Abstract
Purpose
Lymphedema is a potential complication of breast cancer treatment. This longitudinal substudy aimed to prospectively assess arm measurements and symptoms following neoadjuvant chemotherapy and axillary dissection in the ACOSOG/Alliance Z1071 trial to characterize the optimal approach to define lymphedema.
Methods
Z1071 enrolled patients with cT0–4, N1–2, M0 disease treated with neoadjuvant chemotherapy. All patients underwent axillary dissection. Bilateral limb volumes, circumferences, and related symptoms were assessed pre-surgery, 1–2 weeks post-surgery, and semiannually for 36 months. Lymphedema definitions included volume increase ≥10% or limb circumference increase ≥2cm. Symptoms were assessed by the Lymphedema Breast Cancer Questionnaire.
Results
In 488 evaluable patients, lymphedema incidence at 3-years by ≥10%-volume-increase was 60.3% (95% CI: 55.0%−66.2%) and by ≥2cm-circumference increase was 75.4% (95% CI: 70.8%−80.2%). Symptoms of arm swelling and heaviness decreased from post-surgery for the first 18 months and then were relatively stable. The 3-year cumulative incidence of arm swelling and heaviness was 26.0% (95% CI: 21.7%−31.1%) and 30.9% (95% CI: 26.3%−36.3), respectively. There was limited agreement between the two measurements (kappa 0.27) and between symptoms and measurements (kappa coefficients ranging from 0.05–0.09).
Conclusions
Lymphedema incidence by limb volume and circumference gradually increased over 36 months post-surgery, whereas lymphedema symptoms were much lower. These findings underscore the importance of prospective surveillance and evaluation of both limb measurements and symptom assessment. Lymphedema incidence rates varied by definition. We recommend that ≥10% volume change criterion be used for lymphedema evaluation for referral for specialist care.
Keywords: Lymphedema, Clinical trials, Neoadjuvant chemotherapy, Prospective surveillance breast cancer survivorship
Introduction
According to the American Cancer Society, there are approximately 2.9 million breast cancer survivors living in the United States and about one-third to one-half of these survivors are expected to develop upper extremity lymphedema during their lifetime [1]. The impact of lymphedema has largely negative consequences and it is still frequently unrecognized and only treated when more visible stages emerge [2]. It would be advantageous if lymphedema could be identified and diagnosed earlier so lymphedema care providers could initiate effective treatment [2].
Lymphedema is an accumulation of fluid with high protein concentrations in the interstitial spaces [3]. Lymphedema may be caused by a malformation or disruption of the lymphatic system, and can be classified as either primary or secondary [4]. Primary lymphedema can be congenital or develop at any time from puberty or adulthood from intrinsic causes [4]. Secondary or acquired lymphedema arising from extrinsic factors is more common than primary lymphedema. Surgery and radiation for treatment of cancer are the most common causes of secondary lymphedema [3].
Breast cancer-related lymphedema is usually related to specific treatment modalities, in particular axillary lymph node dissection or sentinel lymph node surgery, radiation and chemotherapy [5–8]. The onset of breast cancer-related lymphedema can be gradual or rapid, with 15%−54% of breast cancer survivors developing lymphedema within three years of surgery [9]. One prospective study reported that 75% of the breast cancer-related lymphedema cases were evident in the first year after surgery [10]. Lymphedema after breast cancer treatment can occur in the breast and chest wall, but it predominantly affects the arms. Once lymphedema itself is clinically apparent, various components of the lymphatic system may already be involved and the condition can become chronic [11]. If lymphedema is not appropriately addressed by evidence-based interventions, it can lead to progressive arm swelling, infection, and eventually tissue and neurologic changes [12]. Lymphedema results in not only significant negative consequences physically, but also psychologically, and lymphedema can profoundly negatively affect quality of life of survivors [12].
There are multiple measurement modalities that have been used to assess and diagnose lymphedema. In the past, water displacement volumetry and, most recently, perometry have been common methods to assess for lymphedema in patients presenting with limb swelling [12]. Both have limitations for routine clinical use, particularly related to clinic space limitations [12]. Serial circumferential limb measurements have been used most commonly to assess lymphedema, as this approach is widely available and has no specific space or equipment requirements. Circumferential measurements have been found to be both accurate and reliable when carried out by trained staff [13]. Moreover, studies have shown that self-reported assessment of symptoms (e.g., swelling, heaviness, redness, and tenderness) and limb function change (e.g., reduced range of motion) by breast cancer survivors can be an effective component of assessment for lymphedema [2,3,5,9,11,14]. Patient sensations have also been proposed as early indicators of lymphedema, and it is recommended that both self-reported sensations and limb measurement be assessed at each follow-up visit [2,15–17]. Symptoms of arm swelling and heaviness have been found to be significantly predictive of limb increase by circumferences in early studies [2].
