Abstract
Intracellular Ca2+ ([Ca2+]i) induces platelet aggregation, and influences the activation of aggregation associated-molecules. The increased [Ca2+]i activates both the Ca2+/calmodulin-dependent phosphorylation of the myosin light chain and the diacylglycerol-dependent phosphorylation of pleckstrin to trigger granule secretion (i.e., dense body and α-granule) and platelet aggregation. This study was carried out to elucidate the antagonistic effect of 20(S)-ginsenoside Rg3 (G-Rg3) present in Panax ginseng Mayer on Ca2+. G-Rg3 inhibited thrombin-induced human platelet aggregation in a dose-dependent manner and suppressed thrombin-induced elevation of [Ca2+]i mobilization. G-Rg3 increased the levels of cAMP, and subsequently, elevated the phosphorylation of inositol 1,4,5-triphosphate receptor I (Ser1756) during thrombin-induced human platelet aggregation. Moreover, G-Rg3 inhibited thapsigargin-induced Ca2+ influx and the thrombin-induced elevation of extracellular signal-regulated kinase 2 phosphorylation. G-Rg3 exhibited an inhibitory effect on [Ca2+]i levels leading to granule release and thus a therapeutic potential against platelet-mediated thrombotic disease is suggested.
Keywords: 20(S)-ginsenoside Rg3; inositol 1,4,5-triphosphate receptor I; extracellular signal-regulated kinases; p-selectin expression
INTRODUCTION
Platelets are activated at sites of vascular damage via molecules such as collagen, thrombin, thromboxane A2, and adenosine diphosphate. Complete platelet aggregation is essential for hemostatic plug formation. This physiological event is achieved in presence of free cytosolic Ca2+. Thus, inhibiting intracellular Ca2+ ([Ca2+]i) is useful in preventing platelet-mediated cardiovascular diseases such as thrombosis or myocardial infarction. Elevation of [Ca2+]i levels by agonists is dependent on calcium mobilization from the endoplasmic reticulum (ER) and influx from extracellular spaces. Thrombin, a platelet agonist, is known to stimulate platelet aggregation by binding to the Gq-coupled proteinase-activated receptor activating phospholipase Cβ (PLCβ). The activated PLCβ hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to produce inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (1). IP3 mobilizes free cytosolic Ca2+ from ER by binding to the IP3 receptor type I (IP3RI). The increased Ca2+ stimulates granule secretion (i.e., dense body and α-granule) and platelet aggregation. Another way to increase [Ca2+]i level is via influx from extracellular spaces. Depletion of the intracellular Ca2+ store by IP3 is known to be associated with the influx of extracellular Ca2+, which is stimulated by extracellular signal-regulated kinases (2,3).
Selectins are expressed by activation or inflammatory response of various vascular cells, including platelets, leukocytes and endothelial cells (4,5). L-selectin is expressed by leukocytes, E-selectin is expressed by endothelial cells, and p-selectin is expressed by endothelial cells and platelets. The p-selectin is located in the inner layer of α-granule, which is released by the agonists, and is re-expressed on the platelet surface (6). P-selectin plays an important role in interactions with immune cells (7) and is an indicator of α-granule secretion.
During normal circulation, vascular endothelial cells release both prostaglandin I2 and nitric oxide, which facilitate the production of cyclic AMP (cAMP) and cyclic GMP (cGMP) in the platelets. Elevated cAMP and cGMP levels induce the activation of protein kinase A (PKA) and protein kinase G (PKG), respectively, both of which phosphorylate substrate protein, IP3RI (8). IP3RI becomes inactive after phosphorylation, leading to inhibition of [Ca2+]i mobilization (9,10). Therefore, IP3RI phosphorylation is very useful for evaluating the Ca2+-antagonistic effect of compounds.
Panax ginseng Mayer is used in conventional medicine in Asia owing to its therapeutic effects on thrombosis, hypertension, vasorelaxation, inflammation, oxidation, atherosclerosis, and myocardial infarction (11,12). Because platelets are crucial mediators of cardiovascular diseases, many studies have investigated these functions. With respect to the effects of ginsenosides on platelet aggregation, it has been reported that G-Rg3 has inhibitory effects on collagen-induced blood platelet aggregation (13), thromboxane A2 production, ATP release, and [Ca2+]i mobilization (14). Furthermore, dihydroxyginsenoside Rg3 downregulated extracellular signal-regulated kinase 2 and p38 mitogen-activated protein kinase (15) and ginsenoside Rg3-enriched fraction showed inhibitory effects on collagen-induced rat platelets (16). In our previous study, we also investigated the inhibitory effects of G-Rg3 (20S, 20R) on thrombin-induced human platelets (17). However, the inhibitory mechanism of G-Rg3 on the platelets is still not fully understood. Therefore, in the present study, we characterized the modulatory mechanism associated with the inhibitory effects of Rg3 on human platelet [Ca2+]i levels.
