Abstract
In this study, the antioxidant flavonoid, myricitrin, was isolated and identified from Daebong persimmon peel. The persimmon peel extract was successively fractionated with n-hexane, ethyl acetate, and n-butanol. The ethyl acetate fraction had the strongest antioxidant activities among the solvent fractions and was further fractionated by silica gel and octadecylsilane column chromatography, and preparative high performance liquid chromatography. Three antioxidant compounds were finally isolated, and compound 2 was identified as myricitrin by liquid chromatography/mass spectrometry and 1H and 13C nuclear magnetic resonance. Myricitrin had the strongest antioxidant activities by ferric ion reducing antioxidant power and α,α-diphenyl-2-picrylhydrazyl radical scavenging assays. These results suggested that myricitrin was found as a major antioxidant flavonoid responsible for the strong antioxidant activities of Daebong persimmon peels.
Keywords: antioxidant, myricitrin, persimmon peel, LC/MS, NMR
INTRODUCTION
Free radicals and oxidative stress have been associated with many diseases including atherosclerosis, diabetes mellitus, neurodegenerative diseases, and virus infections (1). Flavonoids are capable of preventing free radical and oxidative stress, thus stopping oxidative damage (2,3). Consequently, flavonoids have become of interest for contemporary health care.
In this respect, persimmon (Diospyros kaki), having various biological effects including antioxidant, anti-diabetic, and anti-cancer (4,5), is one of the useful resources to obtain the natural antioxidant flavonoids. It contains abundant phenolic acids, vitamins, and flavonoids including catechin, epicatechin, and gallocatechin (6). Among the persimmon varieties, Daebong persimmon (Diospyros kaki L. cv. Hachiya) is one of the astringent-type varieties consumed as dried product after removing peels. Consequently, a large amount of persimmon peels are discarded at the production areas. Some researchers had reported persimmon health-benefits include hypocholesterolemic (7), anti-tumor (8), and anti-diabetic effects (9). We also reported the physicochemical properties and pretreatment of Daebong persimmon peel for functional food materials (10), and studied the enzymatic hydrolysis process of Daebong persimmon peels for vinegar fermentation (11).
In this study, we isolated the antioxidant compounds from Daebong persimmon peel by antioxidant activity-guided fractionation and identified the chemical structure by instrumental analyses, including high performance liquid chromatography (HPLC), liquid chromatography/mass spectrometry (LC/MS), 1H and 13C nuclear magnetic resonance (NMR).
MATERIALS AND METHODS
Preparation of Daebong persimmon peel powder and chemicals
Daebong persimmon peels were collected at the production area (Gwangyang, Korea). Persimmon peels were dried in the shade and ground in a roller mill (C.W. Brabender Instruments, Inc., South Hackensack, NJ, USA) at 1,000 rpm with a 0.5-mm screen. α,α-Diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-tris(2-pyridyl)-1,3,5-triazine (TPTZ), gallic acid, and Trolox were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All organic solvents were of analytical grade (Merck KGaA, Darmstadt, Germany), except for HPLC, LC/MS (J.T. Baker Chemical Co., Phillipsburg, NJ, USA), and NMR (Cambridge Isotope Laboratories, Andover, MA, USA).
