Key Teaching Points.
-
•
Cardiac fibromas are relatively frequently associated with ventricular tachycardias, often of multiple morphologies.
-
•
Management of ventricular arrhythmias in unresectable fibromas could be challenging and various strategies may be employed.
-
•
Stereotactic radiosurgery could be considered in case of failure of conventional approaches such as catheter or surgical ablation.
Introduction
Primary cardiac tumors are very rare and may have variable clinical presentation, depending on their nature and location. Recent WHO classification divides them into benign, tumors of uncertain biologic behavior, germ cell tumors, and malignant tumors.1 Sustained or incessant ventricular tachycardia (VT) has been reported relatively infrequently in association with cardiac tumors.2, 3, 4 Interestingly, patients with large fibromas in the pediatric population were at the highest risk, with VT occurring in more than 60% of cases.4 In adults, this type of tumor is very sporadic.5 We present a case of a patient with an intermediate cardiac fibroma triggering recurrent VTs that necessitated the whole spectrum of therapeutic approaches, including stereotactic radiosurgery.
Case report
A 34-year-old patient, a professional taxi driver, with unremarkable family and personal history was admitted in November 2015 to a regional hospital for atypical chest pain that occurred after previous respiratory infection with fever. He presented with hemodynamically tolerated, repetitive monomorphic VTs of several morphologies. The patient was transferred to a tertiary center. A transthoracic echocardiogram showed nondilated left ventricle with normal ejection fraction (left ventricular end-diastolic diameter 51 mm) with large intramyocardial mass in the inferolateral wall (29 × 56 mm) (Figure 1A). This finding was confirmed by magnetic resonance imaging, which showed a well-demarcated, large intramyocardial tumor (60 × 40 × 25 mm). Gadolinium administration resulted in homogeneous enhancement, in both early and late phase. On the lateral margin of the tumor, semilunar envelope without enhancement was observed. Positron emission tomography and computed tomography with F-fluorodeoxyglucose revealed the defect in F-fluorodeoxyglucose accumulation corresponding with an intramyocardial mass inferolaterally (60 × 47 × 30 mm) surrounded on the lateral margin by a hypodense envelope without tracer accumulation (Figure 1B). Selective coronary arteriography documented normal epicardial coronary arteries with pathologic vascularization within the region of the tumor, supplied by 2 large marginal branches and by the left posterolateral branch of the left coronary artery. No other cardiac pathology was found and there was no evidence of extracardiac involvement. After loading with amiodarone, VTs were partly suppressed. The patient was implanted with a single-chamber implantable cardioverter-defibrillator (ICD) and was discharged in stable condition.
