Abstract
Citrus pomace (CP) is a by-product occurred during juice or other products processing. The enormous amount of CP caused serious environmental issues. However, CP is rich in a variety of bioactive compounds. In the present study, a water extract of CP (CPW) was prepared from the by-product and the in vitro and in vivo antioxidant activities of CPW were investigated. The in vitro antioxidant activities of CPW were evaluated by measuring the free radical scavenging activity and protective effects against 2,2-azobis(2-amidinopropane) hydrochloride (AAPH)-induced oxidative stress in Vero cells. CPW scavenges 1,1-diphenyl-2-picrylhydrazyl, alkyl, and hydroxyl radicals at IC50 of 0.16±0.00, 0.31±0.01, and 0.86±0.02 mg/mL, respectively. In addition, CPW improved cell viability and scavenged intracellular reactive oxygen species (ROS) in AAPH-stimulated Vero cells in a dose-dependent manner. The in vivo antioxidant activities of CPW were investigated in a model of AAPH-induced zebrafish embryos. CPW significantly improved the survival rates and reduced heartbeat rates in AAPH-stimulated zebrafish. Furthermore, the intracellular ROS and cell death levels were remarkably decreased in CPW-treated zebrafish. Therefore, the present results indicated that CPW possesses potent in vitro and in vivo antioxidant properties and could be a potential ingredient used in food, pharmaceutical, and cosmetic industries.
Keywords: citrus pomace, water extract, oxidative stress, antioxidant activity, zebrafish
INTRODUCTION
Oxidative stress is an imbalance between intracellular reactive oxygen species (ROS) accumulation and a capacity of the biological system’s scavenging ROS or repairing the resulting damage. In aerobic organisms, ROS are a series of normal products generated during normal cellular metabolic processes such as hydrogen peroxide (H2O2), hypochlorous acid (HClO), and free radicals (1–3). In general, cells maintain a balance between ROS generation and scavenging under the normal physiological conditions (4). However, this balance can be broken by many natural factors including disease, aging, and personal habits such as smoking or drinking. An excess of ROS accumulation in cells can damage the cellular organelles and essential macromolecules including DNA, lipids, and proteins, which lead to various physiological disabilities as well as result in metabolic disorders (5–7).
Antioxidants are the molecules that inhibit the oxidation of other molecules. Both synthetic and natural antioxidants are able to scavenge free radicals and to inhibit oxidation processes (8). Natural compounds from plants and algae possess remarkably high antioxidant activity with low side effects. Therefore, investigating natural antioxidants has become a focus of functional food and pharmaceutical science. Currently, finding cheap, abundant, and renewable resources of antioxidant compounds that originate from the residues of agricultural industries has attracted more attention (9).
Citrus (Citrus unshiu) pomace (CP) is the residue remaining after citrus fruits are processed for juice or other products. Nearly 50% of citrus fruit material is disposed of during industrial citrus juice processing, and the enormous amount of CP causes serious environmental issues (10). However, CP is rich in a variety of phenols and flavonoids that possess various bioactivities including antioxidant, anti-inflammatory, and anti-cancer (10–13). Therefore, investigating the bioactivities of CP will help utilize industry waste more efficiently and alleviate environmental pollution.
Our previous studies had investigated antioxidant effects of CP extracts processed by far-infrared irradiation, heat, and super-heated steam (14,15). However, the antioxidant activities of water extracts of CP has not been investigated so far. The objective of the present study is to evaluate the protective effect of water extract of CP against 2,2-azobis(2-amidinopropane) hydrochloride (AAPH)-induced oxidative stress in vitro in Vero cells and in vivo in zebrafish.
MATERIALS AND METHODS
Chemicals and reagents
Gallic acid, rutin, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 5,5-dimethyl-1-pyrolin N-oxide, AAPH, α-(4-pyridyl-1-oxcide)-N-t-butylnitrone, dimethyl sulfoxide (DMSO), 3-(4–5-dimethyl-2yl)-2–5-diphynyltetrasolium bromide (MTT), phosphate buffered saline (PBS), and 2′,7′-dichlorodihydroflurescin diacetate (DCFH-DA) were purchased from Sigma Co. (St. Louis, MO, USA). Roswell Park Memorial Institute-1640 (RPMI-1640) medium, fetal bovine serum (FBS), penicillin/streptomycin, and trypsin-ethylenediaminetetraacetic acid were purchased from Gibco-BRL (Grand Island, NY, USA). FeSO4·7H2O, H2SO4, and H2O2 were purchased from Fluka Chemie GmbH (Buchs, Switzerland). All other chemicals used in this study were of analytical grade.
