Abstract
Chlorine is a chemical threat agent that can be harmful to humans. Acute inhalation of high levels of chlorine results in the death of airway epithelial cells and can lead to persistent adverse effects on respiratory health, including airway remodeling and hyperreactivity. We previously developed a mouse chlorine exposure model in which animals developed inflammation and fibrosis in large airways. In the present study, examination by laser capture microdissection of developing fibroproliferative lesions in FVB/NJ mice exposed to 240 ppm-h chlorine revealed upregulation of genes related to macrophage function. Treatment of chlorine-exposed mice with the corticosteroid drug budesonide daily for 7 days (30–90 μg/mouse i.m.) starting 1 h after exposure prevented the influx of M2 macrophages and the development of airway fibrosis and hyperreactivity. In chlorine-exposed, budesonide-treated mice 7 days after exposure, large airways lacking fibrosis contained extensive denuded areas indicative of a poorly repaired epithelium. Damaged or poorly repaired epithelium has been considered a trigger for fibrogenesis, but the results of this study suggest that inflammation is the ultimate driver of fibrosis in our model. Examination at later times following 7-day budesonide treatment showed continued absence of fibrosis after cessation of treatment and regrowth of a poorly differentiated airway epithelium by 14 days after exposure. Delay in the start of budesonide treatment for up to 2 days still resulted in inhibition of airway fibrosis. Our results show the therapeutic potential of budesonide as a countermeasure for inhibiting persistent effects of chlorine inhalation and shed light on mechanisms underlying the initial development of fibrosis following airway injury.
Keywords: Chlorine, chemical threat agent, airway fibrosis, corticosteroid, inflammation
INTRODUCTION
Chlorine is an irritant gas used in a variety of industrial processes which has led to the production and transportation of vast amounts of this chemical in the United States (Das and Blanc, 1993; Evans, 2005). Humans may be exposed to chlorine unintentionally through industrial, transportation, or household accidents, or intentionally through warfare attacks or acts of terror (Van Sickle et al., 2009; White and Martin, 2010; Summerhill et al., 2017). Inhalation of high levels of chlorine can lead to acute lung injury as well as persistent adverse effects on respiratory health, including respiratory symptoms, inflammation, airway pathology, and decrements in lung function (Hoyle and Svendsen, 2016). Treatments to prevent such long-term adverse effects are lacking. We have previously shown in animal models that chlorine inhalation results in epithelial cell death and denudation of the airway epithelium (Tian et al., 2008; Musah et al., 2017). Repair of the large airways after chlorine injury is carried out by basal cells that proliferate and differentiate to repopulate the pseudostratified epithelium (Musah et al., 2012; Mo et al., 2013). Some inbred mouse strains possess pseudostratified airway epithelium containing low numbers of basal cells, and in these areas, repair is inefficient leading to the development of airway fibrosis (Musah et al., 2012; Mo et al., 2013; O’Koren et al., 2013).
Fibrosis is a scarring reaction in response to tissue injury that can result in impaired organ function. Fibrosis represents a common endpoint of aberrant repair that can occur following many types of acute or chronic injury. Despite this fact and the availability of a plethora of fibrosis models, the ultimate pathological and molecular triggers of fibrogenesis remain poorly understood. Fibrogenesis is commonly associated with injuries that produce epithelial damage and inflammation. The latter two processes have been proposed to participate in the development of lung fibrosis, and their relative importance in disease development has been debated (King et al., 2011; Mulugeta et al., 2015). Animal models in which the fibrogenic process can be followed from its inception provide an opportunity to examine pathogenic processes underlying the development of fibrosis.
Corticosteroids have been widely used in the treatment of lung injury and disease. Previous studies by us (Chen et al., 2013; Hoyle et al., 2016) and others (Wang et al., 2002; Wang et al., 2005; Kohno et al., 2010; Gao and Ju, 2016; Ju et al., 2016) have shown that administration of corticosteroids inhibits multiple aspects of acute lung injury after chlorine inhalation. We have also shown that inflammatory cells infiltrate the airway epithelium at later times after chlorine exposure when fibrosis is developing (Musah et al., 2012; Mo et al., 2013). The association of inflammation with the development of fibrosis, coupled with previous data showing the upregulation of proinflammatory genes by chlorine exposure (Balakrishna et al., 2014; Hamamoto et al., 2017), suggested that corticosteroid treatment might be a viable countermeasure for preventing chlorine-induced airway fibrosis. In the current study, we evaluated the effect of budesonide on the development of airway fibrosis after chlorine inhalation.
MATERIALS AND METHODS
Animals and chlorine exposure.
Experiments involving animals were approved by the University of Louisville Institutional Animal Care and Use Committee and were performed in accordance with the Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). FVB/NJ mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and housed under specific pathogen free conditions. Most experiments were performed using male mice. Pilot studies (not shown) indicated that chlorine exposure in female mice produced fibrotic airway lesions that were indistinguishable from those in male mice, and one experiment (the effect of delayed budesonide treatment) was done using female mice. Mice were randomly assigned to exposure and treatment groups. Mice at 8–10 weeks of age weighing 18–27g were exposed to chlorine using a whole-body chlorine exposure system. The exposure system consisted of a whole-body inhalation exposure chamber situated inside a secondary containment chamber, a chlorine generation and dilution system, and an exhaust scrubber, chlorine sampler for integrative analysis, and gas analyzer for monitoring of chlorine concentration during exposures. The exposure chamber was a clear polyester cabinet of volume 54 L (51.8 cm H X 34. 1 cm W X 41.4 cm D) (Bel-Art Products, Pequannock, NJ; Cat. No. F420741000J). Overall flow through the exposure chamber was provided by a Teflon bellows exhaust pump operating at a flow rate of approximately 50 L/min, thus providing about 1 chamber air change per minute. Chlorine was generated from a compressed gas cylinder containing 10,030 ppm Cl2 in dry nitrogen (Scott Specialty Gases, Pasadena, TX) and was diluted with room air inside an 8-port Teflon manifold prior to entering the exposure chamber through 8 inlets distributed across the face of one sidewall of the chamber. All flow lines and compression fittings in the system were made of fluorocarbon polymer or stainless steel. Exhaust from the chamber passed through a Greenburg Smith impinger containing 400 mL of 10% aqueous sodium carbonate for removal and scavenging of chlorine prior to the exhaust pump. After placing mice in the exposure chamber, the exposure period commenced with the initiation of the chlorine flow which was stopped after exactly 1 hour. The system was then ventilated for an additional 10 minutes prior to removal of the animals, resulting in a 70-minute exposure period. The total chlorine dose for the exposure was determined by sampling using a modified version of an ASTM method for airborne chlorine (Rando and Hammad, 1990). Sample air was passed through a 250-mL fritted glass bubbler containing 100 mL 1% sulfamic acid solution at a sample flow rate of 1 L/min. Chlorine concentration in the collected sample was measured by iodometric analysis as described (Rando and Hammad, 1990), except that chlorine levels (as produced iodine) were measured spectrophotometrically at 405 nm, rather than by specific-ion electrode. Chamber chlorine concentration was also continuously monitored during exposures using an X-Stream 2 gas analyzer calibrated against a standardized span gas (Rosemount Analytical, Solon, OH). Chamber chlorine concentrations reached 95% of the target concentration within 6 min of the start of exposure.
The target chlorine exposure dose was 240 ppm-hr. This dose was chosen based on previous results showing that it produced widespread epithelial damage and fibrosis in large airways with relatively low mortality within 7 days of exposure (Musah et al., 2012; Mo et al., 2013). The actual chlorine dose determined from integrative sampling averaged 240±4 ppm-h (mean±SD, n=5 exposures). In the current studies, a mortality rate of 8% within 7 days after chlorine exposure was observed. Mice were exposed in groups of 36 at a time; the quantitative analyses shown in the figures show comparisons of mice all exposed together.
Budesonide treatment.
After exposure to chlorine, mice were treated with either budesonide or vehicle by intramuscular injection. For intramuscular treatment, spray-dried budesonide formulation (Hoyle et al., 2016) was suspended in vehicle solution (0.1% Tween-80 in calcium/magnesium-free Dulbecco’s phosphate buffered saline). We previously showed anti-inflammatory effects of intramuscular budesonide treatment in a dose range of 30–90 μg/mouse (Hoyle et al., 2016), and doses in this range were used in the present study.
Histology and immunofluorescence.
