Abstract
Rumex crispus (Rc) and Cordyceps militaris (Cm) mixture (Rc-Cm; AST2017-01) ameliorated production of proinflammatory cytokines, inflammation-related genes, and nitric oxide (NO) induced by lipopolysaccharide (LPS) in mouse splenocytes. Rc-Cm (6:4) and Taemyeongcheong (commercial healthy drink containing Rc-Cm) were co-administered along with LPS. Rc-Cm inhibited production of tumor necrosis factor α, interferon γ, interleukin (IL)-1β, and IL-6 in LPS-induced splenocytes. However, levels of inflammatory cytokines were elevated in the absence of LPS treatment. Rc-Cm significantly suppressed mRNA expression of IL-1β, IL-6, and the inflammation-related genes inducible NO synthase (iNOS) and cyclooxygenase 2 (COX-2), as well as NO production upon LPS co-treatment. Whereas Rc-Cm increased mRNA expression of IL-1β, and IL-6, but did not up-regulate expression of iNOS and COX-2, or increase NO production without LPS co-treatment. Therefore, treatment of Rc-Cm to LPS-induced splenocytes ameliorated induction of pro-inflammatory cytokines, inflammation-related genes, and NO production. In the absence of LPS, Rc-Cm treatment up-regulated pro-inflammatory cytokines but did not alter expression of the inflammation-related genes iNOS and COX-2 or NO production. These results indicate that the natural phytochemicals chrysophanol and cordycepin in Rc-Cm promote anti-inflammatory activities and immune cell responses.
Keywords: Rumex crispus, Cordyceps militaris, splenocytes, LPS
INTRODUCTION
Chronic inflammatory reactions induce metabolic diseases in various organs, resulting in cancer, obesity, diabetes, cardiovascular disease (CVD), neurodegeneration, and asthma (1). Lipopolysaccharide (LPS) stimulates LPS-binding protein (LBP), toll-like receptor-4 (TLR4) (2,3), and nuclear factor κB (NF-κB). NF-κB promotes production of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, tumor necrosis factor α (TNF-α), type 1 interferons (IFNs) such as IFN-β and IFN-γ and inflammation-related enzymes such as inducible nitric oxide (NO) synthase (iNOS), and cyclooxygenase-2 (COX-2) in the nucleus (4–6). NO has been reported to increase production of iNOS and reactive oxygen species (ROS), resulting in activation of inflammation (7).
Naïve T cells are transformed into four different helper T (Th) cell types (Th1, 2, 17, and Treg) by various cytokines (8). Each Th cell plays a different role depending on its type. Th1 regulates cellular immunity and intracellular pathogen clearance, Th2 regulates humoral immunity, extracellular pathogen clearance, and allergies, Th17 regulates tissue information and autoimmunity, and Treg regulates immune suppression (8). Th1 and Th2 are well-known factors, and the balance between Th1 and Th2 is important (9).
Rumex crispus (Rc) is a perennial plant belonging to the Polygonaceae family. The roots and leaves of Rc have been reported to have functional activities (10). Rc is commonly consumed as a dried or processed herb or used as a medicinal product (11), and is known to inhibit arachidonic acid induced inflammation in mice (12). Rc also protects against liver injury (12) and exhibits antioxidant (13), anti-cancer (14) and anti-obesity (15) effects. Similarly, Cordyceps militaris (Cm) has been used since ancient times as an herbal ingredient (16) and has been reported to have anti-inflammatory and anti-cancer effects (11,17). Both Rc and Cm have been shown to have increased anti-inflammatory effects when mixed at various ratios. Specifically, Rc-Cm mixture (6:4), a product named AST2017-01, was shown to be the most effective in inducing synergistic anti-inflammatory effects in a human mast cell line (HMC-1) (18). Jeong et al. (18) reported that Rc-Cm reduced production of IL-1β, IL-6, and TNF-α in phorbol 12-myristate 13-acetate and calcium ionophore A23187 (PMACI)-treated HMC-1 cells.
Beopje is a processing method consisting of washing, steaming, dehydration, parching (19), and processing of herbal ingredients for removal of anti-nutrients and enhancement of phytochemicals (20). RC made using the Beopje method has been shown to have greater anti-inflammatory effects than that without Beopje in LPS-induced RAW 264.7 cells (data not shown) and mouse splenocytes (21).
