Abstract
Probiotics may offer a safe intervention for diarrheal diseases. The aim of the present study was the assessment of the antidiarrheal property of probiotic bacteria. For their antidiarrheal efficacy assessment, yogurt was prepared using the isolated bacteria from selective regional yogurt of Bangladesh, and mice model trails were conducted using castor oil induced diarrheal mice. The probiotic treatment was applied on three mice groups, each having 6 mice and their respective doses were 50 mL/kg body weight in treatment group (TG) 1, 100 mL/kg body weight in TG2, and 150 mL/kg body weight in TG3. A four week treatment of probiotic significantly (P<0.001) reduced the percentage (67.37%) of diarrhea in TG3 (150 mL yogurt/kg body weight). All the treatment groups showed a significant (P<0.001) increase in the latent periods, reduced the total fecal output, and frequency and fecal water content compared to the negative control group. Serum electrolytes (Na+ and K+) and total protein levels were higher in the TG3 compared to the negative control group. Further research regarding molecular characterization and identification of specific genes and proteins of interest may help to develop the next generation bacteriocins and antidiarrheal drugs.
Keywords: diarrhea, yogurt, probiotics, electrolytes, treatment
INTRODUCTION
Diarrhea is defined as the passage of three or more loose or liquid stools per day (1,2). Diarrhea can also be defined as the alteration in the bowel movement which is characterized by an increase in the volume, frequency, and weight of stools (3,4). Diarrhea is usually a symptom of an infection in the intestinal tract, which can be caused by a variety of bacterial, viral, and parasitic organisms. Infection is spread through contaminated food or drinking-water, or from person to person as a result of poor hygiene. Another cause of diarrhea is the use of a broad spectrum of antibiotics for different purposes. Antibiotic-associated diarrhea (AAD) can be seen most frequently in older patients, and the risk increases progressively with longer treatment courses. The main mechanism of AAD is thought to be through impaired resistance to pathogens and as a result of disruption of the gut microbial flora and subsequent changes in the metabolism of carbohydrates, short chain fatty acids, and bile acids (5). Depending on the duration, diarrhea can be classified into 3 categories which are: (I) acute diarrhea, when the duration is less than 2 weeks, (II) persistent diarrhea, when the duration varies from 2 to 4 weeks, and (III) chronic diarrhea, when the duration is more than four weeks (6).
Two major functions of the small intestinal epithelial cells are the secretion and absorption of fluids and electrolytes. About 99% of the overall fluid is absorbed by small intestine and colon (7). Active transport of Na+, Cl−, and HCO3− among others results in the net fluid movement across the gastrointestinal epithelium cells. Any genetic modification, infection or dysregulation in this active transport often results in diarrhea (8). Secretion of chloride or bicarbonate ions or inhibition of absorption of Na+ is the basic mechanism for secretory diarrhea (9). Activation of chloride channels in the apical membrane of enterocytes such as calcium-activated chloride channels and cystic fibrosis transmembrane regulator increase secretion in much secretory diarrhea (10). Many reasons such as non-osmotic laxative abuse, bacterial toxins, ileal bile, acid malabsorption, lymphocytic colitis, and diverticulitis are involved in secretory diarrhea (11).
For the prevention and control of diarrhea, rehydration therapy is thought to be the best treatment (12). Oral rehydration solutions are used for the treatment of diarrhea and prevention of fluid loss and shorten the illness duration. In recent years, their acceptance has been decreased because of their inability to reduce bowel movement and frequency of fluid loss. Nowadays, antibiotics are used in the treatment of diarrhea, but higher doses of antibiotics can cause the appearance of Clostridium difficile infection (13). The gut microbiota has an effective role in the cure of gastrointestinal conditions like diarrhea (14). This introduces a new practice for diarrhea treatment by the use of fermented foods with probiotic bacteria. Probiotics are defined as live microorganisms, exerting beneficial health effects on the host. Their consumption in the general population is primarily believed to contribute to the individual maintenance of gastrointestinal and general health. Probiotics may be an effective solution to the treatment of acute diarrhea. A recent meta-analysis has shown that used alongside rehydration therapy, probiotics appear to be safe and have clear beneficial effects in shortening the duration and reducing stool frequency in diarrhea (15). When diarrhea lasts not more than 14 days and three or more stools per day, then it is called acute diarrhea. A milk drink fermented with Lactobacillus rhamnosus GG (LGG), Lactobacillus acidophilus La-5, and Bifidobacterium lactis Bb-12 significantly reduced the induction of AAD in the treatment group (16). Considering this perspective, the present research was conducted to assess the antidiarrheal efficacy of probiotic bacteria isolated from selective regional yogurt.
