Abstract
Volatile flavor compounds created in the mixture of enzymatic hydrolysates of soy sauce residue and defatted soybean by reaction flavor technology (RFT) were analyzed and compared using solid phase micro-extraction/gas chromatography/mass selective detector to develop a seasoning sauce. Using response surface methodology analysis, RFT was performed by adding 0.50% fructose, 0.33% glutamic acid, 0.68% arginine, 0.37% methionine, and 0.86% glycine in the base and reaction conditions at 93°C for 120 min. A total of 57 compounds, 43 in RFT and 45 in control, were detected, including 8 aldehydes and ketones, 6 aromatic hydrocarbons, 3 acids, 12 alcohols, 6 esters, 4 furans, 9 nitrogen-containing compounds, 4 sulfur-containing compounds, and 5 miscellaneous. In RFT samples, aldehydes and ketones, aromatic hydrocarbons, alcohols, esters, and S-containing compounds were significantly increased. Sulfur-containing compounds were increased by 687 fold compared to control samples (P<0.05). Among these, the main contributors to the aroma activity of RFT samples were considered to be, with a very low threshold, the newly generated compounds, dimethyl disulfide (roasted onion/garlic-like/meaty odor), dimethyl trisulfide (roasted garlic-like/meaty odor), and methional (roasted potato/potato chip-like odor).
Keywords: soy sauce residue, enzymatic hydrolysate, response surface methodology, reaction flavor
INTRODUCTION
Soy sauce has been flavored as a liquid condiment for a long time in Asia. In Korea, soy sauces can be roughly split into two categories by their ingredients: Korean-style soy sauce (hansik ganjang) and modernized soy sauce (gaeryang ganjang) (1,2). The Korean Ministry of Food and Drug Safety Food Code classifies modernized soy sauce into four categories by their different processing methods: brewed soy sauce, acid-hydrolyzed soy sauce, enzyme-hydrolyzed soy sauce, and blended soy sauce (1,2). Brewed soy sauce is traditionally made from soybean and wheat. Nowadays, defatted soybean is widely used as a cost-saving ingredient because the necessary component for soy sauce production is the protein, not the oil. The rich fermented soybean flavor and the savory soy sauce taste make brewed soy sauce widely accepted by consumers. The consumption of brewed soy sauce has shown rapid growth, and the Korea Agro-Fisheries and Food Trade Corporation has reported that consumption in the domestic market was 64,500 tons in 2014 (3).
Soy sauce residue (SSR) is a by-product of the brewed soy sauce manufacturing process. However, it is usually discarded without suitable application or is partially used as feedstuff. Assuming 13~15% of total soy sauce is converted to SSR as a by-product, approximately 9,000 tons of SSR are produced every year by the soy sauce industry of Korea. SSR still contains many nutrients such as crude protein (23.09 g/100 g) and amino-N (788.00 mg/100 g) (4).
Chen et al. (5) investigated the hydrolysis efficiency of SSR using ultrasonic probe-assisted enzymolysis technology. Gao et al. (6) researched the isolation, identification and amino acid composition of proteins in SSR. However, most previous studies on SSR have focused on the extraction of high value-added compounds and the hydrolysis condition. There is limited research on SSR protein as an effective resource. If SSR can be used to partly replace defatted soybean in the production of seasoning sauce, and the sensory quality of the product made with SSR can be improved, this should prove advantageous for reducing the cost of developing flavoring agents.
Reaction flavor technology (RFT) has been widely used in food production for improving sensory quality (7–9). Kim and Kim (7) developed meat flavor extract by using non-enzymatic browning of reaction precursors. Using reaction flavor, Kim and Beak (8) developed a baked beef flavor, and Kim et al. (9) developed a boiled-type shrimp flavor. These thermally induced flavor compounds are mainly concerned with development of cooked, nutty, and meat-like odor notes, and mainly consisted of pyrazines, aldehydes, and sulfur-containing compounds (10). Hwang et al. (11) studied the relative reactivities of amino acids in pyrazine formation using glycine, glutamic acid, and arginine, and reported that a large amount of pyrazines are generated by adding glycine. Hou et al. (12) also reported that glycine is the simplest amino acid to generate pyrazines from and is usually used to induce meat-like flavor, and Yu and Ho (13) reported that sulfur-containing compounds can be generated from a thermal reaction of methionine. Therefore, the objective of this study was to develop and compare reaction flavor compounds in seasoning sauce by applying RFT to the base of enzymatic hydrolysates of SSR and defatted soybean (DS).
MATERIALS AND METHODS
Materials
SSR (moisture: 32.91%, crude protein: 23.09%, crude lipid: 7.68%, and crude ash: 8.85%) (4) and DS (moisture: 6.95%, crude protein: 51.58%, crude lipid: 1.35%, and crude ash: 6.12%) were donated by Sungsim Master Food Co. (Changnyeong, Korea). SSR was packaged by 2-layer polyethylene film, stored in a low temperature room (5~8°C), and crushed before use. DS was crushed and boiled in an autoclave (Gaon Science, Bucheon, Korea) at 120°C for 15 min, dried, crushed again, and stored in a low temperature room (5~8°C) until use. The commercial proteases Alcalase® 2.4 L, Protamex®, and Flavourzyme® 500 MG were produced by Novozymes (Copenhagen, Demark) and donated by Biosis Co., Ltd. (Busan, Korea). The amino acids arginine (purity: >98.5%, Ajinomoto Co., Inc., Tokyo, Japan), glycine (purity: >98.5%, Ajinomoto Co.), methionine (purity: >99.0%, DL-methionine, Dongeun Co., Pyeongtek, Korea), glutamic acid (purity: >99.0%, Dongeun Co.), and fructose (ADM Decatur, Decatur, IL, USA) used for reaction flavor experiments were purchased from a local food additives shop.