The majority of studies of lymphedema after breast cancer treatment have focused on patients treated with surgical resection followed by adjuvant treatment. There is a paucity of prospective data on lymphedema rates in women treated with neoadjuvant chemotherapy. With increasing use of chemotherapy in the neoadjuvant setting, especially in node positive disease, this is an opportunity to prospectively evaluate lymphedema in a contemporary cohort all receiving systemic therapy prior to surgery. The American College of Surgeons Oncology Group (ACOSOG) Z1071 trial was designed to evaluate the role of sentinel lymph node (SLN) surgery in patients who presented with node positive breast cancer and were treated with neoadjuvant chemotherapy [18]. A lymphedema substudy was incorporated into the Z1071 trial to examine the incidence of lymphedema in breast cancer survivors following neoadjuvant chemotherapy and axillary dissection by measuring limb volume, circumferences, and reported symptoms of breast cancer survivors and to compare these methods of assessment for lymphedema. ACOSOG is now a part of the Alliance for Clinical Trials in Oncology.
Methods
Eligibility Criteria
The ACOSOG Z1071 study enrolled women older than 18 years of age with cT0-T4,N1–2,M0 breast cancer who had fine-needle aspiration or core needle biopsy of an axillary node documenting nodal metastasis at diagnosis, prior to neoadjuvant chemotherapy [18]. Patients were excluded if they had prior ipsilateral axillary surgery or prior SLN surgery/excisional lymph node biopsy for pathologic confirmation of axillary status. For the lymphedema substudy, patients who had bilateral breast cancer, current limb infection, lymphangitis, or any other condition that would affect testing were excluded. Only those with lymphedema data at baseline and at least one follow-up time-point after the post-operative period were included in this analysis. All patients were treated with neoadjuvant chemotherapy followed by breast surgery and SLN surgery with completion axillary dissection. The protocol was approved by each institutional review board of the participating sites and informed consent was obtained from all participants. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center.
Measurement and Assessment of Lymphedema
Limb measurements were taken on both arms at five anatomic locations: (1) the axilla, (2) halfway from the antecubital fossa to the axilla, (3) antecubital fossa, (4) halfway from the antecubital fossa to the wrist, and (5) the wrist, with volume calculated using the truncated cone formula [14]. Symptoms were assessed by the Lymphedema Breast Cancer Questionnaire (LBCQ) [2,19]. LBCQ is a validated and reliable self-report questionnaire to assess indicators of lymphedema [2]. There are 19 symptoms that are components of the LBCQ lymphedema assessment. Measurements were obtained and LBCQ symptom questionnaires were administered at the following time-points: prior to surgery (after completion of neoadjuvant chemotherapy); 1–2 weeks after surgery, and 6, 12, 18, 24, and 36 months post-surgery.
Lymphedema definitions used were volume increase ≥10% or limb circumference increase ≥2cm, as compared to baseline and/or the contralateral limb [14,19]. Both the volume measure and any 2cm measure were corrected for any change in the contralateral arm from baseline at the same time-point. Therefore, for the ‘any 2cm increase,’ the increase was calculated as (ipsilateral time point measurement – ipsilateral baseline measurement) – (contralateral time point measurement – contralateral baseline measurement). This number had to be 2cm or more to count as a 2cm increase. A similar calculation was done for the volume measure. Additionally, rates of LBCQ symptoms were assessed, with particular focus on arm swelling and heaviness.
Statistical analysis
The objective in this analysis was to estimate the incidence of lymphedema among patients undergoing axillary lymph node dissection. The level of agreement for determining whether a patient had developed lymphedema among the different measurements for lymphedema (arm volume, arm circumference, arm heaviness, arm swelling) was evaluated using Cohen’s kappa coefficient. A binomial estimate and 95% confidence interval (CI) were used to summarize the lymphedema rates at 2 weeks post-surgery. The cumulative incidence rates at later time-points did not include the 2-week post-surgery evaluations, as swelling at this time may be confounded with temporary post-surgical change. Cumulative incidence rates over time were determined with the Kaplan-Meier estimator. Cumulative incidence analyses were then repeated using death as a competing risk with minimal differences found (with results not shown). The database used for these analyses was locked May 1, 2013. Statistical analyses were carried out using SAS (SAS Institute Inc, version 9.2).
Results
Lymphedema data were available on 488 patients, 70% of the eligible patients in the parent study (N=701). The median age was 49 years (range 23–78). There were no differences in baseline characteristics between the patients included in this analysis and those not included. Applying the criterion of ≥10% limb volume increase, the cumulative lymphedema incidence at 36 months was 60.3% (95% CI: 55.0%−66.2%). Using the ≥2-cm circumference increase criterion, the cumulative lymphedema incidence was 75.4% (95% CI: 70.8%−80.2%) (Table 1). The cumulative incidence by the ≥2cm circumference criterion increased from 6 months to 3 years, with lymphedema rates consistently greater than that determined using the 10% volume change definition at the same time-points (Figure 1). The weighted kappa coefficient comparing lymphedema by volume increase ≥10% to lymphedema by circumference increase ≥2cm was 0.27 (95% CI: 0.18–0.36), indicating slight-to-fair agreement between the two criteria (Table 2).
Table 1.