Preparation of water extract of CP
CP (0.1 g) was extracted with 10 mL distilled water in a shaking incubator (100 rpm) in the dark at 25°C for 24 h. Then the extracts were centrifuged at 1,000 g for 15 min to remove the residue, and then the supernatant was filtered through Whatman No. 1 filter paper. After freeze drying, the water extract of CP was obtained and named CPW. The sample was kept at −20°C until use.
Measurement of total phenolic content (TPC) and total flavonoid content (TFC) of CPW
TPC and TFC of CPW were measured by colorimetric assay. The phenolic content of CPW was calculated as gallic acid equivalent (GAE); and the flavonoid content was calculated as rutin equivalent (RE). The methods were described in our previous studies (14).
Determination of the free radical scavenging activity of CPW
The free radical scavenging activity of CPW was measured using an electron spin resonance (ESR) spectrometer (JES-FA; JEOL, Tokyo, Japan). DPPH, hydroxyl, and alkyl radical scavenging activities of CPW were determined using the methods described by Heo et al. (16).
Cell culture
Monkey kidney fibroblast cells (Vero cells, ATCC® CCL-81TM, American Type Culture Collection, Manassas, VA, USA) were maintained in RPMI-1640 medium containing 10% heat-inactivated FBS, penicillin (100 unit/mL), and streptomycin (100 μg/mL) at 37°C under a humidified atmosphere containing 5% CO2. Cells were sub-cultured every 3 days and seeded in a 24-well plate at a concentration of 1×105 cells/well for experiments.
Determination of cytotoxicity of CPW on Vero cells
Cytotoxicity of CPW on Vero cells was determined by the MTT assay. Cells were seeded in a 24-well plate. After 16 h incubation, the cells were treated with various concentrations (25, 50, and 100 μg/mL) of CPW and incubated for 24 h. Then, MTT solution (100 μL, 2 mg/mL in PBS) was added to each well. After 3 h incubation, the supernatants were aspirated and the formazan crystals were dissolved in DMSO. The amount of formazan was determined by measuring the absorbance at 540 nm using a microplate reader (SynergyTM HT, BioTek® Instruments, Inc., Winooski, VT, USA).
Determination of the effects of CPW on AAPH-induced cytotoxicity and intracellular ROS generation in Vero cells
Vero cells were seeded and incubated for 16 h, and then treated with various concentrations (25, 50, and 100 μg/mL) of CPW. After 1 h incubation, AAPH (5 mM) was introduced to the cells. The viabilities of cells stimulated with AAPH or treated with CPW were measured by the MTT assay. The intracellular ROS levels were measured by the DCF-DA assay using the protocol described by Heo et al. (17).
Nuclear staining with Hoechst 33342
To measure the apoptotic bodies formation induced by AAPH, a fluorescent probe dye Hoechst 33342 was introduced to the AAPH-stimulated Vero cells. Nuclear staining was performed using the procedure described by Fernando et al. (18). The apoptotic body was observed under a fluorescence microscope (Olympus, Tokyo, Japan).
Maintenance of zebrafish
The adult zebrafish were purchased from a commercial market (Seoul Aquarium, Seoul, Korea). The fish were kept in 3 L acrylic tanks (10 fish per tank) and maintained at 28.5°C with a 14/10-h light/dark cycle. The fish were fed TetraMin flake food (That fish place, Lancaster, PA, USA) supplemented with live brine shrimp (Artemia salina) two times a day, 6 days a week. Embryos were obtained from natural spawning that was induced by turning on the light in the morning. The collection of embryos was completed within 30 min.
Application of CPW and AAPH to the embryos
The embryos were collected and transferred to individual wells on a 12-well plate (15 embryos/group) at 7~9 h post-fertilization (hpf). The embryos were incubated with CPW (25, 50, and 100 μg/mL) for 1 h and then treated with 15 mM AAPH.
Measurement of the heart-beat rate, ROS generation, and cell death in zebrafish
The heart-beat rate of both atrium and ventricle of zebrafish was recorded at 48 hpf for 1 min under the microscope. AAPH-induced intracellular ROS generation and cell death in zebrafish were analyzed with a fluorescent probe dye, DCFH-DA and acridine orange staining, respectively. The zebrafish larva was stained with the fluorescent probe dye and observed under a fluorescence microscope (Olympus, Tokyo, Japan) at 72 hpf using the methods described by Kim et al. (19). The fluorescence intensity of the individual zebrafish was quantified using the ImageJ program (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All experiments were conducted in triplicate and the data were expressed as the mean±standard error (SE). A one-way ANOVA test was used to compare the mean values of each treatment. Significant differences between the means of parameters were determined by the Tukey’s test. A P<0.05 was considered significantly different. The significance differences were established as *P<0.05 and **P<0.01 compared to the AAPH-treated group and ##P<0.01 compared to control group.