Preparation for the enzymatic hydrolysates of SSR and DS
To enzymatically hydrolyze SSR and DS, three commercial enzymes (Alcalase® 2.4 L, Protamex®, and Flavourzyme® 500 MG) were selected and used under their respective optimum conditions, as provided by Novozymes (14). Hydrolysis was conducted with a double-jacket reactor made of pyrex (500 mL capacity, custom-production). Optimal hydrolysis conditions for SSR have been described by Cha and Wang (14) and are as follows: combine 8.79% (w/v) SSR and 100 mL distilled water in a reactor, adjust pH to 7.0 using 0.1 N NaOH, hydrolyze with 0.40% (v/w) Alcalase® 2.4 L at 50°C under agitation. After 2 h, 0.43% of mixed enzymes (Flavourzyme ® 500 MG : Protamex®=1:1 w/w) are put into the reactor and hydrolyzed for 4.43 h. Optimal hydrolysis conditions described for DS by Cha and Wang (15) are as follows: combine 10% (w/w) pretreated DS powder, 0.40% (w/w) Protamex®, and 100 mL distilled water in a reactor, hydrolyze at 50°C for 4 h under agitation, hydrolyze with 0.40% (w/w) Flavourzyme® 500 MG for 5 h. The reactants are then inactivated at 98°C for 10 min and the enzymatic hydrolysates of SSR and DS were obtained after filtration. The mixture of hydrolysates from SSR and DS (1:1, v/v) were used as a base for sensory evaluation (data not shown).
Establishment of optimal reaction flavor conditions by response surface methodology (RSM)
To induce formation of reaction flavor in the base, four amino acids such as glutamic acid, methionine, glycine, and arginine, and the sugar fructose were selected through sensory evaluation (odor) in the preliminary experiments.
The optimal reaction conditions were established by RSM and consist of a central composite design with three independent variables coded at five levels (−2, −1, 0, 1, and 2), as described by Gontard et al. (16). The independent variables were set as the concentration of arginine, methionine, and glycine (Table 1). The dependent variables were expressed as scores of odor and taste from 19 treatments by quantitative description analysis (QDA).
Table 1.
Coded level of independent variables in experimental design1)
| Coded units | Arg | Met | Gly |
|---|---|---|---|
| −2 | 0.67 | 0.17 | 0.33 |
| −1 | 0.83 | 0.25 | 0.67 |
| 0 | 1.00 | 0.33 | 1.00 |
| 1 | 1.17 | 0.42 | 1.33 |
| 2 | 1.33 | 0.50 | 1.67 |
The content (w/v) was g, % of each additive per 100 mL of the mixture of enzymatic hydrolysates of soy sauce residue and defatted soybean (1:1 ratio, v/v).
Two reactants, 0.33% (w/v) glutamic acid and 0.50% (w/v) fructose, were selected as the precursors in preliminary experiments, and were put into the base in advance. After adding optimal concentrations of the other 3 amino acids (Arg, Met, and Gly), thermal reaction was carried out at 93°C for 2 h.
QDA
Odor and taste profiles for QDA were assessed by a 10-member panel (2 males and 8 females), consisting of undergraduates, graduates and staff, who had trained for over 3 months. The panelists first analyzed the samples individually with two commercial seasoning sauces (S and D companies) as references. The panel leader recorded and compiled the results. Three to five open discussions were held to clarify the QDA results, and five expressions of odors and tastes about target samples were established. The scores for odor and taste notes were based on a 9-point scale (1: not detectable, 9: extremely intense). A transparent glass cup (60 mL capacity) was used as the sample vessel and the samples were coded with 3 randomly selected 3-digit numbers. Participants were served a cup of water to rinse after the test. All procedures were approved by the institution review board of Changwon National University (104027-201607-HR-016).
Solid phase microextraction (SPME)/gas chromatography (GC)/mass selective detector (MSD) analysis
A SPME device was used with a SupelcoTM solid phase microextraction fiber holder (Supelco Inc., Bellefonte, PA, USA) and polydimethylsiloxane/divinylbenzene fiber (0.65 μm coating thickness, Supelco Inc.). The sample (6 mL) and 1 μL hexyl acetate (91.11 ng) (Sigma-Aldrich Co., St. Louis, MO, USA) as an internal standard (IS) compound were put into a 20 mL headspace glass vial (Supelco Inc.). Extraction was carried out at 40°C for 25 min using a magnetic stirrer. Further details have been described by Cha et al. (4). Extractions were performed on each sample in triplicate.
The GC/MS system consisted of a Perkin Elmer clarus 600T GC/MSD (Perkin Elmer Inc., Fremont, CA, USA) with splitless mode equipped with a DB-WAXTM capillary column (60 m length×0.25 mm I.D.×0.25 μm film thickness, J&W Scientific, Folsom, CA, USA). The oven temperature was initially programmed at 40°C (held for 5 min) and then increased to 220°C (held for 10 min) at a rate of 4°C/min. The MSD condition was as follows: He carrier gas flow 1.0 cm/s; capillary direct interface temperature 220°C; ion source temperature 204°C; ionization energy 70 eV; mass range 33~350 amu; electron multiplier voltage 1,500 V.
Identification and relative abundance of volatile flavor compounds
Volatile flavor compounds were identified by comparing retention indices (RI) and using NIST (version 2.0 g, The National Institute of Standards and Technology, Gaithersburg, MD, USA) standard MS library data (Perkin Elmer Inc.). The concentration (ng/g) of tentatively identified compounds were calculated as a relative content to the amount of IS put into the samples.