MATERIALS AND METHODS
Materials
We purchased 20(S)-ginsenoside Rg3 from the Ambo Institute (Daejon, Korea) and thrombin and all materials for platelet aggregation from Chrono-Log Corporation (Havertown, PA, USA). We purchased all materials for buffer solution and Rp-8-Br-cAMPS, Rp-8-Br-cGMP, p-chlorophenylthio (CPT)-cAMP, 8-Br-cGMP from Sigma (St. Louis, MO, USA) and Fura 2-acetoxymethyl (AM) from Invitrogen (Eugene, OR, USA). Thapsigargin and cAMP/cGMP enzyme immunoassay kit were obtained from Cayman Chemical (Ann Arbor, MI, USA). Anti-IP3-receptor type I, anti-phosphor-IP3-receptor type I (Ser1756), anti-extracellular signal-regulated kinase (ERK) (1/2), anti-phosphor-ERK (1/2), anti-rabbit IgG-horseradish peroxidase, and cell lysis buffer were obtained from Cell Signaling (Beverly, MA, USA). Anti-β-actin was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal to CD62P (p-selectin) antibody was purchased from Biolegend (San Diego, CA, USA). Polyvinylidene difluoride (PVDF) membrane and enhanced chemiluminesence solution (ECL) were purchased from General Electric Healthcare (Chalfont St. Giles, Buckinghamshire, UK).
Preparation of washed human platelets
Human platelet-rich plasma (PRP) was obtained from the Korean Red Cross Blood Center (Changwon, Korea), and centrifuged for 10 min at 1,300 g. The platelet-containing pellet was then washed twice with washing buffer solution (138 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.36 mM NaH2PO4, 5.5 mM glucose, and 1 mM Na2EDTA, pH 6.5), and resuspended in suspension buffer solution (138 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.36 mM NaH2PO4, 0.49 mM MgCl2, 5.5 mM glucose, and 0.25% gelatin, pH 6.9). The platelet concentration was adjusted to a final concentration of 5×108/mL. All aforementioned procedures were performed at 25°C. Experimental approval (PIRB12-072) was obtained from the Public Institutional Review Board at the National Institute for Bioethics Policy (Seoul, Korea).
Measurement of platelet aggregation
Platelets (108/mL) were preincubated with or without G-Rg3 in 2 mM CaCl2 for 3 min at 37°C and then stimulated by thrombin (0.05 U/mL). The platelet aggregation assay was performed for 5 min using an aggregometer (Chrono-Log Corporation). Platelet aggregation rate (%) was determined as an increase in light transmission. G-Rg3 was dissolved in 0.1% dimethyl sulfoxide (DMSO).
Measurement of cAMP and cGMP
Platelet aggregation was terminated by adding 80% ice-cold ethanol to the platelet suspension. Next, cAMP and cGMP were extracted three times from the suspension by using 80% ice-cold ethanol. The extracts were dried by using nitrogen gas and subsequently dissolved in an assay buffer from a cAMP/cGMP enzyme immunoassay kit. The levels of cAMP and cGMP were determined using SynergyTM HT Multi-Model Microplate Reader (BioTek Instruments, Winoosku, VT, USA).
Determination of Ca2+ mobilization and influx
PRP was incubated with 5 μM of Fura 2-AM at 37°C for 60 min. The washed platelets (108/mL), loaded with Fura 2-AM, were prepared using the procedure described above preincubated with G-Rg3 for 3 min at 37°C in the presence of 100 μM of ethylene glycol bis(2-aminoethyl) tetraacetic acid, and stimulated with thrombin (0.05 U/mL) for Ca2+ mobilization and thapsigargin (1 μM) for Ca2+ influx. After thapsigargin stimulation, 2 mM of calcium was added at 3 min. Fura 2-AM fluorescence was measured using a spectrofluorometer (SFM-25; BioTek Instruments) at an excitation wavelength that changed every 0.5 s from 340 to 380 nm; and an emission wavelength set at 510 nm. The [Ca2+]i values were calculated using the Grynkiewicz method (18).
Western blot for analysis of IP3RI- and ERK-phosphorylation
Washed human platelets (108/mL) were preincubated with or without G-Rg3 in 2 mM CaCl2 for 3 min at 37°C and then stimulated by thrombin (0.05 U/mL). The platelet aggregation assay was performed for 5 min then terminated by addition of 1× lysis buffer. The platelet lysates were then measured for total protein concentration using a BCA protein assay kit (Pierce Biotechnology Inc., Rockford, IL, USA). Proteins (15 μg) were separated using 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto PVDF membranes. The dilutions for the primary and secondary antibodies were 1:1,000 and 1:10,000, respectively. The protein bands were visualized using the ECL reagent (General Electric Healthcare, Buckinghamshire, UK).
Determination of p-selectin release
Washed human platelets (108/mL) were preincubated with G-Rg3 in the presence of 2 mM CaCl2 for 3 min at 37°C followed by thrombin stimulation (0.05 U/mL). The platelets were reconstituted in 250 μL of ice-cold phosphate-buffered saline (PBS) and incubated with 10 μL of Alexa Fluor 488 anti-human CD62P in PBS containing 0.09% sodium azide and 0.2% bovine serum albumin for 60 min at 4°C in a dark room. The platelets were then washed thrice with ice-cold PBS and resuspended in 0.5% paraformaldehyde in PBS. The platelet-bound Alexa Fluor 488 anti-human CD62P was determined using flow cytometry (BD Biosciences, San Diego, CA, USA) and the data were analyzed using the BD cellQuestTM software.