Taemyeongcheong (TMC) is a health functional beverage made using traditional methods in Korea, and is commonly found in various foods and herbal ingredients. TMC inhibits the allergic effects induced by 48/80 (22) and acetaminophen-induced hepatic damage in vivo (23). We have previously reported that TMC inhibited Th2 cytokines such as IL-1β, IL-6, and IL-10 as well as iNOS and COX-2 in LPS-induced RAW 264.7 cells (24).
Therefore, we aimed to determine whether the natural product of Rc and Cs contributes to regulation of inflammation dependent on time and the presence of LPS in mice splenocytes. The healthy drink TMC, which contains a Rc-Cm mixture, was used as a positive control. In this study, we investigated the anti-inflammatory and immune cell regulatory responses of Rc-Cm and TMC treatment on pro-inflammatory cytokine protein levels (TNF-α, IFN-γ, IL-1β, and IL-6) and mRNA expression levels (IL-1β, IL-6, iNOS, and COX-2), as well as NO production in LPS-induced mice splenocytes.
MATERIALS AND METHODS
Sample preparation
Rc, Cm, and TMC were provided by Gawha Wellfood Co. (Jincheon, Chungbuk, Korea), and were freeze-dried. The Rc-Cm samples were mixed at a ratio of 6:4. TMC was made from the following ingredients: Rc (13.62%), Cm (11.14%), Saururus chinensis (11.14%), Viscum album (11.14%), Houttuynia cordata (11.14%), Atractylodes ovata (9.90%), Capsella bursa-pastoris (8.67%), Cornus officinalis (8.67%), Phyllostachys bambusoides leaf (7.43%), and Cordyceps militaris (7.15%). Processed Rc-Cm (Beopje) was prepared by washing, steaming, dehydrating, parching, and then dehydrating again. Rc-Cm was made by the same method as AST2017-01 (18).
Ex vivo splenocytes collection and culture
Mouse splenocytes were collected and cultured with slight modification to the methods described in Vitetta et al. (25) and Amrouche et al. (26). Ten 5-week-old C57BL/6 mice were purchased from Orient Bio (Seongnam, Gyeonggi, Korea) and used in the experiment. Mouse spleens were aseptically removed and immersed in Dulbecco Modified Eagle’s Medium (DMEM, Sigma-Aldrich Co., St. Louis, MO, USA). To make single cell suspensions, spleens were chopped into small pieces using sterilized scissors. Cells were centrifuged at 1,500 rpm for 10 min to pellet, and resuspended to free cells. To quantify splenocyte proliferation, cells were counted using a hemocytometer and dispersed at a concentration of 2×106 cells/mL in 6-well plates. Cells were cultured in DMEM containing 10% fetal bovine serum (Sigma-Aldrich Co.) and 100 units/mL of penicillin-streptomycin (PS, Welgene Inc., Gyeongbuk, Korea). Cells were cultured at 37°C with 5% CO2 (20). This experiment was approved by the CHA University Animal Ethics Committee (IACUC-170149).
Quantitation of pro-inflammatory cytokines by enzymelinked immunosorbent assay (ELISA)
Splenocytes plated in 6-well plates were incubated for 24 h. After the medium was removed, 0.05 mg/mL of Rc-Cm and 0.1 mg/mL of TMC were added to each well. To induce inflammation, each well was treated with LPS (2 μg/mL, Sigma-Aldrich Co.). After incubation for 24, 48, and 72 h, the medium was collected. Concentrations of IL-6, IL-1β, IFN-γ, and TNF-α were measured in collected medium using an ELISA kit (BioLegend, San Diego, CA, USA). This experiment was performed according to the method provided by the manufacturer (19).
mRNA quantitation of pro-inflammatory cytokines and inflammation-related genes in splenocytes by real time-quantitative polymerase chain reaction (RT-qPCR)
Splenocytes plated in 6-well plates were incubated for 24 h. After the medium was removed, 0.05 mg/mL of filtered (0.2 μm, GVS Filtration Inc., Bloomer, WI, USA) and freeze-dried Rc-Cm powder and 0.1 mg/mL of filtered (0.2 μm, GVS Filtration Inc.) and freeze-dried TMC powder were added to each well. To induce inflammation, each well was treated with LPS (2 μg/mL). After 48 h incubation, RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The isolated RNA was quantified using a NanoDrop ND-1000 (NanoDrop Technologies Inc., Wilmington, DE, USA). Quantified RNA was reverse transcribed using Superscript II reverse transcriptase (Invitrogen) and synthesized into cDNA. The synthesized cDNA was amplified using a thermal cycler BioRad CFX-96 real time system (BioRad Laboratories, Hercules, CA, USA), and quantified. The primers used amplified 18s rRNA, IL-1β, IL-6, iNOS, and COX-2, and the primer sequences were as follows: 18s rRNA forward 5′-TCG AGG CCC TGT AAT TGG AA-3′ and reverse 5′-CCC TCC AAT GGA TCC TCG TT-3′, IL-1β forward 5′-AAG GGC TGC TTC CAA AC-3′ and reverse 5′-CTC CAC AGC CAC AAT GA-3′, IL-6 forward 5′-ATG AAG TTC CTC TCT GCA A-3′ and reverse 5′-AGT GGT ATC CTC TGT GAA G-3′, iNOS forward 5′-ATG GCT TGC CCC TGG AA-3′ and reverse 5′-TAT TGT TGG GCT GAG AA-3′, COX-2 forward 5′-GGC AGC AAA TCC TTG C-3′ and reverse 5′-TAT TGT TGG GCT GAG AA-3′ (23).