Following euthanization of mice, lungs were inflated with 10% neutral buffered formalin at a pressure of 20 cm water for 10 min at room temperature and thereafter fixed by immersion in 10% neutral buffered formalin at 4 °C overnight. Tissues were processed, embedded in paraffin, and sectioned at 5 μm onto slides. Right lung lobes were oriented during embedding to produce sections containing cross sections of the lobar bronchi. Hematoxylin and eosin staining was performed on tissue sections for histological examination. Immunofluorescent staining was performed for epithelial cell markers as described (Musah et al., 2012). Antibody against keratin 5 (K5; basal cell marker) was obtained from Covance (Princeton, NJ, catalog # AF 138) and used at a dilution of 1:1,000. Antibody against club cell secretory protein (CCSP, club cell marker) was generously provided by Dr. Gurmukh Singh (VA Medical Center, Pittsburgh, PA) and used at a dilution of 1:1,000. Antibody against acetylated tubulin (AcTub, ciliated cell marker) was obtained from Sigma-Aldrich (St Louis, MO, catalog # T7451) and used at a dilution of 1: 20,000. Immunofluorescent staining for the M2 macrophage marker arginase 1 (Arg1) was performed using antibody from Santa Cruz Biotechnology (Dallas, TX, catalog # SC-18351) at a dilution of 1:50.
Picrosirus red staining and quantification of collagen.
Picrosirus red (PSR) staining was performed to examine collagen deposition in mouse lung sections. Tissue sections were deparaffinized, partially rehydrated in ethanol, incubated in 0.2% phosphomolybdic acid for 2 min, rinsed in water, and incubated in 0.1% Sirius red (Direct Red 80, Sigma-Aldrich) solution for 1 h at room temperature. Tissue sections were thereafter washed in 3% acetic acid, dehydrated in ethanol, changed to xylene, and mounted in Permount.
Digital images of the lobar bronchi of the right lungs were captured, and morphometric analysis was performed using ImageJ (http://rsbweb.nih.gov/ij/). Images were converted to RGB stacks, and analysis was performed on the green channel for optimum discrimination of the PSR signal. The analysis was limited to the lobar bronchi (i.e. the largest airway in each lobe) in the right upper, middle, and lower lobes, as these sites consistently developed airway fibrosis in chlorine-exposed mice. Images were thresholded using the ImageJ Moments option, and the area of PSR-stained pixels was determined. PSR-stainable collagen content was quantified as the area of collagen staining normalized to airway perimeter. Image analysis was performed by investigators who were blinded as to the identity of the samples and the nature of the treatment groups.
Laser capture microdissection and microarray analysis.
Frozen sections were prepared from distal trachea collected from five mice 4 days after chlorine exposure and from five unexposed mice. Laser capture microdissection with an Arcturus PixCell IIe instrument was used to isolate subepithelial tissue from hematoxylin and eosin-stained frozen sections from chlorine-exposed and unexposed mice based on published methods (Andres and Wittliff, 2011). Tissue was dissected from 10 sections per mouse. RNA was prepared from dissected tissue using an Arcturus® PicoPure® extraction kit (ThermoFisher Scientific, Waltham, MA) and analyzed on an Agilent 2100 Bioanalyzer (Santa Clara, CA). For transcript expression analysis by microarray, 250 ng of total RNA from each sample was amplified and labeled according to the GeneChip® WT PLUS Reagent kit protocol from Affymetrix (ThermoFisher, Waltham, MA), followed by hybridization to Affymetrix Mouse Gene 2.0 ST® arrays. The arrays were processed following the manufacturer recommended wash and stain protocol on an Affymetrix FS-450 fluidics station and scanned on an Affymetrix GeneChip® 7G scanner using Command Console 4.0. Gene expression data from the microarray analysis were submitted to the NCBI Gene Expression Omnibus (GEO) repository with accession number GSE109365.
mRNA expression of macrophage-associated genes.
qRT-PCR was performed with cDNA prepared from tissue collected by laser capture microdissection from unexposed and chlorine-exposed mice. qRT-PCR was performed using the TaqMan Gene Expression Assay (Foster City, CA) with probe/primer pairs for macrophage function related genes Arginase 1 (Arg1), Mannose Receptor C-Type 1 (Mrc1), and Chitinase-like 3 (Chil3) with beta-actin as control.
Lung function measurements.
Lung function measurements at baseline and after challenge with increasing doses of inhaled methacholine (PCCA, Houston, TX) were performed by forced oscillation technique using a flexiVent system (SCIREQ, Montreal, Quebec, Canada) as previously described (Hoyle et al., 2010).
Data analysis.
CEL files from gene chip scanning were imported into Partek Genomics Suite 6.6, and transcripts were normalized on a gene level using RMA as normalization and background correction method. Exposed and unexposed groups were compared using one-way ANOVA. Step-Up False Discovery Rate was chosen as the multiple test correction for the resulting p-values. Other data were analyzed using Prism 6.0f (GraphPad; La Jolla, CA) and presented as group means ± standard error of the mean (SEM). Effects of treatments on collagen staining were analyzed by one-way ANOVA with Tukey’s multiple comparisons test. Effects of treatments and methacholine dose on lung mechanics parameters were analyzed by two-way repeated measures ANOVA. The criterion for significance in the ANOVA tests was set at p<0.05.
RESULTS
Gene expression profiling in developing fibrotic lesions.
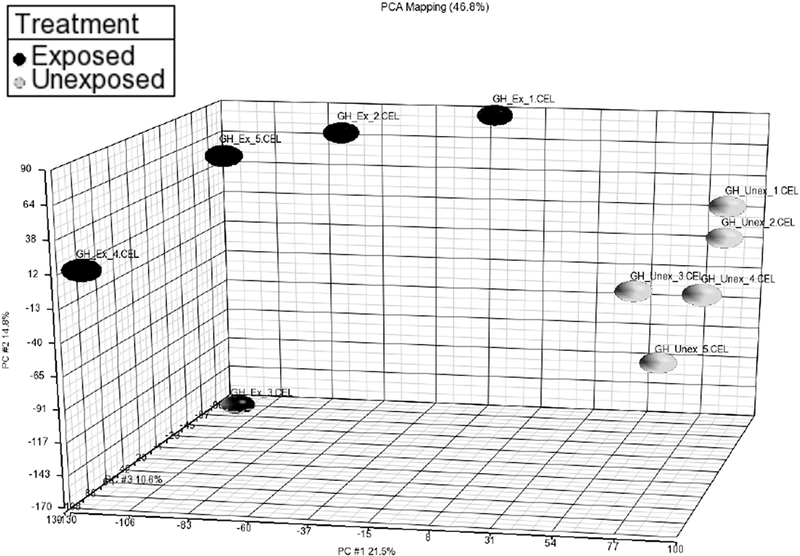
In order to identify genes that may be involved in the initial development of fibrosis, gene expression profiling was performed in tissue from developing fibrotic lesions 4 days after chlorine exposure. At this time, proliferation and expansion of the mesenchyme in injured areas has begun, but there is as yet minimal increase in collagen deposition (Mo et al., 2013). Laser-capture microdissection was used to isolate mesenchymal tissue from tracheas from chlorine-exposed and unexposed mice, and gene expression profiling was performed via microarray analysis. Principal component analysis (Fig. 1) showed that chlorine-exposed and unexposed samples were well separated based on the expression data. The microarray analysis identified 1,596 unique genes whose expression was altered between chlorine-exposed and unexposed mice using a false discovery rate (FDR) threshold of 5%. Of these, 364 genes had expression ratios (exposed:unexposed) of >2.0, and 190 genes had expression ratios of <0.50. These results were indicative of substantial transcriptomal changes in concert with the pronounced pathology observed histologically in the distal trachea. Table 1 shows the results of MetaCore pathway analysis, and lists pathways linked to the altered genes with FDR <5%. The pathways most tightly associated with the observed changes in gene expression were related to cell cycle; these included the top 7 pathways and 12 pathways overall. Identification of cell cycle pathways likely stems from the significant increase in cell proliferation that occurs in the airways during repair of chlorine-induced injury and development of fibrotic lesions (Musah et al., 2012; Mo et al., 2013). Other types of pathways commonly observed were related to immune response (7 pathways), development (5 pathways), DNA damage (4 pathways), and transcription (3 pathways).
Figure 1. Principal component analysis for laser-capture microdissection microarray.
FVB/NJ mice were exposed to chlorine and tracheas were collected 4 days after exposure. Mesenchymal tissue from developing fibrotic lesions was isolated from frozen tracheal sections using laser-capture microdissection. RNA levels were determined by microarray analysis.
Table 1.