Statistical analysis
Statistical analysis of RSM design data was analyzed through Statistical Analysis System version 19 (SAS institute Inc., Cary, NC, USA). Statistical analysis of other experimental data was conducted using SPSS (version 22.0, SPSS Inc., Chicago, IL, USA), with a 95% confidence level used to indicate statistical significance.
RESULTS AND DISCUSSION
Establishment of optimal reaction flavor conditions by RSM
Scores of odor and taste from the 19 treatments are shown in Table 2. In the QDA analysis, five kinds of odor (savory, smoke, nutty, salty, and sweet) and five kinds of taste (savory, dried shrimp-like, nutty, smoke, and acidic) were described during the sensory evaluation (data not shown). During SAS analysis, savory for odor and dried shrimp-like for taste were considered suitable for RSM and were therefore chosen for analysis.
Table 2.
Response surface methodology (RSM) of dependent variables for making seasoning sauce from the mixture of enzymatic hydrolysates of soy sauce residue (SSR) and defatted soybean (DS)
| Design point | Independent variables1) | Dependent variables2) | |||
|---|---|---|---|---|---|
|
|
|
||||
| Arg | Met | Gly | Odor | Taste | |
| 1 | −1 | −1 | −1 | 4.97±0.21 | 4.93±0.35 |
| 2 | 1 | −1 | −1 | 4.37±0.15 | 5.10±0.72 |
| 3 | −1 | 1 | −1 | 5.33±0.42 | 4.73±0.25 |
| 4 | 1 | 1 | −1 | 4.77±0.12 | 5.10±0.17 |
| 5 | −1 | −1 | 1 | 5.20±0.46 | 5.20±0.26 |
| 6 | 1 | −1 | 1 | 5.07±0.25 | 5.47±0.42 |
| 7 | −1 | 1 | 1 | 5.23±0.32 | 4.87±0.15 |
| 8 | 1 | 1 | 1 | 5.00±0.61 | 5.23±0.15 |
| 9 | 2 | 0 | 0 | 4.50±0.52 | 5.23±0.31 |
| 10 | −2 | 0 | 0 | 5.67±0.32 | 4.33±0.12 |
| 11 | 0 | 2 | 0 | 4.97±0.47 | 5.03±0.45 |
| 12 | 0 | −2 | 0 | 5.13±0.06 | 4.83±0.31 |
| 13 | 0 | 0 | 2 | 5.10±0.10 | 5.03±0.65 |
| 14 | 0 | 0 | −2 | 4.63±0.59 | 5.00±0.17 |
| 15 | 0 | 0 | 0 | 4.50±0.30 | 4.90±0.44 |
| 16 | 0 | 0 | 0 | 4.50±0.26 | 4.70±0.26 |
| 17 | 0 | 0 | 0 | 4.47±0.25 | 4.77±0.15 |
| 18 | 0 | 0 | 0 | 4.50±0.15 | 4.80±0.52 |
| 19 | 0 | 0 | 0 | 4.70±0.35 | 4.60±0.81 |
The coded levels of independent variables are the same as represented in Table 1.
Odor, savory odor value; Taste, dried shrimp-like taste value (mean value, n=3).
Odor and taste values analyzed using SAS and statistical results are shown in Table 3. Considering that the purpose of this study was to generate reaction flavor from the mixture of enzymatic hydrolysates from SSR and DS (1:1 ratio, v/v), we focused on odor over taste with RFT, since adjusting taste is possible using food additives in later process whereas flavor is hard to change. The odor results are mainly discussed here for establishing the optimal conditions of reaction flavors.
Table 3.
Model coefficients estimated by multiple linear regression for dependent variables for making seasoning sauce from the mixture of enzymatic hydrolysates of soy sauce residue and defatted soybean
| Factor | Odor | Taste |
|---|---|---|
| Constant | 4.558** | 4.799** |
| Linear | ||
| [Arg] | −0.241** | 0.186* |
| [Met] | 0.025 | −0.023 |
| [Gly] | 0.125** | 0.061 |
| Quadratic | ||
| [Arg]2 | 0.147** | 0.023 |
| [Met]2 | 0.138* | 0.061 |
| [Gly]2 | 0.092* | 0.082 |
| Crossproduct | ||
| [Arg]×[Met] | −0.008 | 0.036 |
| [Arg]×[Gly] | 0.100 | 0.011 |
| [Met]×[Gly] | −0.100 | −0.046 |
| Model | ||
| Linear | <0.001 | 0.030 |
| Quadratic | 0.002 | 0.275 |
| Crossproduct | 0.134 | 0.882 |
| R-Square | 0.915 | 0.682 |
| Total model | 0.001 | 0.136 |
| Lack of fit | 0.113 | 0.062 |
P <0.05 and
P <0.01.
The model coefficients are shown in Table 3. In linear, [Arg] and [Gly] were significant at a 99% confidence level; in quadratic, [Arg]2 was significant at a 99% confidence level, and [Met]2 and [Gly]2 were significant at a 95% confidence level; neither were considered significant in crossproduct. This response model was determined as an adequate model because R2 was 0.915, near to 1. Lack of fit (0.113) showed P>0.05 and the total model (0.001) showed P<0.05. Therefore, the model equation obtained from the SAS result was: odor score =4.558−0.241[Arg]+0.025[Met]+0.125[Gly]+0.147 [Arg]2+0.138[Met]2+0.092[Gly]2−0.008[Arg][Met]+0.100[Arg][Gly]−0.100[Met][Gly]. The predicted odor score was 4.27 during the stationary point. From the ridge analysis for accepting high scores, the optimal flavoring conditions were set as 0.68% (w/v) arginine, 0.37% (w/v) methionine and 0.86% (w/v) glycine of the base, and a high odor score (5.66) was obtained.