NO production
NO production was measured by the nitrite concentration in the media using Griess reagent (Sigma-Aldrich Co.). Splenocytes plated in 6-well plates were incubated for 24 h. The medium was removed and exchanged for medium without FBS and PS for 24 h to induce starvation. After the medium was removed, 0.05 mg/mL of Rc-Cm and 0.1 mg/mL of TMC were added to each well. To induce inflammation, each well was treated with LPS (2 μg/mL). After 24, 48, and 72 h incubation, the medium was collected. The collected media were treated with Griess reagent, and the absorbance was measured at 550 nm using a Wallac Victor3 1420 Multilabel Counter (Perkin-Elmer, Wellesley, MA, USA) (23).
Statistical analysis
All data are presented as the mean±standard deviation (SD). Differences between the mean values for individual groups were assessed by one-way analysis of variance (ANOVA) of Duncan’s multiple range test. Differences were considered significant when P<0.05. The SPSS version 18 statistical software package (SPSS Inc., Westlands, Hong Kong) was used to perform analyses (24).
RESULTS
Levels of pro-inflammatory cytokines TNF-α, IFN-γ, IL-1β, and IL-6 in mice splenocytes
Treatment with Rc-Cm at a concentration of 0.005~0.05 mg/mL did not significantly affect proliferation of RAW 264.7 cells after 24, 48, or 72 h of incubation (data not shown). In a previous study, TMC showed no cytotoxicity in RAW 264.7 cells at a concentration of 0.01~0.1 mg/mL (24).
TNF-α and IFN-γ
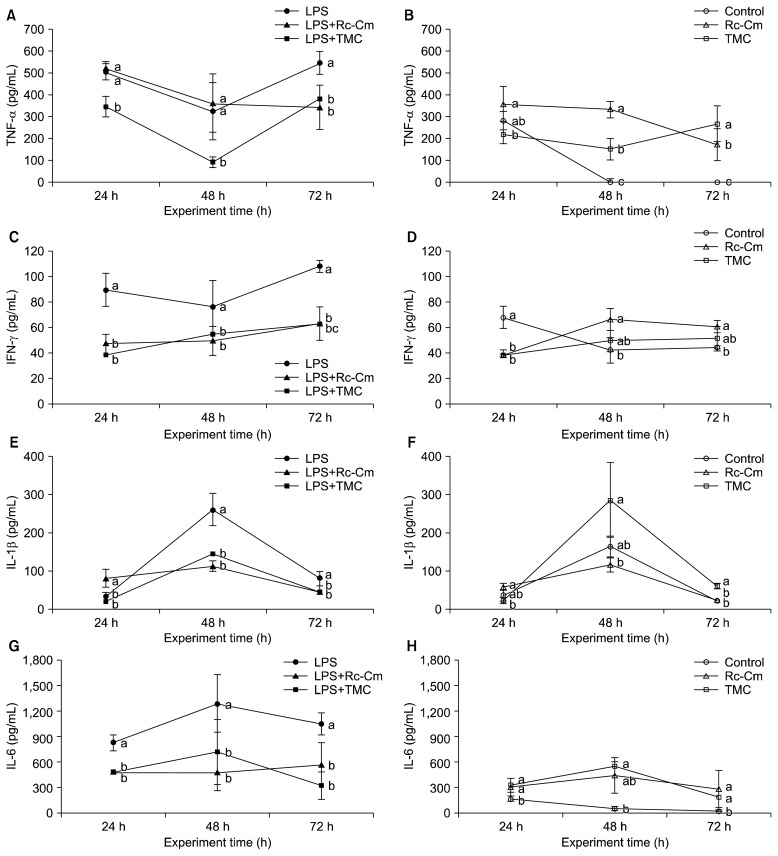
LPS treatment significantly increased TNF-α, and IFN-γ levels compared to the control (P<0.05) (Fig. 1A and 1C). LPS+TMC (91.7±23.9 pg/mL) treatment significantly reduced TNF-α protein levels compared to LPS (322.1±130.4 pg/mL) at 48 h (P<0.05) (Fig. 1A). However, in the absence of LPS, Rc-Cm (334.8±37.0 pg/mL) and TMC (154.2±48.4 pg/mL) treatments significantly increased TNF-α protein levels compared to the control (2.0±2.5 pg/mL) at 48 h (P<0.05) (Fig. 1B). LPS+Rc-Cm (49.5±11.5 pg/mL) and LPS+TMC (54.7±2.4 pg/mL) treatments significantly reduced IFN-γ protein levels compared to LPS (76.5±20.8 pg/mL) at 48 h (P<0.05) (Fig. 1C). However, Rc-Cm (66.6±8.7 pg/mL) treatment significantly increased IFN-γ protein levels compared to the control (42.5±10.3) at 48 h (P<0.05) (Fig. 1D). Moreover, LPS+Rc-Cm treatment resulted in a similar TNF-α level compared to LPS treatment at 48 h.