Pathways Altered in Chlorine-Exposed Mice (FDR-adjusted p value<0.05)
| Pathway | FDR |
|---|---|
| Cell cycle: Chromosome condensation in prometaphase | 3.0E-16 |
| Cell cycle: The metaphase checkpoint | 7.5E-14 |
| Cell cycle: Role of APC in cell cycle regulation | 5.1E-09 |
| Cell cycle: Start of DNA replication in early S phase | 6.6E-07 |
| Cell cycle: Spindle assembly and chromosome separation | 8.7E-07 |
| Cell cycle: Initiation of mitosis | 2.9E-06 |
| Cell cycle: Transition and termination of DNA replication | 4.2E-04 |
| Ligand-independent activation of androgen receptor in prostate cancer | 4.2E-04 |
| Immune response: Antigen presentation by MHC class I - cross-presentation | 1.5E-03 |
| Protein folding and maturation: Bradykinin/kallidin maturation | 1.7E-03 |
| DNA damage: ATM / ATR regulation of G2 / M checkpoint | 1.7E-03 |
| Reproduction: Progesterone-mediated oocyte maturation | 2.2E-03 |
| Cell cycle: Sister chromatid cohesion | 2.7E-03 |
| Immune response: IL-6 signaling pathway via JAK/STAT | 2.7E-03 |
| Androgen receptor activation and downstream signaling in prostate cancer | 4.1E-03 |
| Glucocorticoid-induced elevation of intraocular pressure as glaucoma risk factor | 6.8E-03 |
| Cell cycle: Role of Nek in cell cycle regulation | 6.8E-03 |
| Development: Regulation of lung epithelial progenitor cell differentiation | 1.0E-02 |
| Cell cycle: Cell cycle (generic schema) | 1.1E-02 |
| Immune response: IFN-alpha/beta signaling via PI3K and NF-kB pathways | 1.3E-02 |
| DNA damage: Brca1 as a transcription regulator | 1.8E-02 |
| Transcription: Ligand-dependent activation of the ESR1/SP pathway | 1.8E-02 |
| Apoptosis and survival: Granzyme A signaling | 1.8E-02 |
| Transcription: Androgen receptor nuclear signaling | 2.0E-02 |
| CFTR folding and maturation (normal and CF) | 2.0E-02 |
| Immune response: IL-4-induced regulators of cell growth, survival, differentiation and metabolism | 2.0E-02 |
| Aberrant B-Raf signaling in melanoma progression | 2.1E-02 |
| Cytoskeleton remodeling: TGF, WNT and cytoskeletal remodeling | 2.2E-02 |
| DNA damage: ATM/ATR regulation of G1/S checkpoint | 2.2E-02 |
| Ovarian cancer: Main signaling cascades | 2.3E-02 |
| Transcription: Epigenetic regulation of gene expression | 2.5E-02 |
| Immune responseIL-13 signaling via PI3K-ERK pathway | 3.1E-02 |
| Cell cycle: Nucleocytoplasmic transport of CDK/cyclins | 3.5E-02 |
| Immune response: Substance P-stimulated expression of proinflammatory cytokines via MAPKs | 3.6E-02 |
| Mitogenic action of Estradiol / ESR1 (nuclear) in breast cancer | 3.9E-02 |
| Development: Ligand-independent activation of ESR1 and ESR2 | 4.0E-02 |
| Development: WNT signaling pathway | 4.2E-02 |
| Apoptosis and survival: DNA-damage-induced apoptosis | 4.3E-02 |
| Development: Adiponectin signaling | 4.3E-02 |
| Cell cycle: Role of SCF complex in cell cycle regulation | 4.3E-02 |
| Blood coagulation: Platelet microparticle generation | 4.3E-02 |
| Role of IL-23/ T17 pathogenic axis in psoriasis | 4.3E-02 |
| Immune response: Oncostatin M signaling via JAK-Stat | 4.3E-02 |
| Regulation of GSK3 beta in bipolar disorder | 4.5E-02 |
| Development: Thrombopoietin-regulated cell processes | 4.5E-02 |
| DNA damage: Role of Brca1 and Brca2 in DNA repair | 4.5E-02 |
| Substance P-mediated inflammation and pain in sickle cell disease | 4.5E-02 |
| Neurophysiological process: Circadian rhythm | 4.9E-02 |
Inspection of altered genes related to immune response revealed a number of upregulated genes associated with macrophage function (Table 2). Included within these were genes expressed in alternatively activated M2 macrophages, such as the macrophage mannose receptor (Mrc1/CD206), arginase 1 (Arg1), Chil3 (YM-1), and IL4 receptor alpha (Martinez and Gordon, 2015; Guo et al., 2017). The expression of selected genes from this group was assessed by qRT-PCR. Mrc1 was upregulated 2.2±1.5 fold, Arg1 was upregulated 7.2±1.5 fold, and Chil3 was upregulated 14±1.7 fold by this analysis. These results were of interest in view of the participation of macrophages in lung injury caused by other chemical threat agents (Malaviya et al., 2016; Venosa et al., 2016).
Table 2.
Genes associated with macrophage function that were upregulated in developing fibroproliferative lesions in chlorine-exposed mice.
| Gene | Fold change | p value | FDR-adjusted p value |
|---|---|---|---|
| Serpinb2 (PAI-2) | 26.9 | 4.7E-04 | 0.020 |
| Ccl8 | 15.8 | 5.5E-08 | 0.00073 |
| Chil3 (YM-1) | 9.5 | 9.8E-05 | 0.0088 |
| Plac8 | 9.0 | 1.1E-05 | 0.0034 |
| Ccl12 | 7.6 | 7.7E-05 | 0.0082 |
| Clec4d (Dectin-3) | 7.6 | 1.6E-04 | 0.011 |
| Ifi204 | 6.2 | 3.0E-05 | 0.0051 |
| Ccl6 | 6.0 | 3.0E-05 | 0.0051 |
| Slpi | 5.8 | 8.0E-06 | 0.0029 |
| Ccl7 | 5.7 | 2.7E-03 | 0.055 |
| Il6 | 5.4 | 6.6E-05 | 0.0076 |
| Ccl2 | 4.8 | 1.3E-03 | 0.036 |
| Mrc1 (CD206) | 2.7 | 2.1E-03 | 0.045 |
| Siglec1 (CD169) | 2.7 | 1.2E-05 | 0.0034 |
| Il4ra | 2.2 | 3.0E-4 | 0.015 |
| Arg1 (Arginase 1) | 2.0 | 4.9E-04 | 0.021 |
Arg1 expression in airway fibrotic lesions in chlorine-exposed mice.
To examine the location of a potential marker of alternatively activated macrophages within the lung, we performed immunostaining for Arg1 (Fig. 2). Arg1 is a marker for M2 macrophages and also has been implicated as a mediator in the development of fibrosis (McLarren et al., 2011; Pera et al., 2014). No staining for Arg1 was detected in trachea (Fig. 2A) or lungs (Fig. 2D) of unexposed mice. Increased expression of Arg1 was observed after chlorine exposure in both trachea (Fig. 2B and 2C) and lobar bronchi (Fig. 2E and 2F) 4 and 7 days after exposure. Staining was present in cells with the morphologic appearance of macrophages lining the airway lumen and within developing fibrotic lesions. These results were consistent with the gene expression analysis and suggest the presence of M2 macrophages in areas of airway fibrosis that develop after chlorine exposure.
Figure 2. Effect of chlorine exposure on Arg1 expression.
Chlorine-exposed FVB/NJ mice were euthanized for collection of lungs and trachea 4 or 7 days after exposure. Immunofluorescence staining was performed for Arg 1 in the trachea (A-C) and lobar bronchi (D-F). Arrow shows fibroproliferative lesion with Arg 1 staining in the trachea (in red). Arrowhead shows repaired tracheal epithelium devoid of Arg1 staining. Scale bar in panel C represents 200 μm in A-C and 100 μm in D-F.
Inhibition of chlorine-induced airway fibrosis by budesonide.
