SUMMARY
Background
MYC gene rearrangement (MYC-R) is present in approximately 10% of aggressive B-cell lymphomas with approximately half harboring a BCL2 gene rearrangement (BCL2-R). Multiple retrospective studies of R-CHOP show an inferior outcome in patients with MYC-R, both alone and with BCL2-R and/or BCL6-R, and suggest better outcomes with more aggressive treatment. In the current study, we aimed to determine the outcome of DA-EPOCH-R, an aggressive infusional treatment regimen, in untreated MYC-R aggressive B-cell lymphomas.
Methods
Final analysis of a prospective multi-center study of DA-EPOCH-R (dose-adjusted treatment: etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituxiomab) in 53 patients with untreated MYC-R aggressive B-cell lymphomas. DAEPOCH-R was scheduled to be administered with central nervous system prophylaxis for 6 cycles. Primary endpoints included event-free and overall survival. The study was registered at ClinicalTrials.gov (NCT01092182).
Findings
Patient characteristics included median age 61 (range 29–80) years, stage III/IV disease in 43 (81%), and high-intermediate/high international prognostic score (IPI) in 26 (49%) patients. Characteristics were similar among patients with confirmed MYC-R single-hit (n=19) versus those with a BCL2-R and/or BCL6-R, termed double-hit, (n=24) lymphomas. With a median follow up of 55.6 (Interquartile range: 50.5–61.1) months, the 48-month EFS and OS for all patients was 71% and 77%, respectively, with no differences among patients with single versus double-hit tumors or age < versus ≥ 60 years. The EFS at 48-months for low/low-intermediate (0–2) versus high-intermediate/high (3–5) IPI was 89% versus 50%, respectively, for all patients, and 92% versus 55% for double-hit patients. Toxicity included grade 4 neutropenia and thrombocytopenia on 53% and 13% of cycles, respectively, and fever with neutropenia occurred on 19% of cycles. There were 3 treatment related deaths.
Interpretation
In this study, DA-EPOCH-R produced durable remissions in MYC-R aggressive B-cell lymphomas and should be considered for the treatment of these diseases.
Funding
Cancer Trials Support Unit and Center for Cancer Research, National Cancer Institute, USA, and Genentech Inc.
Keywords: MYC, BCL2, double-hit lymphoma, DA-EPOCH-R
INTRODUCTION
Diffuse large B-cell lymphomas are molecularly heterogeneous and vary in their cell of origin, oncogenic mutations and deregulated signaling pathways(1–3). A relatively frequent molecular event is a MYC 8q24 rearrangement (MYC-R), often accompanied by translocations involving BCL2 and/or BCL6, and present in around 10% of diffuse large B-cell lymphoma(4, 5). These rearrangements have been associated with a poor prognosis in multiple retrospective/observational studies of R-CHOP chemotherapy in diffuse large B-cell lymphoma(5–11). In one of the first studies to assess the clinical impact of MYC-R, patients with tumors that contained a MYC-R, some of which also had a BCL2 rearrangement, had a significantly shorter survival following R-CHOP treatment compared to patients without a translocation(6). While the presence of rearrangements involving BCL2 and/or BCL6 may contribute to the poor prognosis, it has been shown that MYC-R alone (single-hit) is associated with a significantly worse prognosis(7, 8, 12, 13).
The recognition of MYC-R in diffuse large B-cell lymphoma and its association with an inferior prognosis led to the establishment of a new category of high-grade B-cell lymphoma with MYC-R and BCL2 and/or BCL6 translocations, termed high-grade Bcell lymphoma double-hit, in the 2016 revision of the World Health Organization (WHO) classification of lymphoid neoplasms(14). Although “double-hit” high-grade B-cell lymphoma is now recognized as a specific entity, these tumors are pathologically and clinically heterogeneous. MYC-R can be found in tumors with morphological features of diffuse large B-cell lymphoma and high-grade B-cell lymphoma not otherwise specified (HGBL NOS), which are mostly derived from germinal B-cells, and pasmablastic lymphoma, a post-germinal center subtype(4, 14).
The inferior prognosis of MYC-R aggressive B-cell lymphomas with R-CHOP chemotherapy has prompted the use of aggressive treatments such as Burkitt lymphoma regimens and stem cell transplantation as outlined in the National Comprehensive Cancer Network (NCCN) guidance (version 4.2018)(5, 15–17). As there are no prospective studies in untreated MYC-R aggressive B-cell lymphomas, there is an unmet medical need to evaluate dose-intense treatment in these patients. DA-EPOCH-R is a dose-intense immuno-chemotherapy platform in which the continuous infusion of 3 of its components and pharmacodynamic dose-adjustments may be particularly important for highly proliferative lymphomas such as MYC-R aggressive B-cell and Burkitt lymphoma(18, 19). We hypothesized that DA-EPOCH-R would overcome the negative prognostic impact of MYC-R alone and in double-hit aggressive B-cell lymphomas and conducted a prospective multicenter study.
PATIENTS AND METHODS
Study Design and Participants
We conducted a prospective 13-center non-randomized phase 2 study of DA-EPOCH-R in untreated de novo MYC-R aggressive B-cell lymphomas including high-grade B-cell lymphoma double-hit, high-grade B-cell lymphoma, high-grade B-cell lymphoma not otherwise specified, diffuse large B-cell lymphoma and plasmablastic lymphoma to obtain an estimate of its efficacy (Appendix list participating sites). These patients were enrolled on a protocol of DA-EPOCH-R that included two separate cohorts; one for MYC-R aggressive B-cell lymphomas and one for Burkitt lymphomas. The two cohorts had independent accrual goals, study objectives, and differences in treatment algorithms. The two cohorts were to be separately reported but combined into a single protocol based on the use of DA-EPOCH-R and overlapping study centers. Thus, as intended, patients in the Burkitt lymphoma cohort will be independently reported.