Fig. 1.
The effect of Rumex crispus (Rc)-Cordyceps militaris (Cm) and Taemyeongcheong (TMC) on the production of pro-inflammatory cytokines of tumor necrosis factor α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-1β, and IL-6 in mice splenocytes with LPS and without LPS at different time points. LPS, 2 μg/mL of LPS; LPS+Rc-Cm, 2 μg/mL LPS+0.05 mg/mL of Rc-Cm; LPS+TMC, 2 μg/mL of LPS+0.1 mg/mL TMC; Control, no treatment; Rc-Cm, 0.05 mg/mL of Rc-Cm; TMC, 0.1 mg/mL of TMC. Means with different letters (a–c) at each time are significantly different (P <0.05) by Duncan’s multiple range test.
IL-1β and IL-6
LPS significantly increased IL-1β and IL-6 levels compared to the control (P<0.05) (Fig. 1E and 1G). LPS+Rc-Cm (113.8±13.8 pg/mL) and LPS+TMC (145.9±11.5 pg/mL) treatments significantly reduced IL-1β protein levels compared to LPS (260.9±42.3 pg/mL) at 48 h (P<0.05) (Fig. 1E), whereas TMC (287.5±97.2 pg/mL) treatment significantly increased IL-1β protein levels compared to the control (165.1±27.1 pg/mL) at 48 h (Fig. 1F). LPS+Rc-Cm (476.8±214.7 pg/mL) and LPS+TMC (720.9±159.8 pg/mL) treatments significantly reduced IL-6 protein levels compared to LPS (1,291.1±339.3 pg/mL) at 48 h (P<0.05) (Fig. 1G), whereas Rc-Cm (446.2±209.4 pg/mL) and TMC (550.4±57.6 pg/mL) treatments significantly increased IL-6 protein levels compared to the control (54.2±21.3 pg/mL) at 48 h (P<0.05) (Fig. 1H). Thus, Rc-Cm and TMC reduced production of pro-inflammatory cytokines in LPS-induced splenocytes but increased pro-inflammatory cytokine production in naïve splenocytes.
mRNA expression levels of pro-inflammatory cytokines IL-1β and IL-6 in mice splenocytes
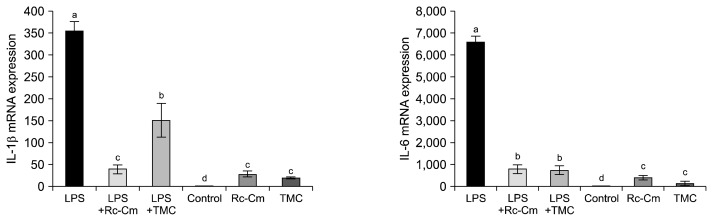
Previously, we reported that TMC inhibited Th2 cytokines in RAW 264.7 cells (21). Therefore, in this study, we investigated whether Rc-Cm and TMC interfere with mRNA expression of the Th2 cytokines IL-1β and IL-6 in splenocytes. The mRNA expression levels of IL-1β and IL-6 are shown in Fig. 2. Rc-Cm and TMC significantly increased IL-1β and IL-6 levels compared to the control, which is similar to the results shown in Fig. 1. LPS increased mRNA levels of these cytokines in splenocytes compared to the control (P<0.05). In addition, LPS+Rc-Cm and LPS+TMC significantly reduced expression levels of IL-1β (0.27 and 0.08) and IL-6 (0.12 and 0.13) compared to LPS (1.00) (P<0.05). However, expression levels of pro-inflammatory cytokines were significantly elevated in the absence of LPS (Fig. 2).