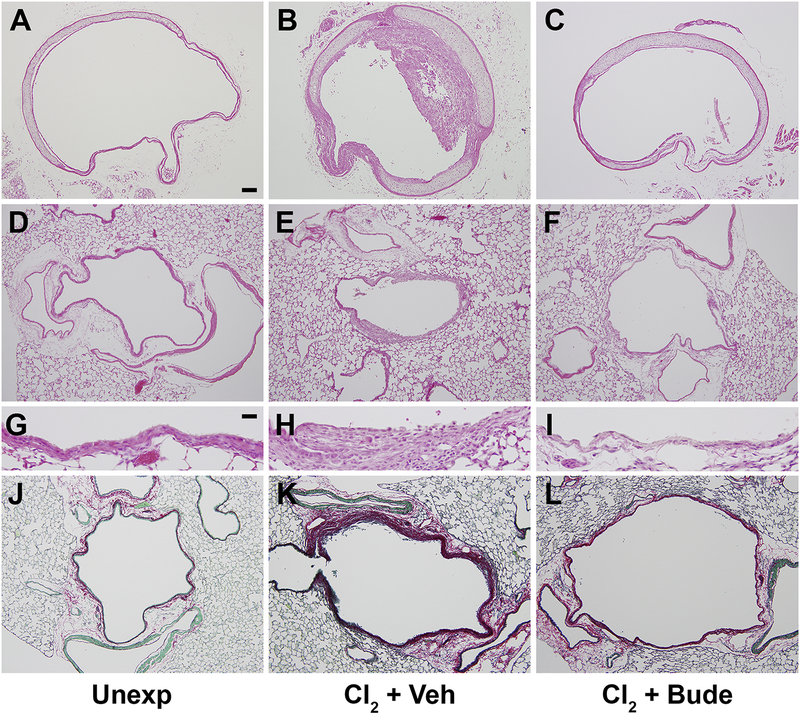
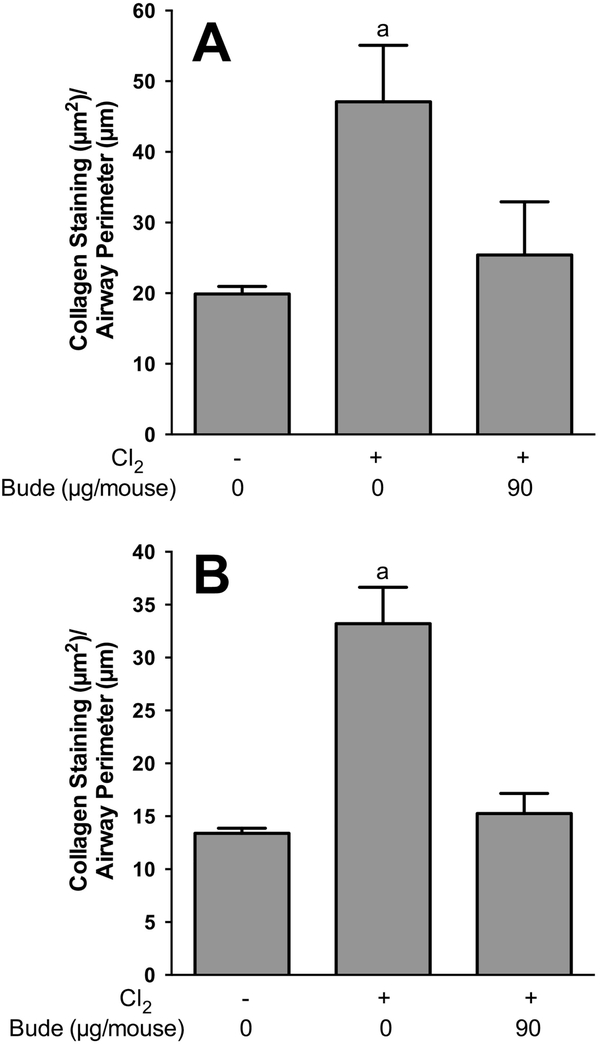
Based on our previous results showing beneficial effects of anti-inflammatory corticosteroid therapy on acute chlorine-induced lung injury (Chen et al., 2013; Hoyle et al., 2016), we examined whether treatment of chlorine-exposed mice with budesonide affected the development of airway fibrotic lesions. We previously developed a spray-dried budesonide formulation that was suitable for intramuscular treatment (Hoyle et al., 2016), and this formulation was used in the present study. In tracheal sections from unexposed mice, a typical pseudostratified epithelium was observed (Fig. 3A). In chlorine-exposed mice that received vehicle treatment, partial occlusion of the trachea by fibrotic lesions was observed 7 days after chlorine exposure (Fig. 3B). Budesonide treatment prevented the development of tracheal fibrosis (Fig. 3C); however, the epithelium was not fully repaired and appeared simple. In the lung, the epithelium in lobar bronchi of unexposed mice appeared intact as observed by hematoxylin and eosin staining (Fig. 3D and 3G), and there was minimal PSR collagen staining in the thin mesenchyme immediately beneath the epithelium (Fig. 3J). Chlorine exposure with vehicle treatment resulted in epithelial damage, aberrant repair, and a thickening of the mesenchyme in the airway walls (Fig. 3E and 3H) that was accompanied by abundant collagen staining (Fig. 3K). Intramuscular budesonide treatment was effective in preventing the development of airway thickening (Fig. 3F and 3I) and PSR-stained collagen deposition (Fig. 3L) in lobar bronchi. Although the large airways of chlorine-exposed mice treated with budesonide did not develop fibrosis, the epithelium at these sites was poorly repaired with large areas of the airway that were denuded of epithelium (Fig. 3I). Quantification of airway fibrosis was performed by image analysis of PSR collagen staining (Fig. 4). Chlorine exposure significantly increased collagen staining in lobar bronchi and trachea, and budesonide treatment reduced the increase in collagen staining by 80% in trachea (Fig. 4A) and by 91% in lobar bronchi (Fig. 4B).
Figure 3. Effect of budesonide intramuscular treatment on airway fibrosis.
FVB/NJ mice were exposed to chlorine and treated with budesonide or vehicle intramuscularly for 7 days beginning 1 h after exposure. Mice were euthanized for tissue collection 7 days after exposure (1 day after the last budesonide treatment). Hematoxylin and eosin staining was performed in unexposed (A), chlorine-exposed, vehicle-treated (B), and chlorine-exposed, budesonide treated (C) tracheal sections or in lung sections (D-I). PSR staining was performed in lungs from unexposed (J), chlorine-exposed, vehicle treated (K), and chlorine-exposed, budesonide treated (L) mice. Scale bar in panel A represents represent 100 μm in panels A-F and J-L; scale bar in panel G represents 25 μm in panels G-I.
Figure 4. Effect of intramuscular budesonide treatment on airway collagen staining.
Chlorine-exposed mice were treated with either vehicle or 90 μg/mouse budesonide for 7 days starting 1 h after exposure. Mice were euthanized for tissue collection 7 days after chlorine exposure. PSR staining was performed on tracheal and lung tissue sections, and quantification of collagen staining was analyzed by ImageJ. (A) Quantification of PSR staining in trachea. a, p<0.05 vs other groups. n=6–8 mice per group. (B) Quantification of PSR staining in lobar bronchi. a, p<0.001 vs other groups. n=6–8 mice per group.
Intramuscular budesonide treatment inhibits Arg1 staining.
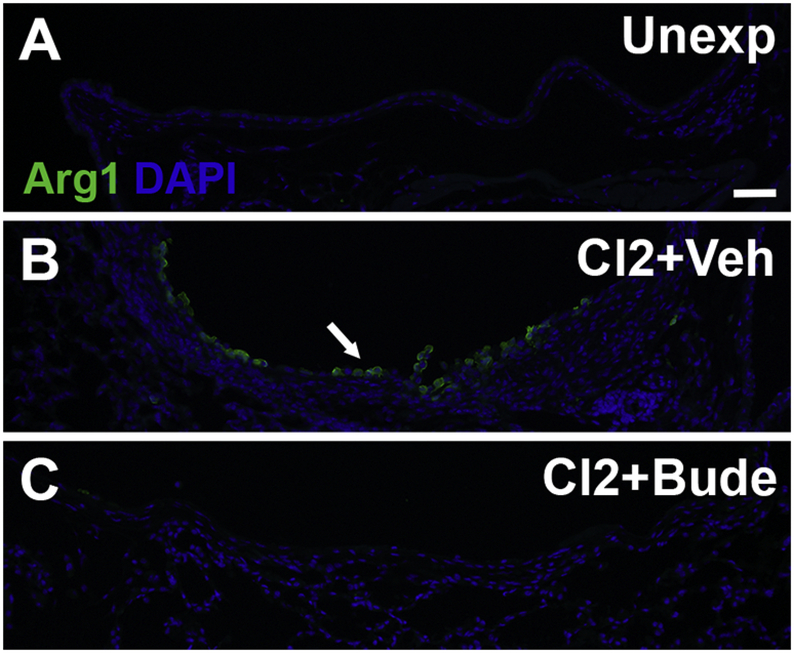
To investigate the effect of treatment with budesonide on the presence of M2 macrophages, Arg1 immunostaining was performed on lungs collected from mice 7 days after chlorine exposure. In unexposed mice, Arg1-expressing cells were generally absent in the large airways (Fig. 5A). In chlorine-exposed mice treated with vehicle, an abundance of Arg1-expressing cells was observed 7 days after chlorine exposure (Fig. 5B). In mice exposed to chlorine and treated intramuscularly with budesonide, very few to no Arg1-expressing cells were observed at this time (Fig. 5C). These results suggest that budesonide treatment prevents the influx of M2 macrophages to sites of airway injury following chlorine exposure and are consistent with known anti-inflammatory effects of budesonide.
Figure 5. Effect of budesonide treatment on Arg1 expression.