Extraction and isolation of antioxidant compounds
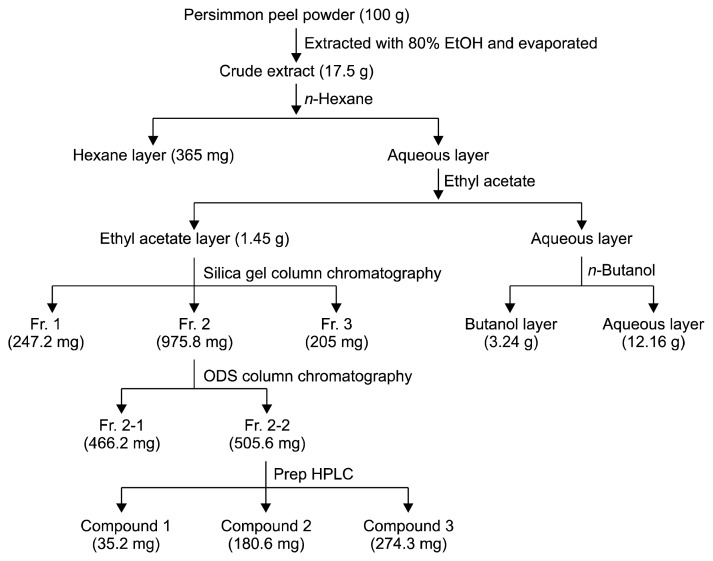
The isolation scheme of antioxidant from Daebong persimmon peel is shown in Fig. 1. Briefly, persimmon peel powders were extracted with 80% ethanol under reflux for 12 h. This ethanol extract was filtered and concentrated to remove ethanol using rotary evaporation. The resultant extract was successively fractionated with n-hexane, ethyl acetate, and n-butanol. The n-hexane, ethyl acetate, and n-butanol extracts were separately evaporated, whereas the aqueous layer was lyophilized to dryness. Among them, greater antioxidant activity was observed in the ethyl acetate fraction compared with other fractions. The ethyl acetate fraction was subjected to silica gel column chromatography, and was eluted with solvents of increasing polarities. Fraction 2 was further isolated using octadecylsilane (ODS) column chromatography. Finally, fraction 2 was purified by preparative HPLC, and compounds 1, 2, and 3 were obtained. Isolated compounds were analyzed using an HPLC (1260 Infinity Quaternary LC System, Agilent Technologies, Palo Alto, CA, USA) equipped with a ultraviolet (UV) detector at 280 nm (C18 column, Develosil ODS-HG-5, 4.6×150 mm, Nomura Chemical Co., Ltd., Seto, Japan).
Fig. 1.
Isolation schemes of antioxidant from Daebong persimmon peels.
Instrumental analyses
UV/visible (Vis) analysis was conducted to confirm the polyphenol type of fractions using UV/Vis spectrophotometer (UV 1601 PC, Shimadzu Co., Kyoto, Japan) in the wavelength range of 200~600 nm. LC/MS analysis was conducted to identify the molecular weight of isolated compounds. Determination was performed with a 6410 Triple Quadrupole LC/MS system (Agilent Technologies). Electro spray ionization-MS was performed in the positive ion mode (600°C, 20 psi). Data were collected using a mass scan from 0 to 460 m/z, at 1.5 s per scan. 1H and 13C NMR spectra of isolated compounds were recorded on a NMR spectrometer (400 MHz for 1H NMR and 100 MHz for 13C NMR; Bruker Avance Digital 400, Bruker, Karlsruhe, Germany). Chemical shift (δ) for 1H and 13C NMR are recorded in parts per million (ppm) relative to solvent signals (methanol-d4: δH 3.30 and δC 49.0) as internal standards.
Antioxidant activities
To test the antioxidant activities, DPPH radical scavenging activity (12) and ferric ion reducing antioxidant power (FRAP) assays (13) were conducted. The DPPH radical scavenging activities of the extracts and fractions were determined by measuring the decrease in absorbance of methanolic DPPH solution at 517 nm in the presence of the sample. The initial concentration of DPPH was 0.1 mM and the reading was taken after allowing the solution to stand for 30 min. In cases where the absorbance decreased too much (when the solution turned yellow) before the 30 min period, the sample was appropriately diluted. The results are expressed in μM gallic acid equivalents (μM GAE).
The FRAP assay was performed using TPTZ solution. The working solution was prepared by mixing 25 mL of acetate buffer (pH 3.6), 2.5 mL of TPTZ solution (10 mM), and 2.5 mL of FeCl3·6H2O solution (20 mM), and warmed at 37°C before every determination. Then, 175 μL of this working solution was mixed with 25 μL of the sample solution. After incubation at 37°C for 30 min in the dark, the absorbance of the sample solution was measured at 590 nm. Results are expressed in μM trolox equivalents (μM TE). All of the tests were performed in triplicate.