RESULTS
TPC, TFC, and the free radical scavenging activities of CPW
TPC and TFC of CPW is summarized in Table 1. The phenolic and flavonoid content of CPW is 3.35±0.00 g/100 g GAE and 4.07±0.73 g/100 g RE, respectively. CPW scavenged DPPH, alkyl, and hydroxyl radicals at the half maximal inhibitory concentration (IC50) of 0.16±0.00, 0.31±0.01, and 0.86±0.02 mg/mL, respectively (Table 1). These results show that CPW possesses strong free radical scavenging activity, especially for DPPH and alkyl radicals. Based on these results, AAPH was selected as the stimulator to induce oxidative stress in Vero cells and zebrafish to evaluate the in vitro and in vivo antioxidant activities of CPW.
Table 1.
Total phenolic content (TPC), total flavonoid content (TFC), and free radical scavenging activities of water extract of citrus pomace (CPW)
| Sample | TPC (g/100 g GAE) | TFC (g/100 g RE) | IC50 (mg/mL) | ||
|---|---|---|---|---|---|
|
| |||||
| DPPH | Alkyl | Hydroxyl | |||
| CPW | 3.35±0.00 | 4.07±0.73 | 0.16±0.00 | 0.31±0.01 | 0.86±0.02 |
The results are expressed as the mean±SE (n=3).
GAE, gallic acid equivalent; RE, rutin equivalent; DPPH, 1-diphenyl-2-picrylhydrazyl radical scavenging activity; Alkyl, alkyl radical scavenging activity; Hydroxyl, hydroxyl radical scavenging activity.
Cytotoxicity of CPW
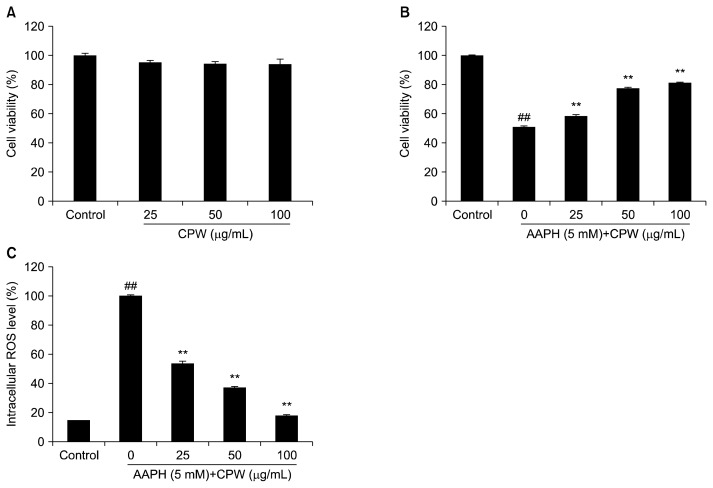
The cytotoxicity of CPW in Vero cells was determined by the MTT assay. The results show that the viabilities of cells treated with different contractions of CPW were higher than 90% (Fig. 1A). This shows that CPW was not cytotoxic under these concentrations.
Fig. 1.
Cytotoxicity and effects of water extract of citrus pomace (CPW) on 2,2-azobis(2-amidinopropane) hydrochloride (AAPH)-stimulated Vero cells. (A) Cytotoxicity of CPW on Vero cells; (B) protective effect of CPW against AAPH-induced cell death; (C) protective effect of CPW against AAPH-induced intracellular reactive oxygen species (ROS) generation in Vero cells. Cell viability was measured by MTT assay and intracellular ROS level was analyzed by DCF-DA assay. The results are expressed as the mean±SE (n=3). The significant differences were established as **P <0.01 as compared to AAPH-treated group and ##P <0.01 as compared to control group.
Protective effects of CPW against AAPH-induced oxidative stress
The protective effect of CPW against AAPH-induced cell death and intracellular ROS generation were measured by the MTT assay and DCF-DA assay, respectively. As the results show (Fig. 1B and C), the viability of cells treated with AAPH was significant decreased, and the intracellular ROS level was increased. However, CPW remarkably improved cell viability and reduced intracellular ROS levels in AAPH-stimulated Vero cells in a dose-dependent manner.