Statistical analyses
The experimental results are reported as the mean±standard deviation accompanied by the number of observations. The data were compared with analysis of variance (ANOVA). Significant differences among the group means were compared using the Newman-Keuls method. Statistical analysis was performed using SPSS 21.0.0.0 (SPS Inc., Chicago, IL, USA). P<0.05 was considered to be statistically significant.
RESULTS
Effects of G-Rg3 on thrombin-induced human platelet aggregation
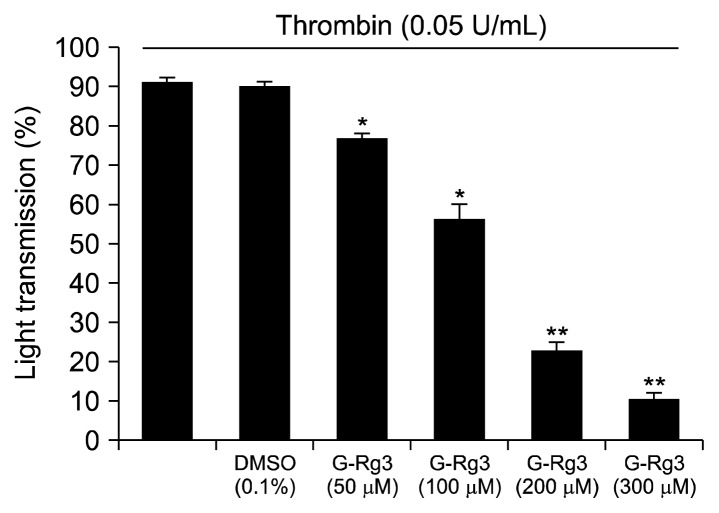
Based on a previous report, 0.05 U/mL of thrombin was used as it maximally aggregates human platelets (17). Therefore, thrombin (0.05 U/mL) was used as the human platelet agonist in this study. When washed human platelets (108/mL) were activated with thrombin, the aggregation rate increased by up to 92.5±1.3%. However, various concentrations of G-Rg3 (50, 100, 200, and 300 μM) reduced thrombin-stimulated platelet aggregation in a dose-dependent manner (Fig. 1). DMSO (0.1%) did not influence the thrombin-induced platelet aggregation (Fig. 1).
Fig. 1.
Effects of ginsenoside Rg3 (G-Rg3) on thrombin-induced human platelet aggregation. Measurement of platelet aggregation was carried out as described in “MATERIALS AND METHODS” section. The rate of inhibition by G-Rg3 was recorded as the percentage of the thrombin induced aggregation rate. The data are expressed as the mean±standard deviation (n=4). *P <0.05 and **P <0.01 versus the thrombin-stimulated human platelets.
Effects of G-Rg3 on cAMP and cGMP production
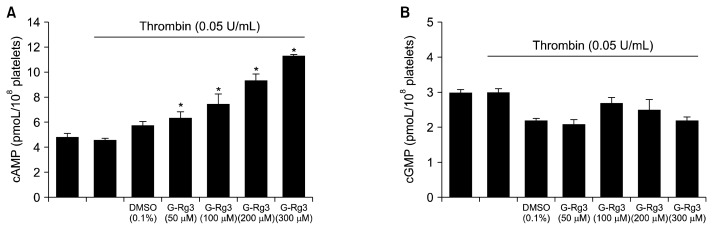
Next, we investigated whether G-Rg3 enhanced cAMP and cGMP production in thrombin-induced human platelet aggregation. As shown in Fig. 2A, thrombin mildly downregulated cAMP levels, but G-Rg3 (50 to 300 μM) increased thrombin-attenuated cAMP levels in a dose-dependent manner. Thrombin did not alter cGMP levels as compared with its basal levels. On the contrary, cGMP levels appear to be downregulated by G-Rg3 (Fig. 2B).
Fig. 2.
Effects of ginsenoside Rg3 (G-Rg3) on cyclic AMP (cAMP) and cyclic GMP (cGMP) production. Effects of G-Rg3 on cAMP (A) and (B) cGMP production in thrombin-induced platelets. cAMP and cGMP were determined as described in the “MATERIALS AND METHODS” section. The data are expressed as the mean±standard deviation (n=4). *P <0.05 versus the thrombin-stimulated human platelets.