Figure 1.
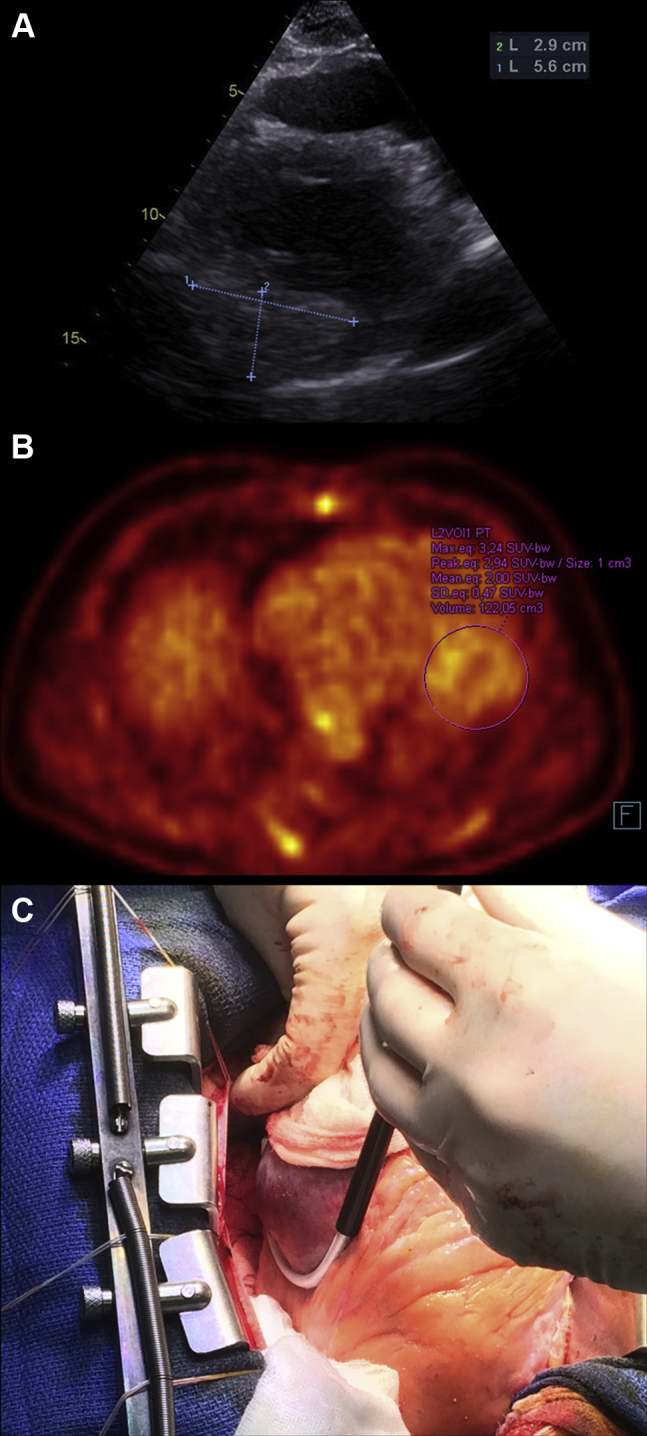
A: A 2-dimensional echocardiogram (modified parasternal long-axis view) depicting the extent of the intramyocardial cardiac mass localized inferolaterally. B: Positron emission tomography and computed tomography with F-fluorodeoxyglucose image showing low uptake of F-fluorodeoxyglucose within the tumor (maximum standardized uptake value of 3.24 and mean value of 2.0 are within the range typical for benign tumors). C: The extent of the tumor as seen during cardiac surgery and cryoablation probe at the border zone of the tumor.
The patient was readmitted in April 2016 for recurrent ICD shocks (a total of 7). The predominant rhythm was monomorphic VT of 170 beats/min, which was terminated by intravenous amiodarone. The patient was transferred to our center for consideration of VT ablation. On amiodarone therapy, VT had a repetitive pattern with a rate around 130 beats/min. Based on the decision of the heart team, the patient was referred to cardiac surgery with a prospect of resection or partial resection of the tumor and/or cryoablation of the arrhythmia substrate. The surgery was performed from a medial sternotomy. The large and very stiff tumor was revealed, involving the entire inferolateral wall of the heart. The structure and extent of the tumor, and its location, did not allow even partial resection. Fast epicardial mapping with a multipolar electrode array was performed after multiple puncture biopsies were taken from the tumor. Only far-field signals were recorded above the mass. Therefore, encircling cryoablation was performed with the prospect of modification of the border zone around the tumor (Figure 1C). The recovery was uneventful and the occurrence of arrhythmias decreased significantly, and only isolated ventricular ectopic beats (14% in a 24-hour recording) or very short runs of slow monomorphic VT were observed. The patient continued with metoprolol 50 mg and amiodarone 200 mg daily. Histology revealed fibrous tissue with sporadic hypertrophic cardiomyocytes and 2 vascular foci of low proliferative activity (proliferation marker Ki-67 positive in less than 5% of endothelial cells). Vascular proliferation with low and no mitotic activity was observed. A more accurate classification was not possible from available samples.