Statistical analysis
The values were expressed as the mean±standard deviation. The results were evaluated through analysis of variance with Duncan’s multiple range test (P<0.05) using Statistical Analysis System software version 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
Isolation of antioxidant compounds
Isolation process of antioxidant compounds is shown in Fig. 1. The extraction yield was 17.5 g of extract per 100 g of persimmon peel powder. The ethanol extract was fractionated with n-hexane, ethyl acetate, and n-butanol by their polarity. The yields of fractions including n-hexane fraction, ethyl acetate fraction, n-butanol fraction, and aqueous fraction were 365 mg, 1.45 g, 3.24 g, and 12.16 g, respectively. Among them, the ethyl acetate fraction showed the strongest antioxidant activity. Thus, antioxidant compounds of ethyl acetate fraction were isolated in further experiments. The ethyl acetate fraction was separated with chloroform/methanol by step-wise elution using a silica gel column chromatography, and three fractions were obtained. Among them, fraction 2 showed the strongest antioxidant activity and the highest amount (975.8 mg). Thus, fraction 2 was separated by ODS column chromatography, and fraction 2-1 (466.2 mg) and 2-2 (505.6 mg) were obtained. Finally, prep-HPLC was conducted to completely separate with Fr. 2-2.
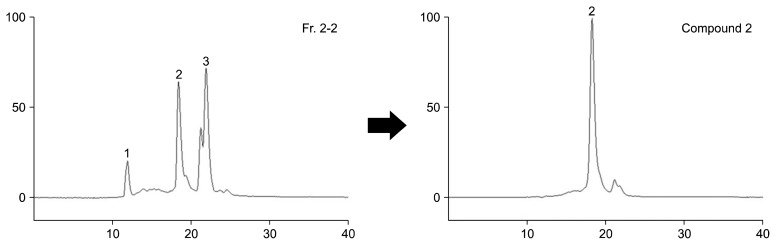
As a result, fraction 2-2 was separated to three compounds (compound 1, 35.2 mg; compound 2, 180.6 mg; compound 3, 274.3 mg). In HPLC chromatogram (Fig. 2), it was supposed that compound 2 was pure.
Fig. 2.
High performance liquid chromatography (HPLC) chromatograms of compound 2 from Daebong persimmon peels. Fr. 2-2, ODS column chromatography fractions; Compound 2, prep-HPLC fraction from fraction 2-2.
Identification of isolated compound
To identify the chemical structure of compound 2, UV/Vis absorption spectrum, LC/MS, 1H and 13C NMR analyses were conducted. The instrumental data are shown in Fig. 3 and described as follows:
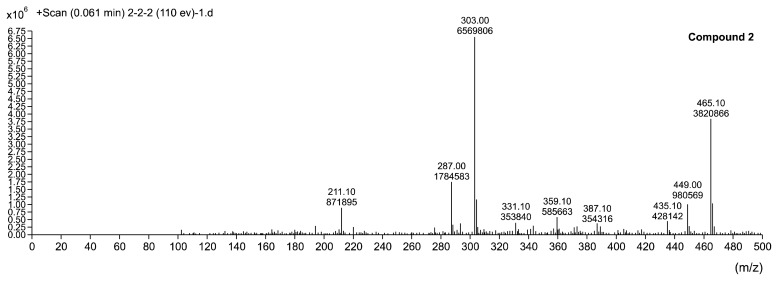
Fig. 3.
Liquid chromatography/mass spectrometry spectrum of compound 2 from Daebong persimmon peel.