Effects of G-Rg3 on elevation of [Ca2+]i mobilization and IP3RI phosphorylation
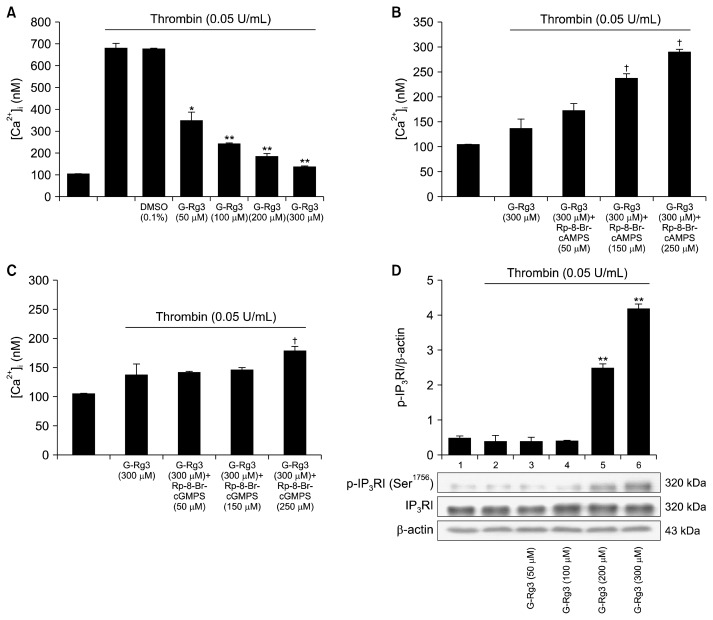
Because [Ca2+]i is essential for platelet activation, we investigated the effects of G-Rg3 on Ca2+ antagonistic activity. As shown in Fig. 3A, [Ca2+]i level was increased from 101.5±1.2 nM to 680.3±20.3 nM by thrombin (0.05 U/mL) (Fig. 3A). However, G-Rg3 decreased thrombin-elevated [Ca2+]i level in a dose-dependent manner (50 to 300 μM) (Fig. 3A). If G-Rg3-mediated decrease in [Ca2+]i level is mediated via cAMP/PKA or cGMP/PKG pathway, the G-Rg3-mediated downregulation of [Ca2+]i level could be reverted with the aid of PKA or PKG inhibitors. As shown in Fig. 3B, the PKA inhibitor Rp-8-Br-cAMPS (50 to 250 μM) increased the [Ca2+]i level in a dose-dependent manner when used along with G-Rg3 (300 μM) (Fig. 3B). There was a 111.2% increase in [Ca2+]i levels after treatment with G-Rg3 (300 μM) plus Rp-8-Br-cAMPS (250 μM). However, Rp-8-Br-cGMPS (250 μM) mildly enhanced G-Rg3 (300 μM)-mediated decrease in [Ca2+]i levels (Fig. 3C). There was a 30% increase in [Ca2+]i levels after treatment with G-Rg3 (300 μM) plus Rp-8-Br-cGMPS (250 μM). The inhibitory effects of Rp-8-Br-cGMPS on G-Rg3 action were less intense compared to Rp-8-Br-cAMPS. Next, we investigated the IP3RI phosphorylation-associated changes in [Ca2+]i level. G-Rg3 upregulated IP3RI (Ser1756) phosphorylation during thrombin-induced human platelet aggregation in a dose-dependent manner (50 to 300 μM) (Fig. 3D). This result suggested an increase in cAMP levels by G-Rg3 (Fig. 2A). The increased levels of cAMP would have contributed to the phosphorylation of IP3RI. Therefore, G-Rg3-indcued increase in IP3RI (Ser1756) phosphorylation was mediated via cAMP/PKA-pathway.
Fig. 3.
Effects of ginsenoside Rg3 (G-Rg3) on thrombin-induced [Ca2+]i mobilization and inositol 1,4,5-trisphosphate receptor type I (IP3RI) phosphorylation. Effects of G-Rg3 on (A) thrombin-induced [Ca2+]i mobilization, (B) [Ca2+]i mobilization in the presence of A-kinase inhibitor (Rp-8-Br-cAMPS), (C) [Ca2+]i mobilization in the presence of G-kinase inhibitor (Rp-8-Br-cGMPS), and (D) thrombin-induced IP3RI phosphorylation. Measurement of [Ca2+]i and Western blotting were determined as described in the “MATERIALS AND METHODS” section. The data are expressed as the mean±standard deviation (n=4). *P <0.05 and **P <0.01 versus the thrombin-stimulated human platelets, †P <0.05 versus the thrombin-stimulated human platelets in the presence of G-Rg3 (300 μM).
Effects of G-Rg3 inhibitors on [Ca2+]i influx and ERK dephosphorylation
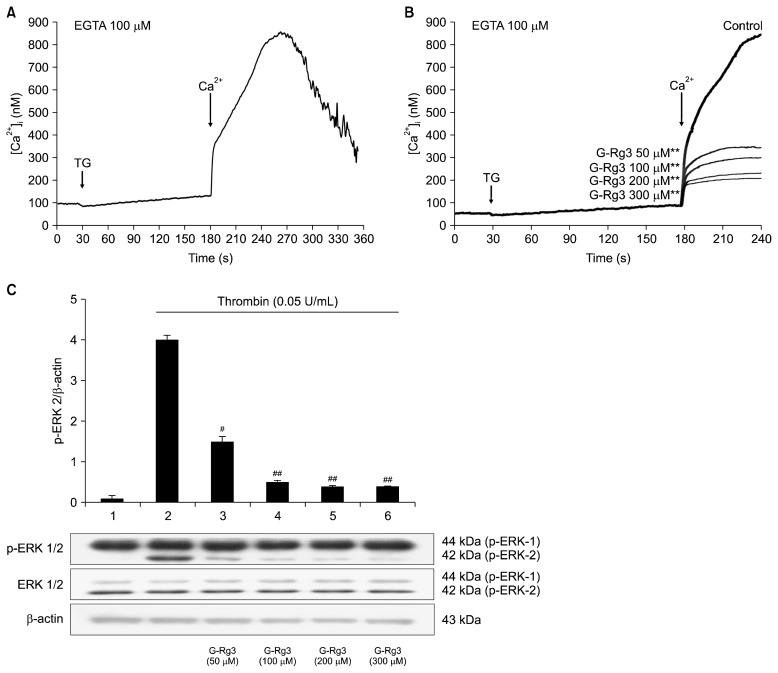
Thapsigargin is an inhibitor of the sarco/ER Ca2+ ATPase, and triggers Ca2+ influx from extracellular spaces. After adding 2 mM CaCl2, 1 μM of thapsigargin mediated an increase in the Ca2+ influx from 101.5±1.2 nM (the basal level) to 860.5±21.2 nM (Fig. 4A). However, G-Rg3 (50 to 300 μM) inhibited Ca2+ influx in a dose-dependent manner (Fig. 4B). There was 76.9% decrease in Ca2+ influx after the treatment with G-Rg3 (300 μM). It is known that Ca2+, mobilized from ER, is involved in ERK phosphorylation which leads to influx of extracellular Ca2+ (2,3); thus, we investigated the effects of G-Rg3 on dephosphorylation of ERK (1/2). As shown in Fig. 4C, ERK 2 phosphorylation was upregulated in presence of thrombin (0.05 U/mL) compared with control platelets. However, G-Rg3 inhibited thrombin-induced phosphorylation of ERK 2 (42 kDa) in a dose-dependent manner (50 to 300 μM).