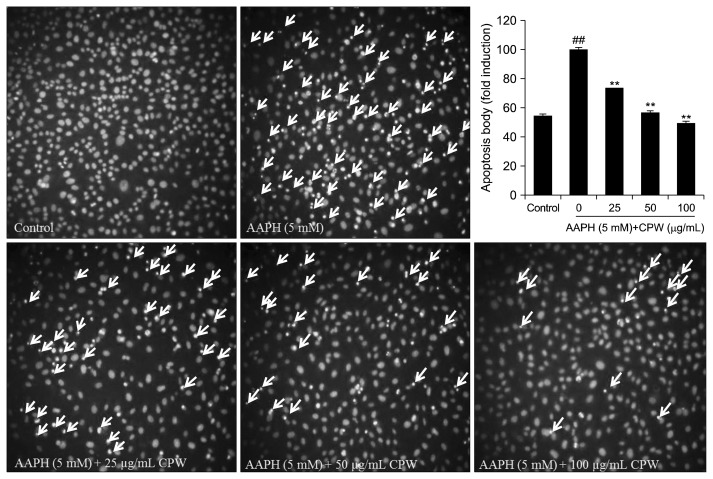
Protective effect of CPW against AAPH-induced apoptosis
As shown in Fig. 2, AAPH significantly induced apoptotic body formation. However, the amount of apoptotic bodies was remarkably reduced in the CPW-treated cells. Furthermore, the numbers of apoptotic in the cells were decreased as the CPW concentration increased.
Fig. 2.
Protective effects of water extract of citrus pomace (CPW) against 2,2-azobis(2-amidinopropane) hydrochloride (AAPH)-induced apoptotic body formation in Vero cells. Apoptotic body formation was observed under a fluorescence microscope after Hoechst 33342 staining. Apoptosis levels were measured by ImageJ software. The results are expressed as the mean±SE (n=3). **P <0.01 as compared to AAPH-treated group and ##P <0.01 as compared to control group.
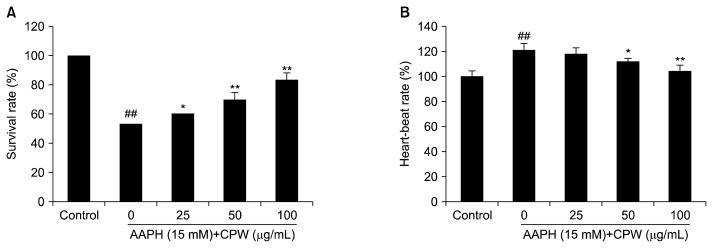
Survival rate and heart-beat rate of zebrafish
The survival rate and heart-beat rate of zebrafish were measured. As shown in Fig. 3, AAPH significantly reduced the survival rate and elevated the heart-beat rate of zebrafish. However, the survival rates of zebrafish pretreated with CPW were significantly increased in a dose-dependent manner. In addition, the heart-beat rates of CPW-treated zebrafish decreased with increasing concentrations of CPW.
Fig. 3.
The survival rate and heart-beat rate after pretreatment with water extract of citrus pomace (CPW) and/or treated with 2,2-azobis(2-amidinopropane) hydrochloride (AAPH). (A) The survival rate; (B) the heart-beat rate. The results are expressed as the mean±SE (n=15). *P <0.05 and **P <0.01 as compared to AAPH-treated group and ##P <0.01 as compared to control group.
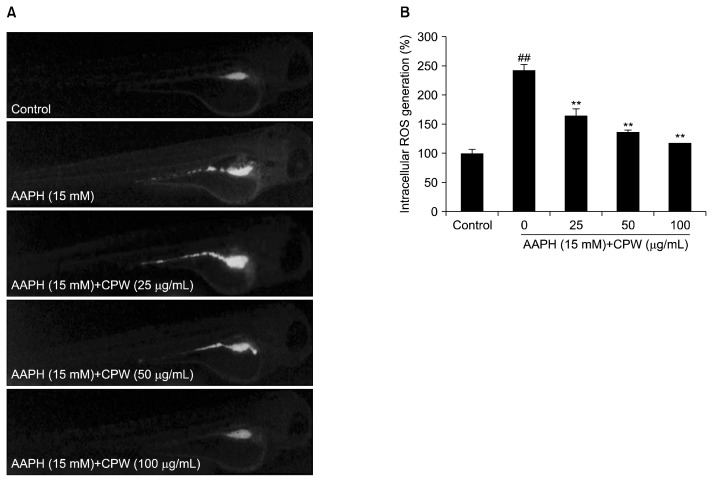
Effects of CPW against AAPH-induced intracellular ROS generation in zebrafish
The intracellular ROS generation of AAPH-stimulated zebrafish was analyzed by detection of DCF-DA. As shown in Fig. 4, the intracellular ROS levels of the non-treated zebrafish with AAPH was referred as 100%, and the ROS levels of AAPH-stimulated zebrafish was 242.2%. Furthermore, ROS levels of CPW-treated zebrafish were significantly decreased in a dose-dependent manner (Fig. 4B).
Fig. 4.
The protective effect of water extract of citrus pomace (CPW) against 2,2-azobis(2-amidinopropane) hydrochloride (AAPH)-induced intracellular reactive oxygen species (ROS) generation in zebrafish. (A) Zebrafish under fluorescence microscope; (B) the levels of intracellular ROS. Intracellular ROS levels were evaluated using ImageJ software. The results are expressed as the mean±SE (n=3). **P <0.01 as compared to AAPH-treated group and ##P <0.01 as compared to control group.