In conclusion, the optimal reaction flavor conditions were as follows: the optimum condition of the precursors was 0.33% (w/v) glutamic acid, 0.50% (w/v) fructose, 0.68% (w/v) arginine, 0.37% (w/v) methionine, and 0.86% (w/v) glycine. The reactions were then carried out in a shaking water bath for 2 h at 93°C.
Volatile flavor compounds of seasonings produced with and without RFT
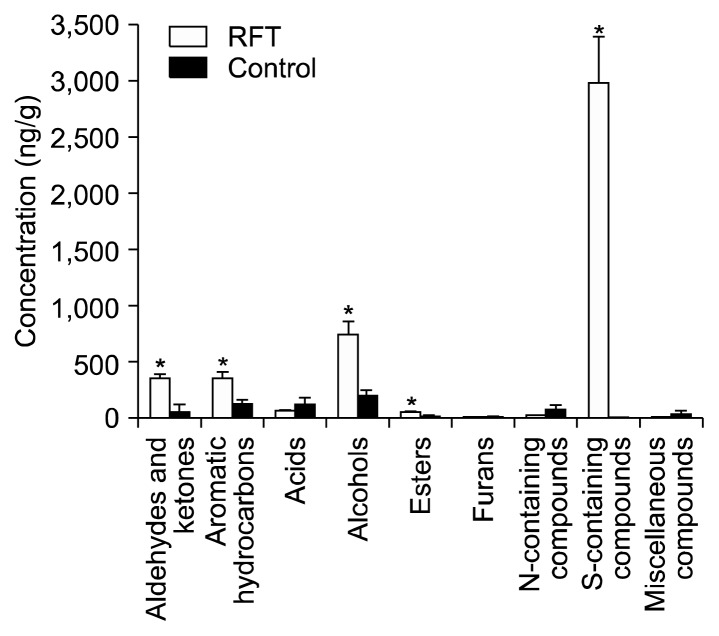
Volatile compounds in the seasonings produced with and without RFT were analyzed by SPME and GC/MSD. A total of 57 compounds, 43 in RFT and 45 in control, were detected in samples, including 8 aldehydes and ketones, 6 aromatic hydrocarbons, 3 acids, 12 alcohols, 6 esters, 4 furans, 9 nitrogen-containing compounds, 4 sulfur-containing compounds and 5 miscellaneous compounds (Table 4). Comparison of the mean amounts of each compound group detected with and without RFT are shown in Fig. 1.
Table 4.
Volatile flavor compounds of the mixture of enzymatic hydrolysates of soy sauce residue and defatted soybean with and without reaction flavor technology1) (unit: ng/g)
| Compounds | RI | RFT | Control | t-value |
|---|---|---|---|---|
| Aldehydes and Ketones (8) | 351.72±35.63 | 56.73±65.73 | 13.05* | |
| 2-Methylbutanal | 846 | 17.03±10.53 | 4.40±2.87 | 2.05 |
| 3-Methylbutanal | 858 | 35.66±28.76 | 7.48±6.62 | 1.65 |
| Hexanal | 1,085 | 45.44±28.47 | −2) | 2.77 |
| 2-Heptanone | 1,189 | 10.78±12.76 | 16.12±14.11 | −0.49 |
| 3-Octanone | 1,259 | 2.39±0.51 | 11.93±1.32 | −11.67* |
| 2-Octanone | 1,290 | 63.98±9.49 | 6.01±0.83 | 10.54* |
| 3-Hydroxybutanone | 1,296 | 0.44±0.34 | 2.62±0.74 | −4.92* |
| Benzaldehyde | 1,542 | 176.00±4.13 | 8.17±3.79 | 51.87* |
| Aromatic hydrocarbons (6) | 355.57±56.12 | 117.68±44.80 | 5.74* | |
| Methylbenzene | 1,045 | − | 2.65±1.44 | −3.18* |
| 2-Methoxyphenol | 1,880 | 30.71±4.11 | 19.09±6.83 | 2.52 |
| 2-Phenylethanol | 1,929 | 73.41±15.25 | 33.60±12.17 | 3.54* |
| 2-Phenylbutanol | 2,002 | 0.12±0.04 | 2.89±1.21 | −3.97* |
| Phenol | 2,024 | 5.01±0.38 | 4.11±1.99 | 0.77 |
| 4-Ethyl-2-methoxyphenol | 2,051 | 246.32±48.65 | 55.34±22.76 | 6.16* |
| Acids (3) | 63.96±6.13 | 124.23±53.43 | −1.94 | |
| Acetic acid | 1,462 | 36.25±3.66 | 43.55±18.65 | −0.67 |
| 2-Methylpropanoic acid | 1,576 | − | 18.85±6.15 | −5.31* |
| 3-Methylbutanoic acid | 1,676 | 27.71±2.57 | 61.83±28.76 | −2.05 |
| Alcohols (12) | 757.63±128.90 | 198.15±45.07 | 6.82* | |
| Cyclopropyl methanol | 1,101 | 66.52±41.59 | − | 1.59 |
| 3-Methyl-1-butanol | 1,211 | 626.85±129.66 | 79.68±15.80 | 7.26* |
| Pentanol | 1,253 | − | 1.30±0.94 | −2.39 |
| 2-Heptanol | 1,320 | 5.32±1.00 | 2.46±0.38 | 4.64* |
| 3,4-Dimethyl-1-pentanol | 1,355 | 23.53±0.99 | 16.38±4.26 | 2.83* |
| 3-Octanol | 1,395 | − | 74.99±20.63 | −6.30* |
| 1-Octen-3-ol | 1,452 | 3.08±0.14 | 8.61±2.64 | −3.62* |
| Heptanol | 1,457 | 8.29±4.40 | − | 3.26* |
| 2-Ethyl-1-hexanol | 1,491 | 16.17±1.27 | 5.37±1.88 | 8.25* |
| Octanol | 1,560 | 2.19±0.55 | 6.33±3.75 | −1.89 |
| (Z)-2-Octen-1-ol | 1,620 | 1.51±0.02 | 3.03±1.11 | −2.38 |
| Undecanol | 1,899 | 4.17±4.20 | − | 1.72* |
| Esters (6) | 50.29±10.18 | 18.65±6.40 | 4.56* | |
| Ethyl 2-hydroxypropanoate | 1,351 | − | 2.14±0.60 | −6.11* |
| Diisopropyl carbonate | 1,585 | 2.87±0.46 | − | 10.85* |
| Cyclopentyl 4-ethylbenzoate | 1,756 | 12.72±7.28 | − | 3.02* |
| Ethylbezene acetate | 1,803 | 20.13±3.48 | 4.22±1.85 | 7.00* |
| Methylbenzene propanoate | 1,835 | − | 2.07±0.67 | −5.40* |
| Tetrahydro-2-furanmethanol acetate | 1,887 | 14.57±0.86 | 10.22±3.62 | 2.03 |
| Furans (4) | 6.34±0.85 | 10.42±2.77 | −2.76 | |