Fig. 4.
Effects of ginsenoside Rg3 (G-Rg3) on Ca2+ influx and extracellular signal-regulated kinase (ERK) 1/2 phosphorylation. (A) Thapsigargin-induced Ca2+ influx in the presence of ethylene glycol bis(2-aminoethyl)tetraacetic acid (EGTA) (100 μM). Effects of G-Rg3 on (B) thapsigargin-induced Ca2+ influx and (C) ERK 1/2 phosphorylation. Ca2+ influx and Western blotting were determined as described in the “MATERIALS AND METHODS” section. The data are expressed as the mean±standard deviation (n=4). **P <0.01 versus the thapsigargin-stimulated human platelets. #P <0.05 and ##P <0.01 versus the thrombin-stimulated human platelets. TG, thapsigargin; Ca2+, 2 mM CaCl2.
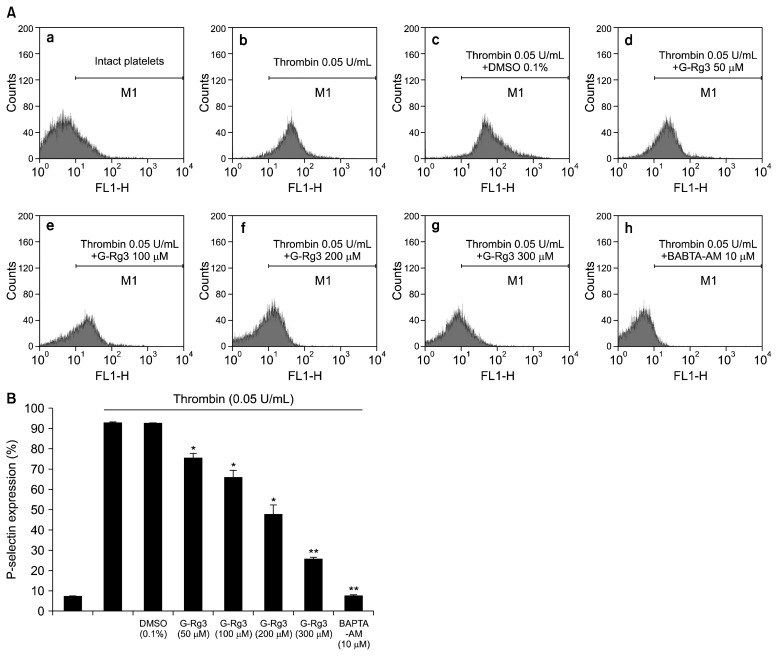
Effects of G-Rg3 on p-selectin expression
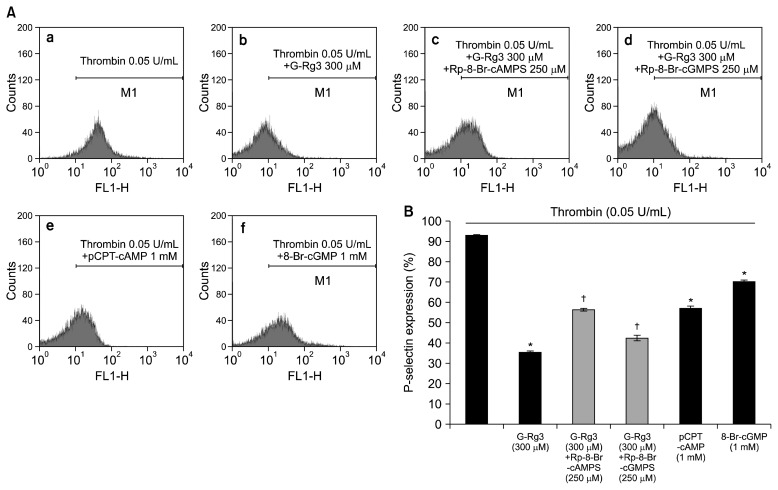
In order to verify the inhibitory effects of G-Rg3 on α-granule release, we investigated p-selectin expression. Thrombin upregulated the expression of p-selectin (Fig. 5A-b) as compared with control platelets (Fig. 5A-a). However, G-Rg3 inhibited thrombin-induced increase in p-selectin expression in a dose-dependent manner (50 to 300 μM) (Fig. 5A-d~g, 5B). Moreover, intracellular Ca2+ chelator, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM, also significantly inhibited thrombin-induced p-selectin expression (Fig. 5A-h and 5B), which proved the involvement of intracellular Ca2+ in the granule release signaling pathways. Next, we investigated whether the granule release was regulated by cAMP/PKA or cGMP/PKG pathway. As shown in Fig. 6A, the PKA inhibitor Rp-8-Br-cAMPS enhanced the G-Rg3 (300 μM) mediated decreased p-selectin expression by 57.5% (Fig. 6A-c, 6B), but PKG inhibitor Rp-8-Br-cGMPS enhanced the G-Rg3 (300 μM)-mediated p-selectin expression by 18.7% (Fig. 6A-d, 5B). In addition, cAMP activator, p-CPT-cAMP (1 mM), and cGMP activator, 8-Br-cGMP (1 mM), affected thrombin-elevated p-selectin expression (Fig. 6B).
