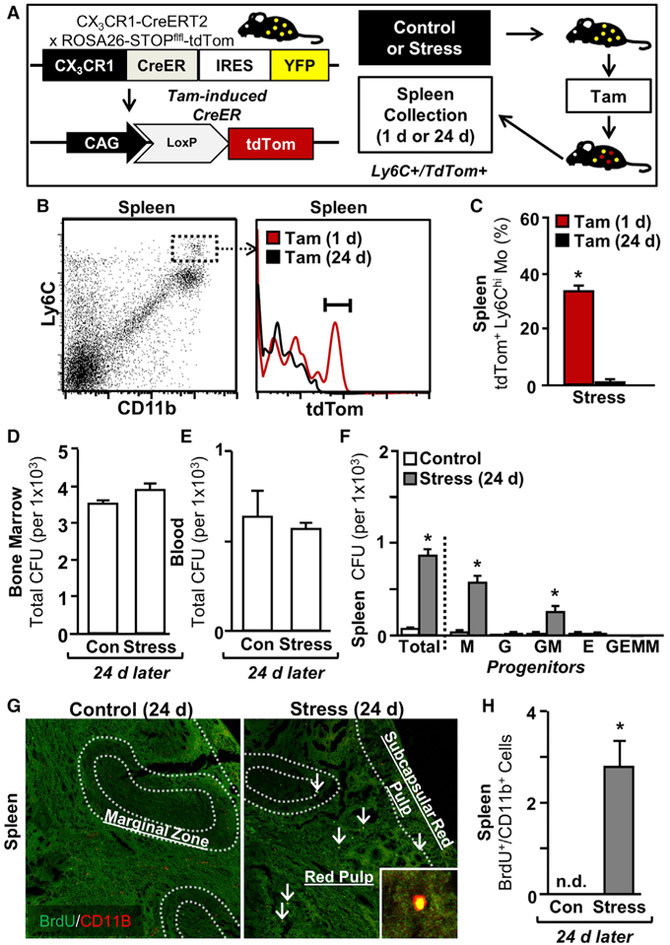
Persistence of Extramedullary Myelopoiesis 24 Days after Social Stress
We previously found that stress induces a releasable reservoir of splenic Mos that persisted for at least 24 days (McKim et al., 2016b; Wohleb et al., 2014), but it remains unclear whether this stems from ongoing myelopoietic output or increased myeloid cell lifespan. To address this question, a fate mapping study using CX3CR1-CreERT2/Stopflfl-tdTomato mice (Figure 6A) was completed in which Mos express yellow fluorescent protein (YFP) under the control of the CX3CR1+ promoter and CX3CR1+ cells are subsequently induced to permanently express tdTomato (tdTom) following injection of tamoxifen (Tam). Here, mice were subjected to social stress and then injected with Tam either 1 or 24 days later. Tam injection induced ~40% recombination efficiency in Mos, as tracked by tdTomato expression 24 hr post-injection (Figure 6B). tdTomato+ Mos were detected in the spleen 1 day after stress (p < 0.05), but they were not detected at 24 days later (Figures 6B and 6C). These data indicate that the Mos that remain in the spleen 24 days after stress were not the same ones generated initially with stress. Thus, these cells may be the result of the proliferation of the progenitor cells that engrafted in the spleen.
Figure 6. Persistence of Extramedullary Myelopoiesis 24 Days after Social Stress.
(A) Male CX3CR1(CreERT2)/ROSA26(STOPflfl-tdTom) were exposed to 6 repeated cycles of social defeat stress (Stress) or left undisturbed as controls. Next, mice were injected with Tam (1 mg i.p.) 1 or 23 days later. At the 24-day endpoint, spleens were collected and tdTomato expression was determined in Mos.
(B) Gating strategy for tdTomato+ Mos in the spleen.
(C) Percentage of tdTomato+ Mos in the spleen 24 days later.