MATERIALS AND METHODS
Experimental animals
Healthy Swiss albino mice of either sex weighting 15~25 g and aged 5~6 weeks were used for the experiment. The mice were obtained from the animal house of International Center for Diarrheal Diseases Research, Bangladesh. The animals were kept in cages at room temperature and on a 12 h light/dark cycle. All the animals were fed standard diet and water ad libitum. All the mice were acclimated for one week prior to the experiment. The care and handling were according to the ethical guidelines approved by Animal Ethics Committee, Khulna University, Khulna, Bangladesh (Research Ref. No.: KUAEC-2017/07/01), which agrees with the EU Directive 2010 for animal experiments.
Grouping and dosing
Thirty six mice (18 male and 18 female) were assigned for the study. Male and female mice were separately grouped into six groups each having six mice. The groups were randomly assigned to the study design. All groups were provided their respective treatment orally. The first group was assigned as a standard group and received only basal diet with water during the study period. The second group was assigned as a negative control group and received basal diet and castor oil. The third group was assigned as positive control group and standard drug, loperamide (3 mg/kg) was administered orally for all the test. For the rest three groups, three doses of yogurt (50 mL/kg, 100 mL/kg, and 150 mL/kg) per mice per day were determined respectively for the treatment group 1 (TG1), treatment group 2 (TG2), and treatment group 3 (TG3). Yogurt was started to administer from the day of the experiment until the 27th day of the experiment in order to increase the probiotic bacteria in the guts of mice.
Yogurt preparation
Ten probiotic bacteria were isolated and biochemically characterized according to the method described by Hoque et al. (17) from regional yogurt of Bangladesh through their morphological (size, shape, and motility), biochemical (Gram staining and catalase test), and physiological (pH tolerance, bile salt tolerance, NaCl tolerance, phenol tolerance, and antimicrobial activity) properties (17). Cow milk was collected from a regional farm and was heated at 100°C for 15 min and then cooled to 43°C. Then inoculated with a 5% (v/v) liquid culture of the isolated probiotic mixture. The inoculated milk was poured into containers and incubated at the anaerobic condition at 37°C for 8 h. After coagulation, fresh yogurt were preserved in 4°C for further usage.
Castor oil induced diarrhea
The method used in this study was described by Umer et al. with slight modifications (18). Briefly, Swiss albino mice of the described group were kept fasted for 24 h with excess to water. Mice in the positive control group were administered (3 mg/kg) loperamide 1 h before administration of castor oil. All mice were given 0.5 mL of castor oil orally and white blotting paper was lined under each of the cages. Diarrhea was considered by the presence of stool or any fluid materials that stained the blotting paper placed under every cage lined with the floor. When the feces became unformed, muddy or watery was considered to as diarrhea. Time taken before the first defecation was considered as the ‘latent period’. Total numbers of fecal output as well as the diarrheic feces (muddy or watery feces) excreted by the experimental animal for a period of 4 h after the latent period was determined. After 4 h of observation, blood was drawn from each of the mice for determination of electrolytes fluctuation from the baseline.
During the observation period of 4 h, latent period (time interval between the administration of castor oil and the first defecation in a minute), total fecal output, and fecal water content were recorded for an individual mouse. Because only after castor oil induction the feces of mice were converted to muddy or watery than the normal state in castor oil induced mice. The time period from day 1 to day 26 was considered only for increasing of probiotic bacteria in guts of mice. This period was not considered for data collection.
Percentages of diarrheal inhibition, as well as the weight of total and wet fecal output was determined according to the formula follows:
where ATFPC is average number of wet feces in the positive control group, ATFT is average number of total feces in the test group, and ATFNC is average number of wet feces in the positive negative group.