Fig. 5.
Effects of ginsenoside Rg3 (G-Rg3) on p-selectin expression. (A) The flow cytometry histograms on p-selectin expression. a, Intact platelets (base); b, thrombin (0.05 U/mL); c, thrombin (0.05 U/mL)+0.1% dimethyl sulfoxide; d, thrombin (0.05 U/mL)+G-Rg3 (50 μM); e, thrombin (0.05 U/mL)+G-Rg3 (100 μM); f, thrombin (0.05 U/mL)+G-Rg3 (200 μM); g, thrombin (0.05 U/mL)+G-Rg3 (300 μM); h, thrombin (0.05 U/mL)+1,2-bis (2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-acetoxymethyl (AM) (10 μM). (B) Effects of G-Rg3 on thrombin-induced p-selectin expression (%). Determination of p-selectin expression was carried out as described in the “MATERIALS AND METHODS” section. The data are expressed as the mean±standard deviation (n=4). *P <0.05 and **P <0.01 versus the thrombin-stimulated human platelets.
Fig. 6.
Effects of ginsenoside Rg3 (G-Rg3) on p-selectin expression in the presence of A-kinase inhibitor or G-kinase inhibitor. (A) The flow cytometry histograms on p-selectin expression. a, Thrombin (0.05 U/mL); b, thrombin (0.05 U/mL)+G-Rg3 (50 μM); c, thrombin (0.05 U/mL)+G-Rg3 (300 μM)+A-kinase inhibitor (Rp-8-Br-cAMPS) (250 μM); d, thrombin (0.05 U/mL)+G-Rg3 (300 μM)+G-kinase inhibitor (Rp-8-Br-cGMPS) (250 μM); e, thrombin (0.05 U/mL)+p-chlorophenylthio (CPT)-cyclic AMP (cAMP) (1 mM); f, thrombin (0.05 U/mL)+8-Br-cyclic GMP (cGMP) (1 mM). (B) Effects of G-Rg3 in the presence of Rp-8-Br-cAMPS or Rp-8-Br-cGMPS on thrombin-induced p-selectin expression (%). Determination of p-selectin expression was carried out as described in the “MATERIALS AND METHODS” section. The data are expressed as the mean±standard deviation (n=4). *P <0.05 versus the thrombin-stimulated human platelets, †P <0.05 versus the thrombin-stimulated human platelets in the presence of G-Rg3 (300 μM).
DISCUSSION
Prostacyclin and nitric oxide are involved in the synthesis of cAMP and cGMP, respectively, which inhibit platelet functions via PKA and PKG, respectively (9). Both PKA and PKG have two major substrates in platelets: IP3RI and vasodilator-stimulated phosphoprotein. The IP3RI is located on the ER surface, and [Ca2+]i mobilization by binding with IP3 (10). The increased [Ca2+]i causes phosphorylation of both the myosin light chain (20 kDa) and pleckstrin (47 kDa) to trigger granule secretion (19,20). However, the phosphorylation of IP3RI (Ser1756) inhibits its activity (10). In this report, we confirmed that G-Rg3 inhibited [Ca2+]i mobilization via IP3RI (Ser1756) phosphorylation by cAMP/PKA, which is supported from the result that cAMP inhibitor Rp-8-Br-cAMPS inhibited G-Rg3-mediated elevation in the phosphorylation of IP3RI (Ser1756) in thrombin-induced human platelet aggregation.
It has been reported that stromal interaction molecule 1 (STIM1) is located in the membrane of endoplasmic reticulum and has an N-terminal EF hand domain (23). STIM1 recognizes the depletion of Ca2+ in endoplasmic reticulum and accelerates Ca2+ influx. Recently, It has been reported that STIM1 have phosphorylation sites (24,25), and reported that tyrosine phosphorylation of STIM1 is activated by thapsigargin in human platelets (24). In addition, another research has demonstrated that both ERK1/2 and the Src family are involved in STIM1 phosphorylation (24,26). As shown in Fig. 4B, G-Rg3 suppressed the thapsigargin-induced Ca2+ influx and ERK 2 phosphorylation. These results suggested that the inhibition of Ca2+ influx by G-Rg3 may be associated with STIM1 suppression. The specific interaction between G-Rg3 and STIM1 will be studied in the future.