Rates of lymphedema at study timepoints
| Baseline (rate) | 1–2 weeks (rate) | 6- month Cum Inc | 12month Cum Inc | 18-month Cum Inc | 24-month Cum Inc | 36-month Cum Inc | |
|---|---|---|---|---|---|---|---|
| ≥10% limb volume increase | --- | 73/365 (20.0%) | 4.6% (3.0% – 7.3%) 370 | 30.7% (26.4% – 35.8%) 241 | 45.0% (40.1% – 50.5%) 175 | 53.9% (48.8% – 59.5%) 126 | 60.3% (55.0% – 66.2%) 63 |
| ≥2-cm circumference increase | --- | 125/383 (32.6%) | 7.7% (5.5% – 10.8%) 372 | 45.1% (40.4% – 50.3%) 205 | 58.3% (53.5% – 63.5%) 141 | 69.8% (65.2% – 74.8%) 90 | 75.4% (70.8% – 80.2%) 52 |
| Arm heaviness symptom | 27/409 (6.6%) | 105/427 (24.6%) | 1.6% (0.7% – 3.4%) 380 | 12.0% (9.1% – 15.8%) 316 | 20.4% (16.6% – 25.0%) 261 | 22.6% (18.7% – 27.5%) 224 | 26.0% (21.7% – 31.1%) 130 |
| Arm swelling symptom | 13/411 (3.2%) | 100/426 (23.5%) | 2.1% (1.0% – 4.1%) 379 | 13.6% (10.5% – 17.6%) 309 | 23.2% (19.2% – 28.0%) 249 | 27.8% (23.4% – 32.9%) 203 | 30.9% (26.3% – 36.3%) 124 |
Cum Inc: cumulative incidence
Figure 1.
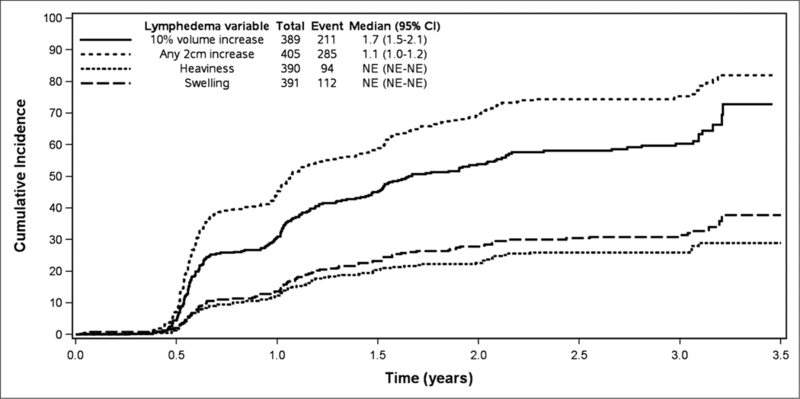
Cumulative incidence of ≥2cm circumference increase, ≥10% volume increase, self-reported heaviness, and self-reported swelling, adjusted for ipsilateral and contralateral limb change from baseline.
Table 2.
Concordance of lymphedema measures (≥10% volume increase and any ≥2 cm circumferential increase)
| Lymphedema by ≥10% volume increase |
Lymphedema by any location ≥2-cm increase | |
|---|---|---|
| Yes | No | |
| Yes | 178 (45.6%) | 33 (8.5%) |
| No | 103 (26.5%) | 75 (19.3%) |
Kappa=0.27 (95% CI: 0.18 – 0.36) p<0.0001
Self-reported symptoms peaked in the immediate post-operative period, then declined over the subsequent 18 months and remained relatively stable after 18 months. The 36-month cumulative incidence of lymphedema based on symptoms of arm heaviness was 26.0% (95% CI: 21.7, 31.1) and/or arm swelling was 30.9% (95% CI: 26.3, 36.3). Lymphedema incidence rates were significantly lower when using the definition based on arm heaviness and/or swelling symptoms compared to either arm circumference ≥2cm or arm volume ≥10% increase measurements. There was limited agreement, with weighted kappa coefficients ranging from 0.05 to 0.09, comparing volume ≥10% increase or circumference ≥2cm increase to reported symptoms of arm heaviness and/or arm swelling.
The median time to lymphedema development by ≥10% limb volume increase was 1.7 years (95% CI: 1.5–2.1) and by any ≥2 cm circumference increase was 1.1 years (95% CI: 1.0–1.2). The median was not reached on symptom report of heaviness or swelling since less than half of the women reported these symptoms cumulatively over the three years.
A variety of symptoms were reported from baseline through 36 months; the incidence rates of the 19 symptoms assessed by the LBCQ are shown in Table 3. Sixty-three percent (95% CI: 58.5–68.1) of participants reported ‘no symptoms’ at baseline (pre-operatively), however, 6.7% (95% CI: 5.4–8.0) experienced 6 or more symptoms at baseline (Table 4). At 1–2 weeks after surgery, 87.8% (95% CI: 84.6–91.0) of patients reported 1 or more symptoms, with 47.0% (95% CI: 42.1–51.9) reporting 6 or more symptoms. No symptoms were reported in 50.9% (95% CI: 44.2–57.5) at 36 months, whereas 13.6% (95% CI: 9.1–18.1) reported six or more symptoms at 36 months. Overall, presence of any symptoms gradually decreased from 87.8% (95% CI: 84.6–91.0) at the post-operative time-point to 49.1% (95% CI: 42.5–55.7) at 36 months. Of the 30 patients with six or more signs/symptoms at 36 months, only four (13%) had six or more signs/symptoms at baseline.