(D–F) Quantification of CFUs in the (D) BM, (E) blood, and (F) spleen 24 days after social defeat. In a separate experiment, male C57BL/6 mice were subjected to 6 repeated cycles of social defeat (stress) or left undisturbed as controls. Next, mice were injected with BrdU 24 days after social defeat, and myeloid cell proliferation was determined in the spleen.
(G) Representative BrdU (green) and CD11b (red) labeling in the spleen.
(H) Number of CD11b+/BrdU+ cells per field of view in the spleen 24 days after stress.
Data from (B) and (C) are derived from 1 experiment (n = 6), (D)–(F) from 1 experiment (n = 3), and (G) and (H) from 1 experiment (n = 6). Bars represent means ± SEMs. Means with asterisks are significantly different from control (p < 0.05).
To confirm that a splenic Mo reservoir was maintained by extramedullary myelopoiesis after stress, CFU and BrdU pulse chase experiments were conducted at 24 days after cessation of stress. At this time point, the number of progenitors present in BM and blood did not differ as a function of stress (Figures 6D and 6E), but the splenic CFU remained highly elevated (Figure 6F, p < 0.0001). Increased total CFU stemmed primarily from the increased presence of GM- and M-CFU in the spleen (Figure 6F, p < 0.0001 for each). In a separate experiment, mice were pulsed with BrdU 24 days following the cessation of stress, and results showed a significant population of BrdU+/CD11b+ Mos in splenic red pulp that was not detected in control mice (Figures 6G and 6H, p < 0.05). These cells were likely locally produced because BM myelopoiesis had returned to baseline by this time point (Wohleb et al., 2014) and because of the short latency between pulse and tissue collection. Thus, the HSPC that engraft in the spleen after social stress maintain the capacity to produce myeloid cells for at least 24 days afterward.
DISCUSSION
Chronic stress is associated with increased Mo and neutrophil production (Wohleb et al., 2015) that can in turn contribute to an array of poor health outcomes, ranging from anxiety (Wohleb et al., 2013, 2014; McKim et al., 2016b, 2018) to atherosclerosis (Heidt et al., 2014; Tawakol et al., 2017) and metastatic cancer (Cole et al., 2015b; Antoni et al., 2006, 2016). Here, we identify a major biological mechanism of such effects by revealing that repeated social stress mobilizes BM HSPCs to circulate, engraft into the spleen, and establish a persistent extramedullary hematopoietic depot that selectively enhances the production of erythroid and myeloid lineage blood cells. Similar dynamics were observed after repeated pharmacologic stimulation of β-adrenergic receptors. This indicates a key role for SNS activation in mediating long-term hematopoietic adaptation to chronic stress. These findings are consistent with previous observations that linked other sources of chronic sympathetic activation, including myocardial infarction to induction of a splenic myeloid cell pool that can exacerbate inflammatory pathology (Emami et al., 2015; Dutta et al., 2012; Leuschner et al., 2012; Robbins et al., 2012). Furthermore, our previous studies document a diverse array of exacerbated pathologies associated with the persistent induction of Mo production following social stress. For instance, repeated social defeat stress exacerbated lupus nephritis (Aqel et al., 2017) and allergen-induced airway inflammation (Bailey et al., 2009) and promoted the reactivation of latent herpes simplex virus infections (Padgett et al., 1998). Moreover, social defeat stress increased susceptibility to endotoxic shock (Quan et al., 2001), caused increased lung inflammation (Curry et al., 2010), promoted allodynia and hyperalgesia (Sawicki et al., 2018), impaired learning and memory (McKim et al., 2016a), and prolonged anxiety (McKim et al., 2016b, 2016a). Our findings here significantly diverge from previous reports that stress transiently increased myeloid cell production in the BM (Heidt et al., 2014; Powell et al., 2013) by showing that stress also mobilizes HSPCs to the spleen that generate myeloid cells over protracted durations. Fundamentally, these results define the hematological basis for the pro-inflammatory effects of chronic stress and identifies targets for interventions to block those effects and protect health in the face of adverse social conditions.