Effects of CPW against AAPH-induced cell death in zebrafish
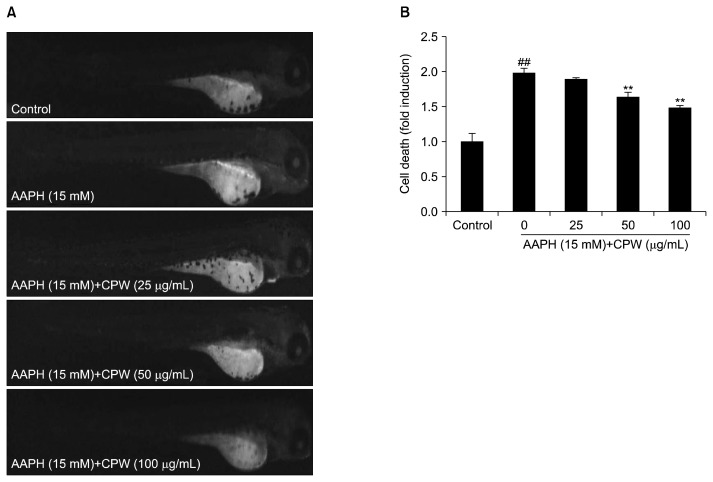
In order to evaluate the protective effects of CPW against AAPH-induced cell death in zebrafish, zebrafish were dyed with acridine orange, and the fluorescent intensities were measured. The results showed that CPW remarkably and dose-dependently reduced AAPH-induced cell death in zebrafish (Fig. 5).
Fig. 5.
The protective effect of water extract of citrus pomace (CPW) against 2,2-azobis(2-amidinopropane) hydrochloride (AAPH)-induced cell death in zebrafish. (A) Zebrafish under fluorescence microscope; (B) the levels of cell death. Cell death levels were evaluated using ImageJ software. The results are expressed as the mean±SE (n=3). **P <0.01 as compared to AAPH-treated group and ##P <0.01 as compared to control group.
DISCUSSION
Research suggests that overproduction and/or accumulation of ROS are associated with oxidative stress and inflammatory responses (20–24). Oxidative stress plays an important role in human chronic diseases such as obesity, cardiovascular diseases, diabetes, and neurodegenerative diseases (25–29). Therefore, a ROS scavenger that is not only effective to scavenge ROS but is also non-toxic may be an ideal candidate to help prevent these diseases. Many synthetic compounds such as butylhydroxyanisole (BHA) and butylhydroxytoluene (BHT), and natural compounds like vitamin C and E have been studied for their antioxidant activities (30–32). The toxicity of BHA and BHT has also been reported. Some reports suggest that BHA and BHT are tumor promoters (33,34). Because of the side-effects of synthetic antioxidants, natural compounds, especially from food ingredients, are thought to be safe antioxidants.
Natural compounds such as polyphenols, flavonoids, polysaccharides, and proteins possess strong antioxidant activities (35). Lee et al. (36) separated the polyphenol compound dieckol from Ecklonia cava and evaluated its protective effects against high glucose-induced oxidative stress. Ko et al. (20) purified peptides from flounder fish and determined their protective effects against AAPH-induced oxidative stress in Vero cells.
Citrus fruits are one of the most flavorful fruits in many parts of the world. The production of citrus fruits in the world is nearly 80 million tons per year. During beverage processing, the juice yield of citrus fruits is about half of the fruit weight (38). An enormous amount of by-products like CP are produced every year. Many researches have reported that CP is rich in phenolic acids and flavonoids that are bioactive (8,38–40). Our previous study investigated the effects of a super-heated steam process on the antioxidant contents of CP and evaluated the antioxidant activity of CP (14). However, the protective effects of water extract of CP against AAPH-induced oxidative stress in vitro in Vero cells and in vivo in zebrafish has not been reported. Therefore, we investigated the antioxidant activity of CPW in vitro in Vero cells and in vivo in zebrafish.