Fecal output and fecal water content in mice
After administration of castor oil, when the faces became unformed, muddy, or watery was considered to as diarrhea. All of the faces were collected after each defecation and put into a covered vessel for each animal to prevent the faces from drying. All the faces collected over a 4 h period were dried for about 1 h at 100°C in a ventilated oven. Fecal water content was determined according to the following formula:
The effect in serum metabolites
After 4 h of observation, all the animals were sacrificed, and blood was obtained by heart puncture. Then the blood was kept in the anticoagulant tube and analyzed for determining total protein, albumin, sodium, potassium, calcium, phosphate, white blood cell (WBC), and hemoglobin.
Statistical analysis
All the statistical analysis were performed using Statistical Analysis System for analysis of variance (SAS Institute, Cary, NC, USA) and Graphpad Prism (version 5.0, GraphPad Software, San Diego, CA, USA).
RESULTS AND DISCUSSION
Isolation of probiotic bacteria
Bacterial characteristics presented in the Table 1, show that, all the isolated bacteria were gram positive, catalase negative, rod shaped, non-motile, and coagulase positive. All the bacteria were tolerant to harsh condition at low pH, NaCl (1~8%), phenol (0.1~0.4%), and bile salt (0.3%). All the results showed that the bacteria were typical Lactobacillus spp.
Table 1.
Biochemical and physiological characteristics of the isolated bacteria
| Isolate no. | Shape | Gram staining | Catalase | Coagulase | Motility | pH tolerance | Bile tolerance | NaCl tolerance | Phenol tolerance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rods | + | − | + | − | pH 3.0 | 0.3% | 2% | 4% | 6% | 8% | 0.1% | 0.2% | 0.3% | 0.4% |
| 2 | rods | + | − | + | − | ++ | ++ | ++ | ++ | + | − | ++ | ++ | ++ | ++ |
| 3 | rods | + | − | + | − | ++ | ++ | ++ | ++ | + | − | ++ | ++ | ++ | ++ |
| 4 | rods | + | − | + | − | ++ | ++ | ++ | ++ | + | − | ++ | ++ | ++ | ++ |
| 5 | rods | + | − | + | − | ++ | ++ | ++ | ++ | + | − | ++ | ++ | ++ | ++ |
| 6 | rods | + | − | + | − | ++ | ++ | ++ | ++ | + | − | ++ | ++ | ++ | ++ |
| 7 | rods | + | − | + | − | ++ | ++ | ++ | ++ | + | − | ++ | ++ | ++ | ++ |
| 8 | rods | + | − | + | − | ++ | ++ | ++ | ++ | + | − | ++ | ++ | ++ | ++ |
| 9 | rods | + | − | + | − | ++ | ++ | ++ | ++ | + | − | ++ | ++ | ++ | ++ |
| 10 | rods | + | − | + | − | ++ | ++ | ++ | ++ | + | − | ++ | ++ | ++ | ++ |
+, the excellent tolerant against the condition; −, no tolerance against the condition.
Effects of probiotic on castor oil induced a diarrheal model
In the castor oil induced diarrheal model as presented in Table 2, probiotic yogurt significantly prolonged the onset of diarrhea and reduced the frequency and weight of total stool (Fig. 1) compared with the negative control. All the probiotic fed the treatment groups showed statistically significant (P<0.001) effects on the onset of diarrhea, the total number of feces, the average weight of total feces, and average fecal water content compared to the negative control group. TG2 (100 mL/kg body weight) and TG3 (150 mL/kg body weight) showed significant (P<0.001) differences with TG1 (50 mL/kg body weight) regarding onset of diarrhea. Moreover, a significance difference (P<0.05) was observed in onset of diarrhea when compared with TG3 and TG2. The total number of feces was significantly (P<0.001) reduced in all of the groups compared with the negative control group. However, no significance difference was observed in the total number of feces in the all of the treatment groups compared with the positive control group.
Table 2.