Mice were exposed to chlorine and treated with vehicle or budesonide intramuscularly for 7 days starting 1 h after exposure. Mice were euthanized for tissue collection 7 days after exposure. Immunofluorescence staining for Arg 1 staining was performed on lung tissue sections containing lobar bronchi from unexposed (A), chlorine-exposed, vehicle treated (B), and chlorine-exposed, budesonide treated (C) mice. Arrow indicates Arg 1-expressing-cells in airway fibrotic lesion. Scale bar represents 50 μm for all panels.
Budesonide treatment improves lung function.
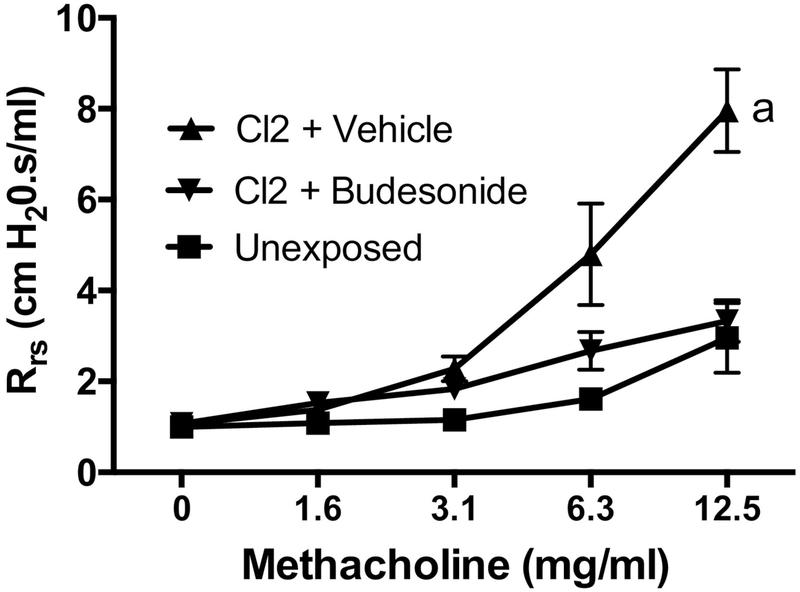
To determine the effect of budesonide treatment on pulmonary function, lung mechanics measurements were performed 7 days after chlorine exposure. Chlorine exposure produced airway hyperreactivity to inhaled methacholine, and this exaggerated response was almost completely prevented by intramuscular budesonide treatment (Fig. 6).
Figure 6. Effect of budesonide treatment on lung function.
Mice were exposed to chlorine and treated with vehicle or budesonide intramuscularly for 7 days starting 1 h after exposure. Lung function was measured 7 days after exposure. a, dose-response curve p<0.05 vs other treatments. n=4–7 mice per group.
Airway histology after cessation of treatment.
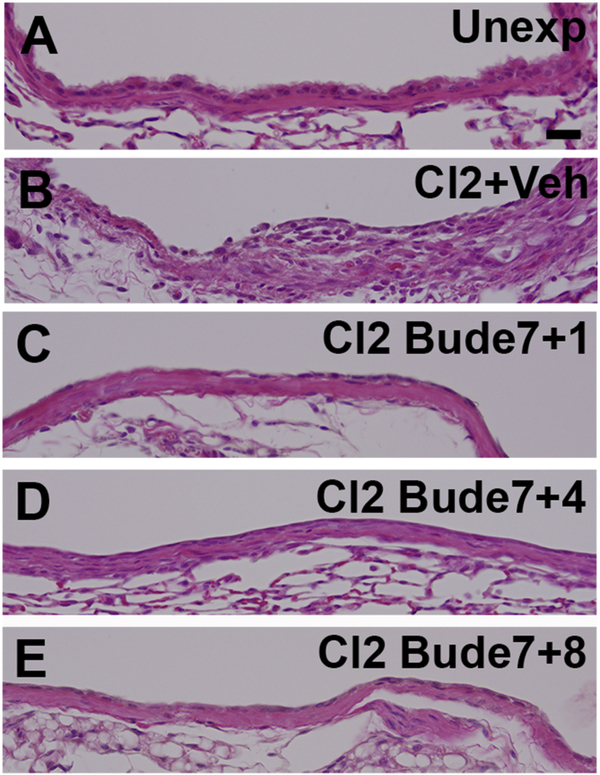
We and others have shown previously that airway fibrotic lesions develop following chlorine exposure in areas in which there is poor epithelial repair (Musah et al., 2012; Mo et al., 2013; O’Koren et al., 2013). Since lobar bronchi in budesonide-treated mice still contained large areas devoid of epithelial cells, this observation raised the question of whether fibrosis might develop at these sites once budesonide treatment was stopped. To investigate this, mice were treated with budesonide daily for 7 days starting 1 h after chlorine exposure and then analyzed 7, 10 or 14 days after exposure (i.e. 1, 4, and 8 days after the last budesonide treatment). Similar to previous results, chlorine inhalation produced airway fibrosis (Fig. 7B) that was prevented by budesonide treatment (Fig. 7C). In budesonide-treated mice 7 days after chlorine exposure, most of the airway was denuded and only a minority of the airway lumen was covered with epithelium. Analysis performed 10 days after chlorine exposure (i.e. 4 days after the last budesonide treatment) showed that much of the airway epithelium was now covered by a single layer of cells and that fibrosis had not developed (Fig. 7D). Examination of histological sections from lungs collected 14 days after chlorine exposure (i.e. 8 days after the last budesonide treatment) revealed that the airway lumen was fully covered by a layer of epithelial cells (Fig. 7E). This layer contained apparently undifferentiated squamous and cuboidal epithelial cells, most of which did not have the morphological appearance of club or ciliated cells. Quantification of collagen staining revealed there was no significant difference in PSR-stained collagen deposition between lungs analyzed 1 (86% inhibition), 4 (77% inhibition), or 8 days (76% inhibition) after the last budesonide treatment (Fig. 8). These results indicated that the initial 7 days of budesonide treatment were sufficient to prevent the development of fibrosis until the damaged airways were re-epithelialized.
Figure 7. Airway histology after cessation of budesonide treatment.
Mice were exposed to chlorine and treated with either vehicle or budesonide intramuscularly for 7 days starting 1 h after exposure. Lungs were collected 7, 10, or 14 after exposure (1, 4, or 8 days after cessation of budesonide treatment). Histology of lobar bronchi is shown. Scale bar in panel A represents 25 μm for all panels.
Figure 8. Airway collagen staining after cessation of budesonide treatment.
Mice were exposed to chlorine and treated with either vehicle or budesonide intramuscularly for 7 days starting 1 h after exposure. Lungs were collected 7, 10, or 14 after exposure (1, 4, or 8 days after cessation of budesonide treatment). PSR staining was performed on lung tissue sections and collagen staining in lobar bronchi was quantified using ImageJ. a, p<0.01 vs other groups. n=6–8 mice per group.
Immunofluorescence staining for epithelial cell markers in the airway epithelium was performed to track the progress of epithelial repair in injured airways. Lobar bronchi in unexposed mice showed sparse staining (absent from most sections) for the basal cell marker keratin 5 (Fig. 9A). This was in agreement with our previous study showing a paucity of basal cells in lobar bronchi from FVB/NJ mice (Mo et al., 2013). These airways exhibited an even distribution of interspersed club and ciliated cells (Fig. 9B). Seven days after chlorine exposure and treatment with vehicle, most areas of lobar bronchi were devoid of basal, club, and ciliated cells (Fig. 9C and 9D). In budesonide-treated mice analyzed 7 days after exposure, isolated clusters containing a single layer of keratin 5-stained cells were observed (Fig. 9E). There were still no club or ciliated cells observed at this time (Fig. 9F). Four days after cessation of budesonide treatment, an expansion of keratin 5-stained cells had occurred to cover more of the airway lumen, and the clusters contained multiple layers of cells (Fig. 9G). Some minimal differentiation of repaired airway epithelium had occurred as indicated by sporadic club cells (Fig. 9H), although their distribution was abnormal compared with unexposed. Eight days after the last budesonide treatment, most of the airway lumen was covered by keratin 5-stained cells that often were arranged in multiple layers (Fig. 9I). In addition to sporadic club cells (not shown), only a few ciliated cells were observed (Fig. 9J). Taken together, these results indicate that although the development of fibrosis continued to be inhibited after budesonide treatment had ended, the differentiation of the newly repaired epithelium was still in progress.
Figure 9. Distribution of airway epithelial cells after cessation of budesonide treatment.
Mice were exposed to chlorine and treated with either vehicle or budesonide intramuscularly for 7 days starting 1 h after exposure. Lungs were collected 7, 10, or 14 after exposure (1, 4, or 8 days after cessation of budesonide treatment). Immunofluorescence staining was performed on lung sections for keratin 5 (basal cell marker), club cell secretory protein (CCSP; club cell marker), and acetylated tubulin (ciliated cell marker). Arrows in panels E and G indicate clusters of K5-stained epithelial cells. Arrow in panel H indicates a cluster of CCSP-stained cells. Arrow in panel J indicates isolated cells that stain for acetylated tubulin. Scale bar in panel A represents 50 μm for all panels.