Stress enhancement of BM myelopoiesis occurred at the cost of lymphopoiesis and erythropoiesis (Figure S1, top box). Our observation of suppressed B cell production may help explain previous observations of stress-induced reduction of humoral reactions to vaccines and infections (Kiecolt-Glaser et al., 1996). Similarly, the observed suppression of BM E production may contribute to stress-induced anemia and thereby explain clinical observations linking stress and trauma to increased incidence of anemia (Weisberg et al., 2002). Moreover, stress-induced suppression of BM erythropoiesis was accompanied by a robust expansion of E proliferation in the spleen (Figure S1, bottom left box) that constitutes the primary cellular driver of the stress-induced splenomegaly. Splenectomy significantly aggravated stress-induced anemia. These data indicate that the establishment of a splenic erythropoietic depot represents an adaptive response to compensate for stress-induced effects on BM hematopoiesis. Despite this, the pathways mediating stress-induced erythropoietic shifts are as yet unknown. However, stress-induced hypoferremia and anemia have been linked to the HPA-interleukin-6 (IL-6)-hepcidin axis (Wei et al., 2008; Zhao et al., 2008).
Our previous work shows that enhanced Mo trafficking from the spleen to the brain after acute stress contributes to recurring anxiety 24 days after exposure to repeated social defeat stress (i.e., stress sensitization). The relevance of this finding was that stress caused protracted immune sensitization that persisted well beyond the cessation of the stressor. Here, we identify the biological basis for that persisting enhancement of Mo trafficking in the establishment of a stable myelopoietic depot in the spleen. For instance, fate mapping confirmed that the Mos present in the spleen 24 days after social defeat stress were not the same ones generated earlier during stress exposure. Instead, these cells were recent myelopoietic progeny, and their elevated numbers likely stem from the ongoing activity of the hematopoietic system induced in the spleen by chronic stress (Figure S1, bottom). This suggests that persistent splenic generation of Mos contributes to recurring anxiety following acute stress (McKim et al., 2016b; Wohleb et al., 2014). For example, splenectomy either before or after stress blocked Mo trafficking and the recurrence of anxiety in stress-sensitized mice. Mo trafficking to the brain represents an independent axis of immune-to-brain signaling that contributes to negative behavioral adaptations to stress (Wohleb et al., 2015). For instance, increased perivascular Ms were observed in the brains of depressed individuals who committed suicide (Torres-Platas et al., 2014). The present results advance our understanding of those dynamics by establishing one hematological pathway by which social stress persistently alters circulating Mo population dynamics and thereby influences myeloid cell inflammatory signaling in the brain over an extended period.
Another important aspect of the present study is that our results are consistent with previous data indicating that β-adrenergic signaling is the key mediator of chronic stress effects on inflammation and myeloid cell population dynamics (Powell et al., 2013; Hanke et al., 2012; Heidt et al., 2014). Consistent with activation of the SNS, we previously reported that social stress increased norepinephrine in plasma and spleen (Hanke et al., 2012). Elevations in plasma persisted only 20 min, while elevations in spleen persisted >24 hr. Here, 6 days of isoprenaline injections (non-selective β-adrenergic agonist) was sufficient to induce a splenic extramedullary hematopoietic depot. Nonetheless, 1 or 3 days of isoprenaline injections had no effect (data not shown); thus, there is a requirement for chronic or repeated β-adrenergic signaling in driving extramedullary hematopoiesis in the spleen. This observation parallels our results using 6 days of repeated social defeat stress. Similarly, 1 and 3 days of social defeat stress were insufficient to increase spleen weight (Wohleb et al., 2014). The present agonist studies establish β-adrenergic signaling alone as sufficient to induce splenic myelopoiesis, and they complement previous studies with β-adrenergic antagonists that have identified adrenergic signaling as a necessary mediator of stress-induced myelopoiesis (Powell et al., 2013; Heidt et al., 2014; Hanke et al., 2012). Relevant to this, circadian fluctuations in HSPC circulatory dynamics (Lucas et al., 2008) were mediated by sympathetic tone in the BM (Méndez-Ferrer et al., 2008). In the chronic variable stress model, β3 adrenergic antagonists inhibited myelopoietic response to stress (Heidt et al., 2014). In addition, we have reported that neutralization of GM-colony-stimulating factor (CSF) similarly prevented increased myelopoiesis during social defeat stress (Powell et al., 2013) and that inhibition of corticosterone synthesis partially blocked stress-induced monopoiesis (Niraula et al., 2018). However, the specific role of adrenergic receptor subtypes was not assessed here. Ongoing studies are addressing the multifaceted neuroendocrine signaling pathways that mediate stress-induced myelopoiesis in the spleen and BM. It should be noted that stress-induced myelopoiesis is undoubtedly dependent on CNS threat appraisal. For instance, the blockade of central responses to social defeat stress by benzodiazepines completely prevented both stress-induced myelopoiesis and splenomegaly (Ramirez et al., 2016; McKim et al., 2018). These data support the conclusion that robust activation of the CNS threat appraisal circuitry is the primary source of the SNS outflow to the immune system (McKim et al., 2018). These results may explain recent data correlating human amygdalar positron emission tomography (PET) activity and self-reported stress with increased splenic hematopoiesis (Tawakol et al., 2017).