| Furfural | 1,479 | − | 3.87±1.32 | −5.10* |
| 2-[(Methylthio)methyl]furan | 1,519 | 0.29±0.04 | − | 12.56* |
| 2-Furanmethanol | 1,670 | 1.28±0.70 | 3.48±1.08 | −2.96* |
| 3-Phenylfuran | 1,876 | 4.77±0.24 | 3.07±1.19 | 2.43 |
| Nitrogen-containing compounds (9) | 24.53±2.89 | 71.70±43.37 | −1.88 | |
| 1,3-Propanediamine | 1,079 | − | 12.90±4.60 | −4.86* |
| 2,5-Dimethylpyrazine | 1,331 | 0.15±0.06 | 8.01±1.86 | −7.33* |
| 2,6-Dimethylpyrazine | 1,337 | 2.48±0.39 | − | 10.94* |
| 2-Acetyl-1-pyrroline | 1,338 | − | 1.32±0.30 | −7.73* |
| 2-Ethyl-6-methylpyrazine | 1,401 | 0.44±0.22 | 1.11±0.51 | −2.09 |
| Trimethylpyrazine | 1,413 | 8.78±1.08 | 7.88±2.53 | 0.56 |
| 2-Ethyl-5,6-dimethylpyrazine | 1,471 | − | 1.90±0.61 | −5.39* |
| Tetramethylpyrazine | 1,482 | 12.52±2.00 | 14.65±5.98 | −0.58 |
| 3,5-Diethyl-2-methylpyrazine | 1,520 | 0.16±0.09 | 23.93±35.61 | −1.16 |
| Sulfur-containing compounds (4) | 2,980.09±415.99 | 4.34±1.11 | 12.34* | |
| Dimethyl disulfide | 1,078 | 1,405.91±258.22 | − | 9.34* |
| Dimethyl trisulfide | 1,396 | 1,558.53±154.84 | − | 17.43* |
| Methional | 1,470 | 8.29±4.40 | − | 3.26* |
| 2-Acetylthiazole | 1,666 | 7.36±0.50 | 4.34±1.11 | 4.28* |
| Miscellaneous compounds (5) | 3.17±0.45 | 39.06±27.44 | −2.27 | |
| Limonene | 1,200 | − | 25.18±22.54 | −1.94 |
| Trimethyl oxazole | 1,202 | 3.17±0.45 | − | 12.34* |
| γ-Terpinene | 1,249 | − | 6.63±5.82 | −1.97 |
| 3-Methyl-6-(1-methylethylidene)cyclohexene | 1,287 | − | 4.09±0.37 | −19.20* |
| β-Bisabolene | 1,736 | − | 3.16±0.93 | −5.91 |
Data presented as mean±SD. (n=3).
RI, retention index on Supelcowax 10TM column; RFT, reaction flavor technology.
P <0.05 by t -test.
Concentration (ng/g) of each compound was calculated as a relative content to hexyl acetate (91.12 ng/g) put in sample (factor=1).
Not detected.
Fig. 1.
Mean amounts of group compounds detected in the mixture of enzymatic hydrolysates of soy sauce residue and defatted soybean with reaction flavor technology (RFT) and without (Control). Mean values within the same group with asterisks are significantly different (P <0.05).
Aldehydes
Four aldehydes (2-methylbutanal, 3-methylbutanal, hexanal, and benzaldehyde) were identified in RFT. Among these, the most abundant compound was benzaldehyde (176.00 ng/g), which has a characteristic almond-like odor, and was increased 21.54 fold greater than the control (8.17 ng/g) (Table 4). Benzaldehyde has been reported to have a high odor activity value in Japanese soy sauce (17). The second most abundant compound was hexanal (45.44 ng/g), which has a fruity odor. Hexanal has been reported to be generated by RFT via lipid oxidation (18), and has been identified in a koji-fermented soy sauce (18,19). Two compounds, 2-methylbutanal (roasted cocoa-like odor) and 3-methylbutanal (banana-like odor), regularly found in soy sauces (18–21) were detected, and were increased 3.87 and 4.77 fold by RFT, respectively. The formation of 2-methylbutanal and 3-methylbutanal can be explained by degradation of the amino acids isoleucine and leucine, respectively (17,18), and each consist of one less carbon than the original amino acids. Degradation of isoleucine and leucine has been reported as a pathway of the Maillard reaction (22,23).
Ketones and aromatic hydrocarbons
Four ketones (2-heptanone, 3-octanone, 2-octanone, and 3-hydroxybutanone) were identified in both the RFT and control samples. The abundance of 2-octanone (63.98 ng/g) was increased 10.65 fold in RFT samples compared with controls (6.01 ng/g). Ketones are one kind of decomposed product of fat acidification and have herb and flower-like odors (24).