Effect of delayed budesonide treatment.
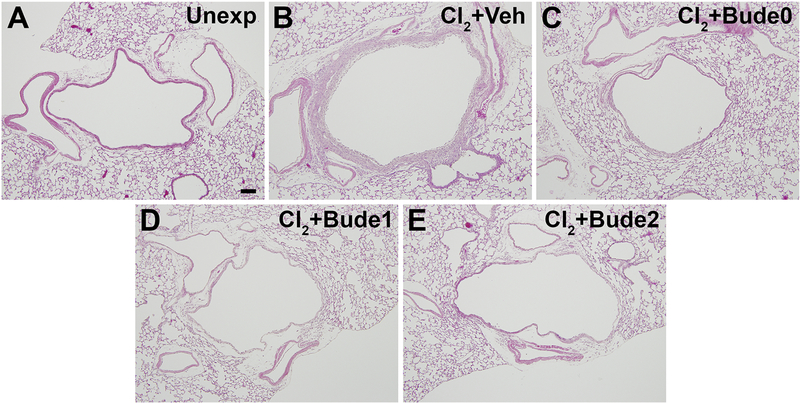
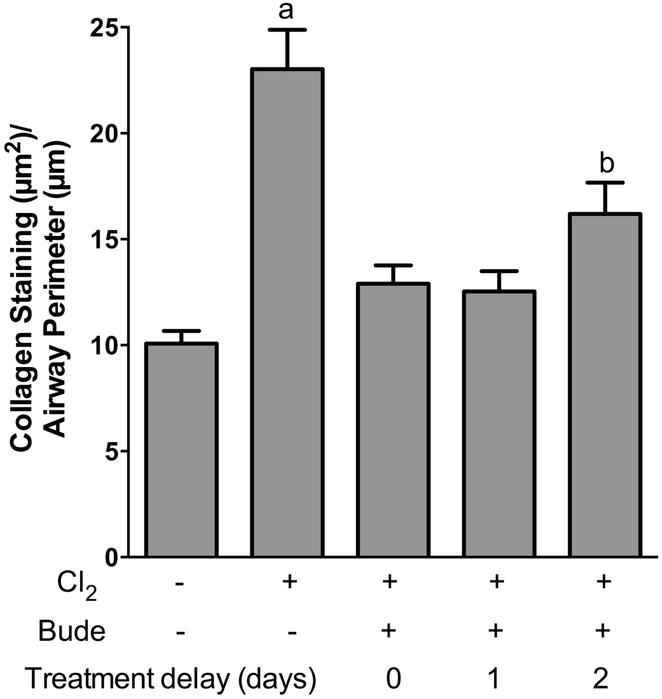
To determine if delaying the start of budesonide treatment would still result in the inhibition of airway fibrosis, treatment with budesonide commenced either 1 hr, 1 day, or 2 days after chlorine exposure. Chlorine exposure plus vehicle treatment resulted in the development of airway fibrosis (Fig. 10B). Treatment with budesonide starting 1 hr, 1 day, or 2 days after exposure resulted in similar effects including inhibition of fibrosis and limited epithelial repair (Fig. 10C–10E). Quantification of collagen staining revealed a significant reduction of 78%, 80%, and 62% for treatment starting 1 hr, 1 day, and 2 days after exposure, respectively (Fig. 11). Thus, the results show that a delay in the start of budesonide treatment for up to 2 days still results in significant benefit for preventing the development of airway fibrosis.
Figure 10. Effect of delayed budesonide treatment on airway fibrosis.
Mice were exposed to chlorine and treated intramuscularly with vehicle or budesonide beginning 1 h after exposure, 1 day after exposure, or 2 days after exposure and treated for 7, 6, or 5 days respectively. Lungs were collected for histological analysis 7 days after chlorine exposure. Hematoxylin and eosin staining of sections containing lobar bronchi are shown. Bude0, Bude1, and Bude2 indicate the start of budesonide treatment either 0 (i.e. the day of exposure), 1, or 2 days after chorine exposure. Scale bar in panel A represents 100 μm for all panels.
Figure 11. Effect of delayed budesonide treatment on airway collagen staining.
Mice were exposed to chlorine and treated intramuscularly with vehicle or budesonide beginning 1 h after exposure, 1 day after exposure, or 2 days after exposure and treated for either 7, 6, or 5 days respectively. Lungs were collected for histological analysis 7 days after chlorine exposure. PSR staining was performed on tissue sections, and quantification of collagen staining was performed using ImageJ. a, p<0.01 vs other groups. b, p<0.05 vs unexposed. n=7–9 mice per group.
DISCUSSION
Dysfunctional epithelium or aberrant epithelial repair after injury has been associated with the development of fibrosis, yet the underlying mechanisms by which these processes are linked remain obscure. We developed a murine model in which chlorine inhalation in mice produces denudation of airway epithelium and development of fibrotic lesions by 7 days after exposure. The rapid and reproducible development of airway disease in this model affords the opportunity to investigate mechanisms of fibrogenesis. Examination of the developing fibroproliferative lesions in the current study by laser-capture microdissection allowed gene expression at the exact sites of developing lesions to be analyzed. These lesions were isolated for analysis at a time when proliferation in the mesenchyme has commenced (Musah et al., 2012; Mo et al., 2013), and the identification of the most tightly associated gene expression pathways related to cell cycle correlates with this observation. One possible consequence of the excessive proliferation observed during this reparative phase is that the exaggerated signal from this process may have dwarfed the identification of other signaling pathways that may also be involved in the initial development of airway fibroproliferative lesions. Microarray analysis of fibroproliferative lesions revealed an upregulation in a number of genes associated with immune response and macrophage function. Upregulation of inflammatory genes following chlorine exposure is likely influenced by differences in the variety of cell types between chlorine-exposed and unexposed mice and is consistent with the localization of inflammatory cells observed within airway lesions. Lung injury caused by other chemical threat agents such as sulfur mustard and nitrogen mustard has also been associated with inflammatory mediators and immune cells such as macrophages with an attendant development of fibrosis (Malaviya et al., 2016; Venosa et al., 2016). Genes related to macrophage function, such as YM1, CD206, and IL6, that were implicated in mustard lung injury were also altered following exposure to chlorine. Overall, results from the microarray analysis identified genes associated with inflammatory pathways as being upregulated in developing fibroproliferative lesions and support the strategy of anti-inflammatory therapy for inhibiting chlorine-induced airway fibrosis.
Corticosteroids have been used as anti-inflammatory treatments for a variety of acute and chronic pulmonary conditions. Treatment with corticosteroids has been investigated previously in animal models of chlorine-induced lung injury. Budesonide and betamethasone administered in a pig model of chlorine-induced acute lung injury improved arterial oxygenation and lung compliance (Wang et al., 2005), while budesonide treatment alone in this same model inhibited chlorine-induced increases in lung wet:dry weight ratio (Wang et al., 2002). Administration of dexamethasone in rat models of chlorine injury was effective in inhibiting acute inflammation (Demnati et al., 1998; Wigenstam et al., 2016). We have previously shown that some acute effects of chlorine inhalation, including neutrophil influx and pulmonary edema, were inhibited by treatment with mometasone and budesonide in FVB/NJ mice (Chen et al., 2013; Hoyle et al., 2016). Treatment of chlorine-exposed BALB/C mice with dexamethasone was shown to produce partial inhibition of acute neutrophilic inflammation and airway hyperreactivity but nearly complete inhibition of airway hyperreactivity measured 14 days after exposure (Jonasson et al., 2013). In addition, other types of anti-inflammatory treatments inhibited acute inflammation and airway hyperreactivity caused by chlorine inhalation (McGovern et al., 2015; Hamamoto et al., 2017). In the current study, budesonide treatment inhibited multiple aspects of chlorine-induced injury evident 7 days after exposure, including inflammation, airway fibrosis, and airway hyperreactivity. Because budesonide inhibited all three of these aspects of airway injury in the current study, it was not possible to understand from these results how the processes may be connected. However, the pronounced structural changes to the airways seem likely to underlie the observed functional change, i.e. hyperreactivity, and this result is consistent with the kinetics of disease development, in which these two aspects of pathology develop in parallel (Mo et al., 2013). By contrast, chlorine-induced airway hyperreactivity was evident in BALB/C mice 14 days after exposure in the absence of any obvious structural abnormalities in the lungs, and this was inhibited by early dexamethasone treatment (Jonasson et al., 2013). Because of the generalized anti-inflammatory nature of corticosteroids, it was not possible in the current analysis to draw conclusions about which immune cell type(s) may be involved in the development of chlorine-induced airway fibrosis. Arginase 1 was identified as a convenient indicator of chlorine-induced macrophage inflammation, but multiple cell types, such as M1 and M2 macrophages, neutrophils, or others, may potentially be involved in the development of fibrosis in this model.