Patients with MYC-R aggressive B-cell lymphoma were enrolled between March 25, 2010 and February 20, 2014, and the data was locked in November 15, 2017. Eligibility included a confirmed histological diagnosis of a MYC-R aggressive B-cell lymphoma. Patient histology is presented according to the modified World Health Organization Classification of Lymphoid Neoplasms 2016(14, 20). The histology was confirmed at each participating institution with central review of all histological reports by the National Cancer Institute. Additional eligibility included age 18 years or older, no prior systemic chemotherapy, all disease stages, any performance status, adequate organ function unless related to disease, and a negative pregnancy test in women of child bearing potential. Patients with central nervous system (CNS) leptomeningeal involvement and human immunodeficiency virus (HIV) infection were eligible. Pretreatment evaluation included standard laboratory investigations, whole body computed tomography (CT) scans, bone marrow aspirate and biopsy, cerebral spinal fluid (CSF) analysis by flow cytometry and cytology, and as clinically indicated, brain MRI/CT(21). Tumor response was assessed per the revised response criteria for malignant lymphoma(22). All patients received an interim FDG (fluorodeoxyglucose)-PET (positron emission tomography) scan after two cycles of treatment as a research endpoint. The FDG-PET scans were interpreted by each institution and were not used for medical decisions. The study was approved by the investigational review board of each institution and all patients provided written informed consent.
Procedures
DA-EPOCH-R (starting doses: etoposide 50 mg/m2/day infusion x 96 hours (days 1–5); doxorubicin 10 mg/m2/day infusion x 96 hours (days 1–5); vincristine 0.4 mg/m2/day infusion x 96 hours (days 1–5); cyclophosphamide 750 mg/m2 intravenous (day 5); prednisone 60 mg/m2/bid orally (days 1–5); rituximab 375 mg/m2 intravenous (day 1); and filgrastrim 5 μg/kg/day subcutaneously (day 6 until absolute neutropils are > 5000/μl past the nadir) was administered for 6 cycles as previously described and in the outpatient setting when feasible(23). It was recommended that HIV-positive patients not receive antiretroviral therapy during chemotherapy. DA-EPOCH-R was pharmacodynamically dose-adjusted based on the neutrophil nadir, which was checked twice weekly. Patients received filgrastim beginning 24 hours after the last dose of chemotherapy and continued through the neutrophil nadir until absolute neutrophil recovery, defined as 5000 cells per cubic millimeter or higher. Pegylated filgrastim was allowed. Bactrim® DS was administered twice daily for 3 days per week. Cycles were begun every 21 days providing the ANC ≥ 1000/μl and platelets ≥ 100,000/μl.
If below these limits, counts were checked daily until recovery and G-CSF was administered as indicated. Patients without evidence of leptomeningeal involvement received prophylactic intrathecal methotrexate 12 mg on days 1 and 5 of cycles 3 to 6 of DA-EPOCH-R for a total of 8 doses. Patients with leptomentingeal involvement received active treatment with methotrexate 12 mg intrathecally or 6 mg via Ommaya reservoir twice weekly for 4 weeks, then weekly for 6 weeks, and then monthly for 4 months. This study employed commercial chemotherapy drugs, which were provided by each institution. The supplier of the drugs may vary by institution based on pharmacy purchasing practices. Filgrastim was provided by Genetech.
Patients underwent staging of involved sites by CT scan after 2 and 6 cycles and every 4 months for 2 years and then yearly for 3 years until disease progression. FDG PET scans were performed after cycle 2 and 6 (if positive after cycle 2) and as clinically indicated thereafter. Radiological scans were reviewed at each participating site and were not centrally reviewed.
MYC-rearrangement by fluorescence in-situ hybridization (FISH) using (8q24) break apart probes or conventional cytogenetics was required and performed at the participating sites. Rearrangement of BCL2 was assayed by FISH with probes from the regions IgH (14q32) and BCL2 (18q21), and BCL6 was assayed using (3q27) break apart probes or cytogenetics but were not eligibility requirements.
Outcomes
The primary study objectives included event-free survival (EFS) and overall survival (OS) with analysis in single and double/triple-hit patients. EFS was determined from the on-study date until the date of progression, documentation of disease following the last treatment cycle, death, or last follow-up, and OS was calculated from the on-study date until date of death or last follow-up using the Kaplan-Meier method. Secondary outcomes included assessment of outcomes on interim (after cycle 2) FDG-PET/CT scans and toxicity of DA-EPOCH-R.
Statistical Analysis
Due to the absence of comparator prospective trials, the study endpoint was to estimate event-free (EFS) and overall survival (OS) of DA-EPOCH-R in patients with MYC-R aggressive B-cell lymphoma for a total of 53 patients. All patients were included for analysis of outcome and toxicity. Exploratory analyses for differences in EFS and OS were assessed in patients with single-hit and double/triple-hit lymphomas (termed double-hit), age < and >= 60 years, international-prognostic index, and histology highgrade B-cell lymphoma double-hit/not otherwise specified versus diffuse large B-cell lymphoma. The statistical significance of the difference between a pair of Kaplan-Meier curves was determined by an exact log-rank test. All p-values are two-tailed and presented without adjustment for multiple comparisons. We did statistical analyses with SAS (version 9.3). The study registered at ClinicalTrials.gov (NCT01092182).
Role of the Funding Source
The Cancer Therapy Evaluation Program provided input into the study design and approved the protocol as the study sponsor. Data management was provided by the Cancer Therapy Support Unit data operations of the Cancer Therapy Evaluation Program. All sites submitted data and responded to queries using the Medidata Rave Clinical Data Management System. The funding source was not involved in interpretation of the data or writing of the report. The funding source was provided a copy of the manuscript for submission but was not required for approval. The communicating author had full access to the data and final responsibility for submission.