Compound 2 (myricitrin, myricetin 3-O-rhamnoside; 180.6 mg). UV, λmax nm 257, 360 (MeOH); LC/MS, m/z 303, 465 (M+H)+; 1H NMR (in CD3OD), δ 6.19 (1H, d, 1.8 Hz, H-6), 6.35 (1H, d, 2.3 Hz, H-8), 6.94 (2H, s, H-2′, 6′), 5.30 (1H, d, 1.8 Hz, H-1″), 4.21 (1H, dd, 3.2, 1.8 Hz, H-2″), 3.76~3.78 (1H, dd, 9.4, 3.4 Hz, H-3″), 3.31~3.34 (1H, m, H-4″), 3.48~3.54 (1H, m, H-5″), 0.94~0.96 (3H, m, H-6″); 13C NMR (in CD3OD), δ 159.2 (C-2), 136.1 (C-3), 179.5 (C-4), 163.1 (C-5), 99.7 (C-6), 164.0 (C-7), 94.6 (C-8), 158.4 (C-9), 105.6 (C-10), 121.7 (C-1′), 109.6 (C-2′, 6′), 146.7 (C-3′, 5′), 137.7 (C-4′), 103.5 (C-1″), 71.7 (C-2″), 72.0 (C-3″), 73.2 (C-4″), 71.9 (C-5″), 17.5 (C-6″).
UV/Vis absorption spectra of compound 2 showed specific absorption peaks at 257 and 360 nm (data not shown). This result indicated that compound 2 could be assumed as a flavonol (14,15). Molecular mass of compound 2 could be assumed as a myricitrin (Fig. 3) (C21H20O12, myricetin 3-O-rhamnoside, m/z 303, 465 [M+H]+), consistent with previous studies (16–18). The fragment ions at m/z 303 might be the aglycone ion from myricitrin whose rhamnosyl group was cleaved ([M-(C6H12O5)+H]+). Collision induction dissociation of the clusters led to the fragment at m/z 465, [M+H]+, which, in turn, gave rise to the aglycone myricetin after rhamnose loss. Considering this result and previous research (19), electrospray proved to be the most efficient mass ionization method for the identification of biologically active substances.
The data of 1H and 13C NMR indicated 21 carbon signals and the typical flavonol α-L-rhamnopyranoside pattern; δ 5.30 (1H, d; H-1″), δ 4.21 (1H, dd; H-2″), δ 3.76~3.78 (1H, dd; H-3″), δ 3.31~3.34 (1H, m; H-4″), δ 3.48~3.54 (1H, m; H-5″), and δ 0.94~0.96 (3H, m; H-6″). Moreover, the data showed chemical shift values of a sugar moiety and a singlet of two protons at δ 6.94 (2H, s; H-2′ and 6′) with pair of doublets at δ 6.19 (1H, d; H-6) and δ 6.35 (1H, d; H-8). It demonstrated the presence of myricetin structure and O-glycosidic linkage due to the rhamnopyranoside at C-3 position (δ 136.1). These NMR data were in good agreement with those of previous research (18,20). Thus, compound 2 was identified as a myricetin 3-O-rhamnoside, commonly known as a myricitrin.
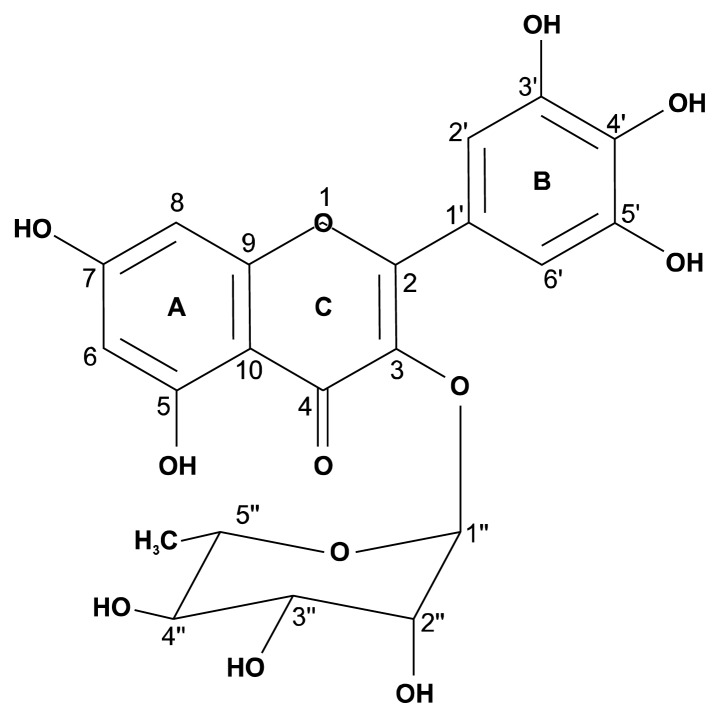
The chemical structure of the compound 2 (myricitrin) is shown in Fig. 4. It has hydroxyl groups at 5 and 7 positions, and three continuous hydroxyl groups at 3′, 4′, and 5′ positions. Rhamnose was attached at C-3 position, and it has three hydroxyl groups. Flavonoid glycosides such as astragalin, isoquercitrin, and myricitrin are contained in persimmon leaves (21). In addition, myricitrin was also isolated from Nymphaea lotus (22), Chrysobalanus icaco (23), and Polygonum aviculare (24).