Fig. 2.
mRNA levels of pro-inflammatory cytokines interleukin (IL)-1β and IL-6 in mice splenocytes at 48 h. LPS, 2 μg/mL of lipopolysaccharide (LPS); LPS+Rc-Cm 2 μg/mL LPS+0.05 mg/mL of Rumex crispus (Rc)-Cordyceps militaris (Cm); LPS+TMC, 2 μg/mL of LPS+0.1 mg/mL Taemyeongcheong (TMC); Control, no treatment; Rc-Cm, 0.05 mg/mL of Rc-Cm; TMC, 0.1 mg/mL of TMC. The mRNA expression levels were calculated based on 18s rRNA, which was used as a control (control fold ratio=1). Means with different letters (a–d) on the bar are significantly different (P <0.05) by Duncan’s multiple range test.
mRNA expression of inflammation-related genes in mice splenocytes
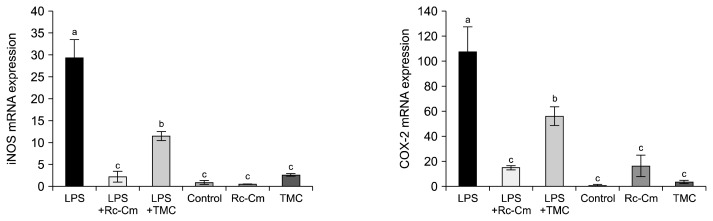
mRNA expression levels of iNOS and COX-2 are shown in Fig. 3. Rc-Cm and TMC treatments did not significantly affect iNOS and COX-2 expression compared to the control. LPS-induced mice splenocytes showed significantly increased mRNA levels compared to the control. LPS+Rc-Cm and LPS+TMC treatments significantly reduced iNOS (0.39 and 0.08) and COX-2 (0.52 and 0.14) levels compared to LPS (1.00) (P<0.05). Thus, Rc-Cm and TMC reduced inflammation-related genes in LPS-induced splenocytes but not in naïve splenocytes.
Fig. 3.
mRNA levels of inflammation-related genes inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) in mice splenocytes at 48 h. LPS, 2 μg/mL of lipopolysaccharide (LPS); LPS+Rc-Cm 2 μg/mL LPS+0.05 mg/mL of Rumex crispus (Rc)-Cordyceps militaris (Cm); LPS+TMC, 2 μg/mL of LPS+0.1 mg/mL Taemyeongcheong (TMC); Control, no treatment; Rc-Cm, 0.05 mg/mL of Rc-Cm; TMC, 0.1 mg/mL of TMC. The mRNA expression levels were calculated based on 18s rRNA, which was used as a control (control fold ratio=1). Means with different letters (a–c) on the bar are significantly different (P <0.05) by Duncan’s multiple range test.
NO production in mice splenocytes
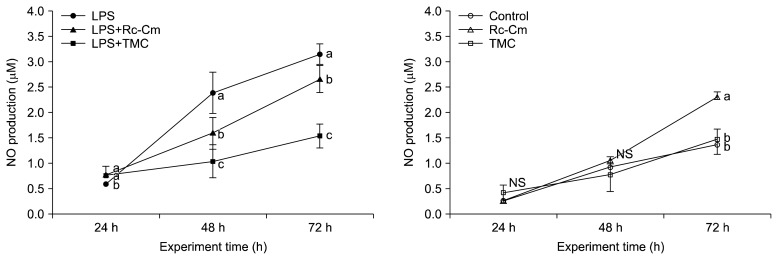
LPS-treated splenocytes showed significant time-dependent elevation of NO production compared to the control (Fig. 4). Splenocytes treated with LPS+Rc-Cm (1.59±0.31 μM) and LPS+TMC (1.04±0.33 μM) showed significantly lower NO levels than LPS-treated splenocytes (2.39±0.51 μM) at 48 h (P<0.05). Rc-Cm (1.06±0.07 μM) and TMC (0.79±0.34 μM) treatments did not significantly affect NO levels compared to the control (0.93±0.08 μM) at 48 h.
Fig. 4.