Table 3.
Self-reported lymphedema symptoms
| Baseline (rate) | 1–2 weeks (rate) | 6 -month Cum Inc | 12- month Cum Inc | 18-month Cum Inc | 24- month Cum Inc | 36-month Cum Inc | |
|---|---|---|---|---|---|---|---|
| Limited movement of shoulder | 23/413 (5.6%) | 188/430 (43.7%) | 3.6% (2.2% – 6.0%) 372 | 19.4% (15.8% – 23.9%) 288 | 25.0% (20.9% – 29.8%) 244 | 28.6% (24.2% – 33.7%) 202 | 32.5% (27.9% – 38.1%)1 22 |
| Limited movement of elbow | 13/411 (3.2%) | 56/430 (13.0%) | 1.5% (0.7% – 3.4%) 381 | 5.6% (3.7% – 8.4%) 338 | 8.5% (6.0% – 11.9%) 295 | 10.1% (7.4% – 13.8%) 245 | 12.2% (9.1% – 16.3%) 151 |
| Limited movement of wrist | 13/410 (3.2%) | 14/428 (3.3%) | 0.8% (0.2% – 2.4%) 384 | 6.9% (4.8% – 10.0%) 333 | 11.6% (8.7% – 15.4%) 285 | 13.3% (10.2% – 17.4%) 237 | 16.0% (12.5% – 20.6%) 144 |
| Limited movement of fingers | 43/409 (10.5% ) | 40/430 (9.3% ) | 1.3% (0.5% – 3.1% ) 378 | 9.4% (6.9% – 12.9%) 320 | 15.1% (11.8% – 19.3%) 267 | 18.1% (14.5% – 22.7%) 217 | 22.0% (17.8% – 27.1%) 124 |
| Does your arm or hand feel weak? | 50/409 (12.2%) | 155/430 (36.1%) | 5.2% (3.4% – 8.0%) 364 | 20.0% (16.4% – 24.5%) 266 | 29.4% (25.0% – 34.5%) 228 | 34.7% (30.0% – 40.1%) 185 | 37.8% (32.9% – 43.4%) 107 |
| Have you experienced breast tenderness? | 76/413 (18.4%) | 275/428 (64.2%) | 6.5% (4.5% – 9.6%) 355 | 22.8% (18.7% – 27.4% ) 276 | 31.5% (27.1% – 36.7% _ 221 | 35.2% (30.5% – 40.6% ) 178 | 38.0% (33.2% – 43.6% ) 105 |
| Have you experienced arm tenderness? | 37/411 (9.0% ) | 274/431 (63.6% ) | 4.7% (3.0% – 7.3%) 365 | 23.4% (19.4% – 28.1%) 272 | 31.6% (27.2% – 36.9%) 218 | 36.7% (31.9% – 42.1%) 177 | 40.6% (35.6% – 46.2%) 97 |
| Have you experienced redness? | 19/411 (4.6%) | 60/431 (13.9%) | 2.1% (1.0% – 4.1%) 373 | 8.6% (6.2% – 12.0%) 322 | 11.5% (8.6% – 15.2%) 279 | 12.8% (9.8% – 16.8%) 236 | 15.6% (12.2% – 20.1%) 147 |
| Have you experienced blistering? | 8/412 (1.9%) | 11/430 (2.6%) | 1.3% (0.5% – 3.1%) 379 | 4.5% (2.8% – 7.2%) 338 | 4.8% (3.1% – 7.6%) 304 | 4.8% (3.1% – 7.6%) 256 | 6.0% (4.0% – 9.2%) 161 |
| Have you experienced firmness/tightness? | 26/411 (6.3%) | 183/431 (42.5%) | 6.4% (4.4% – 9.3%) 364 | 32.8% (28.4% – 37.8%) 247 | 42.1% (37.4% – 47.5%) 195 | 45.8% (41.0% – 51.2%) 164 | 50.8% (45.7% – 56.4%) 98 |
| Have you experienced increased temperature in your arm? | 10/409 (2.4%) | 36/425 (8.5%) | 0.8% (0.3% – 2.4%) 382 | 3.5% (2.0% – 5.9% 341 | 6.7% (4.5% – 9.9% 297 | 7.0% (4.8% – 10.2% 295 | 9.0% (6.4% – 12.7%) 217 |
| Arm heaviness symptom | 27/409 (6.6%) | 105/427 (24.6%) | 1.6% (0.7% – 3.4%) 380 | 12.0% (9.1% – 15.8%) 316 | 20.4% (16.6% – 25.0%) 261 | 22.6% (18.7%- 27.5%) 224 | 26.0% (21.7% – 31.1%) 130 |
| Have you experienced numbness? | 54/408 (13.2%) | 243/426 (57.0%) | 6.2% (4.2% – 9.1%) 363 | 36.3% (31.7% – 41.8%) 230 | 47.7% (42.8% – 53.1%) 170 | 52.1% (47.1% – 57.6%) 140 | 55.9% (50.8% – 61.5%) 79 |
| Have you experienced stiffness? | 23/405 (5.7%) | 193/426 (45.3%) | 2.9% (1.6% – 5.1%) 372 | 19.6% (15.9% – 24.1%) 287 | 26.6% (22.4% – 31.6%) 239 | 29.9% (25.5% – 35.1%) 199 | 34.2% (29.4% – 39.7%) 121 |
| Have you experienced aching? | 53/411 (12.9%) | 201/428 (47.0%) | 3.1% (1.8% – 5.4%) 369 | 18.1% (14.6% – 22.5%) 292 | 27.7% (23.4% – 32.7%) 239 | 30.5% (26.1% – 35.7%) 205 | 33.2% (28.6% – 38.7%) 119 |
| Have you experienced chest wall swelling? | 7/410 (1.7%) | 79/426 (18.5%) | 1.6% (0.7% – 3.5%) 377 | 8.0% (5.7% – 11.3%) 325 | 11.9% (9.0% – 15.7%) 281 | 13.2% (10.1% – 17.3%) 234 | 15.2% (11.8% – 19.6%) 143 |
| Have you experienced breast swelling? | 18/413 (4.4%) | 127/428 (29.7%) | 2.1% (0.1% – 4.0%) 374 | 10.4% (7.7% – 14.0%) 316 | 14.6% (11.3% – 18.7%) 270 | 16.3% (12.8% – 20.6%) 227 | 18.3% (14.6% – 23.0%) 140 |