RESULTS
Clinical Characteristics
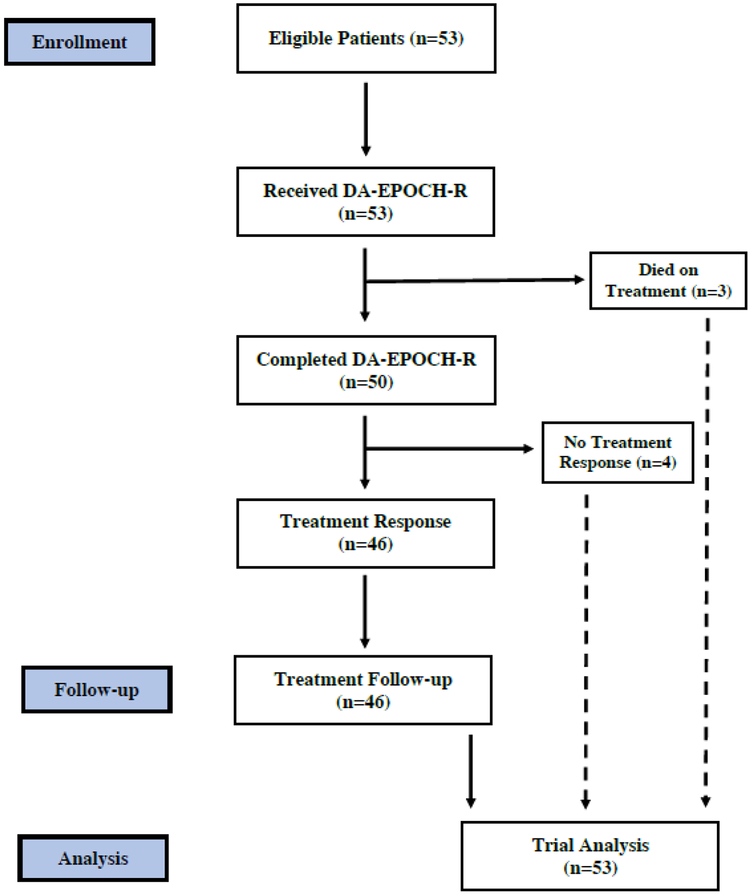
Fifty-three untreated patients with MYC-R aggressive B-cell lymphomas were enrolled Figure 1. Clinical characteristics include a median age of 61 (range 29–80) years, male sex in 40 (75%), stage III or IV disease in 43 (81%), and elevated lactate dehydrogenase (LDH) in 31 (61%) patients (Table 1). Three (6%) patients had CSF involvement at diagnosis and 5 (9%) patients were HIV-positive. Based on the international prognostic index (IPI), 26 (49%) patients had high-intermediate or high risk disease. Histology included high-grade B-cell lymphoma double-hit in 24 (45%), high-grade B-cell lymphoma not otherwise specified in 10 (19%) and diffuse large B-cell lymphoma in 18 (34%) of patients(14). All patients had MYC-R, which included a BCL2-R in 22 (42%) and BCL6-R in 5 (16%) patients. Due to the lack of tissue, BCL2 and BCL6 FISH were not performed in 1 and 21 cases, respectively. Considering all cases, single-hit and double-hit lymphoma (which includes 3 patients with triple-hit) was confirmed in 19 and 24 patients, respectively. There were no significant differences in clinical characteristics among patients with single versus double-hit lymphoma with the exception of a worse performance status in the former group (Table 1).
Figure 1.
Flow diagram of patients enrolled on study. The analysis of survival outcome includes all patients enrolled. The analysis of treatment response excludes 3 patients who died prior to restaging.
Table 1:
Patient and Tumor Characteristics
| Characteristics | All MYC-R | MYC-R Only |
MYC-R + BCL2-R/ BCL6-R |
P value3 | |||
|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | ||
| Total Patients | 53 | 100 | 19 | 591 | 24 | 462 | |
| Age Median (Range) | 61 (29–80) | 63 (36–80) | 62 (35–76) | ||||
| Male sex | 40 | 75 | 16 | 84 | 14 | 58 | 0.10 |
| Stage III or IV | 43 | 81 | 14 | 74 | 20 | 83 | 0.48 |
| Elevated LDH | 31 (n=51) | 61 | 11 | 58 | 14 | 58 | 1.00 |
| ECOG PS 2–4 | 11 | 21 | 13 | 68 | 4 | 17 | 0.0013 |
| Extranodal sites ≥ 2 | 12 | 23 | 5 | 26 | 6 | 25 | 1.00 |
| Bone marrow positive | 9 | 17 | 3 | 16 | 5 | 21 | 1.00 |
| CSF positive | 3 (n=44) | 7 | 2 (17) | 12 | 1 (21) | 5 | 0.58 |
| IPI Score | |||||||
| 0–1 | 12 | 23 | 4 | 21 | 6 | 25 | 0.76 |
| 2 | 15 | 28 | 5 | 26 | 7 | 29 | |
| 3 | 17 | 32 | 7 | 37 | 8 | 33 | |
| 4–5 | 9 | 17 | 3 | 16 | 3 | 13 | |
| HIV Positive | 5 | 9 | 3 | 16 | 0 | 0 | 0.08 |
| Histology | |||||||
| HGBL DH | 24 | 45 | 0 | 0 | 24 | 100 | NA |
| HGBL NOS | 10 | 19 | 6 | 32 | 0 | 0 | NA |
| DLBCL | 18 | 34 | 13 | 68 | 0 | 0 | NA |
| Plasmablastic | 1 | 2 | 0 | 0 | 0 | 0 | NA |
| Translocations | |||||||
| MYC | 53 (n=53) | 100 | 19 | 100 | 24 | 100 | NA |
| BCL2 | 22 (n=52) | 42 | 0 | 0 | 22 | 92 | NA |
| BCL6 | 5 (n=32) | 16 | 0 | 0 | 5 | 21 | NA |
Based on 32 cases with BCL2 and BCL6 FISH analysis.