The effect of Rumex crispus (Rc)-Cordyceps militaris (Cm) and Taemyeongcheong (TMC) on the production of nitric oxide (NO) in mice splenocytes with lipopolysaccharide (LPS) (A) and without LPS (B) at different time points. LPS, 2 μg/mL of LPS; LPS+Rc-Cm 2 μg/mL LPS+0.05 mg/mL of Rc-Cm; LPS+TMC, 2 μg/mL of LPS+0.1 mg/mL TMC; Control, no treatment; Rc-Cm, 0.05 mg/mL of Rc-Cm; TMC, 0.1 mg/mL of TMC. Means with different letters (a–c) at each time are significantly different (P <0.05) by Duncan’s multiple range test. NS, not significantly different.
DISCUSSION
LPS stimulates mammalian cells to interact with multiple proteins such as LBP and TLR4 (2,3). TLR4 activated by LPS induces NF-κB, and activated NF-κB promotes transcription of the pro-inflammatory cytokines IL-1β, IL-6, TNF-α, type 1 IFNs, iNOS, and COX-2 in the nucleus (4–6). NO is recognized as one of the most multifunctional players in the immune system and is derived from the amino acid L-arginine via the enzymatic activity of iNOS (27). Patients with cancer, obesity, and type 2 diabetes exhibit increased circulating levels of IL-1, IL-6, and TNF-α (28,29), and NO is involved in tumor formation, chronic degenerative disease, autoimmune processes, and control of infectious diseases (27). Upon initiation of inflammation, an immune response is triggered, and a variety of immune cells are activated. Th cells are important in the immune response and produce various cytokines (30). Th can be classified based on the type of cytokine they produce. Th1 produce TNF-α, IFN-γ, and IL-12, whereas Th2 produce IL-4, IL-5, IL-6, and IL-10 (30). IL-1β stimulates Th2 and promotes cytokine production by Th2 (31).
Rc-Cm inhibited the pro-inflammatory cytokines TNF-α and IFN-γ for Th1 and IL-1β and IL-6 for Th2. These results indicate that Rc-Cm suppresses pro-inflammatory cytokines activated by LPS and reduced inflammation. It was previously reported that TMC reduces production of Th2 cytokines (IL-1β, IL-6, and IL-10) (24). Thus, a health beverage containing Rc-Cm (TMC) may inhibit both Th1 and Th2 inflammatory activities through suppression of pro-inflammatory cytokines.
Rc has previously been shown to inhibit inflammation in arachidonic acid and carrageenan-induced mice (12). Cm has been shown to inhibit iNOS and COX-2 in rats (32) and as iNOS and COX-2 in LPS-induced RAW 264.7 cells (33). In this study, Rc-Cm and TMC suppressed iNOS and COX-2 mRNA expression and NO production in LPS-induced splenocytes. Rc-Cm demonstrated better anti-inflammatory effects than TMC (Fig. 2 and 3) due to differences between the ingredient mixture and healthy drink. These results indicate that Rc-Cm reduced production of both of Th1 and Th2 cytokines and decreased expression of the inflammation-related genes iNOS and COX-2 in LPS-induced mice splenocytes. Rc-Cm in TMC are likely to be the major ingredients with anti-inflammatory effects.
Rc with processing (120 μg/g) showed increased production of the active compound chrysophanol compared to Rc without Beopje (70.8 μg/g) (data not shown). Cm contains cordycepin, which has been shown to suppress phytohemagglutinin-induced inflammation in peripheral blood mononuclear cells (34). Joeng et al. (18) reported that a 6:4 ratio of Rc-Cm and chrysophanol had anti-inflammatory effects in PMACI-treated HMC-1 cells. Thus, a 6:4 ratio had synergistic anti-inflammatory effects since chrysophanol and cordycepin regulate inflammation. Based on these results, a 6:4 ratio and phytochemicals of chrysophanol and cordycepin in Rc-Cm are optimal for development of immunoregulatory (atopic dermatitis, asthma, and allergy) products.
Rc has been shown to reduce NO production in LPS-induced RAW 264.7 cells (35), and Cm has been shown to reduce NO production in rat brain microvascular endothelial cells injured by oxygen-glucose deprivation (36). In a previous study, TMC reduced NO production in RAW 264.7 cells (21). In this study, Rc-Cm decreased NO production in LPS-induced splenocytes at 48 and 72 h. Therefore, Rc-Cm suppressed pro-inflammatory cytokines, iNOS, COX-2, and NO production activated by LPS. Thus, Rc-Cm decreased inflammatory stimuli in toxic agent (i.e., LPS)-induced immune cells.