Fig. 4.
Chemical structure of compound 2 from Daebong persimmon peels.
Antioxidant activities of isolated compounds
Antioxidant activities of isolated compounds were determined by DPPH radical scavenging activity and FRAP assay. The values of DPPH and FRAP were ranged from 105.18~165.75 μM GAE and 682.79~1,609.56 μM TE, respectively (Table 1). Compound 1 and 3 had similar activities and showed no significant differences. Compound 2, identified as a myricitrin, showed the strongest antioxidant activities. Thus, myricitrin is a compound that has the greatest impact on the antioxidant activities of Daebong persimmon peel. Myricitrin is rhamnose glycoside of myricetin contained in various plants, and it has been reported that has strong antioxidant activity as radical scavenger and metal-ion chelator (25). In addition, myricitrin has been reported to inhibit the aldose reductase activity (18) and the oxidation of low-density lipoprotein (26), and has anti-malarial effect (27). It also showed lower IC50 values for ·OH and ·O2− than ascorbic acid in radical scavenging activity by electron spin resonance measurement (20). The reason of these activities is that flavonoids having hydroxyl groups in the B-ring can act as strong radical scavengers through formation of a hydrogen bond with the semiquinone radical of the B-ring (28). In a previous study, the pyrogallol substituents in the B-ring of myricitrin also showed stronger inhibition effects of low-density lipoprotein oxidation induced by copper ion and α,α’-azobis-(2-amidinopropane dihydrochloride than the O-dihydroxy-substituted flavonoid (20). In terms of the importance of the B-ring, other researchers reported that myricetin reacts 6 times more quickly than quercetin to the galvinoxyl radical and concluded that flavonoid reactivity depends highly on the substitution pattern of OH groups in the B-ring (29). Moreover, flavonol glycosides showed stronger antioxidant activity than that of aglycones due to the number and localization of hydroxyl groups as well as hydrogen bonds (30). The role of the hydrogen bonds is to repair the damage in the structure of these flavonols when they donate hydroxyl hydrogens to free radicals. It is of crucial importance for human health because it can prevent the formation of new and perhaps more potent free radicals (31). Thus, myricitrin-rich persimmon peel can be utilized as a natural and strong antioxidant food material.
Table 1.
Antioxidant activities of isolated compounds from Daebong persimmon peels
| Samples (1 mg/mL) | DPPH (μM GAE) | FRAP (μM TE) |
|---|---|---|
| Compound 1 | 138.27±3.46b | 703.09±90.18b |
| Compound 2 | 165.75±1.57a | 1,609.56±90.88a |
| Compound 3 | 105.18±5.03c | 682.79±40.87b |
DPPH, α,α-diphenyl-2-picrylhydrazyl radical scavenging activity; FRAP, ferric ion reducing antioxidant power; GAE, gallic acid equivalent; TE, trolox equivalent.