| Arm swelling symptom | 13/411 (3.2%) | 100/426 (23.5%) | 2.1% (1.0% −4.1%) 379 | 13.6% (10.5% – 17.6%) 309 | 23.2% (19.2% – 28.0%) 249 | 27.8% (23.4% – 32.9%) 203 | 30.9% (26.3% −36.3%) 124 |
| Have you experienced pockets of fluid development? | 8/407 (2.0%) | 82/423 (19.4%) | 2.6% (0.1% – 4.8%) 375 | 11.7% (8.9% – 15.5%) 316 | 17.0% (13.5% – 21.3%) 266 | 22.2% (18.2% – 27.1%) 216 | 26.2% (21.8% – 31.4%) 131 |
Table 4.
Patients reporting multiple lymphedema signs and symptoms
| Number of Symptoms | Baseline (rate) | 1–2 weeks (rate) | 6 -month Cum Inc | 12-month Cum Inc | 18-month Cum Inc | 24-month Cum Inc | 36-month Cum Inc |
|---|---|---|---|---|---|---|---|
| 1 or more | 142/387 (36.7%) | 353/402 (87.8%) | 14.8% (11.6% – 18.8%) 321 | 61.9% (57.2% – 67.1%) 139 | 76.2% (71.9% – 80.7%) 83 | 80.9% (76.9% – 85.1%) 61 | 83.3% (79.4% – 87.4%) 34 |
| 6 or more | 26/387 (6.7%) | 189/402 (47.0%) | 3.0% (1.7% – 5.3%) 359 | 16.9% (13.4% – 21.2%) 285 | 24.2% (20.1% – 29.1%) 235 | 28.2% (23.8% – 33.5%) 198 | 31.5% (26.8% – 37.1%) 113 |
Discussion
The current study reports on prospective surveillance of lymphedema using limb volume and circumference measurements and self-reported symptoms from patients enrolled in ACOSOG Z1071. There were 70% of the participants from the parent study who enrolled in the lymphedema substudy and they had similar clinical and pathologic characteristics as the patients who did not enroll. This rigorous prospective study design from pre-operative baseline through 3 years of survivorship is necessary for enhancing the understanding of breast cancer treatment sequelae, such as lymphedema. This study provides evidence of lymphedema occurrence and symptom experience in patients treated with neoadjuvant chemotherapy and axillary dissection. The overall findings of this study show that at 36 months post-surgery, lymphedema incidence was: 60.3% (95% CI: 55.0%−66.2%) by the criterion of ≥10% limb volume increase; and 75.4% (95% CI: 70.8%−80.2%) by ≥2cm circumference increase criterion. Arm heaviness and arm swelling had a 3-year 25–31% cumulative incidence, respectively. Lymphedema symptoms were relatively stable after 18 months.
As breast cancer management becomes more targeted and less invasive, it is anticipated that rates of lymphedema will decrease. Our ability to standardize measurement approaches and timelines, as was done in this trial, will allow for comparisons of the impact of treatment on the development of lymphedema across studies.
The cumulative incidences of post-operative breast cancer lymphedema are higher than some reported studies. We posit several reasons for these findings, including the homogeneous patient population and the rigorous measurement protocols. The patient population was a high-risk group of women who all had node-positive disease at baseline, were treated with neoadjuvant chemotherapy, and had complete axillary node dissection. We instituted rigorous measurement protocols with baseline measures for comparison and we followed patients over 36 months, a longer time than some other studies have reported. We also used multiple measures, which have been shown to contribute to a range of findings. We included assessment of pre-operative baseline symptoms following neoadjuvant chemotherapy, which has not been commonly reported in the literature. However, lack of baseline measurements prior to neoadjuvant chemotherapy means we are unable to discern whether patients were experiencing one or more lymphedema symptoms associated with having received chemotherapy.