Rc-Cm and TMC increased cytokine levels in mouse splenocytes but had no effect on production of iNOS, COX-2, and NO in the absence of LPS. Compound A induced Th2 cell activity in splenocytes in the absence of LPS (37). In addition, naringenin activated Th cells in splenocytes in the absence of LPS (38). Therefore, Rc-Cm stimulated pro-inflammatory cytokine production in splenocytes in the absence of LPS. Taken together, Rc-Cm inhibited toxic agent (i.e., LPS)-induced inflammation but promoted immune cell activities. Elevation of immune cell activities did not affect inflammation since levels of iNOS, COX-2, and NO production were not increased in mouse splenocytes in the absence of LPS. Therefore, Rc-Cm can regulate inflammation and immune cell responses.
In conclusion, Rc-Cm decreased production of the LPS-induced pro-inflammatory cytokines TNF-α, IFN-γ, IL-1β, and IL-6 and inhibited expression of LPS-induced inflammation-related enzymes iNOS and COX-2, and NO production. However, Rc-Cm regulated immune cell (Th1 and Th2) responses were not associated with elevation of inflammation-related factors in mouse splenocytes in the absence of LPS. These results indicate that Rc-Cm is rich in health beneficial phytochemicals (chrysophanol and cordycepin), and that these phytochemicals regulate LPS-induced inflammation and immune cell responses. Therefore, consumption of the natural anti-inflammation ingredients Rc and Cm could be effective for treatment of atopic dermatitis, asthma, and allergies, and they could be used to make various products (drugs, beverages, etc.).
ACKNOWLEDGEMENTS
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program (or Project), funded by Ministry of Agriculture, Food and Ruarl Affairs (MAFRA) (116169-3).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2007;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 2.Gioannini TL, Weiss JP. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol Res. 2007;39:249–260. doi: 10.1007/s12026-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 3.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 4.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 6.Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, Kim DS, Park JS, Cho HJ. Spinal NF-κB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci. 2004;19:3375–3381. doi: 10.1111/j.0953-816X.2004.03441.x. [DOI] [PubMed] [Google Scholar]
- 7.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 8.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 10.Chang SW, Kim IH, Han TJ. Anthraquinone productivity by the cultures of adventitious roots and hairy from curled dock (Rumex crispus) Korean J Plant Tissue Culture. 1999;26:7–14. [Google Scholar]
- 11.Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- 12.Lee SS, Kim DH, Yim DS, Lee SY. Anti-inflammatory, analgesic and hepatoprotective effect of semen of Rumex crispus. Korean J Pharmacogn. 2007;38:334–338. [Google Scholar]
- 13.Jeong GT, Lee KM, Park DH. Study of antimicrobial and antioxidant activities of Rumex crispus extract. Korean Chem Eng Res. 2006;44:81–86. [Google Scholar]
- 14.Shiwani S, Singh NK, Wang MH. Carbohydrase inhibition and anti-cancerous and free radical scavenging properties along with DNA and protein protection ability of methanolic root extracts of Rumex crispus. Nutr Res Pract. 2012;6:389–395. doi: 10.4162/nrp.2012.6.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SJ, Choi JH, Jung YS, Yu MH. Inhibitory effect of Rumex crispus L. fraction on adipocyte differentiation in 3T3-L1 cells. Korean J Food Sci Technol. 2013;45:90–96. doi: 10.9721/KJFST.2013.45.1.90. [DOI] [Google Scholar]
- 16.Zhu JS, Halpern GM, Jones K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: part I. J Altern Complement Med. 1998;4:289–303. doi: 10.1089/acm.1998.4.3-289. [DOI] [PubMed] [Google Scholar]
- 17.Rao YK, Fang SH, Tzeng YM. Evaluation of the anti-inflammatory and anti-proliferation tumoral cells activities of Antrodia camphorata, Cordyceps sinensis, and Cinnamomum osmophloeum bark extracts. J Ethnopharmacol. 2007;114:78–85. doi: 10.1016/j.jep.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Jeong HJ, Kim HY, Kim HM. Molecular mechanisms of anti-inflammatory effect of chrysophanol, an active component of AST2017-01 on atopic dermatitis in vitro models. Int Immunopharmacol. 2018;54:238–244. doi: 10.1016/j.intimp.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Kim YG, Park KY. Inhibitory effects of ginger and processed (Beopje) ginger extracts on HCl-ethanol induced gastritis in rats. J Korean Soc Food Sci Nutr. 2012;41:1528–1533. doi: 10.3746/jkfn.2012.41.11.1528. [DOI] [Google Scholar]