Antidiarrheal effects of probiotics against castor oil induced diarrheal mice
| Groups | The onset of diarrhea (min) | Total number of feces | Average weight of total feces (g) | Average fecal water content (g) | Total fecal output (%) | Reduction (%) |
|---|---|---|---|---|---|---|
| Std | 42.50±1.839 | 6.50±0.76 | 0.214±0.010 | 0.020±0.003 | ||
| NC | 22.00±1.18 | 15.83±1.49*** | 0.59±0.008*** | 0.090±0.006*** | ||
| PC | 120.50±4.02*** | 4.67±0.21 | 0.20±0.010 | 0.018±0.001 | 33.99 | 73.68 |
| TG1 | 74.00±2.42*** | 6.83±0.79 | 0.33±0.004 | 0.028±0.002 | 56.29 | 56.84 |
| TG2 | 87.00±1.10***§§§ | 6.167±1.10 | 0.295±0.011 | 0.020±0.002 | 50.14 | 61.05 |
| TG3 | 105.33±3.31***§§§† | 5.167±0.47 | 0.238±0.011 | 0.015±0.001 | 40.48 | 67.37 |
NC, negative control group; PC, positive control group; Std, standard group; TG1, treatment group 1; TG2, treatment group 2; TG3, treatment group 3.
Values are mean±SEM (n=6).
Analysis was performed using one way ANOVA followed by Tukey’s adjustment.
P <0.001 compared with NC group,
P <0.001 compared with TG1 group, and
P <0.05 compared with TG2 group.
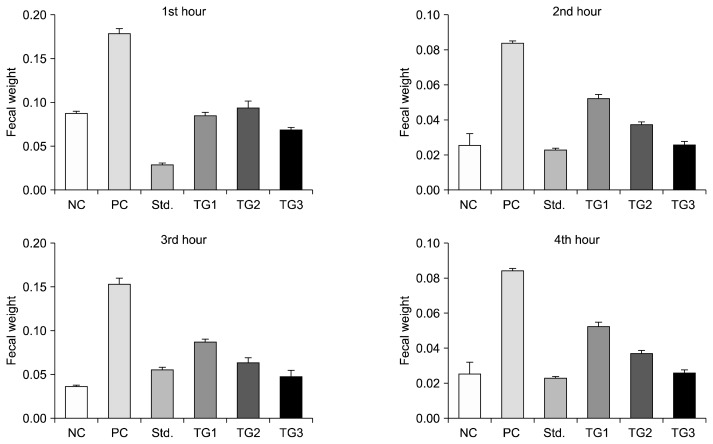
Fig. 1.
Mean fecal output (g) from 1st to 4th hour after induction of castor oil. The feces were collected from the blotting paper lined under the cage after administration of castor oil orally and weighted in order to measure the total amount of defecation in the mice groups. Values are mean±SEM. NC, negative control; PC, positive control; Std., standard; TG1, treatment group with 50 mL/kg of yogurt; TG2, treatment group with 100 mL/kg of yogurt; TG3, treatment group with 150 mL/kg of yogurt.
Total stools weight (Fig. 1), TG3 and the positive control group showed significant (P<0.001) reduction of total stool weight compared with TG1 and TG2. When total fecal weight was compared within the treatment groups as well as the positive control group, no significance differences were observed. Besides, the results revealed that, the percentage of diarrheal inhibitions were 56.84%, 61.05%, 67.37%, and 73.68%, in TG1, TG2, TG3, and positive control group, respectively. And the percentages of total fecal output were 56.29%, 50.14%, 40.48%, and 33.99%, in TG1, TG2, TG3, and the positive control group, respectively.
Effects of probiotics on blood serum electrolytes and metabolites
At the time of diarrhea electrolytes are known to be decreased with fecal output and duration of diarrhea. The effects of probiotic yogurt on serum electrolytes, sodium, potassium, and chloride were measured (Table 3). Serum total protein level, albumin, globulin, WBC, and hemoglobin were also measured. Sodium levels in all of the groups were significantly (P<0.001) different compared with the negative control group. TG2 was significantly (P<0.01) different compared with the standard group, and TG3 was different compared to the standard group (P<0.001). Moreover, TG3 was significantly (P<0.001) different compared to TG1 and the positive control group (P<0.05).
Table 3.