In the present study, CP was extracted with distilled water. The in vitro antioxidant activity of CPW was evaluated by measuring the free radical scavenging activities using ESR spectroscopy, and the protective effects against AAPH-induced oxidative stress in Vero cells were measured. The in vivo antioxidant activity of CPW was investigated in AAPH-stimulated zebrafish embryos. CPW contained 3.35±0.00 g/100 g GAE phenolic content and 4.07±0.73 g/100 g RE flavonoid content, and scavenged DPPH, alkyl, and hydroxyl radicals at the IC50 of 0.16±0.00, 0.31±0.01, and 0.86±0.02 mg/mL, respectively (Table 1). Our previous study reported that 70% ethanol extract of CP (CPE) contained 2.69±0.10 g/100 g GAE phenolic content and 1.27±0.46 g/100 g RE flavonoid content, respectively. In addition, CPW scavenged DPPH, alkyl, and hydroxyl radicals at the IC50 of 0.65±0.02, 0.33±0.02, and 0.67±0.01 mg/mL, respectively (14). These results show that CPW contains higher phenolic and flavonoid contents and possesses stronger DPPH and alkyl scavenging activities than CPE. Further results indicated that CPW significantly improved cell viability and reduced intracellular ROS levels in AAPH-induced Vero cells in a dose-dependent manner (Fig. 1). As shown in Fig. 2, AAPH significantly induced apoptotic body formation in Vero cells. However, CPW remarkably reduced AAPH-induced apoptotic body formation in Vero cells in a dose-dependent manner.
Zebrafish is a popular model in toxicological and pharmacological studies. Zebrafish stimulated with AAPH was successfully used to evaluate the antioxidant activity in our previous studies (4,19,41). Thus, we selected AAPH-stimulated zebrafish to evaluate the in vivo antioxidant activity of CPW. As shown in Fig. 3, the survival rate was decreased by 46.67%, and the heart-beat rate was increased by 20.62% in the AAPH-stimulated zebrafish compared to the control group. The survival rates were improved by 6.66, 16.66, and 30%, and the heartbeat rates were decreased by 3.27, 8.68, and 16.15% in the zebrafish treated with 25, 50, and 100 μg/mL of CPW, respectively. Furthermore, AAPH significantly induced intracellular ROS generation and cell death in zebrafish (Fig. 4 and 5). However, the intracellular ROS levels and cell death were remarkably decreased in CPW-treated zebrafish in a dose-dependent manner. These results showed that CPW possesses protective effects against AAPH-induced oxidative stress in vivo in zebrafish.
In conclusion, CP is rich in phenolic and flavonoid compounds and possesses potent antioxidant activities for improving cell viability, reducing intracellular ROS level, and decreasing the apoptotic body formation in AAPH-stimulated Vero cells, as well as improving survival rate, reducing heart-beat rate, and decreasing intracellular ROS levels and cell death in AAPH-stimulated zebrafish. These results suggest that CP possesses in vitro and in vivo antioxidant properties and may be a potential ingredient that could be used in the food, pharmaceutical, and cosmetic industries.
ACKNOWLEDGEMENTS
This research was supported by the 2017 scientific promotion program funded by Jeju National University.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Fernando IPS, Sanjeewa KKA, Samarakoon KW, Lee WW, Kim HS, Kim EA, Gunasekara UKDSS, Abeytunga DTU, Nanayakkara C, de ilva ED, Lee HS, Jeon YJ. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. Algae. 2017;32:75–86. doi: 10.4490/algae.2017.32.12.1. [DOI] [Google Scholar]
- 3.Lee JH, Ko JY, Oh JY, Kim EA, Kim CY, Jeon YJ. Evaluation of phlorofucofuroeckol-A isolated from Ecklonia cava (Phaeophyta) on anti-lipid peroxidation in vitro and in vivo. Algae. 2015;30:313–323. doi: 10.4490/algae.2015.30.4.313. [DOI] [Google Scholar]
- 4.Kang MC, Kim SY, Kim EA, Lee JH, Kim YS, Yu SK, Chae JB, Choe IH, Cho JH, Jeon YJ. Antioxidant activity of polysaccharide purified from Acanthopanax koreanum Nakai stems in vitro and in vivo zebrafish model. Carbohydr Polym. 2015;127:38–46. doi: 10.1016/j.carbpol.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Ryu BM, Kim WS, Kim GH, Jeon YJ. Protective effect of gallic acid derivatives from the freshwater green alga Spirogyra sp. against ultraviolet B-induced apoptosis through reactive oxygen species clearance in human keratinocytes and zebrafish. Algae. 2017;32:379–388. doi: 10.4490/algae.2017.32.11.29. [DOI] [Google Scholar]