In summary, our work demonstrates that social stress dramatically skews medullary hematopoiesis to myelopoiesis, induces HSPC mobilization from the BM, and promotes long-lasting extramedullary hematopoiesis in the spleen (Figure S1). These findings provide a clear biological mechanism for understanding the complex effects of stress on hematologic and immune function, and they suggest targets for interventions to protect health against the risks associated with chronic stress and adverse social conditions.
STAR★METHODS
CONTACT FOR REAGENTS AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, John Sheridan (Sheridan.1@osu.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Male C57BL/6 (6–8 weeks old) and CD-1 (12 months old) mice were purchased from Charles River Breeding Laboratories (Wilmington, MA) and allowed to acclimate to their surroundings for 7–10 d before initiation of any experimental procedures. C57BL/6 mice were housed in cohorts of three and CD-1 mice were singly housed. CAG-EGFP (stock #006567), Cx3cr-CreER (stock #021160), Rosa26-Stopflfl-tdTom Ai9 (stock #007909), and CD45.1 (stock #002014) mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed in 11.5 × 7.5 × 6 inch polypropylene cages. Rooms were maintained at 21°C under a 12 h light/dark cycle with ad libitum access to water and rodent chow. All procedures were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
Repeated Social Defeat (stress)
In brief, an aggressive intruder male CD-1 mouse (retired breeder) was introduced into cages of established male cohorts (three per cage) of C57BL/6 mice for 6 consecutive nights between 1700 and 1900 (2 h). During each cycle, submissive behaviors including upright posture, fleeing, and crouching were observed to ensure that the resident mice showed subordinate behavior. If the intruder did not initiate a defeat within 5–10 min or was defeated by any of the resident mice, then a new intruder was introduced. At the end of the 2 h period, the intruder was removed and the residents were left undisturbed until the following day when the paradigm was repeated. Different intruders were used on consecutive nights. The health status of the mice was examined throughout the paradigm and injured or moribund mice were removed from the study. Consistent with previous studies using stress, < 5% of mice met the early removal criteria (Wohleb et al., 2013). Control mice were left undisturbed in their home cages. Tissue collection occurred between 0800 and 0930.
METHOD DETAILS
BrdU & EdU injections
Mice were injected i.p. with 50 mg/kg BrdU or EdU either 30 minutes or 18 hours prior to tissue collection.
Tamoxifen injections
Tamoxifen (2 mg) was injected i.p. into CX3CR1-CreERT2xROSA26-STOPflfl-tdTomato either on the last night of stress or 23 days after stress. tdTomato fluorescence in splenic monocytes was assessed by flow cytometry 24 days after the final cycle of stress.