Different letters (a–c) in a column are significantly different at P <0.05 by Duncan’s multiple range test.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th ed. Oxford University Press; New York, NY, USA: 2006. p. 199. [Google Scholar]
- 2.Niki E. Do free radicals play causal role in atherosclerosis? Low density lipoprotein oxidation and vitamin E revisited. J Clin Biochem Nutr. 2011;48:3–7. doi: 10.3164/jcbn.11-007FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Liao L, Moore J, Wu T, Wang Z. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.) Food Chem. 2009;113:160–165. doi: 10.1016/j.foodchem.2008.07.061. [DOI] [Google Scholar]
- 4.Choi JH, Lee EY, Kim GJ, Park IH, Kim JS, Choi GB, Jung SG, Ham YS. Physicochemical properties and physiological activities of Ulsan sweet persimmon peel·flesh according to cultivars. J Korean Soc Appl Biol Chem. 2006;49:309–314. [Google Scholar]
- 5.Kim SK, Lee GD, Chung SK. Monitoring on fermentation of persimmon vinegar from persimmon peel. Korean J Food Sci Technol. 2003;35:642–647. [Google Scholar]
- 6.Nakatsubo F, Enokita K, Murakami K, Yonemori K, Sugiura A, Utsunomiya N, Subhadrabandhu S. Chemical structures of the condensed tannins in the fruits of Diospyros species. J Wood Sci. 2002;48:414–418. doi: 10.1007/BF00770702. [DOI] [Google Scholar]
- 7.Gorinstein S, Kulasek GW, Bartnikowska E, Leontowicz M, Zemser M, Morawiec M, Trakhtenberg S. The influence of persimmon peel and persimmon pulp on the lipid metabolism and antioxidant activity of rats fed cholesterol. J Nutr Biochem. 1998;9:223–227. doi: 10.1016/S0955-2863(98)00003-5. [DOI] [Google Scholar]
- 8.Kawase M, Motohashi N, Satoh K, Sakagami H, Nakashima H, Tani S, Shirataki Y, Kurihara T, Spengler G, Wolfard K, Molnár J. Biological activity of persimmon (Diospyros kaki) peel extracts. Phytother Res. 2003;17:495–500. doi: 10.1002/ptr.1183. [DOI] [PubMed] [Google Scholar]
- 9.Lee SO, Chung SK, Lee IS. The antidiabetic effect of dietary persimmon (Diospyros kaki L. cv. Sangjudungsi) peel in streptozotocin-induced diabetic rats. J Food Sci. 2006;71:S293–S298. doi: 10.1111/j.1365-2621.2006.tb15656.x. [DOI] [Google Scholar]
- 10.Hwang IW, Jeong MC, Chung SK. The physicochemical properties and the antioxidant activities of persimmon peel powders with different particle sizes. J Korean Soc Appl Biol Chem. 2011;54:442–426. doi: 10.3839/jksabc.2011.068. [DOI] [Google Scholar]
- 11.Hwang IW, Chung SK, Jeong MC, Chung HS, Zheng HZ. Optimization of enzymatic hydrolysis of persimmon peels for vinegar fermentation. J Korean Soc Appl Biol Chem. 2013;56:435–440. doi: 10.1007/s13765-013-3036-6. [DOI] [Google Scholar]
- 12.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 13.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 14.Cockell CS, Knowland J. Ultraviolet radiation screening compounds. Biol Rev Camb Philos Soc. 1999;74:311–345. doi: 10.1017/S0006323199005356. [DOI] [PubMed] [Google Scholar]
- 15.Anouar EH, Gierschner J, Duroux JL, Trouillas P. UV/Visible spectra of natural polyphenols: a time-dependent density functional theory study. Food Chem. 2012;131:79–89. doi: 10.1016/j.foodchem.2011.08.034. [DOI] [Google Scholar]