Based on 52 cases with BCL2 and/or BCL6 FISH analysis.
MYC-R only versus MYC-R + BCL2-R and/or BCL6-R (Fisher exact for all except Cochran-Armitage trend test for comparison of IPI).
Abbreviations: LDH-lactate dehydrogenase; ECOG-Eastern Cooperative Oncology Group; IPI-international prognostic index; CSF-cerebral spinal fluid-leptomenigeal disease; HIV-human immunodeficiency virus.
HGBL DH-high grade B-cell lymphoma, double-hit, with MYC and BCL2 and/or BCL6 rearrangements. HGBL NOS-High grade B-cell lymphoma, not otherwise specified, that have feature intermediate between diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL) but do not harbor a genetic double hit. Synonym is B-cell lymphoma, unclassifiable with features intermediate between DLBCL and BL. DLBCL-diffuse large B-cell lymphoma. NA-not applicable.
Clinical Outcome
Considering all 53 patients, 39 achieved a CR and 7 achieved a PR for an overall response rate of 87%. Four patients did not respond and 3 patients were inevaluable due to infectious-related deaths on study before staging could be performed. The 3 patients with leptomenigeal disease at diagnosis died, one from progressive disease and 2 from treatment-related infections. Four patients in CR and one patient in PR, but without documentation of active disease, underwent an autologous (4) or allogeneic (1) bone marrow transplant following therapy Two patients who were in CR received consolidation radiation following DA-EPOCH-R.
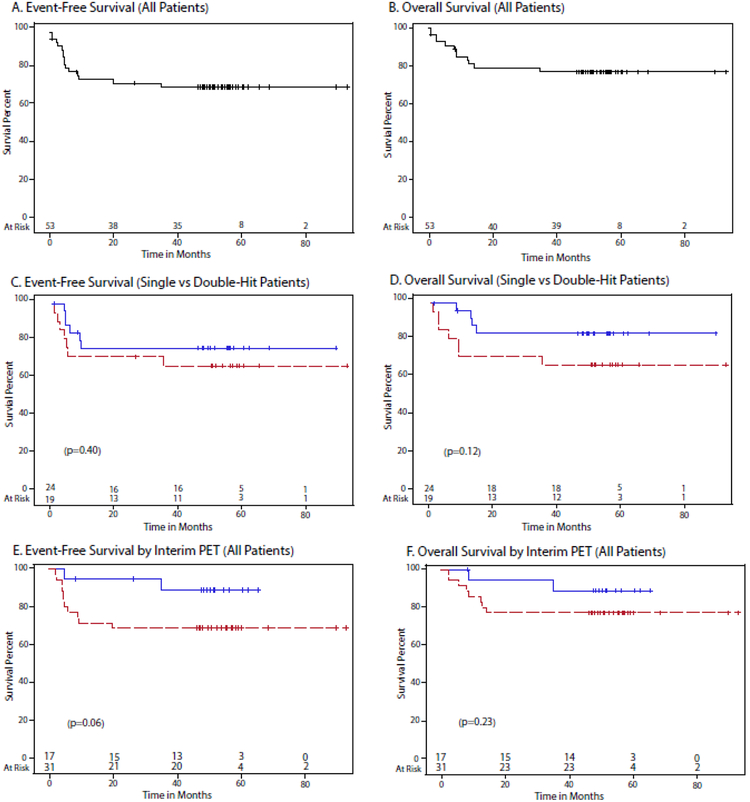
The study median potential follow-up is 55.6 (Interquartile range: 50.5–61.1) months. For all patients, the 48 month EFS and OS were 71% (95% CI: 56.5–81.4%) and 76.7 % (95% CI: 62.6–86.1%) respectively (Figure 2A and 2B). When considering confirmed single and double-hit patients (Table 1 footnote), the EFS at 48-months was 62.7% (95% CI: 37.2–80.2%) and 73.4% (95% CI: 50.1–87.1%) (p=0.40), respectively, and the OS was 63.2% (95% CI: 38–80.4%) and 82% (95% CI: 58.8–92.8%) (p=0.12), which was not statistically different (Figures 2C and 2D).
Figure 2.
Kaplan–Meier Estimates of event-free (EFS) and overall survival (OS) of MYC-R lymphoma. A. EFS of 53 patients with MYC-R lymphoma at 48-months was 71% (95% confidence interval [CI], 56.5–81.4%). B. OS of 53 patients with MYC-R lymphoma at 48-months was 76.7 (95% CI: 62.6–86.1%). C. EFS of single-hit (n=19) versus double-hit (n=24) lymphoma at 48-months was 62.7% (95% CI: 37.2–80.2%) and 73.4% (95% CI: 50.1–87.1%), respectively. D. OS of single-hit (n=19) versus double-hit (n=24) lymphoma at 48-months was 63.2% (95% CI: 38–80.4%) and 82% (95% CI: 58.892.8%), respectively. E. EFS of MYC-R lymphoma by negative (n=17) verus positive (n=31) interim FDG-PET at 48-months was 87.4% (95% CI: 58.1–96.7%) and 64.5% (95% CI: 45.2–78.5%), respectively. F. OS of MYC-R lymphoma by negative (n=17) verus positive (n=31) interim FDG-PET at 48-months was 87.5% (95% CI: 58.6–96.7%) and 74.2% (95% CI: 55–86.2%), respectively.