Effects of probiotics on blood serum electrolytes and metabolites
| Groups | Sodium (mmol/L) | Potassium (mmol/L) | Chloride (mmol/L) | Total protein (mg/dL) | Albumin (mg/dL) | Globulin (mg/dL) | WBC (103/mm3) | Hemoglobin (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| Std | 135.83±0.70*** | 3.60±0.05* | 98.20±0.62 | 6.61±0.14 | 3.48±0.14 | 3.13±0.08 | 7.90±0.25 | 10.46±0.19 |
| NC | 129.67±1.45 | 3.10±0.05 | 95.67±1.14 | 5.83±0.08 | 3.27±0.06 | 2.57±0.06 | 8.80±0.45 | 9.18±0.45 |
| PC | 141.50±1.31*** | 4.47±0.18*** | 99.67±1.43 | 7.20±0.20** | 4.15±0.09*** | 3.05±0.16 | 10.78±0.31* | 12.30±1.70 |
| TG1 | 138.17±1.42*** | 4.07±0.18** | 100.50±1.47 | 7.05±0.19** | 3.87±0.12* | 3.18±0.27 | 9.73±0.35 | 10.78±0.50 |
| TG2 | 142.67±1.28***‡‡ | 3.77±0.04*§ | 100.50±1.20 | 7.01±0.23** | 3.91±0.07** | 3.10±0.17 | 10.23±0.38 | 9.93±0.67 |
| TG3 | 147.67±0.84***#§§§‡‡‡ | 4.55±0.24***§ | 99.83±1.49 | 6.78±0.24* | 3.72±0.17 | 3.07±0.09 | 10.53±0.50* | 9.60±0.37 |
NC, negative control group; PC, positive control group; Std, standard group; TG1, treatment group 1; TG2, treatment group 2; TG3, treatment group 3.
Values are mean±SEM (n=6).
Analysis was performed using one way ANOVA followed by Tukey’s adjustment.
P <0.05,
P <0.01, and
P <0.001 compared with NC group,
P <0.05 compared with PC group,
P <0.01 and
P <0.001 compared with TG1 group, and
P <0.01 and
P <0.001 compared with Std group.
Serum potassium levels were compared with the negative control group and all the treatment groups and standard groups. TG3 and the positive control group were statistically significant (P<0.001) different from the negative control group. But TG1 and TG2 showed a difference at the level of significance that was P<0.01 and P<0.05, respectively. The difference was found between the TG3 and TG2 at P<0.05 level of significance. No differences were found between the TG3 and positive control group. Moreover, the probiotic yogurt showed no effects on serum chloride levels. When the total protein level of all the groups was compared with the negative control group, there was a significant difference between the groups. The level of significance was P<0.01, P<0.01, and P<0.05 for TG1, TG2, and TG3, respectively. The positive control group showed the highest level of significance (P<0.001) compared with the negative control group. There was no statistically significance difference between the treatment groups and positive control group. The positive control group, TG1, and TG2 showed significant differences with the negative control group regarding total albumin levels, and the levels of significance were P<0.001, P<0.05 and P<0.01, respectively. Whereas there was no significant difference between the groups for globulin and hemoglobin. When the WBC count was compared to the positive control group, the positive control group was significant (P<0.05) compared to the negative control group. There were no significant differences in the treatment groups compared to the standard group.
DISCUSSION
All the isolated bacteria showed probiotic characteristics. Isolates were isolated using de Man, Rogosa, and Sharpe (MRS) media. Some of the components of MRS culture media such as sodium acetate, magnesium sulfate, and Tween-80 are known to act as special growth factors for the growth of lactic acid bacteria. Among them, Tween-80 is a surfactant which facilitates the uptake of nutrients and sodium acetate, and ammonium citrate act as selective agents (19). In the present study, the isolates were sub cultured six times and incubated anaerobically which might decrease the many fastidious unwanted microorganisms and favor the growth of lactic acid bacteria. From their colony morphology, physiological characteristics, and biochemical characteristics (Gram positive, catalase negative, non-motile, sugar fermentation pattern, and resistance to inhibitory substances such as pH 2.2, 0.3% bile acid, 0.1~0.4% phenol, and 1~4% NaCl) all the ten isolates were considered probiotic bacteria. Hoque et al. (17) identified Lactobacillus species from yogurt samples by observing their morphological characteristics and different biochemical characteristics such as Gram positive, catalase negative, non-motile, sugar fermentation pattern, and resistance to inhibitory substances such as pH 2.2, 0.3% bile acid, 0.1~0.4% phenol, and 1~10% NaCl. Microscopically the isolated bacteria were Gram positive, rod shaped, non-motile, catalase negative, and coagulase positive. Therefore, the results of the presents study were similar to Hoque et al. (17).