Isolation of Cells from BM, Spleen, and Blood
Tissues were collected immediately following CO2 asphyxiation. Whole blood was collected with EDTA-lined syringes by cardiac puncture. Spleens were collected in ice-cold Hanks’ balanced salt solution (HBSS). Spleens were then scored with a surgical blade and gently pressed through a 70 uM strainer to obtain a single cell suspension. To collect bone marrow, the epiphyses of femurs were cut off and the marrow was flushed out with ice-cold HBSS. Cell pellets were washed, filtered through a 70-μM nylon cell strainer, and then the total number of cells was determined with a LUNA Automated Cell Counter (Logos, Anyang, South Korea).
Flow Cytometry
In brief, Fc receptors were blocked with anti-CD16/CD32 antibody (eBioscience). Cells were washed and then incubated with the appropriate antibodies for 40 minutes at 4°C. Nonspecific binding was assessed using isotype-matched antibodies. BrdU antibody labeling occurred following permeablization with 0.1% Triton-X, fixation with 1% paraformaldehyde, and incubation with DNAase at 37°C. Antigen expression was determined using a modified a DxP 9 (Cytek, Fremont, CA) Becton Dickinson FACSCalibur 9-color 3 laser cytometer (BD Biosciences, Franklin Lakes, NJ). DAPI fluorescence was analyzed with the violet laser, and DAPI-Widthhi doublet events were excluded from cell cycle analysis. Data were analyzed using FlowJo software (Tree Star) and positive labeling for each antibody was determined based on isotype stained controls.
Immunohistochemistry
Mice were transcardially perfused with cold saline followed by ice cold 4% PFA. Spleens were post fixed in 4% PFA at 4°C for 24 hours followed by 30% sucrose for 48 hours. Tissue was then embedded in OCT and flash frozen is super cooled isopentane and cryosectioned at 12 uM. Sections were incubated in primary antibodies (rat anti-CD11b clone M1/70, rat anti-ter119 clone ter119; rat anti-BrdU clone BU1/75) followed by appropriate fluorochrome conjugated secondary antibodies.
Colony-forming unit assay
Colony-forming unit (CFU) assay was completed using Methocult M3434 media (Stem Cell Technology) following the manufacturer’s protocol. Cells were resuspended in Iscove’s Modified Dulbecco’s Medium supplemented with 2% FBS. Colonies were counted using a low magnification inverted microscope.
Adoptive transfer
1 × 106 CD45.1+ splenocytes from control or stressed mice were injected together with 1 × 106 GFP+bone marrow competitor cells (from JAX strain 006567) intravenously into CD45.2 host mice 48 hours following myeloablation. Myeloablation was achieved by injecting CD45.2 mice with 30 mg/kg busulfan twice 24 hours apart. Relative engraftment of CD45.1+ splenocytes was assessed 1 and 4 months later via mandibular blood and flow cytometry.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical Analysis
To test for normal distribution, data were subjected to Shapiro–Wilktest using Statistical Analysis Systems (SAS) statistical software. To determine significant main effects and interactions between main factors, data were analyzed using two-way (stress × treatment) ANOVA using the General Linear Model procedures of SAS. When there was a main effect of experimental treatment or a treatment interaction effect, differences between group means were evaluated using a Bonferroni adjusted probability thresholds. Post hoc analysis results are depicted graphically in figures. All data are expressed as treatment means ± SEM.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rat anti-CD16/CD32 | BD Biosciences | 2.4G2 |
| Rat anti-CD11b | ThermoFisher | M1/70 |
| Rat anti-Ly6G | ThermoFisher | 1A8 |
| Rat anti-Ly6C | ThermoFisher | HK1.4 |
| Rat anti-TER119 | ThermoFisher | TER119 |
| Rat anti-CD3 | ThermoFisher | 17A2 |
| Rat anti-B220 | ThermoFisher | RA3-6B2 |
| Rat anti-cKit | ThermoFisher | 2B8 |
| Rat anti-Sca1 | ThermoFisher | D7 |
| Mouse anti-CD45.1 | ThermoFisher | A20 |
| Mouse anti-CD45.2 | ThermoFisher | 104 |
| Rat anti-BrdU | ThermoFisher | BU20A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Methocult | Stem Cell | GF M3434 |
| BrdU | Sigma | 19-160 |
| EdU | Sigma | 900584 |
| Busulfan | Sigma | B1170000 |
| Tamoxifen | Sigma | T5648 |
| 123count eBeads Counting Beads | eBioscience | 01-1234-42 |
| Isoprenaline | Sigma | I5627 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6NCrl | Charles River | 027 |
| Retired CD1 Breeder | Charles River | 022 |
| B6.SJL-Ptprca Pepcb/BoyJ | Jackson Laboratory | 002014 |
| C57BL/6-Tg(CAG-EGFP)1Osb/J | Jackson Laboratory | 003291 |
| B6.129P2(Cg)-Cx3cr1tm2.1(cre/ERT2)Litt/WganJ | Jackson Laboratory | 021160 |
| B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J | Jackson Laboratory | 007909 |
| Software and Algorithms | ||
| Flowjo software | Treestar | RRID: SCR_008520 |
| Equipment | ||
| DxP9 Cytometer | Cytek | Dxp9 |
| Luna Cell Counter | Logos Biosystems | L10001 |
| Microscope | Leica | DM5000B |
Highlights.