Prognostic Analysis
We performed exploratory analyses of clinical and histological variables. We were interested in the effect of IPI and age as older patients often do not tolerate aggressive immunochemotherapy and have a worse outcome(5). Analysis of double-hit patients by low/low-intermediate (0–2) and high-intermediate/high (3–5) risk IPI showed an EFS at 48-months of 91.7% (95% CI: 53.9–98.8%) and 54.5% (95% CI: 22.9–78%) (p=0.049), respectively, and an OS 90.9% (95% CI: 50.8–98.7%) and 72.2% (95% CI: 37.190.3%), indicating that high risk patients can achieve durable remissions (Supplementary appendix Figure 1A and 1B). We also analyzed the outcome of double-hit patients by age < 60 and >= 60 years and showed no difference with an EFS at 48-months of 71.6% (95% CI: 35–89.9%) and 75% (95% CI: 40.8–91.2%) (p=0.85), respectively, and an OS 70.7% (95% CI: 33.7–89.5%) and 91.7% (95% CI: 53.998.9%) (Supplementary appendix 1C and 1D). When all patients with MYC-R were considered, there was also no significant differences by age in EFS or OS (data not shown). We also examined if histology was associated with outcome. Considering all patients, the EFS of high-grade B-cell lymphoma double-hit/not otherwise specified versus diffuse large B-cell lymphoma yielded an EFS at 48-months of 70.8% (95% CI: 51.3–83.6%) and 69.6% (95% CI: 44.5–85.1%) (p=0.95), respectively, and OS of 77% (95% CI: 57.7–88.3%) and 75% (95% CI: 50–88.7%), which showed no differences in outcome (Supplementary appendix Figure 1E and 1F).
A pre-planned analysis was performed to assess if an interim FDG-PET identified patients at significant risk of treatment failure with DA-EPOCH-R, and could be used to direct adaptive treatment strategies. Considering patients with negative (Deauville 1–3) and positive (Deauville 4–5) scans, the 48-month EFS was 87.4% (95% CI: 58.196.7%) and 64.5% (95% CI: 45.2–78.5%)(p=0.057) and the OS was 87.5% (95% CI: 58.6–96.7%) and 74.2% (95% CI: 55–86.2%)(p=0.23), respectively (Figure 2E and 2F).
Although patients with an interim positive FDG-PET scan had a worse outcome, over 60% of these patients nonetheless achieved durable remissions.
Dose-Intensity and Toxicity
Toxicity was assessed in all 53 patients and on all 301 cycles (Table 2). Considering all cycles, the median (range) dose-level administered was 1 (−5 to 6). Furthermore, the maximum dose-level achieved in each patient was level 1 in 19 (36%), level 2 in 14 (26%), level 3 in 14 (26%), level 4 in 3 (6%), level 5 in 2 (4%) and level 6 in 1 (2%) patient. Forty-five patients received all 6 cycles of treatment, and 3 patients received five cycles of treatment due to concerns over tolerance. Six patients did not complete treatment due to on treatement death in 3 and progressive disease in three patients. Grade 4 neutropenia and thrombocytopenia occurred on 160 (53%) and 40 (13%) cycles, respectively and fever and neutropenia occurred on 56 (19%) cycles. Grade 2 and 3 motor neurotoxicity occurred in 3 and 4 patients, respectively, and grade 2 and 3 sensory neurotoxicity occurred in 11 and 4 patients. There were 3 treatment-related infectious deaths in patients who were 60, 66 and 75 years old with an IPI of 3, 4, and 5, respectively. These infectious deaths were due to respiratory failure/septic shock, sepsis and multi-organ failure/sepsis respectively.
Table 2:
DA-EPOCH-R Toxicity
| Toxicity | Total Nos. |
Grade 2 Nos. (%) |
Grade 3 Nos. (%) |
Grade 4 Nos. (%) |
Grade 5 Nos. (%) |
|---|---|---|---|---|---|
| Nos. of Patients | |||||
| Nos. of Cycles | |||||
| Haematological Toxicity (% cycles) | |||||
| Neutropenia | 189 | - | 29 (10%) | 160 (53%) |
0 |
| Thrombocytopenia | 99 | - | 59 (20%) | 40 (13%) | 0 |
| Infection (% cycles) | |||||
| Fever and Neutropenia | 56 | 0 | 54 (18%) | 2 (1%) | 0 |
| Other | 24 (8%) | 15 (5%) | 1 (0.3%) | 0 | |
| Gastrointestinal (% cycles) | |||||
| Mucositis | 44 | 26 (9%) | 18 (6%) | 0 | 0 |
| Constipation | 30 | 30 (10%) |
0 | 0 | 0 |
| Neurological (% patients) | |||||
| Sensory | 15 | 11 (21%) |
4 (8%) | 0 | 0 |
| Motor | 7 | 3 (6%) | 4 (8%) | 0 | 0 |
| Treatment-related death (% patients) | |||||
| Sepsis/organ failure | 3 (6%) |
DISCUSSION
To our knowledge, this is the first prospective study of chemotherapy in MYC-R aggressive B-cell lymphoma. In this trial, we assessed the outcome of DA-EPOCH-R to address an important unmet clinical need(24). With a median follow-up of 55.6 months, this multicenter study yielded a 48-month EFS and OS of 71% and 77%, respectively, in patients with MYC-R aggressive B-cell lymphomas. The results were similar in the patients with a double-hit lymphoma, which showed an EFS and OS of 73.4% and 82%, respectively, at 48-months. Notably, patients with MYC-R alone had a marginally worse outcome compared to double-hit patients. These results are similar to those achieved in the Cancer and Leukemia Group B multicenter study of DA-EPOCH-R in de novo diffuse large B-cell lymphoma, which is consistent with our earlier findings that suggest DA-EPOCH-R obviates the adverse effect of MYC-R(19, 25).