- 6.Cha SH, Lee JH, Kim EA, Shin CH, Jun HS, Jeon YJ. Phloroglucinol accelerates the regeneration of liver damaged by H2O2 or MNZ treatment in zebrafish. RSC Adv. 2017;7:46164–46170. doi: 10.1039/C7RA05994A. [DOI] [Google Scholar]
- 7.Lee JH, Han JW, Ko JY, Lee W, Ahn G, Kim CY, Kim GH, Jeon YJ. Protective effect of a freshwater alga, Spirogyra sp., against lipid peroxidation in vivo zebrafish and purification of antioxidative compounds using preparative centrifugal partition chromatography. J Appl Phycol. 2016;28:181–189. doi: 10.1007/s10811-015-0548-y. [DOI] [Google Scholar]
- 8.Hayat K, Zhang X, Farooq U, Abbas S, Xia S, Jia C, Zhong F, Zhang J. Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chem. 2010;123:423–429. doi: 10.1016/j.foodchem.2010.04.060. [DOI] [Google Scholar]
- 9.Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, Núñez MJ, Parajó JC. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- 10.Sreenath HK, Crandall PG, Baker RA. Utilization of citrus by-products and wastes as beverage clouding agents. J Ferment Bioeng. 1995;80:190–194. doi: 10.1016/0922-338X(95)93218-9. [DOI] [Google Scholar]
- 11.Madeira JV, Jr, Nakajima VM, Macedo JA, Macedo GA. Rich bioactive phenolic extract production by microbial bio-transformation of Brazilian Citrus residues. Chem Eng Res Des. 2014;92:1802–1810. doi: 10.1016/j.cherd.2014.07.014. [DOI] [Google Scholar]
- 12.Anagnostopoulou MA, Kefalas P, Papageorgiou VP, Assimopoulou AN, Boskou D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis) Food Chem. 2006;94:19–25. doi: 10.1016/j.foodchem.2004.09.047. [DOI] [Google Scholar]
- 13.Faltin Z, Camoin L, Ben-Hayyim G, Perl A, Beeor-Tzahar T, Strosberg AD, Doron H, Eshdat Y. Cysteine is the presumed catalytic residue of Citrus sinensis phospholipid hydroperoxide glutathione peroxidase over-expressed under salt stress. Physiol Plant. 1998;104:741–746. doi: 10.1034/j.1399-3054.1998.1040432.x. [DOI] [Google Scholar]
- 14.Wang L, Jo MJ, Katagiri R, Harata K, Ohta M, Ogawa A, Kamegai M, Ishida Y, Tanoue S, Kimura S, Lee SC, Jeon YJ. Antioxidant effects of citrus pomace extracts processed by super-heated steam. LWT. 2018;90:331–338. doi: 10.1016/j.lwt.2017.12.024. [DOI] [Google Scholar]
- 15.Kim JW, Jeon YJ, Lee JH, Lee SC. Effect of far-infrared irradiation and heat treatment on the antioxidant activity of extracts from citrus pomaces. J Korean Soc Appl Biol Chem. 2006;49:60–64. [Google Scholar]
- 16.Heo SJ, Park PJ, Park EJ, Kim SK, Jeon YJ. Antioxidant activity of enzymatic extracts from a brown seaweed Ecklonia cava by electron spin resonance spectrometry and comet assay. Eur Food Res Technol. 2005;221:41–47. doi: 10.1007/s00217-005-1187-3. [DOI] [Google Scholar]
- 17.Heo SJ, Ko SC, Cha SH, Kang DH, Park HS, Choi YU, Kim D, Jung WK, Jeon YJ. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol In Vitro. 2009;23:1123–1130. doi: 10.1016/j.tiv.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Fernando IPS, Sanjeewa KKA, Kim HS, Wang L, Lee WW, Jeon YJ. Apoptotic and antiproliferative properties of 3β-hydroxy-Δ5-steroidal congeners from a partially purified column fraction of Dendronephthya gigantea against HL-60 and MCF-7 cancer cells. J Appl Toxicol. 2018;38:527–536. doi: 10.1002/jat.3559. [DOI] [PubMed] [Google Scholar]
- 19.Kim EA, Lee SH, Ko CI, Cha SH, Kang MC, Kang SM, Ko SC, Lee WW, Ko JY, Lee JH, Kang N, Oh JY, Ahn G, Jee YH, Jeon YJ. Protective effect of fucoidan against AAPH-induced oxidative stress in zebrafish model. Carbohydr Polym. 2014;102:185–191. doi: 10.1016/j.carbpol.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Shahidi F, Kamil J, Jeon YJ, Kim SK. Antioxidant role of chitosan in a cooked cod (Gadus morhua) model system. J Food Lipids. 2002;9:57–64. doi: 10.1111/j.1745-4522.2002.tb00208.x. [DOI] [Google Scholar]
- 21.Ko JY, Lee JH, Samarakoon K, Kim JS, Jeon YJ. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food Chem Toxicol. 2013;52:113–120. doi: 10.1016/j.fct.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 22.Wang YZ, Fu SG, Wang SY, Yang DJ, Wu YHS, Chen YC. Effects of a natural antioxidant, polyphenol-rich rosemary (Rosmarinus officinalis L.) extract, on lipid stability of plant-derived omega-3 fatty-acid rich oil. LWT. 2018;89:210–216. doi: 10.1016/j.lwt.2017.10.055. [DOI] [Google Scholar]