Social stress mobilizes hematopoietic progenitors from the bone marrow to the spleen
Splenic HSPCs proliferate and differentiate into multiple erythromyeloid cell types
Ectopic splenic erythropoiesis abrogates stress-induced anemia
Extramedullary production of CD11b+ cells persists for at least 24 days
ACKNOWLEDGMENTS
This study was supported by National Institute of Mental Health (NIMH) grants R01-MH-093473 and R01-MH097243 to J.F.S. and National Institute on Aging grants R01-AG033028 to J.P.G. and R01-AG043404 to S.W.C. D.B.M. was supported by National Institute of Dental and Craniofacial Research (NIDCR) Training Grant T32-DE-014320 and NIMH F31-MH109234. We thank The Ohio State University Comprehensive Cancer Center’s (OSUCCC) Analytical Cytometry Shared Resources.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes one figure and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.10.102.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- American Psychiatric Association; (2000). Diagnostic and Statistical Manual of Mental Health Disorders, Fourth Edition, Text Revision (American Psychiatric Association). [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, and Sood AK (2006). The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Cancer 6, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Bouchard LC, Jacobs JM, Lechner SC, Jutagir DR, Gudenkauf LM, Carver CS, Lutgendorf S, Cole SW, Lippman M, and Blomberg BB (2016). Stress management, leukocyte transcriptional changes and breast cancer recurrence in a randomized trial: an exploratory analysis. Psychoneuroendocrinology 74, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqel SI, Hampton JM, Bruss M, Jones KT, Valiente GR, Wu LC, Young MC, Willis WL, Ardoin S, Agarwal S, et al. (2017). Daily Moderate Exercise Is Beneficial and Social Stress Is Detrimental to Disease Pathology in Murine Lupus Nephritis. Front. Physiol. 8, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Kierstein S, Sharma S, Spaits M, Kinsey SG, Tliba O, Amrani Y, Sheridan JF, Panettieri RA, and Haczku A (2009). Social stress enhances allergen-induced airway inflammation in mice and inhibits corticosteroid responsiveness of cytokine production. J. Immunol. 182, 7888–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, and Turner RB (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. USA 109, 5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, and Cacioppo JT (2011). Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc. Natl. Acad. Sci. USA 108, 3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Capitanio JP, Chun K, Arevalo JM, Ma J, and Cacioppo JT (2015a). Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc. Natl. Acad. Sci. USA 112, 15142–15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, and Sood AK (2015b). Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 15, 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry JM, Hanke ML, Piper MG, Bailey MT, Bringardner BD, Sheridan JF, and Marsh CB (2010). Social disruption induces lung inflammation. Brain Behav. Immun. 24, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Malarkey WB, Neri E, and McEwen BS (2012). Stress-induced redistribution of immune cells–from barracks to boulevards to battlefields: atale ofthree hormones–Curt RichterAward winner. Psychoneuroendocrinology 37, 1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, et al. (2012). Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, Fayad ZA, Lehrer-Graiwer J, Korsgren M, Figueroa AL, et al. (2015). Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc. Imaging 8, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke ML, Powell ND, Stiner LM, Bailey MT, and Sheridan JF (2012). Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav. Immun. 26, 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, et al. (2014). Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, and Feuerer M (2013). Origin of monocytes and macrophages in a committed progenitor. Nat. Immunol. 14, 821–830. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, and Sheridan J (1996). Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc. Natl. Acad. Sci. USA 93, 3043–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt BA, Worthman CM, Adhikari RP, Luitel NP, Arevalo JM, Ma J, McCreath H, Seeman TE, Crimmins EM, and Cole SW (2016). Psychological resilience and the gene regulatory impact of posttraumatic stress in Nepali child soldiers. Proc. Natl. Acad. Sci. USA 113, 8156–8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, et al. (2012). Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 209, 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D, Battista M, Shi PA, Isola L, and Frenette PS (2008). Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell 3, 364–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, and Godbout JP (2016a). Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. J. Neurosci. 36, 2590–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, and Sheridan JF (2016b). Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol. Psychiatry 79, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, Ramirez-Chan K, Wang Y, Roeth RM, Sucaldito AD, et al. (2018). Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 23, 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Lucas D, Battista M, and Frenette PS (2008). Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447. [DOI] [PubMed] [Google Scholar]
- Miller GE, Murphy ML, Cashman R, Ma R, Ma J, Arevalo JM, Kobor MS, and Cole SW (2014). Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav. Immun. 41, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niraula A, Wang Y, Godbout JP, and Sheridan JF (2018). Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression. J. Neurosci. 38, 2328–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett DA, Sheridan JF, Dorne J, Berntson GG, Candelora J, and Glaser R (1998). Social stress and the reactivation of latent herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA 95, 7231–7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, et al. (2017). The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 214, 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, and Cole SW (2013). Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. USA 110, 16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PT, and Sheridan JF (2001). Social stress increases the susceptibility to endotoxic shock. J. Neuroimmunol. 115, 36–45. [DOI] [PubMed] [Google Scholar]
- Ramirez K, Niraula A, and Sheridan JF (2016). GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav. Immun. 51, 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, and Sheridan JF (2015). Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience 289, 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, et al. (2012). Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 125, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N (2012). Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems–2011 Curt Richter Award Winner. Psychoneuroendocrinology 37, 307–316. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Marin TJ, Ma R, and Miller GE (2009). Biologic cost of caring for a cancer patient: dysregulation of pro- and anti-inflammatory signaling pathways. J. Clin. Oncol. 27, 2909–2915. [DOI] [PubMed] [Google Scholar]
- Sawicki CM, Kim JK, Weber MD, Jarrett BL, Godbout JP, Sheridan JF, and Humeidan M (2018). Ropivacaine and Bupivacaine prevent increased pain sensitivity without altering neuroimmune activation following repeated social defeat stress. Brain Behav. Immun. 69, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, et al. (2017). Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 389, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, and Mechawar N (2014). Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun 42, 50–59. [DOI] [PubMed] [Google Scholar]
- Wei C, Zhou J, Huang X, and Li M (2008). Effects of psychological stress on serum iron and erythropoiesis. Int. J. Hematol 88, 52–56. [DOI] [PubMed] [Google Scholar]
- Weisberg RB, Bruce SE, Machan JT, Kessler RC, Culpepper L, and Keller MB (2002). Nonpsychiatric illness among primary care patients with trauma histories and posttraumatic stress disorder. Psychiatr. Serv 53, 848–854. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, and Sheridan JF (2011). β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci 31, 6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, and Sheridan JF (2013). Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci 33, 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, and Godbout JP (2014). Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry 75, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Sheridan JF, and Godbout JP (2015). Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci 8, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. (2013). Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Chen J, Wang W, Wang L, Ma L, Shen H, and Li M (2008). Psychological stress induces hypoferremia through the IL-6-hepcidin axis in rats. Biochem. Biophys. Res. Commun 373, 90–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.