When we initiated this study in 2010, several retrospective studies had identified MYC-R as an adverse biomarker for R-CHOP treatment(5–7). Hence, we choose to include all patients with MYC-R, irrespective of secondary hits with BCL2 and/or BCL6. However, the most recent WHO classification (2016) only identified MYC-R tumors with a BCL2 and/or BCL6 rearrangement double-hit high-grade B-cell lymphomas as a specific entity based on their poor prognosis(14). Despite the exclusion of patients with single-hit MYC-R from the classification, they have an adverse prognosis as well(5, 6, 8, 12, 13). Further, the literature remains unclear on the contribution of BCL2-R and/or BCL6-R to the adverse prognosis of single-hit MYC-R aggressive B-cell lymphomas. It is of interest that alternative mechanisms of increased protein production other than rearrangement may also confer a poor prognosis with R-CHOP treatment. Notably, a recent retrospective study compared patients with typical double-hit lymphomas to patients with atypical double-hit lymphomas that included cases with MYC-R and extra copies of BCL-2; BCL-2-R rearrangements with extra copies of MYC; and cases with only extra copies of MYC and BCL-2(26). Patients with atypical and typical double-hits had a similar 2-year overall survival of 54% and 49%, respectively, which were significantly inferior to patients without abnormalities. These results suggest that the alternative mechanisms of MYC and/or BCL-2 expression may also confer an inferior outcome.
The outcome of MYC-R lymphomas is dependent on the IPI with all risk groups showing a worse outcome compared to non-rearranged patients as shown in a retrospective study of R-CHOP(5). In the present study, 26 (49%) patients had high-intermediate or highrisk disease with advanced stage in 43 (81%) and age over 60 years in 27 (50%) patients, characteristics that were similar in patients with single and double-hit lymphomas. When the outcome of double-hit lymphomas was analyzed by IPI groups, the EFS at 48-months in patients with low/low-intermediate disease was 92% compared to 55% in patients with high-intermediate/high-risk disease. When patients with single-hit lymphoma are included, similar results were observed (data not shown). Unlike what has been observed with R-CHOP, older patients faired as well as younger ones(5). Given the older age of many patients with MYC-R lymphomas and the accepted need for more aggressive treatment, the absence of an age effect in this study suggests that DA-EPOCH-R is effective in older patients and, unlike more aggressive “Burkitt-like” regimens, has acceptable tolerance (NCCN Guideline 4.2018). We also assessed if interim FDG-PET scans could identify patients unlikely to achieve durable remissions with DA-EPOCH-R. While almost all FDG-PET negative patients achieved durable remissions, more than 60% of FDG-PET positive patients also had durable remissions, suggesting interim FDG-PET scans are not highly specific with DA-EPOCH-R.
Our study has a number of limitations. Five patients underwent consolidation with transplantation (4 autologous and 1 allogeneic) following DA-EPOCH-R in the absence of documented disease. Although these interventions may have altered the disease course, a recent retrospective study showed no benefit of autologous transplant following DA-EPOCH-R in double-hit lymphomas(27). In comparing single and double-hit cases, it is notable that patients with single-hit lymphomas had a significantly worse performance status, suggesting that double-hit patients could have a worse outcome with DA-EPOCH0R if this characteristic was balanced. The need for specialized tests to detect MYC-R will delay study enrollment and likely biased accrual toward lower risk patients in our study(28). While this is also an issue for standard of care, the results we present may well over estimate the benefit of DA-EPOCH-R in an unselected population of patients. Our study also did not employ central histological review, raising the possibility that some patients did not have de novo MYC-R aggressive B-cell lymphomas. However, all patients were required to have a MYC-R, which is the unifying molecular characteristic of these patients. The exploratory analyses of prognostic variables are limited by relatively small numbers and should be interpreted with caution. We observed 3 treatment-related deaths, which is higher than we and others have reported with DA-EPOCH-R, indicating these patients are at risk of severe toxicity(23).
These results suggest DA-EPOCH-R may improve upon the outcome of R-CHOP. One of the early observational studies that assessed the prognostic impact of MYC-R evaluated 245 biopsies from de novo diffuse large B-cell lymphoma patients treated with RCHOP(5). Among 35 patients (14%) with MYC-R, which included 26 (74%) patients with double-hit BCL2-R, the 2-year overall survival was 35% compared to 61% for patients without MYC-R. A similar result was reported from a retrospective cohort of 135 de novo diffuse large B-cell lymphoma in which the 5-year overall survival of patients with and without a MYC-R was 33% and 72%, respectively(6). In this series, only 25% of MYC-R patients also harbored a BCL2-R. Not all studies, however, have found MYC-R confers as dismal a prognosis following R-CHOP treatment(12). In a retrospective analysis of 36 patients from a phase III randomized study of 14-day versus 21-day R-CHOP, the 2-year overall survival of MYC-R patients was 75% versus 85% in non-rearranged patients (p=0.016)(12). In that series, 16 patients with BCL2-R doublehit lymphoma had a 2-year overall survival of 63% compared to 84% in non-rearrange patients, which was not statistically different. In this subset analysis, the authors did not report the IPI risk category of their double-hit patients, which strongly influence outcome. Furthermore, one should consider that phase III clinical trials may be subject to entry bias toward more favorable patients, as suggested by the unusually high EFS of 85% in the non-MYC-R patients in that study(12, 29). Although we cannot rule out bias toward better prognosis patients in our study, the distribution of prognostic factors was similar to an observational study that showed a 2-year EFS of 23% for R-CHOP treated single-hit MYC-R patients compared to 59% for more aggressively treated patients(8). A comparison of our results to these retrospective studies of R-CHOP in MYC-R aggressive B-cell lymphomas must be tempered by potential differences in distribution of high-risk IPI patients and accrual biases.