A total of 5 aromatic hydrocarbons were identified in the RFT samples and 6 in the controls. Methylbenzene, which was found in the control but not the RFT samples, may be catalytically oxidized to benzaldehyde. The most abundant aromatic hydrocarbon in the RFT samples was 4-ethyl-2-methoxyphenol (246.32 ng/g), and was 4.45 fold greater than in controls. 4-Ethyl-2-methoxyphenol has been reported to be a significant contributor to the flavor of Japanese soy sauce because of its very strong soy sauce-like odor (17), and can be produced from both lignin pyrolysis and 2-methoxyphenol (25). Four aromatic hydrocarbons, 4-ethyl-2-methoxyphenol, 2-phenylethanol (73.41 ng/g, floral odor), 2-methoxyphenol (30.71 ng/g, smoky odor) and phenol (5.01 ng/g, green odor), have been reported in other soy sauces (19,20).
Acids
Two acids were found in the RFT samples, compared to three in the controls. These can be attributed to the acidic sensory of soy sauce. Acetic acid, a product of microbiology fermentation (26), was found to be the major acid in soy sauce. A similar results has been observed for Korean soy sauce (17). 3-Methylbutanoic acid (sweat/strong cheese-like odor) was the most abundant acid in control samples (61.83 ng/g), and was decreased to 27.17 ng/g following RFT. 2-Methylpropanoic acid, attributable to a rotten butter-like and sweat odor, was found at 18.85 ng/g in control samples, but was not present following RFT. Compounds such as 2-methylpropanoic acid and 3-methylpropanoic acid are reported to be mainly produced from branched-chain amino acids via the Ehrlich pathway by the action of various fungal enzymes during fermentation (27).
Alcohols
Twelve alcohols were overall identified in this study (10 in the RFT samples and 9 in the control samples). In both the RFT and control samples, 3-methyl-1-butanol (isoamyl alcohol/fruity/kiwi-like odor) showed the highest abundance (626.85 ng/g and 79.68 ng/g, respectively), increased 7.87 fold by reaction flavor. 1-Octen-3-ol (mushroom-like odor) has regularly been reported as one of the major constituents in soy sauce has a favorable impact on soy sauce products (20,21). 3-Octanol (cheese/mint-like odor), which is the dominant constituent in the soy sauce koji, and pentanol (wine-like odor) were not present following RFT. However, cyclopropyl methanol (66.52 ng/g), heptanol (8.29 ng/g), and undecanol (4.17 ng/g) were generated through RFT.
Esters
Six esters were identified in both the RFT and control samples. Control samples consisted of 18.66 ng/g esters, significantly increased by RFT to 50.28 ng/g. Tetrahydro-2-furanmethanol acetate was the most abundant ester in control samples (10.22 ng/g), followed by ethylbenzene acetate (4.22 ng/g), which has a wine-like odor. In RFT samples, ethylbezene acetate was the most abundant ester (20.13 ng/g) followed by tetrahydro-2-furanmethanol acetate (14.57 ng/g). Most of the esters are formed via esterification of fatty acids and alcohols during fermentation (28,29), and can contribute to flavor by minimizing the sharpness and bitterness imparted by amines and fatty acids (30,31).
Furans
Four furans were detected in both the RFT (6.34 ng/g) and control (10.42 ng/g) samples, however no significant differences in their abundances were recorded. The heterocyclics in soy sauce, which include furans and pyrazines, are mainly formed via a Maillard reaction (20). The presence of several furans in soy sauce could result from both glucose pyrolysis and Maillard reactions (17). Generally, furans are formed from decomposition of Amadori and Heyns products in a Maillard reaction (32).
Nitrogen-containing compounds
Almost all the nitrogen-containing compounds detected in RFT and control samples consisted of pyrazines. Pyrazines are an important group of nitrogen-containing flavor compounds, which are generally formed by the reaction of amino acids with a carbonyl group through Maillard reactions (33,34). Most pyrazines are important contributors to the flavors of roasted, toasted, and heated foods (35). A larger number of nitrogen-containing compounds were detected in the control samples than following RFT. These data were thought to be a result of the precursor compounds applied to the reaction flavor system. 2,6-Dimethylpyrazine was detected only in RFT samples, and 1,3-propanediamine and 2-acetyl-1-pyrroline were only detected in control samples. Two pyrazines, 2,5-dimethylpyrazine (meaty/nutty odor) and 2,6-dimethylpyrazine (meaty/nutty/coffee-like odor), are formed from cysteines by thermal treatment (36,37), and are reported to be the main pyrazines in several soy sauces, including Thai soy sauces (26), Korean acid hydrolyzed soy sauces (17), and Japanese soy sauces (38). Trimethylpyrazine (peanut and hazelnut-like odor) and tetramethylpyrazine (coffee/chocolate-like odor) also accounted for a large proportion of the nitrogen-containing compounds in both RFT (21.30 ng/g) and control (22.53 ng/g) samples.
Sulfur-containing compounds
Four sulfur-containing compounds dimethyl disulfide (roasted onion/garlic-like/meaty odor), dimethyl trisulfide (roasted garlic-like/meaty odor), methional (roasted potato/potato chip-like odor) and 2-acetylthiazole were predominantly detected following RFT; of these compounds, only 2-acetylthiazole was found in controls. The amount of sulfur-containing compounds was 687 fold higher following RFT samples compared with control samples (P<0.05) (Fig. 1). Methional is a Strecker aldehyde of methionine, and is detected in cooked potatoes (32). Schutte and Teranishi (39) reported that methional is produced by decomposition of methionine, which was added as the precursor of reaction flavor in this study. Methional was subsequently decomposed into dimethyl sulfide and trimethyl sulfide. These three newly generated compounds (12 ng/g dimethyl disulfide, 0.01 ng/g dimethyl trisulfide, and 0.2 ng/g methional) (40) were, with a very low threshold, considered to be the main contributors to the aroma activity of RFT.