One of the major selection criteria of probiotics is resistance to low pH. Chou and Weimer reported that probiotic bacteria need to be resistant at low pH (pH 1.5~3.0) while they pass from the stomach. The time between the first entrance and release from stomach requires approximately 3 h and by this time probiotic must be viable (20). In most in vitro assays, pH 3.0 has been preferred cause significant decrease in viability is often observed at pH 2.0 or bellow (21).
All of the isolates obtained from the present study were more or less tolerant at pH 3.0. However, all of them showed resistance to low pH after a 4 h of period, which needs to pass the stomach. Bao et al. isolated 90 strains of Lactobacillus fermentum, among them 35 strains showed tolerance against low pH, which indicated the similarity with our findings (22). A similar research was conducted with 13 strains of Lactobacillus plantarum, and 7 strains were highly tolerance against low pH (23). L. acidophilus NIT isolated from infant feces showed resistance to pH 2~4, which directly indicate the similarity with this present study (24).
Probiotics exhibiting antidiarrheal activity may have a potential to retard the onset of diarrhea significantly as seen in TG2 and TG3. According to the WHO criteria, a decrease in consistency and an increase in frequency in bowel movements to greater than 3 stools per day generally describes diarrhea. Even though diarrhea has been defined over time by various scientific groups in different ways, the emphasis is given on the consistency of stools rather than the numbers. Gidudu et al. (25) defined diarrhea, when the water percentages exceed 90% whereas the water percent of stools normally 60~90%. However, the percentages of inhibition were mainly focused on the frequency and total stools as a good marker of antidiarrheal activity.
Diarrhea is also presented as an increase in weight of defecations (3). Accordingly, this study displayed a dose dependent reduction in percentages of total fecal output (P<0.001) indicating the antidiarrheal potential of the study models. This study is concordant with other studies in which Lactobacillus sporogenes significantly reduce the episodes (P<0.002) and shorten the duration (P<0.001) of acute rotavirus diarrhea in infants than placebo (26). Another double-blind randomized study investigated that Lactobacillus reuteri DSM 17938 significantly reduced watery diarrhea than placebo (2.1±1.7 days vs. 3.3±2.1 days; P<0.03) as well as relapse rate of diarrhea (15% vs. 42%; P<0.03) (27). Foster et al. (28) reported that, injection of Lactobacillus preparation in infected ileal loop significantly reduced the enterotoxin response against Escherichia coli enterotoxin-induced diarrhea in the rabbit. Another study reported that Lactobacillus GG reduced the duration of non-bloody diarrhea compared to the control (31% vs 75% at 48 h) admitted for severe diarrhea and malnutrition which explain the similarity of the findings of this present study (29).
A milk drink fermented with LGG, L. acidophilus La-5, and B. lactis Bb-12 was administered to a treatment group in a double-blind design. Only 5.9% patients developed ADD, which was a significant reduction compared to the placebo group (27.6%) (P=0.035) (16). Another randomized double-blind study was conducted to investigate the antidiarrheal efficacy of a mixture of LGG, B. lactis Bb-12, and L. acidophilus La-5. The results showed that there was no incidence of severe diarrhea in the probiotic group compared to 6 cases in the placebo group (P=0.025) even though there was only one episode of minor diarrhea compared to 21 in the placebo group (P<0.001) (30). All the results show similar findings to the present study. For assessing the antidiarrheal activity of probiotic bacteria, an in vivo mice trail was conducted with a castor oil induced diarrheal rat model. In an animal model, latent period, total fecal output, the frequency of defecation, and the total water content of feces were measured. Blood serum levels of electrolytes, total protein, albumin, and globulin were measured. Hematological parameters of hemoglobin level and white blood cell count were also measured. The isolates showed significant antidiarrheal properties in castor oil induced diarrheal mice.
ACKNOWLEDGEMENTS
The authors are grateful to the authority of National Agricultural Technology Program-Phage II (NATP-2), Project Implementation Unit, Bangladesh Agricultural Research Council (BARC) for providing funding under Competitive Research Grant (CRG) by the World Bank.