Several observational studies suggest that aggressive treatment is more effective than R-CHOP in MYC-R lymphomas. In a single center study of 129 patients treated with RCHOP, DA-EPOCH-R or R-HyperCVAD/MA (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, alternating with cytarabine plus methotrexate), patients who received DA-EPOCH-R had a significantly better 2-year EFS of 67% compared to 25% for patients who received R-CHOP(11). In a larger multicenter retrospective study of 311 double-hit lymphomas, patients who received ‘intensive therapy’ with DA-EPOCH-R, R-CODOX-M/IVAC or R-HyperCVAD, had significantly better EFS compared to R-CHOP (median 21.6 versus 7.8 months, respectively)(15). The overall conclusion of these studies is that patients with double-hit lymphoma clinically benefit from more intensive treatment, a finding that has been reached by other studies as well(8–10).
While the optimal intensive regimen for MYC-R aggressive B-cell lymphomas remains undefine, the favorable outcome and relative tolerance of DA-EPOCH-R in MYC-R lymphomas compared to other intensive regimens has led to its acceptance as one standard (NCCN Guidelines 4.2018) with phase II confirmation from the present trial(811, 16, 17). Presently, the NCI National Clinical Trials Network (NCTN) is undertaking a phase I trial of DA-EPOCH-R with venetoclax (NCT03036904) in MYC-R DLBCL in an effort to further improve the outcome of these tumor types.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We performed a Pubmed literature search of clinical reports on MYC-R aggressive Bcell lymphoma published in the last 10 years. All of the published articles presented retrospective analyses/reviews of clinical data and overall showed an inferior prognosis with standard R-CHOP chemotherapy. The association of MYC-R with an inferior prognosis led to the establishment of a new category of high-grade B-cell lymphoma with MYC-R and BCL2 and/or BCL6 translocations, termed high-grade B-cell lymphoma double-hit, in the 2016 revision of the World Health Organization (WHO) classification of lymphoid neoplasms. The inferior prognosis of these lymphomas with R-CHOP chemotherapy prompted the use of aggressive treatments as outlined in the National Comprehensive Cancer Network (NCCN) guidance (version 4.2018). We developed a study of DA-EPOCH-R, a dose-intense immuno-chemotherapy platform, in patients with previously untrearted MYC-R aggressive B-cell lymphomas based on the hypothesis that infusional chemotherapy will overcome the adverse prognosis of highly proliferative lymphomas such as MYC-R aggressive B-cell lymphoma.
Added Value of this Study
To our knowledge, this study provides the first prospective information on the treatment of MYC-R aggressive B-cell lymphomas. The study provides evidence that the majority of patients with MYC-R aggressive B-cell lymphomas, including patients with MYC-R alone or those with additional BCL-2 and/or BCL-6 rearrangements, can achieve durable remissions with DA-EPOCH-R treatment.
Implications of All the Available Evidence
Based on these results and retrospective studies, regimens such as DA-EPOCH-R should be considered for the treatment of these lymphomas. Comparison of DA-EPOCH-R with another aggressive regimen is a relevant future research question.
ACKNOWLEDGEMENT
The Cancer Therapy Evaluation Program, and Center for Cancer Research, National Cancer Institute, National Institutes of Health, USA, provided funding for this study. Genentech Inc provided rituximab for the following institutions: Dana Farber Cancer Institute, Massachusetts General Hospital, and M.D. Anderson Cancer Center. Joseph W. Leach, M.D., Unity Hospital, Minneapolis, is acknowledged for enrolling a patient on the study.
DATA SHARING STATEMENT
De-identified clinical data will be provide to the Protocol Registration and Results System of ClinicalTrials.gov within one year of publication. Information on data sharing may be obtain for ClinicalTrials.gov website.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 56th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 6–9, 2014.
DECLARATION OF INTERESTS
Kieron Dunleavy: Reports other from Abbvie, other from Adaptive Technologies, other from Celgene, other from Amgen, other from Seattle Genetics, other from Kite, other from Jannsen, other from Karyopharm, outside the submitted work.
Michelle A. Fanale: Reports grants and personal fees from Seattle Genetics, grants and personal fees from Celgene, grants and personal fees from Takeda, grants from ADC Therapeutics, grants from Molecular Templates, grants and personal fees from Merck, grants and personal fees from BMS, outside the submitted work.
Jeremy S. Abramson: Reports personal fees from Abbvie, personal fees from Celgene, personal fees from Gilead, personal fees from Humaningen, personal fees from Kite Pharma, personal fees from Juno Therapeutics, personal fees from Novartis, personal fees from Verastem, from Merck, outside the submitted work.
Ariela Noy: Reports grants from NIH, grants from NIH, during the conduct of the study; personal fees from Pharmacyclics, grants from Pharmacyclics, grants from Pharmacyclics, personal fees from Janssen Global, personal fees from Medscape, personal fees from targeted Oncology, and Prime Oncology outside the submitted work.
Paolo Fabrizio Caimi: Reports grants from Eastern Cooperative Oncology Group, during the conduct of the study; grants from Genentech, personal fees from Celgene, personal fees from Kite pharmaceutics, personal fees from Genentech, outside the submitted work.
Stefania Pittaluga: None
Samir Parekh: None
Ann Lacasce: None
John W Hayslip: Reports personal fees from AbbVie, outside the submitted work.
Deepa Jagadeesh: Reports other from null, during the conduct of the study; personal fees from Seattle Genetics, personal fees from Celgene, outside the submitted work.