ACKNOWLEDGEMENTS
This study was supported by Business for Cooperative R&D between Industry, Academy, and Research Institute funded Korea Small and Medium Business Administration in 2015–16 (Grant N0. C0364364).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.MFDS. [accessed Dec 2017];Food Standard Revision Notice. 2015 https://www.mfds.go.kr/brd/m_207/view.do?seq=8853.
- 2.MFDS. Article 5 Standards and Specifications for Each Food Product. Ministry of Food and Drug Safety; Cheongju, Chungbuk, Korea: 2016. [accessed Dec 2017]. 20. Soy sauces or pastes; pp. 104–107. http://www.mfds.go.kr/files/upload/eng/Article_5._Standards_and_Spefications_for_Each_Food_Product.pdf. [Google Scholar]
- 3.Korea Agro-Fisheries & Food Trade Corporation. [accessed Dec 2017];Current Status of Processed Food Market Segmentation: Soy Source Market. 2015 :36–37. https://www.atfis.or.kr/article/M001050000/view.do?articleId=1935&page=6&searchKey=&searchString=&searchCategory=
- 4.Cha YJ, Wang W, Cha HR. Studies on volatile flavor compounds of soy sauce residue. J Korean Soc Food Sci Nutr. 2016;45:1755–1761. doi: 10.3746/jkfn.2016.45.12.1755. [DOI] [Google Scholar]
- 5.Chen X, Luo Y, Qi B, Luo J, Wan Y. Improving the hydrolysis efficiency of soy sauce residue using ultrasonic probe-assisted enzymolysis technology. Ultrason Sonochem. 2017;35:351–358. doi: 10.1016/j.ultsonch.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Gao XL, Zhao SW, Sun PF, Lu J. Isolation, identification and amino acid composition of proteins in soy sauce residue. Mod Food Sci Technol. 2013;10:2512–2516. [Google Scholar]
- 7.Kim DS, Kim JS. Manufacturing of meat flavor extract used for browning reaction. Korean J Food Nutr. 2004;17:313–321. [Google Scholar]
- 8.Kim KW, Beak HH. Development of a burnt beef flavor by reaction flavor technology. Korean J Food Sci Technol. 2003;35:1045–1052. [Google Scholar]
- 9.Kim MC, Oh JH, Kim BY, Cho SM, Lee DS, Nam MH, Lee YB, Kim SB. Development of boiled-type shrimp flavor by Maillard reaction and sensory evaluation. J Food Sci Nutr. 2010;15:304–308. [Google Scholar]
- 10.Kim H. PhD Dissertation. Changwon National University; Changwon, Gyeongnam, Korea: 2003. Functional flavorants produced from seafood by-products by reaction flavoring techniques. [Google Scholar]
- 11.Hwang HI, Hartman TG, Ho CT. Relative reactivities of amino acids in pyrazine formation. J Agric Food Chem. 1995;43:179–184. doi: 10.1021/jf00049a033. [DOI] [Google Scholar]
- 12.Hou L, Xie J, Zhao J, Zhao M, Fan M, Xiao Q, Liang J, Chen F. Roles of different initial Maillard intermediates and pathways in meat flavor formation for cysteine-xylose-glycine model reaction systems. Food Chem. 2017;232:135–144. doi: 10.1016/j.foodchem.2017.03.133. [DOI] [PubMed] [Google Scholar]
- 13.Yu TH, Ho CT. Volatile compounds generated from thermal reaction of methionine and methionine sulfoxide with or without glucose. J Agric Food Chem. 1995;43:1641–1646. doi: 10.1021/jf00054a043. [DOI] [Google Scholar]
- 14.Cha YJ, Wang W. Optimal conditions of enzymatic hydrolysate of soy sauce residue for making savory sauce. J Korean Soc Food Sci Nutr. 2017;46:1517–1522. doi: 10.3746/jkfn.2017.46.12.1517. [DOI] [Google Scholar]
- 15.Cha YJ, Wang WF. Formation of savory flavors through reaction flavor system in the enzymatic hydrolysate of soy sauce residue and defatted soybean. Abstract No AGFD67 presented at 254th ACS National Meeting and Exposition; Washington, DC, USA. 2017. [Google Scholar]
- 16.Gontard N, Guilbert S, Cuq JL. Edible wheat gluten films: influence of the main process variables on film properties using response surface methodology. J Food Sci. 1992;57:190–195. doi: 10.1111/j.1365-2621.1992.tb05453.x. [DOI] [Google Scholar]
- 17.Lee SM, Seo BC, Kim YS. Volatile compounds in fermented and acid-hydrolyzed soy sauces. J Food Sci. 2006;71:C146–C156. doi: 10.1111/j.1365-2621.2006.tb15610.x. [DOI] [Google Scholar]
- 18.Feng Y, Cui C, Zhao H, Gao X, Zhao M, Sun W. Effect of koji fermentation on generation of volatile compounds in soy sauce production. Int J Food Sci Technol. 2013;48:609–619. doi: 10.1111/ijfs.12006. [DOI] [Google Scholar]
- 19.Xu N, Liu Y, Hu Y, Zhou M, Wang C, Li D. Autolysis of Aspergillus oryzae mycelium and effect on volatile flavor compounds of soy sauce. J Food Sci. 2016;81:C1883–C1890. doi: 10.1111/1750-3841.13396. [DOI] [PubMed] [Google Scholar]
- 20.Sun SY, Jiang WG, Zhao YP. Profile of volatile compounds in 12 Chinese soy sauces produced by a high-salt-diluted state fermentation. J Inst Brew. 2010;116:316–328. doi: 10.1002/j.2050-0416.2010.tb00437.x. [DOI] [Google Scholar]