The biomarker of α-granule release used in this study was p-selectin. p-Selectin is located in the inner membrane of α-granule. Therefore, we investigated the association of G-Rg3 with α-granule release. Thrombin-upregulated p-selectin expression (Fig. 5A); however, it was inhibited by G-Rg3 via suppression of [Ca2+]i mobilization and Ca2+ influx. Furthermore, p-selectin is expressed on the platelet membrane and causes in inflammation by binding to the p-selectin glycoprotein ligand-1 receptor on neutrophils and monocytes (6). Such inflammatory responses in association with activation of monocytes and endothelial cells, lead to atherosclerosis (25,26) and these interactions act as important indicators to determine the anti-thrombotic and anti-atherosclerotic effects of a compound. G-Rg3 inhibited thrombin-mediated elevation in [Ca2+]i mobilization by phosphorylating IP3RI (Ser1756) and thapsigargin-induced [Ca2+]i influx by de-phosphorylating ERK 2, which led to the suppression of p-selectin expression (Fig. 5). Because [Ca2+]i is the most important regulator of granule secretion, p-selectin expression is also dependent on [Ca2+]i levels. This result reflects that BAPTA-AM (10 μM) treated platelets significantly downregulated p-selectin expression (Fig. 6). In addition, p-selectin expression was regulated by A-kinase and G-kinase inhibitors and activators (Fig. 6). These results suggested that G-Rg3-increased cAMP levels and influenced p-selectin expression via downregulation of [Ca2+]i levels. Taken together, G-Rg3 inhibited thrombin-induced platelet aggregation by inhibiting both calcium mobilization from ER and calcium influx from external source. G-Rg3 significantly upregulated IP3RI phosphorylation and inhibited phosphorylation of ERK 2. Thus, our data proved that the inhibition of platelet aggregation by G-Rg3 can be caused via the regulation of calcium-related signals, IP3RI and ERK.
It has been reported that the G-Rg3-enriched fraction inhibits platelet activation and thrombus formation. The G-Rg3-enriched fraction (50 to 200 μg/mL) inhibited collagen (1.25 μg/mL)-induced granule secretion, p-selectin expression and fibrinogen binding to αIIb/β3 in a dose-dependent manner (16). Thus, we focused on the inhibitory mechanism of G-Rg3 on platelet functions and our results proved that the inhibitory effects of G-Rg3 were due to the elevation of cAMP levels. Furthermore, it has been reported that antiplatelet compounds, such as Korean red ginseng extract, ginsenoside Rp1 and ginsenoside Ro, increased cAMP levels (27,28), suggesting that the inhibitory effects of ginseng saponins on platelets are caused by increased cAMP levels. Recently, it has been reported that newly isolated ginseng saponins and metabolites (Rk1, Rk3, Rg5, Rg6, Rh4, Rs3, Rs4, Rs5, and F4) exhibit antiplatelet activities (29–31), but their mechanism of action is still unclear. Therefore, whether the new ginseng compounds are involved in cAMP production in human platelets will be investigated in future.
Although many calcium inhibitory effects of G-Rg3 have been identified, our study clearly demonstrates how Rg3 inhibits intracellular calcium concentration. Our results demonstrate that Rg3 inhibits calcium mobilization and calcium influx via phosphorylation of the IP3RI and phosphorylation of ERK, and this is mediated by an increase in cAMP levels. The downregulation of [Ca2+]i levels by G-Rg3 suppressed the granule secretion activity of platelets and resulted in decreased expression of p-selectin. Therefore, our study elucidated the calcium inhibitory mechanism by G-Rg3. Thus, G-Rg3 may be used as a therapeutic agent for prevention of thrombosis and other platelet-mediated cardiovascular diseases.
ACKNOWLEDGEMENTS
This study was supported by a grant (NRF-2011-0012143 to Hwa-Jin Park) from the Basic Science Research Program via the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The author declares no conflict of interest.