The position paper by the National Lymphedema Network on screening and measurement for early detection and treatment of breast-cancer related lymphedema is cited as a resource in the manual for certification of breast centers by the American College of Surgeons’ National Accreditation Program for Breast Centers [20,21]. Although the guidelines developed by experts in the field are deliberately flexible, prospective surveillance of limb volume and lymphedema-associated symptoms, preferably beginning at pre-operative baseline, is the recommended standard of care for breast cancer patients. Clinical trials provide the opportunity to standardize timing and method of measurement across sites.
Early identification of breast cancer-related lymphedema is needed to minimize the impact of lymphedema on function and quality of life. Providing patient education information on lymphedema signs and symptoms to post-surgical patients can be part of management to enhance early diagnosis of lymphedema [11,19]. Screening for modest arm volume changes is common practice before ordering interventions such as complete decongestive therapy [14,22,23]. If there is a 200-ml volume increase, a 10% limb volume increase, or a 2-cm circumferential difference compared to baseline or the contralateral limb, the usual standard for diagnosis of lymphedema has been met [10,14]. Moreover, if limb volume is increased even 5%, it will often be associated with increased report of swelling and heaviness, and also reduced quality of life [24]. Unfortunately, most patients experiencing lymphedema only present when the arm is visibly swollen and by this time there is a risk of more severe consequences. Therefore, prospective surveillance, including pre-operative baseline to sequential periodic assessments such as post-operative to every six months, plays a crucial role in terms of earlier detection and management of lymphedema [17,25].
Singh and colleagues also used prospective monitoring to examine arm morbidity among breast cancer patients [26]. They monitored patients for seven months and reported that prospective monitoring and early detection and intervention can reduce lymphedema incidence rate and improve quality of life of participants. An early small study by Stout-Gergich et al. using prospective monitoring by perometry showed decreased arm volumes after an early compression garment intervention [23]. They showed statistically significant differences from limb volume of 83ml at the onset of swelling (p=0.05) down to 48 ml (p<0.001) within an average of 4.8 months [23]. Chance-Hetzler et al. reported an economic model with lower costs for lymphedema management between $3755 and $6353 (40.9% savings), with early referral (5% limb volume change or patient report of heaviness or swelling) based on prospective surveillance, as compared to historical controls with referral at 10% limb volume change [25]. Moreover, prospective monitoring can help patients recognize and better manage the risk of lymphedema, a worry more stressful than any survivorship outcome other than breast cancer recurrence itself [27].
This study has some limitations. Lymphedema protocol data were unavailable on 30% of the Z1071 study population who elected not to participate in the substudy. Also, since this is a protocol targeting participants completing neoadjuvant chemotherapy, these patients likely entered the study experiencing side effects of chemotherapy, which may have been reported as symptoms on the LBCQ at baseline. These findings may differ from baseline data in studies where participants had not received neoadjuvant chemotherapy. The LBCQ may be sensitive, but not specific, to breast cancer-related lymphedema. Also, the Z1071 trial followed lymphedema outcomes through 36 months post-diagnosis, therefore study findings do not address lymphedema emergence from 36 months post-diagnosis through further years of survivorship. Cases of lymphedema have been documented to emerge even as late as two decades after breast cancer diagnosis and treatment.
When rigorously and systematically assessed, findings show that lymphedema continues to be common after axillary dissection. This is one of the first studies to apply rigorous methods in following lymphedema occurrence in breast cancer survivors after neoadjuvant chemotherapy and axillary dissection. Incidence of limb volume and circumference changes meeting the criteria for lymphedema gradually increased over the first year after surgery with cumulative rates increasing over the 36 months of the study. Lymphedema symptoms decreased from post-op over 18 months after surgery, thereafter remaining stable. Lymphedema incidence varied by criteria (arm measurements/symptoms), indicating both are important to assess. Findings underscore the value of prospective clinical surveillance from pre-surgery to 36 months and the importance of both limb and symptom assessment. Further study beyond 36 months is recommended, since lymphedema is a lifetime risk among breast cancer survivors.
The findings from this study show considerable variability in lymphedema rates depending on the definition used for lymphedema. Assessment of patient symptoms had the lowest rate of lymphedema and lagged behind the more objective definitions using measurements. In this field with such disparity in measurement approaches, timelines, and diagnostic criteria, we sought to contribute to the standardization of the criteria for post-breast cancer lymphedema assessment. These findings provide further evidence earlier reported [14] that the occurrence of lymphedema is dependent on the criteria applied, with ≥2-cm circumference change in arm girth at any anatomic point being considered perhaps too sensitive a measure for whole-limb swelling for general clinical significance. Additionally, the relative increase in size with a 2-cm circumference increase varies by the baseline size of the arm, just as 200-ml change does. The criterion of ≥10% volume change compared to baseline and contralateral limb volume change is a slightly more conservative measure of whole-limb volume change and incorporates initial arm size; this modality is commonly used for referral to further assessment and treatment in the lymphedema clinic. For this reason, we recommend the ≥10% volume change (as compared to pre-op baseline and contralateral limb) criterion be used for future analyses and other clinical trials.