- 21.Gao XL, Cui C, Zhao HF, Zhao MM, Yang L, Ren JY. Changes in volatile aroma compounds of traditional Chinese-type soy sauce during moromi fermentation and heat treatment. Food Sci Biotechnol. 2010;19:889–898. doi: 10.1007/s10068-010-0126-7. [DOI] [Google Scholar]
- 22.Hartman GJ, Scheide JD, Ho CT. Formation of volatile compounds from the reaction of leucine and D-glucose in propylene glycol. Perfumer Flavorist. 1983;8:81–86. [Google Scholar]
- 23.Hall G, Andersson J, Lingnert H, Olofsson B. Flavor changes in whole milk powder during storage. The kinetics of the formation of volatile fat oxidation products and other volatile compounds. J Food Qual. 1985;7:153–190. doi: 10.1111/j.1745-4557.1985.tb01049.x. [DOI] [Google Scholar]
- 24.Cha YJ, Baek HH, Hsieh TCY. Volatile components in flavour concentrates from crayfish processing waste. J Sci Food Agric. 1992;58:239–248. doi: 10.1002/jsfa.2740580212. [DOI] [Google Scholar]
- 25.Nunomura N, Sasaki M. Japanese soy sauce flavor with emphasis on off-flavors. In: Charalambous G, editor. Off-Flavors in Foods and Beverages. Elsevier; New York, NY, USA: 1992. pp. 287–312. [DOI] [Google Scholar]
- 26.Wanakhachornkrai P, Lertsiri S. Comparison of determination method for volatile compounds in Thai soy sauce. Food Chem. 2003;83:619–629. doi: 10.1016/S0308-8146(03)00256-5. [DOI] [Google Scholar]
- 27.Chung HY, Fung PK, Kim JS. Aroma impact components in commercial plain sufu. J Agric Food Chem. 2005;53:1684–1691. doi: 10.1021/jf048617d. [DOI] [PubMed] [Google Scholar]
- 28.van der Sluis C, Tramper J, Wijffels RH. Enhancing and accelerating flavour formation by salt-tolerant yeasts in Japanese soy-sauce processes. Trends Food Sci Technol. 2001;12:322–327. doi: 10.1016/S0924-2244(01)00094-2. [DOI] [Google Scholar]
- 29.Cha YJ, Cadwallader KR. Volatile components in salt-fermented fish and shrimp pastes. J Food Sci. 1995;60:19–24. doi: 10.1111/j.1365-2621.1995.tb05597.x. [DOI] [Google Scholar]
- 30.Curionia PMG, Bosset JO. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int Dairy J. 2002;12:959–984. doi: 10.1016/S0958-6946(02)00124-3. [DOI] [Google Scholar]
- 31.Lee SJ, Ahn B. Comparison of volatile components in fermented soybean pastes using simultaneous distillation and extraction (SDE) with sensory characterisation. Food Chem. 2009;114:600–609. doi: 10.1016/j.foodchem.2008.09.091. [DOI] [Google Scholar]
- 32.Salnias JP, Hartman TG, Karmas K, Lech J, Rosen RT. In: Lipid-derived aroma compounds in cooked potatoes and reconstituted dehydrated potato granules. Ho CT, Hartman TG, editors. American Chemical Society; Washington, DC, USA: 1994. pp. 108–129. (Lipids in Food Flavors: ACS Symposium Series 558). [Google Scholar]
- 33.Lu G, Yu TH, Ho CT. Generation of flavor compounds by the reaction of 2-deoxyglucose with selected amino acids. J Agric Food Chem. 1997;45:233–236. doi: 10.1021/jf960609c. [DOI] [Google Scholar]
- 34.Riha WE, Hwang CF, Karwe MV, Hartman TG, Ho CT. Effect of cysteine addition on the voaltiles of extruded wheat flour. J Agric Food Chem. 1996;44:1847–1850. doi: 10.1021/jf950793m. [DOI] [Google Scholar]
- 35.Hwang HI, Hartman TG, Karwe MV, Izzo HV, Ho CT. In: Aroma generation in extruded and heated wheat flour. Ho CT, Hartman TG, editors. American Chemical Society; Washington, DC, USA: 1994. pp. 144–157. (Lipids in Food Flavors: ACS Symposium Series 558). [Google Scholar]
- 36.Pripis-Nicolau L, de Revel G, Bertrand A, Maujean A. Formation of flavor components by the reaction of amino acid and carbonyl compounds in mild conditions. J Agric Food Chem. 2000;48:3761–3766. doi: 10.1021/jf991024w. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Xing J, Chin CK, Ho CT. Effect of urea on volatile generation from Maillard reaction of cysteine and ribose. J Agric Food Chem. 2000;48:3512–3516. doi: 10.1021/jf991076l. [DOI] [PubMed] [Google Scholar]
- 38.Steinhaus P, Schieberle P. Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. J Agric Food Chem. 2007;55:6262–6269. doi: 10.1021/jf0709092. [DOI] [PubMed] [Google Scholar]
- 39.Schutte L, Teranishi R. Precursors of sulfur-containing flavor compounds. CRC Crit Rev Food Technol. 1974;4:457–505. doi: 10.1080/10408397409527166. [DOI] [Google Scholar]
- 40.Cha YJ, Kim H, Jang SM, Yoo YJ. Identification of aroma-active components in salt-fermented big-eyed herring on the market. J Korean Soc Food Sci Nutr. 1998;27:1053–1058. [Google Scholar]