Fibrosis is a scarring reaction representative of nonproductive repair after injury. Development of fibrosis in the lung can be caused by repeated or persistent injury (Mossman and Churg, 1998; Degryse et al., 2010), severe acute injury (Moore et al., 2013), abnormal repair following alveolar or airway epithelial injury (Osterholzer et al., 2012; Mulugeta et al., 2015) or inflammation (Bringardner et al., 2008). Although the molecular trigger for the development of lung fibrosis is not fully understood, the initial pathogenesis typically involves epithelial injury and associated inflammatory response leading to dysregulated repair, fibroblast proliferation, and increased production of extracellular matrix (Spagnolo et al., 2015). In our model of chlorine-induced airway injury, fibrosis develops rapidly at levels of the airway where repair of the damaged epithelium does not progress efficiently. Budesonide treatment prevented the development of airway fibrosis in these areas that still exhibited negligible epithelial repair 7 days after chlorine exposure. This result indicates that the extended period of epithelial denudation and the lack of epithelial repair per se are not sufficient to initiate fibrogenesis in the absence of other events. The known activity of budesonide as an anti-inflammatory agent suggests that these postulated additional events are related to inflammatory processes. These results can be contrasted with clinical use of antiinflammatory therapy, which has proven ineffective for the treatment of established fibrotic disease such as idiopathic pulmonary fibrosis (Ryu et al., 2014). The observations may reflect a fundamental difference in pathogenesis between chlorineinduced airway injury and idiopathic pulmonary fibrosis or may be related to the ability in the chlorine model to intervene during a window between the acute injury and the development of fibrosis. It is possible that the initial development of fibrosis is dependent on inflammation, but then the pro-fibrotic processes become self-sustaining and can proceed in the absence of continued inflammation. This concept is consistent with the results we obtained following delayed budesonide treatment, which suggested a diminution of efficacy starting two days after exposure. It is also consistent with studies in which fibroblasts cultured from fibrotic lungs retain pathogenic properties for extended periods following removal from the diseased tissue (Raghu et al., 1988; Suganuma et al., 1995; Ramos et al., 2001).
Following chlorine inhalation, repair of the pseudostratified airway epithelium is carried out by basal cells, which proliferate and differentiate to repopulate the airway epithelium (Musah et al., 2012; Mo et al., 2013; O’Koren et al., 2013). A/J mice have abundant basal cells throughout the large airways, and the repair process proceeds efficiently, resulting in rapid re-epithelialization and lack of fibrosis (Mo et al., 2013). FVB/NJ mice have few basal cells in lobar bronchi, and this paucity of progenitor cells is associated with poor epithelial repair and the development of fibrosis. In the current study, we were able to expand on these results to investigate the epithelial repair process in FVB/NJ mice in the absence of the development of airway fibrosis following budesonide treatment. As in A/J mice, repair of the damaged epithelium in budesoni-detreated FVB/NJ mice appeared to occur through the expansion of surviving keratin 5-positive basal cells. However, in the absence of budesonide treatment in chlorine-exposed FVB/NJ mice, the small number of basal cells was not adequate to re-establish the epithelium before the development of a fibrotic scar. The inhibition of fibrosis by budesonide treatment allowed time for isolated surviving basal cells to carry out repair and regenerate the epithelium in larger airways. It was of interest that after cessation of budesonide treatment, fibrogenesis continued to be inhibited and the epithelial regeneration continued such that denuded airways were generally absent by 14 days after exposure. Differentiation of the airway epithelium was still underway at this time, as there were yet few club and ciliated cells. In chlorine-exposed A/J mice, the initial regenerated epithelium contains an abnormal distribution of epithelial cells, but the cell distribution continues to normalize up to 56 days after chlorine inhalation (Mo et al., 2015). The functional consequences of such a change in epithelial structure after chlorine exposure are not known, but an abnormal distribution of cells could potentially impair any of the numerous airway epithelial-related functions such as immune response, microbial defense, or response to subsequent injury.
In humans, chlorine inhalation can result not only in acute lung injury but also in long-lasting adverse effects on the respiratory system (Hoyle and Svendsen, 2016). The observations in the present study that intramuscular budesonide treatment was extremely effective in inhibiting chlorine-induced inflammation, airway fibrosis, and changes in lung function highlight its potential as a medical countermeasure for treating chronic effects following chlorine inhalation. Intramuscular budesonide treatment offers the advantage of rapid administration by personnel with limited medical training in the field as a countermeasure to chlorine exposure in a disaster scenario. We showed that a delay in the start of budesonide treatment for up to 2 days still results in significant benefit for preventing the development of airway fibrosis. These results suggest that initial treatment need not be administered immediately but patients could be transported to the hospital before treatment is commenced. This concept of operations would be consistent with alternative routes of budesonide delivery, including delivery directly to the respiratory tract that could be initiated in a hospital setting before fibrosis is established. With regard to the safety of budesonide treatment, it has been shown that corticosteroids appear to be well tolerated in patients with lung injury or disease. In individuals exposed to chlorine in an accidental train derailment in Graniteville, SC, corticosteroids were administered to the majority of hospitalized patients (Van Sickle et al., 2009). Inhaled budesonide is approved for treatment of asthma and COPD. Budesonide has also been used in premature infants for treatment of bronchopulmonary dysplasia (Bassler et al., 2015; Yeh et al., 2016). Our results therefore identify a potential countermeasure strategy in which post-exposure treatment of chlorine poisoning victims with budesonide could prevent long-term detrimental effects of chlorine in the lung.
Highlights.
Fibrotic lesions in chlorine-exposed mouse lungs had large gene expression changes.
Pro-inflammatory gene expression suggested benefits of anti-inflammatory therapy.
The corticosteroid budesonide inhibited chlorine-induced airway fibrosis in mice.
Budesonide inhibited fibrosis even in the absence of efficient epithelial repair.
Inflammation rather than epithelial damage may drive fibrogenesis in this model.