With the growing focus on patient-reported outcomes, and because objective measures (limb volume and girth) of lymphedema are not always highly correlated with subjective measures (such as symptom report), we highly recommend the inclusion of both an objective and subjective measure for lymphedema assessment in the prospective surveillance model and in clinical practice. Symptoms produce distress and require management, whether or not the lymphedema diagnostic threshold for limb volume/girth is met. Similarly, in the absence of sensation changes and the presence of swelling associated with stagnant protein-rich interstitial lymphatic fluid, there is increased risk of cellulitis, erysipelas, lymphangitis, and septicemia. Management of both symptoms and swelling are crucial to optimal outcomes for breast cancer survivors. Early detection of and intervention for secondary lymphedema decreases untoward outcomes such as tissue changes of fibrosis and infection. Standardized measurement approaches at common timelines with consensus-driven referral and management protocols will go far in optimizing quality of life and functional well-being in the years after cancer treatment. Recent studies, including the Z1071 trial, have substantiated that the prospective surveillance model recommended by leaders in the field [12] is indeed feasible in a variety of clinical oncology settings from community to academic centers and that the data collected are valuable in documenting emergence of post-breast cancer treatment lymphedema. This is the ‘gold standard’ recommended to guide optimal assessment and management of secondary lymphedema after breast cancer treatment.
Table 5.
Reported symptoms at 36 months compared to lymphedema definition (≥10% or ≥2cm)
| Lymphedema by ≥10% volume increase or any ≥2 cm increase (ever) | Number of reported signs/symptoms at 36 months | |||
|---|---|---|---|---|
| 0 | 1 | 2–5 | 6 or more | |
| Yes | 88 (50.9%) | 29 (16.8%) | 37 (21.4%) | 19 (11.0%) |
| No | 19 (55.9%) | 7 (20.6%) | 1 (2.9%) | 7 (20.6%) |
| Missing/Unknown | 5 | 2 | 2 | 4 |
Acknowledgments
ClinicalTrials.gov Identifier: NCT00881361
Support:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA180821, U10CA180882, U10CA180790, U10CA180858, U10CA180868, and U10CA180888. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest. We have no financial relationship with the organization that sponsored the research. The authors have full control of all primary data and we agree to allow the journal to review the source data, if requested.
References
- 1.American Cancer Society; (2014) Breast Cancer Facts & Figures 2013–2014 America Cancer Society. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042725.pdf. Accessed October 14 2014 [Google Scholar]
- 2.Armer JM, Radina ME, Porock D, Culbertson SD (2003) Predicting breast cancer-related lymphedema using self-reported symptoms. Nursing research 52 (6):370–379. doi: 10.1097/00006199-200311000-00004 [DOI] [PubMed] [Google Scholar]
- 3.Poage EG, Singer M, Armer JM, Poundall MD, Shellabarger MJ (2008) Demystifying lymphedema: Development of the lymphedema putting evidence into practice card. Clinical Journal of Oncology Nursing 12 (6):951–964. doi: 10.1188/08.CJON.951-964 [DOI] [PubMed] [Google Scholar]
- 4.Dell DD, Doll C (2006) Caring for a patient with lymphedema. Nursing 36 (6):49–51 [DOI] [PubMed] [Google Scholar]
- 5.Fu MR, Ridner SH, Armer J (2009) Post-breast cancer lymphedema: part 2. Am J Nurs 109 (8):34–41; quiz 42. doi: 10.1097/01.NAJ.0000358492.91678.78 [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TT, Hoskin TL, Habermann EB, Cheville AL, Boughey JC (2017) Breast Cancer-Related Lymphedema Risk is Related to Multidisciplinary Treatment and Not Surgery Alone: Results from a Large Cohort Study. Annals of surgical oncology doi: 10.1245/s10434-017-5960-x [DOI] [PMC free article] [PubMed]
- 7.McLaughlin SA, DeSnyder SM, Klimberg S, Alatriste M, Boccardo F, Smith ML, Staley AC, Thiruchelvam PTR, Hutchison NA, Mendez J, MacNeill F, Vicini F, Rockson SG, Feldman SM (2017) Considerations for Clinicians in the Diagnosis, Prevention, and Treatment of Breast Cancer-Related Lymphedema, Recommendations from an Expert Panel: Part 2: Preventive and Therapeutic Options. Annals of surgical oncology doi: 10.1245/s10434-017-5964-6 [DOI] [PubMed]
- 8.McLaughlin SA, Staley AC, Vicini F, Thiruchelvam P, Hutchison NA, Mendez J, MacNeill F, Rockson SG, DeSnyder SM, Klimberg S, Alatriste M, Boccardo F, Smith ML, Feldman SM (2017) Considerations for Clinicians in the Diagnosis, Prevention, and Treatment of Breast Cancer-Related Lymphedema: Recommendations from a Multidisciplinary Expert ASBrS Panel : Part 1: Definitions, Assessments, Education, and Future Directions. Annals of surgical oncology doi: 10.1245/s10434-017-5982-4 [DOI] [PubMed]