- 23.Aziz M, Karboune S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit Rev Food Sci Nutr. 2018;58:486–511. doi: 10.1080/10408398.2016.1194256. [DOI] [PubMed] [Google Scholar]
- 24.Kamil JYVA, Jeon YJ, Shahidi F. Antioxidative activity of chitosans of different viscosity in cooked comminuted flesh of herring (Clupea harengus) Food Chem. 2002;79:69–77. doi: 10.1016/S0308-8146(02)00180-2. [DOI] [Google Scholar]
- 25.Ko SC, Lee M, Lee JH, Lee SH, Lim Y, Jeon YJ. Dieckol, a phlorotannin isolated from a brown seaweed, Ecklonia cava, inhibits adipogenesis through AMP-activated protein kinase (AMPK) activation in 3T3-L1 preadipocytes. Environ Toxicol Pharmacol. 2013;36:1253–1260. doi: 10.1016/j.etap.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Kang N, Lee JH, Lee W, Ko JY, Kim EA, Kim JS, Heu MS, Kim GH, Jeon YJ. Gallic acid isolated from Spirogyra sp. improves cardiovascular disease through a vasorelaxant and antihypertensive effect. Environ Toxicol Pharmacol. 2015;39:764–772. doi: 10.1016/j.etap.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Lee SH, Park MH, Heo SJ, Kang SM, Ko SC, Han JS, Jeon YJ. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem Toxicol. 2010;48:2633–2637. doi: 10.1016/j.fct.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Heo SJ, Hwang JY, Choi JI, Han JS, Kim HJ, Jeon YJ. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol. 2009;615:252–256. doi: 10.1016/j.ejphar.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Kang SM, Cha SH, Ko JY, Kang MC, Kim D, Heo SJ, Kim JS, Heu MS, Kim YT, Jung WK, Jeon YJ. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ Toxicol Pharmacol. 2012;34:96–105. doi: 10.1016/j.etap.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Sebranek JG, Sewalt VJ, Robbins KL, Houser TA. Comparison of a natural rosemary extract and BHA/BHT for relative antioxidant effectiveness in pork sausage. Meat Sci. 2005;69:289–296. doi: 10.1016/j.meatsci.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 32.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 33.Kahl R, Kappus H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z Lebensm Unters Forsch. 1993;196:329–338. doi: 10.1007/BF01197931. [DOI] [PubMed] [Google Scholar]
- 34.Fukushima S, Shibata M, Kurata Y, Tamano S, Masui T. Changes in the urine and scanning electron microscopically observed appearance of the rat bladder following treatment with tumor promoters. Jpn J Cancer Res. 1986;77:1074–1082. [PubMed] [Google Scholar]
- 35.Carocho M, Ferreira ICFR. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, Han JS, Heo SJ, Hwang JY, Jeon YJ. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol In Vitro. 2010;24:375–381. doi: 10.1016/j.tiv.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Bocco A, Cuvelier ME, Richard H, Berset C. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J Agric Food Chem. 1998;46:2123–2129. doi: 10.1021/jf9709562. [DOI] [Google Scholar]
- 38.Benelli P, Riehl CAS, Smânia A, Jr, Smânia EFA, Ferreira SRS. Bioactive extracts of orange (Citrus sinensis L. Osbeck) pomace obtained by SFE and low pressure techniques: mathematical modeling and extract composition. J Supercrit Fluids. 2010;55:132–141. doi: 10.1016/j.supflu.2010.08.015. [DOI] [Google Scholar]
- 39.Lim J, Yoo J, Ko S, Lee S. Extraction and characterization of pectin from Yuza (Citrus junos) pomace: a comparison of conventional-chemical and combined physical-enzymatic extractions. Food Hydrocolloids. 2012;29:160–165. doi: 10.1016/j.foodhyd.2012.02.018. [DOI] [Google Scholar]
- 40.Tao B, Ye F, Li H, Hu Q, Xue S, Zhao G. Phenolic profile and in vitro antioxidant capacity of insoluble dietary fiber powders from citrus (Citrus junos Sieb. ex Tanaka) pomace as affected by ultrafine grinding. J Agric Food Chem. 2014;62:7166–7173. doi: 10.1021/jf501646b. [DOI] [PubMed] [Google Scholar]
- 41.Kang MC, Kim SY, Kim YT, Kim EA, Lee SH, Ko SC, Wijesinghe WA, Samarakoon KW, Kim YS, Cho JH, Jang HS, Jeon YJ. In vitro and in vivo antioxidant activities of polysaccharide purified from aloe vera (Aloe barbadensis) gel. Carbohydr Polym. 2014;99:365–371. doi: 10.1016/j.carbpol.2013.07.091. [DOI] [PubMed] [Google Scholar]