Acknowledgments
GRANTS
This work was supported by the National Institutes of Health CounterACT Program through the Office of the Director and the National Institute of Environmental Health Sciences (Awards U01 ES015673 and U01 ES022564 to G.W.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health. Microarray analysis was performed with assistance of the UofL Genomics Facility, which is supported by NIH P20GM103436 (KY IDeA Networks of Biomedical Research Excellence), NIH P30GM106396 (UofL J. G. Brown Cancer Center Phase III CoBRE), the J. G. Brown Foundation, and user fees. S.M. was supported by training grant T32 ES011564.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andres SA, Wittliff JL, 2011. Relationships of ESR1 and XBP1 expression in human breast carcinoma and stromal cells isolated by laser capture microdissection compared to intact breast cancer tissue. Endocrine 40, 212–221. [DOI] [PubMed] [Google Scholar]
- Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE, 2014. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307, L158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler D, Plavka R, Shinwell ES, Hallman M, Jarreau PH, Carnielli V, Van den Anker JN, Meisner C, Engel C, Schwab M, Halliday HL, Poets CF, Group NT, 2015. Early Inhaled Budesonide for the Prevention of Bronchopulmonary Dysplasia. N Engl J Med 373, 1497–1506. [DOI] [PubMed] [Google Scholar]
- Bringardner BD, Baran CP, Eubank TD, Marsh CB, 2008. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal 10, 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Mo Y, Schlueter CF, Hoyle GW, 2013. Inhibition of chlorine-induced pulmonary inflammation and edema by mometasone and budesonide. Toxicol Appl Pharmacol 272, 408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Blanc PD, 1993. Chlorine gas exposure and the lung: a review. Toxicol Ind Health 9, 439–455. [DOI] [PubMed] [Google Scholar]
- Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE, 2010. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 299, L442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demnati R, Fraser R, Martin JG, Plaa G, Malo JL, 1998. Effects of dexamethasone on functional and pathological changes in rat bronchi caused by high acute exposure to chlorine. Toxicol Sci 45, 242–246. [DOI] [PubMed] [Google Scholar]
- Evans RB, 2005. Chlorine: state of the art. Lung 183, 151–167. [DOI] [PubMed] [Google Scholar]
- Gao W, Ju YN, 2016. Budesonide Attenuates Ventilator-induced Lung Injury in a Rat Model of Inflammatory Acute Respiratory Distress Syndrome. Arch Med Res 47, 275–284. [DOI] [PubMed] [Google Scholar]
- Guo X, Li T, Xu Y, Xu X, Zhu Z, Zhang Y, Xu J, Xu K, Cheng H, Zhang X, Ke Y, 2017. Increased levels of Gab1 and Gab2 adaptor proteins skew interleukin-4 (IL-4) signaling toward M2 macrophage-driven pulmonary fibrosis in mice. J Biol Chem 292, 14003–14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto Y, Ano S, Allard B, O’Sullivan M, McGovern TK, Martin JG, 2017. Montelukast reduces inhaled chlorine triggered airway hyperresponsiveness and airway inflammation in the mouse. Br J Pharmacol 174, 3346–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle GW, Chang W, Chen J, Schlueter CF, Rando RJ, 2010. Deviations from Haber’s Law for multiple measures of acute lung injury in chlorine-exposed mice. Toxicol Sci 118, 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle GW, Chen J, Schlueter CF, Mo Y, Humphrey DM Jr., Rawson G, Nino JA, Carson KH, 2016. Development and assessment of countermeasure formulations for treatment of lung injury induced by chlorine inhalation. Toxicol Appl Pharmacol 298, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle GW, Svendsen ER, 2016. Persistent effects of chlorine inhalation on respiratory health. Ann N Y Acad Sci 1378, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, C.o.L.S., National Research Council, 1996. Guide for the Care and Use of Laboratory Animals. The National Academies Press, Washington, DC. [Google Scholar]
- Jonasson S, Wigenstam E, Koch B, Bucht A, 2013. Early treatment of chlorine-induced airway hyperresponsiveness and inflammation with corticosteroids. Toxicol Appl Pharmacol 271, 168–174. [DOI] [PubMed] [Google Scholar]
- Ju YN, Yu KJ, Wang GN, 2016. Budesonide ameliorates lung injury induced by large volume ventilation. BMC Pulm Med 16, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TE Jr., Pardo A, Selman M, 2011. Idiopathic pulmonary fibrosis. Lancet 378, 1949–1961. [DOI] [PubMed] [Google Scholar]
- Kohno M, Haramoto M, Nakajima O, Yang L, Hinotsu S, Yokohira M, Imaida K, Kawakami K, 2010. Antedrug budesonide by intrapulmonary treatment attenuates bleomycin-induced lung injury in rats with minimal systemic adverse effects. Biol Pharm Bull 33, 1206–1211. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Sunil VR, Venosa A, Vayas KN, Businaro R, Heck DE, Laskin JD, Laskin DL, 2016. Macrophages and inflammatory mediators in pulmonary injury induced by mustard vesicants. Ann N Y Acad Sci 1374, 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, 2015. The evolution of our understanding of macrophages and translation of findings toward the clinic. Expert Rev Clin Immunol 11, 5–13. [DOI] [PubMed] [Google Scholar]
- McGovern TK, Goldberger M, Allard B, Farahnak S, Hamamoto Y, O’Sullivan M, Hirota N, Martel G, Rousseau S, Martin JG, 2015. Neutrophils mediate airway hyperresponsiveness after chlorine-induced airway injury in the mouse. Am J Respir Cell Mol Biol 52, 513–522. [DOI] [PubMed] [Google Scholar]
- McLarren KW, Cole AE, Weisser SB, Voglmaier NS, Conlin VS, Jacobson K, Popescu O, Boucher JL, Sly LM, 2011. SHIP-deficient mice develop spontaneous intestinal inflammation and arginase-dependent fibrosis. Am J Pathol 179, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y, Chen J, Humphrey DM Jr., Fodah RA, Warawa JM, Hoyle GW, 2015. Abnormal epithelial structure and chronic lung inflammation after repair of chlorine-induced airway injury. Am J Physiol Lung Cell Mol Physiol 308, L168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y, Chen J, Schlueter CF, Hoyle GW, 2013. Differential susceptibility of inbred mouse strains to chlorine-induced airway fibrosis. Am J Physiol Lung Cell Mol Physiol 304, L92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BB, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM, 2013. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol 49, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman BT, Churg A, 1998. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med 157, 1666–1680. [DOI] [PubMed] [Google Scholar]
- Mulugeta S, Nureki S, Beers MF, 2015. Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 309, L507–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musah S, Chen J, Hoyle GW, 2012. Repair of tracheal epithelium by basal cells after chlorine-induced injury. Respir Res 13, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musah S, Schlueter CF, Humphrey DM Jr., Powell KS, Roberts AM, Hoyle GW, 2017. Acute lung injury and persistent small airway disease in a rabbit model of chlorine inhalation. Toxicol Appl Pharmacol 315, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Koren EG, Hogan BL, Gunn MD, 2013. Loss of basal cells precedes bronchiolitis obliterans-like pathological changes in a murine model of chlorine gas inhalation. Am J Respir Cell Mol Biol 49, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholzer JJ, Christensen PJ, Lama V, Horowitz JC, Hattori N, Subbotina N, Cunningham A, Lin Y, Murdock BJ, Morey RE, Olszewski MA, Lawrence DA, Simon RH, Sisson TH, 2012. PAI-1 promotes the accumulation of exudate macrophages and worsens pulmonary fibrosis following type II alveolar epithelial cell injury. J Pathol 228, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera T, Zuidhof AB, Smit M, Menzen MH, Klein T, Flik G, Zaagsma J, Meurs H, Maarsingh H, 2014. Arginase inhibition prevents inflammation and remodeling in a guinea pig model of chronic obstructive pulmonary disease. J Pharmacol Exp Ther 349, 229–238. [DOI] [PubMed] [Google Scholar]
- Raghu G, Chen YY, Rusch V, Rabinovitch PS, 1988. Differential proliferation of fibroblasts cultured from normal and fibrotic human lungs. Am Rev Respir Dis 138, 703–708. [DOI] [PubMed] [Google Scholar]
- Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A, 2001. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 24, 591–598. [DOI] [PubMed] [Google Scholar]
- Rando RJ, Hammad YY, 1990. A Diffusive Sampler for Gaseous Chlorine Utilizing an Aqueous Sulfamic Acid Collection Medium and Specific Ion Electrode Analysis. Applied Occupational and Environmental Hygiene 5, 700–706. [Google Scholar]
- Ryu JH, Moua T, Daniels CE, Hartman TE, Yi ES, Utz JP, Limper AH, 2014. Idiopathic pulmonary fibrosis: evolving concepts. Mayo Clin Proc 89, 1130–1142. [DOI] [PubMed] [Google Scholar]
- Spagnolo P, Maher TM, Richeldi L, 2015. Idiopathic pulmonary fibrosis: Recent advances on pharmacological therapy. Pharmacol Ther 152, 18–27. [DOI] [PubMed] [Google Scholar]
- Suganuma H, Sato A, Tamura R, Chida K, 1995. Enhanced migration of fibroblasts derived from lungs with fibrotic lesions. Thorax 50, 984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerhill EM, Hoyle GW, Jordt SE, Jugg BJ, Martin JG, Matalon S, Patterson SE, Prezant DJ, Sciuto AM, Svendsen ER, White CW, Veress LA, Terrorism ATS, Inhalational Disasters Section of the Environmental, O., Population Health, A., 2017. An Official American Thoracic Society Workshop Report: Chemical Inhalational Disasters. Biology of Lung Injury, Development of Novel Therapeutics, and Medical Preparedness. Ann Am Thorac Soc 14, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW, 2008. Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice. Inhal Toxicol 20, 783–793. [DOI] [PubMed] [Google Scholar]
- Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, Garbe P, Moolenaar RL, 2009. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 27, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venosa A, Malaviya R, Choi H, Gow AJ, Laskin JD, Laskin DL, 2016. Characterization of Distinct Macrophage Subpopulations during Nitrogen Mustard-Induced Lung Injury and Fibrosis. Am J Respir Cell Mol Biol 54, 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Winskog C, Edston E, Walther SM, 2005. Inhaled and intravenous corticosteroids both attenuate chlorine gas-induced lung injury in pigs. Acta Anaesthesiol Scand 49, 183–190. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang L, Walther SM, 2002. Inhaled budesonide in experimental chlorine gas lung injury: influence of time interval between injury and treatment. Intensive Care Med 28, 352–357. [DOI] [PubMed] [Google Scholar]
- White CW, Martin JG, 2010. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc 7, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigenstam E, Elfsmark L, Koch B, Bucht A, Jonasson S, 2016. Acute respiratory changes and pulmonary inflammation involving a pathway of TGF-beta1 induction in a rat model of chlorine-induced lung injury. Toxicol Appl Pharmacol 309, 44–54. [DOI] [PubMed] [Google Scholar]
- Yeh TF, Chen CM, Wu SY, Husan Z, Li TC, Hsieh WS, Tsai CH, Lin HC, 2016. Intratracheal Administration of Budesonide/Surfactant to Prevent Bronchopulmonary Dysplasia. Am J Respir Crit Care Med 193, 86–95. [DOI] [PubMed] [Google Scholar]