Footnotes
AUTHORS CONTRIBUTION
The first three authors drew the plan and performed the full time lab work. The rests are responsible for part time laboratory activity, statistical analysis of the data collected along with the first three authors. The final manuscript is revised and preliminary edited by all the authors participated in the research.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Pesola GE, Hogg JE, Yonnios T, McConnell RE, Carlon GC. Isotonic nasogastric tube feedings: do they cause diarrhea? Crit Care Med. 1989;17:1151–1155. doi: 10.1097/00003246-198911000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Viall C, Porcelli K, Teran JC, Varma RN, Steffee WP. A double-blind clinical trial comparing the gastrointestinal side effects of two enteral feeding formulas. J Parenter Enteral Nutr. 1990;14:265–269. doi: 10.1177/0148607190014003265. [DOI] [PubMed] [Google Scholar]
- 3.Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J Antimicrob Chemother. 2003;51:1339–1350. doi: 10.1093/jac/dkg254. [DOI] [PubMed] [Google Scholar]
- 4.Talley NJ, Weaver AL, Zinsmeister AR, Melton LJ., III Self-reported diarrhea: what does it mean? Am J Gastroenterol. 1994;89:1160–1164. [PubMed] [Google Scholar]
- 5.Allen SJ, Wareham K, Wang D, Bradley C, Hutchings H, Harris W, Dhar A, Brown H, Foden A, Gravenor MB, Mack D. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249–1257. doi: 10.1016/S0140-6736(13)61218-0. [DOI] [PubMed] [Google Scholar]
- 6.Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, Hennessy T, Griffin PM, DuPont H, Sack RB, Tarr P, Neill M, Nachamkin I, Reller LB, Osterholm MT, Bennish ML, Pickering LK. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–351. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 7.Mims BC, Curry CE., Jr . Constipation, diarrhea and irritable bowel syndrome. In: Chisholm-Burns MA, Schwinghammer TL, Wells BG, Malone PM, Kolesar JM, DiPiro JT, editors. Pharmacotherapy: A Pathophysiologic Approach. McGraw-Hill Companies; New York, NY, USA: 2008. pp. 307–320. [Google Scholar]
- 8.Ghishan FK, Kiela PR. Small intestinal ion transport. Curr Opin Gastroenterol. 2012;28:130–134. doi: 10.1097/MOG.0b013e32834e7bc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiller LR. Secretory diarrhea. Curr Gastroenterol Rep. 1999;1:389–397. doi: 10.1007/s11894-999-0020-8. [DOI] [PubMed] [Google Scholar]
- 10.Thiagarajah JR, Donowitz M, Verkman AS. Secretory diarrhoea: mechanisms and emerging therapies. Nat Rev Gastroenterol Hepatol. 2015;12:446–457. doi: 10.1038/nrgastro.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gionchetti P, Rizzello F, Venturi A, Campieri M. Probiotics in infective diarrhoea and inflammatory bowel diseases. J Gastroenterol Hepatol. 2000;15:489–493. doi: 10.1046/j.1440-1746.2000.02162.x. [DOI] [PubMed] [Google Scholar]
- 12.Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cammarota G, Anti M, De Lorenzo A, Pola P, Gasbarrini G, Gasbarrini A. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163–169. doi: 10.1046/j.1365-2036.2001.00923.x. [DOI] [PubMed] [Google Scholar]
- 13.Perdigon G, Nader de Macias ME, Alvarez S, Oliver G, Pesce de Ruiz Holgado AA. Prevention of gastrointestinal infection using immunobiological methods with milk fermented with Lactobacillus casei and Lactobacillus acidophilus. J Dairy Res. 1990;57:255–264. doi: 10.1017/S002202990002687X. [DOI] [PubMed] [Google Scholar]
- 14.Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD003048.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearce JL, Hamilton JR. Controlled trial of orally administered lactobacilli in acute infantile diarrhea. J Pediatr. 1974;84:261–262. doi: 10.1016/S0022-3476(74)80618-9. [DOI] [PubMed] [Google Scholar]