- 16.Yokomizo A, Moriwaki M. Transepithelial permeability of myricitrin and its degradation by simulated digestion in human intestinal Caco-2 cell monolayer. Biosci Biotechnol Biochem. 2005;69:1774–1776. doi: 10.1271/bbb.69.1774. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu R, Shimabayashi H, Moriwaki M. Enzymatic production of highly soluble myricitrin glycosides using β-galactosidase. Biosci Biotechnol Biochem. 2006;70:940–948. doi: 10.1271/bbb.70.940. [DOI] [PubMed] [Google Scholar]
- 18.Mok SY, Lee S. Identification of flavonoids and flavonoid rhamnosides from Rhododendron mucronulatum for. albiflorum and their inhibitory activities against aldose reductase. Food chem. 2013;136:969–974. doi: 10.1016/j.foodchem.2012.08.091. [DOI] [PubMed] [Google Scholar]
- 19.de Oliveira FMG, Romão W, Kuster RM. Identification of phenolic compounds in Eugenia uniflora leaves by FTICR MS in association with different ionization sources. Anal Methods. 2018;10:1647–1655. doi: 10.1039/C8AY00129D. [DOI] [Google Scholar]
- 20.Chung SK, Kim YC, Takaya Y, Terashima K, Niwa M. Novel flavonol glycoside, 7-O-methyl mearnsitrin, from Sageretia theezans and its antioxidant effect. J Agric Food Chem. 2004;52:4664–4668. doi: 10.1021/jf049526j. [DOI] [PubMed] [Google Scholar]
- 21.Moon SH, Park KY. Antimutagenic effects of boiled water extract and tannin from persimmon leaves. J Korean Soc Food Nutr. 1995;24:880–886. [Google Scholar]
- 22.Elegami AA, Bates C, Gray AI, Mackay SP, Skellern GG, Waigh RD. Two very unusual macrocyclic flavonoids from the water lily Nymphaea lotus. Phytochemistry. 2003;63:727–731. doi: 10.1016/S0031-9422(03)00238-3. [DOI] [PubMed] [Google Scholar]
- 23.Barbosa WLR, Peres A, Gallori S, Vincieri FF. Determination of myricetin derivatives in Chrysobalanus icaco L. (Chrysobalanaceae) Rev Bras Farmacogn. 2006;16:333–337. doi: 10.1590/S0102-695X2006000300009. [DOI] [Google Scholar]
- 24.Xu F, Guan H, Li G, Liu H. LC method for analysis of three flavonols in rat plasma and urine after oral administration of Polygonum aviculare extract. Chromatographia. 2009;69:1251. doi: 10.1365/s10337-009-1088-x. [DOI] [Google Scholar]
- 25.Luo XD, Basile MJ, Kennelly EJ. Polyphenolic antioxidants from the fruits of Chrysophyllum cainito L. (star apple) J Agric Food Chem. 2002;50:1379–1382. doi: 10.1021/jf011178n. [DOI] [PubMed] [Google Scholar]
- 26.Yokomizo A, Moriwaki M. Myricitrin degraded by simulated digestion inhibits oxidation of human low-density lipoprotein. Biosci Biotechnol Biochem. 2005;69:693–699. doi: 10.1271/bbb.69.693. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Murakami N, Ji H, Abreu P, Zhang S. Antimalarial flavonol glycosides from Euphorbia hirta. Pharm Biol. 2007;45:278–281. doi: 10.1080/13880200701214748. [DOI] [Google Scholar]
- 28.Guo Q, Zhao B, Shen S, Hou J, Hu J, Xin W. ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochim Biophys Acta. 1999;1427:13–23. doi: 10.1016/S0304-4165(98)00168-8. [DOI] [PubMed] [Google Scholar]
- 29.McPhail DB, Hartley RC, Gardner PT, Duthie GG. Kinetic and stoichiometric assessment of the antioxidant activity of flavonoids by electron spin resonance spectroscopy. J Agric Food Chem. 2003;51:1684–1690. doi: 10.1021/jf025922v. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZY, Chan PT, Ho KY, Fung KP, Wang J. Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem Phys Lipids. 1996;79:157–163. doi: 10.1016/0009-3084(96)02523-6. [DOI] [PubMed] [Google Scholar]
- 31.Leopoldini M, Marino T, Russo N, Toscano M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J Phys Chem A. 2004;108:4916–4922. doi: 10.1021/jp037247d. [DOI] [Google Scholar]