REFERENCES
- 1.Schwartz SM, Heimark RL, Majesky MW. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990;70:1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- 2.Rosado JA, Sage SO. Role of the ERK pathway in the activation of store-mediated calcium entry in human platelets. J Biol Chem. 2001;276:15659–15665. doi: 10.1074/jbc.M009218200. [DOI] [PubMed] [Google Scholar]
- 3.Rosado JA, Sage SO. The ERK cascade, a new pathway involved in the activation of store-mediated calcium entry in human platelets. Trends Cardiovasc Med. 2002;12:229–234. doi: 10.1016/S1050-1738(02)00161-5. [DOI] [PubMed] [Google Scholar]
- 4.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 5.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/S1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 6.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 7.von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz UR, Walter U, Eigenthaler M. Taming platelets with cyclic nucleotides. Biochem Pharmacol. 2001;62:1153–1161. doi: 10.1016/S0006-2952(01)00760-2. [DOI] [PubMed] [Google Scholar]
- 9.Cavallini L, Coassin M, Borean A, Alexandre A. Prostacyclin and sodium nitroprusside inhibit the activity of the platelet inositol 1,4,5-trisphosphate receptor and promoteits phosphorylation. J Biol Chem. 1996;271:5545–5551. doi: 10.1074/jbc.271.10.5545. [DOI] [PubMed] [Google Scholar]
- 10.Quinton TM, Dean WL. Cyclic AMP-dependent phosphorylation of the inositol-1,4,5-trisphosphate receptor inhibits Ca2+ release from platelet membranes. Biochem Biophys Res Commun. 1992;184:893–899. doi: 10.1016/0006-291X(92)90675-B. [DOI] [PubMed] [Google Scholar]
- 11.Lee CH, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohanan P, Subramaniyam S, Mathiyalagan R, Yang DC. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res. 2017;42:123–132. doi: 10.1016/j.jgr.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda H, Kubo M, Tani T, Arichi S, Kitagawa I. Pharmacological study on Panax ginseng C.A. Meyer V.1) Effects of red ginseng on the experimental disseminated intravascular coagulation (4). On ginsenoside-Rg3, Rh1 and Rh2. Jpn J Pharmacogn. 1985;39:123–125. [Google Scholar]
- 14.Lee SR, Park JH, Choi KJ, Kim ND. Inhibitory effects of ginsenoside Rg3 on platelet aggregation and its mechanism of action. Korean J Ginseng Sci. 1997;21:132–140. [Google Scholar]
- 15.Lee WM, Kim SD, Park MH, Cho JY, Park HJ, Seo GS, Rhee MH. Inhibitory mechanisms of dihydroginsenoside Rg3 in platelet aggregation: critical roles of ERK2 and cAMP. J Pharm Pharmacol. 2008;60:1531–1536. doi: 10.1211/jpp.60.11.0015. [DOI] [PubMed] [Google Scholar]
- 16.Jeong D, Irfan M, Kim SD, Kim S, Oh JH, Park CK, Kim HK, Rhee MH. Ginsenoside Rg3-enriched red ginseng extract inhibits platelet activation and in vivo thrombus formation. J Ginseng Res. 2017;41:548–555. doi: 10.1016/j.jgr.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin JH, Kwon HW, Cho HJ, Rhee MH, Park HJ. Inhibitory effects of total saponin from Korean Red Ginseng on [Ca2+]i mobilization through phosphorylation of cyclic adenosine monophosphate-dependent protein kinase catalytic subunit and inositol 1,4,5-trisphosphate receptor type I in human platelets. J Ginseng Res. 2015;39:354–364. doi: 10.1016/j.jgr.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 19.Nishikawa M, Tanaka T, Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980;287:863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- 20.Kaibuchi K, Sano K, Hoshijima M, Takai Y, Nishizuka Y. Phosphatidylinositol turnover in platelet activation; calcium mobilization and protein phosphorylation. Cell Calcium. 1982;3:323–335. doi: 10.1016/0143-4160(82)90020-3. [DOI] [PubMed] [Google Scholar]
- 21.Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7:1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 22.Lopez E, Jardin I, Berna-Erro A, Bermejo N, Salido GM, Sage SO, Rosado JA, Redondo PC. STIM1 tyrosine-phosphorylation is required for STIM1-Orai1 association in human platelets. Cell Signal. 2012;24:1315–1322. doi: 10.1016/j.cellsig.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Lang F, Münzer P, Gawaz M, Borst O. Regulation of STIM1/Orai1-dependent Ca2+ signalling in platelets. Thromb Haemost. 2013;110:925–930. doi: 10.1160/TH13-02-0176. [DOI] [PubMed] [Google Scholar]
- 24.Elvers M, Herrmann A, Seizer P, Münzer P, Beck S, Schönberger T, Borst O, Martin-Romero FJ, Lang F, May AE, Gawaz M. Intracellular cyclophilin A is an important Ca2+ regulator in platelets and critically involved in arterial thrombus formation. Blood. 2012;120:1317–1326. doi: 10.1182/blood-2011-12-398438. [DOI] [PubMed] [Google Scholar]
- 25.Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 26.Jennings LK. Role of platelets in atherothrombosis. Am J Cardiol. 2009;103:4A–10A. doi: 10.1016/j.amjcard.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Endale M, Lee WM, Kamruzzaman SM, Kim SD, Park JY, Park MH, Park TY, Park HJ, Cho JY, Rhee MH. Ginsenoside-Rp1 inhibits platelet activation and thrombus formation via impaired glycoprotein VI signalling pathway, tyrosine phosphorylation and MAPK activation. Br J Pharmacol. 2012;167:109–127. doi: 10.1111/j.1476-5381.2012.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon HW, Shin JH, Lee DH, Park HJ. Inhibitory effects of cytosolic Ca2+ concentration by ginsenoside ro are dependent on phosphorylation of IP3RI and dephosphorylation of ERK in human platelets. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/764906. 764906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JG, Lee YY, Kim SY, Pyo JS, Yun-Choi HS, Park JH. Platelet antiaggregating activity of ginsenosides isolated from processed ginseng. Pharmazie. 2009;64:602–604. [PubMed] [Google Scholar]
- 30.Lee JG, Lee YY, Wu B, Kim SY, Lee YJ, Yun-Choi HS, Park JH. Inhibitory activity of ginsenosides isolated from processed ginseng on platelet aggregation. Pharmazie. 2010;65:520–522. [PubMed] [Google Scholar]
- 31.Ju HK, Lee JG, Park MK, Park SJ, Lee CH, Park JH, Kwon SW. Metabolomic investigation of the anti-platelet aggregation activity of ginsenoside Rk1 reveals attenuated 12-HETE production. J Proteome Res. 2012;11:4939–4946. doi: 10.1021/pr300454f. [DOI] [PubMed] [Google Scholar]