- 16.Wenus C, Goll R, Loken EB, Biong AS, Halvorsen DS, Florholmen J. Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur J Clin Nutr. 2008;62:299–301. doi: 10.1038/sj.ejcn.1602718. [DOI] [PubMed] [Google Scholar]
- 17.Hoque MZ, Akter F, Hossain KM, Rahman MSM, Billah MM, Islam KMD. Isolation, identification and analysis of probiotic properties of Lactobacillus spp. from selective regional yoghurts. World J Dairy Food Sci. 2010;5:39–46. [Google Scholar]
- 18.Umer S, Tekewe A, Kebede N. Antidiarrhoeal and antimicrobial activity of Calpurnia aurea leaf extract. BMC Complement Altern Med. 2013;13:21. doi: 10.1186/1472-6882-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Man JC, Rogosa M, Sharpe ME. A medium for the cultivation of lactobacilli. J Appl Microbiol. 1960;23:130–135. [Google Scholar]
- 20.Chou LS, Weimer B. Isolation and characterization of acid- and bile-tolerant isolates from strains of Lactobacillus acidophilus. J Dairy Sci. 1999;82:23–31. doi: 10.3168/jds.S0022-0302(99)75204-5. [DOI] [PubMed] [Google Scholar]
- 21.Prasad J, Gill H, Smart J, Gopal PK. Selection and characterisation of Lactobacillus and Bifidobacterium strains for use as probiotics. Int Dairy J. 1998;8:993–1002. doi: 10.1016/S0958-6946(99)00024-2. [DOI] [Google Scholar]
- 22.Bao Y, Zhang Y, Zhang Y, Liu Y, Wang S, Dong X, Wang Y, Zhang H. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control. 2010;21:695–701. doi: 10.1016/j.foodcont.2009.10.010. [DOI] [Google Scholar]
- 23.Cebeci A, Gürakan C. Properties of potential probiotic Lactobacillus plantarum strains. Food Microbiol. 2003;20:511–518. doi: 10.1016/S0740-0020(02)00174-0. [DOI] [Google Scholar]
- 24.Pan X, Chen F, Wu T, Tang H, Zhao Z. The acid, bile tolerance and antimicrobial property of Lactobacillus acidophilus NIT. Food Control. 2009;20:598–602. doi: 10.1016/j.foodcont.2008.08.019. [DOI] [Google Scholar]
- 25.Gidudu J, Sack DA, Pina M, Hudson MJ, Kohl KS, Bishop P, Chatterjee A, Chiappini E, Compingbutra A, da Costa C, Fernandopulle R, Fischer TK, Haber P, Masana W, de Menezes MR, Kang G, Khuri-Bulos N, Killion LA, Nair C, Poerschke G, Rath B, Salazar-Lindo E, Setse R, Wenger P, Wong VC, Zaman K Brighton Collaboration Diarrhea Working Group. Diarrhea: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:1053–1071. doi: 10.1016/j.vaccine.2010.11.065. [DOI] [PubMed] [Google Scholar]
- 26.Chandra RK. Effect of Lactobacillus on the incidence and severity of acute rotavirus diarrhoea in infants. A prospective placebo-controlled double-blind study. Nutr Res. 2002;22:65–69. doi: 10.1016/S0271-5317(01)00367-0. [DOI] [Google Scholar]
- 27.Francavilla R, Lionetti E, Castellaneta S, Ciruzzi F, Indrio F, Masciale A, Fontana C, La Rosa MM, Cavallo L, Francavilla A. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhea–a double-blind study. Aliment Pharmacol Ther. 2012;36:363–369. doi: 10.1111/j.1365-2036.2012.05180.x. [DOI] [PubMed] [Google Scholar]
- 28.Foster TL, Winans L, Jr, Carski TR. Evaluation of lactobacillus preparation on eterotoxigenic E. coli-induced rabbit ileal loop reactions. Am J Gastroenterol. 1980;73:238–243. [PubMed] [Google Scholar]
- 29.Zaid MR, Hasan M, Khan AA. Attapulgite in the treatment of acute diarrhoea: a double-blind placebo-controlled study. J Diarrhoeal Dis Res. 1995;13:44–46. [PubMed] [Google Scholar]
- 30.Fox MJ, Ahuja KDK, Robertson IK, Ball MJ, Eri RD. Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double-blind, randomised, placebo-controlled study. BMJ Open. 2015;5:e006474. doi: 10.1136/bmjopen-2014-006474. [DOI] [PMC free article] [PubMed] [Google Scholar]