- 9.Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, Miller LT, DeMichele A, Solin LJ (2009) Lymphedema in Breast Cancer Survivors: Incidence, Degree, Time Course, Treatment, and Symptoms. J Clin Oncol 27 (3):390–397. doi: 10.1200/jco.2008.17.9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boccardo FM, Ansaldi F, Bellini C, Accogli S, Taddei G, Murdaca G, Campisi CC, Villa G, Icardi G, Durando P, Puppo F, Campisi C (2009) Prospective evaluation of a prevention protocol for lymphedema following surgery for breast cancer. Lymphology 42 (1):1–9 [PubMed] [Google Scholar]
- 11.Lawenda BD, Mondry TE, Johnstone PAS (2009) Lymphedema: A primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin 59 (1):8–24. doi: 10.3322/caac.20001 [DOI] [PubMed] [Google Scholar]
- 12.Hayes SC, Johansson K, Stout NL, Prosnitz R, Armer JM, Gabram S, Schmitz KH (2012) Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer 118 (8 Suppl):2237–2249. doi: 10.1002/cncr.27467 [DOI] [PubMed] [Google Scholar]
- 13.Armer JM, Stewart BR, Smith K, Cormier JN (2011) Lymphedema following cancer treatment. In: Lester JL, Schmitt PA (eds) Cancer Rehabilitation and Survivorship: Transdisciplinary Approaches to Personalized Care Oncology Nursing Society, Pittsburgh, [Google Scholar]
- 14.Armer JM, Stewart BR (2005) A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphatic Research and Biology 3 (4):208–217. doi: 10.1089/lrb.2005.3.208 [DOI] [PubMed] [Google Scholar]
- 15.Czerniec SA, Ward LC, Refshauge KM, Beith J, Lee MJ, York S, Kilbreath SL (2010) Assessment of breast cancer-related arm lymphedema—Comparison of physical measurement methods and self-report. Cancer Investigation 28 (1):54–62. doi: 10.3109/07357900902918494 [DOI] [PubMed] [Google Scholar]
- 16.Bulley C, Gaal S, Coutts F, Blyth C, Jack W, Chetty U, Barber M, Tan CW (2013) Comparison of breast cancer-related lymphedema (upper limb swelling) prevalence estimated using objective and subjective criteria and relationship with quality of life. BioMed research international 2013:807569. doi: 10.1155/2013/807569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostby PL, Armer JM, Dale PS, Van Loo MJ, Wilbanks CL, Stewart BR (2014) Surveillance recommendations in reducing risk of and optimally managing breast cancer-related lymphedema. Journal of personalized medicine 4 (3):424–447. doi: 10.3390/jpm4030424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Kuerer HM, Bowling M, Flippo-Morton TS, Byrd DR, Ollila DW, Julian TB, McLaughlin SA, McCall L, Symmans WF, Le-Petross HT, Haffty BG, Buchholz TA, Nelson H, Hunt KK (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. Jama 310 (14):1455–1461. doi: 10.1001/jama.2013.278932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armer JM (2005) The problem of post-breast cancer lymphedema: Impact and measurement issues. Cancer Investigation 23 (1):76–83. doi: 10.1081/CNV-48707 [DOI] [PubMed] [Google Scholar]
- 20.NLN Medical Advisory Committee (2013) Position Paper: Screening and Measurement for Early Detection of Breast Cancer Related Lymphedema. Position Statement of the National Lymphedema Network National Lymphedema Network, San Francisco, CA: https://www.lymphnet.org/resources/position-paper-screening-and-measurement-for-early-detection-of-breast-cancer-related [Google Scholar]
- 21.National Accreditation Program for Breast Centers (2014) NAPBC Standards Manual 2014 Edition American College of Surgeons, Chicago, IL: https://www.facs.org/quality-programs/napbc/standards [Google Scholar]
- 22.Armer JM, Shook RP, Schneider MK, Brooks CW, Peterson J, Stewart BR (2009) Enhancing supportive-educative nursing systems to reduce risk of post-breast cancer lymphedema. Self-Care & Dependent-Care Nursing 17 (1):6–15. doi:PMC3405945 [PMC free article] [PubMed] [Google Scholar]
- 23.Stout NL, Pfalzer LA, Springer B, Levy E, McGarvey CL, Danoff JV, Gerber LH, Soballe PW (2012) Breast cancer-related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Physical therapy 92 (1):152–163. doi: 10.2522/ptj.20100167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cormier JN, Xing Y, Zaniletti I, Askew RL, Stewart BR, Armer JM (2009) Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology 42 (4):161–175 [PMC free article] [PubMed] [Google Scholar]
- 25.Chance-Hetzler J, Armer J, Van Loo M, Anderson B, Harris R, Ewing R, Stewart B (2015) Prospective Lymphedema Surveillance in a Clinic Setting. Journal of personalized medicine 5 (3):311–325. doi: 10.3390/jpm5030311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh C, De Vera M, Campbell KL (2013) The effect of prospective monitoring and early physiotherapy intervention on arm morbidity following surgery for breast cancer: a pilot study. Physiotherapy Canada Physiotherapie Canada 65 (2):183–191. doi: 10.3138/ptc.2012-23O [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binkley JM, Harris SR, Levangie PK, Pearl M, Guglielmino J, Kraus V, Rowden D (2012) Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer. Cancer 118 (8 Suppl):2207–2216. doi: 10.1002/cncr.27469 [DOI] [PubMed] [Google Scholar]