Sunil Nagpal: Reports and Owns Stock in Pfizer, Bristol Myers, Merck, Roche.
Mary Jo Lechowicz: None
Rakesh Gaur: None
Andrea Lucas: None
Christopher Melani: None
Mark Roschewski: None
Seth M. Steinberg: None
Elaine S. Jaffe: None
Brad Kahl: Report grants from National Cancer Institute, during the conduct of the study; personal fees from Genentech, outside the submitted work.
Jonathan W. Friedberg: Reports other from Bayer, other from Astellas, outside the submitted work.
Richard F. Little: None
Nancy L. Bartlett: None
Wyndham H. Wilson: None
REFERENCES
- 1.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–11. [DOI] [PubMed] [Google Scholar]
- 2.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-Bcell lymphoma. N Engl J Med. 2002;346(25):1937–47. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018;378(15):1396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott DW, King RL, Staiger AM, Ben-Neriah S, Jiang A, Horn H, et al. Highgrade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28(20):3360–5. [DOI] [PubMed] [Google Scholar]
- 6.Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large Bcell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114(17):3533–7. [DOI] [PubMed] [Google Scholar]
- 7.Niitsu N, Okamoto M, Miura I, Hirano M. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia. 2009;23(4):777–83. [DOI] [PubMed] [Google Scholar]
- 8.Landsburg DJ, Falkiewicz MK, Petrich AM, Chu BA, Behdad A, Li S, et al. Sole rearrangement but not amplification of MYC is associated with a poor prognosis in patients with diffuse large B cell lymphoma and B cell lymphoma unclassifiable. Br J Haematol. 2016;175(4):631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landsburg DJ, Falkiewicz MK, Maly J, Blum KA, Howlett C, Feldman T, et al. Outcomes of Patients With Double-Hit Lymphoma Who Achieve First Complete Remission. J Clin Oncol. 2017;35(20):2260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma EJ, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319–31. [DOI] [PubMed] [Google Scholar]
- 11.Oki Y, Noorani M, Lin P, Davis RE, Neelapu SS, Ma L, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166(6):891–901. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817–26. [DOI] [PubMed] [Google Scholar]
- 13.Kuhnl A, Cunningham D, Counsell N, Hawkes EA, Qian W, Smith P, et al. Outcome of elderly patients with diffuse large B-cell lymphoma treated with RCHOP: results from the UK NCRI R-CHOP14v21 trial with combined analysis of molecular characteristics with the DSHNHL RICOVER-60 trial. Ann Oncol. 2017;28(7):1540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrich AM, Gandhi M, Jovanovic B, Castillo JJ, Rajguru S, Yang DT, et al. Impact of induction regimen and stem cell transplantation on outcomes in doublehit lymphoma: a multicenter retrospective analysis. Blood. 2014;124(15):2354–61. [DOI] [PubMed] [Google Scholar]
- 16.Friedberg JW. How I treat “Double Hit” lymphoma Blood. 2017. [DOI] [PubMed] [Google Scholar]
- 17.Friedberg JW. Double-hit diffuse large B-cell lymphoma. J Clin Oncol. 2012;30(28):3439–43. [DOI] [PubMed] [Google Scholar]
- 18.Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, et al. Lowintensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369(20):1915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai C, Roschewski M, Melani C, Pittaluga S, Shovlin M, Steinberg SM, et al. MYC gene rearrangement in diffuse large B-cell lymphoma does not confer a worse prognosis following dose-adjusted EPOCH-R. Leuk Lymphoma. 2017:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swerdlow SH CE, Harris NL, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Bosman FTJE, Lakhani SR, Ohgaki H, editor. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 21.Hegde U, Filie A, Little RF, Janik JE, Grant N, Steinberg SM, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood. 2005;105(2):496502. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86. [DOI] [PubMed] [Google Scholar]
- 23.Wilson WH, Dunleavy K, Pittaluga S, Hegde U, Grant N, Steinberg SM, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large Bcell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26(16):2717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowakowski GS, Blum KA, Kahl BS, Friedberg JW, Baizer L, Little RF, et al. Beyond RCHOP: A Blueprint for Diffuse Large B Cell Lymphoma Research. J Natl Cancer Inst. 2016;108(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson WH, Jung SH, Porcu P, Hurd D, Johnson J, Martin SE, et al. A Cancer and Leukemia Group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica. 2012;97(5):758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Seegmiller AC, Lin P, Wang XJ, Miranda RN, Bhagavathi S, et al. B-cell lymphomas with concurrent MYC and BCL2 abnormalities other than translocations behave similarly to MYC/BCL2 double-hit lymphomas. Mod Pathol. 2015;28(2):20817. [DOI] [PubMed] [Google Scholar]
- 27.Chen AI, Leonard JT, Okada CY, Gay ND, Chansky K, Fan G, et al. Outcomes of DA-EPOCH-R induction plus autologous transplant consolidation for double hit lymphoma. Leuk Lymphoma. 2018;59(8):1884–9. [DOI] [PubMed] [Google Scholar]
- 28.Maurer MJ, Ghesquieres H, Link BK, Jais JP, Habermann TM, Thompson CA, et al. Diagnosis-to-Treatment Interval Is an Important Clinical Factor in Newly Diagnosed Diffuse Large B-Cell Lymphoma and Has Implication for Bias in Clinical Trials. J Clin Oncol. 2018;36(16):1603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer MJ, Ghesquieres H, Link BK, Jais JP, Habermann TM, Thompson CA, et al. Diagnosis-to-Treatment Interval Is an Important Clinical Factor in Newly Diagnosed Diffuse Large B-Cell Lymphoma and Has Implication for Bias in Clinical Trials. J Clin Oncol. 2018:JCO2017765198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.