- 20.Lee YM, Kim JS. Studies on the processing of herbal medicines (VI)–HPLC analysis of standard compounds of unprocessed and processed herbal medicines–. Korean J Orient Med. 2003;9:69–72. [Google Scholar]
- 21.Park ES, Song GH, Lee SM, Kim TY, Park KY. Increased anti-inflammatory effects of processed curly dock (Rumex crispus L.) in ex vivo LPS-induced mice splenocytes. J Korean Soc Food Sci Nutr. 2018;47:599–604. doi: 10.3746/jjkfn.2018.47.5.599. [DOI] [Google Scholar]
- 22.Ryu KJ, Yoou MS, Park KY. Inhibitory effect of Taemyeongcheong on allergic reactions. Tang. 2016;6:33–37. [Google Scholar]
- 23.Yi RK, Song JL, Lim YI, Kim YK, Park KY. Preventive effect of the Korean traditional health drink (Taemyeongcheong) on acetaminophen-induced hepatic damage in ICR mice. Prev Nutr Food Sci. 2015;20:52–59. doi: 10.3746/pnf.2015.20.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song GH, Park ES, Lee SM, Kim TY, Park KY. An atopic preventive drink (APD) reduces Th2 cytokines in LPS-treated RAW 264.7 cells. Tang. 2017;7:15.1–15.6. [Google Scholar]
- 25.Vitetta ES, Baur S, Uhr JW. Cell surface immunoglobulin: II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. J Exp Med. 1971;134:242–264. doi: 10.1084/jem.134.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amrouche T, Boutin Y, Prioult G, Fliss I. Effects of bi-fidobacterial cytoplasm, cell wall and exopolysaccharide on mouse lymphocyte proliferation and cytokine production. Int Dairy J. 2006;16:70–80. doi: 10.1016/j.idairyj.2005.01.008. [DOI] [Google Scholar]
- 27.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 28.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creely SJ, McTernan PG, Kusminski CM, Fisher fM, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 30.Grewe M, Bruijnzeel-Koomen CA, Schöpf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998;19:359–361. doi: 10.1016/S0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 31.Helmby H, Grencis RK. Interleukin 1 plays a major role in the development of Th2-mediated immunity. Eur J Immunol. 2004;34:3674–3681. doi: 10.1002/eji.200425452. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Li P, Zhao D, Tang H, Guo J. Anti-inflammation effects of Cordyceps sinensis mycelium in focal cerebral ischemic injury rats. Inflammation. 2011;34:639–644. doi: 10.1007/s10753-010-9273-5. [DOI] [PubMed] [Google Scholar]
- 33.Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, Han SK, Park SM, Park JH, Park HI, Sung JM, Jang Y, Chung N, Hwang KC, Kim TW. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006;545:192–199. doi: 10.1016/j.ejphar.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Luo L, Dressel W, Shadier G, Krumbiegel D, Schmidtke P, Zepp F, Meyer CU. Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis. Am J Chin Med. 2008;36:967–980. doi: 10.1142/S0192415X08006387. [DOI] [PubMed] [Google Scholar]
- 35.Park JA, Choi MO. Antimicrobial activity and anti-inflammation effect to the human skin pathogens by the Rumex crispus L. root extracts. Korean J Anaesth Cosmetol. 2011;9:9–16. [Google Scholar]
- 36.Bai X, Tang Y, Lin Y, Zhao Y, Tan T, Wang S, Liu M, Chang Z, Liu Y, Liu Z. Protective effect of Cordyceps sinensis extract on rat brain microvascular endothelial cells injured by oxygen-glucose deprivation. J Tradit Chin Med Sci. 2018;5:64–71. [Google Scholar]
- 37.Liberman AC, Antunica-Noguerol M, Ferraz-de-Paula V, Palermo-Neto J, Castro CN, Druker J, Holsboer F, Perone MJ, Gerlo S, De Bosscher K, Haegeman G, Arzt E. Compound A, a dissociated glucocorticoid receptor modulator, inhibits T-bet (Th1) and induces GATA-3 (Th2) activity in immune cells. PLoS One. 2012;7:e35155. doi: 10.1371/journal.pone.0035155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang HK, Yeh CH, Iwamoto T, Satsu H, Shimizu M, Totsuka M. Dietary flavonoid naringenin induces regulatory T cells via an aryl hydrocarbon receptor mediated pathway. J Agric Food Chem. 2012;60:2171–2178. doi: 10.1021/jf204625y. [DOI] [PubMed] [Google Scholar]