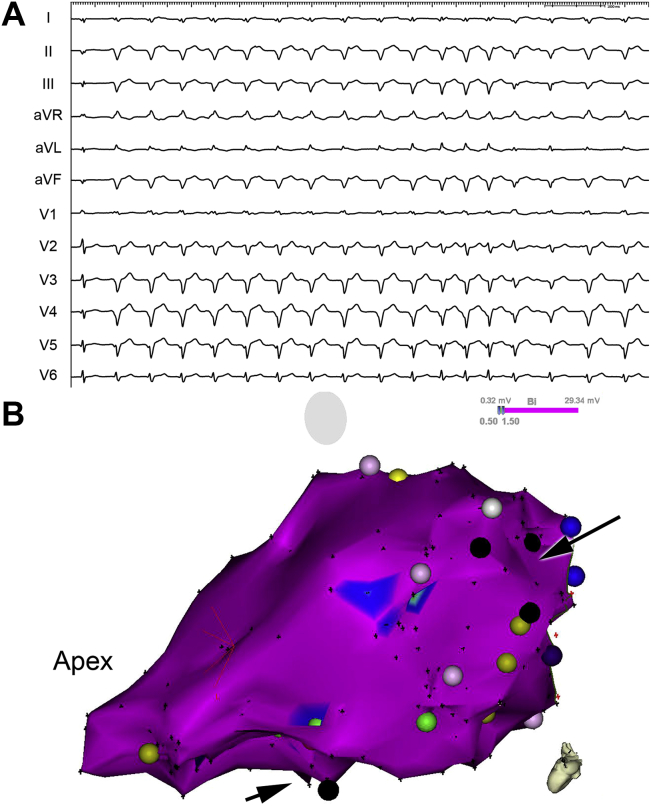
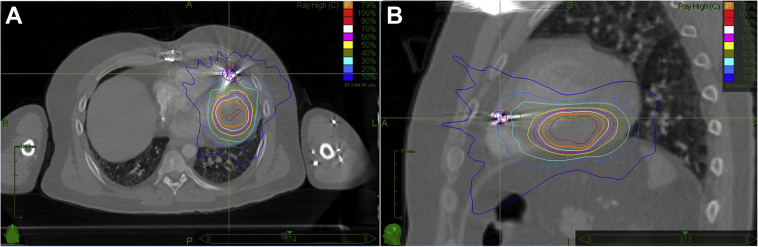
During subsequent follow-up, the patient was feeling well, completely asymptomatic with predominant sinus rhythm. However, 6 months after the procedure, the patient presented with minimally symptomatic, incessant VTs of 2 morphologies, below the detection limit of the ICD (Figure 2A). After he was readmitted to our center, catheter ablation using endocardial approach was considered. Intracardiac echocardiography helped to localize the extent of the tumor and detailed substrate mapping was performed. Bipolar voltage was above 1.5 mV within the entire left ventricle. Owing to the occurrence of 2 morphologies of VT, 2 regions were targeted using activation sequence mapping and pace-mapping (Figure 2B). One was in the basal part of the ventricle at the periphery of the tumor, where local abnormal ventricular activity and slow conduction during pacing was observed, corresponding to subendocardial scar seen on an intracardiac echocardiogram. Ablation in this area resulted in the termination of VT with right bundle branch block morphology and a rightward frontal axis. The other region was between the septum and posteromedial papillary muscle. Corresponding VT appeared to be focal in origin. However, the far-field character of the local electrograms and absent effect of radiofrequency current delivery (40 W at 30 mL/min perfusion rate) suggested deep intramyocardial origin close to the tumor. To assess the therapeutic potential of coiling or alcohol instillation into the tumor vasculature, subsequent selective cannulation of the above branches of the left coronary artery was performed with instillation of cold saline after temporary occlusion by the angioplasty balloon. No effect was noticed on the occurrence of incessant VT. For almost incessant VT of 1 morphology, radiosurgical therapy of the tumor using the stereotactic radiosurgery system CyberKnife (Accuray Inc, Sunnyvale, CA) was discussed with radiation oncologists. The treatment plan of 25 Gy dose in a single outpatient treatment to the 75% isodose line was designed (Figure 3), encompassing the inferolateral wall from base to apex. The dose was centered into the tumor region within the central mid-myocardial layer. After the procedure, the patient still had multiple runs of minimally symptomatic, repetitive VT. Within 8 months of follow-up, VTs gradually disappeared, as documented with Holter recordings and from the ICD memory.
Figure 2.
A: Electrocardiogram recordings of predominant clinical ventricular tachycardia with right bundle branch block morphology and left axis deviation, suggestive of origin close to the lower septum. B: Three-dimensional electroanatomical bipolar voltage map of the left ventricle (modified left lateral view) showing normal voltage (violet color) and 2 regions of catheter ablation close to the posteromedial papillary muscle and the other superiorly in the basal part of ventricle (black arrows).
Figure 3.
Stereotactic radioablation using acquired computed tomography image in transverse (A) and lateral view (B). Orange boundary indicates distribution zone targeted to the tumor.
Discussion
According to our best knowledge, this is the first reported case of cardiac fibroma associated with multiple VT morphologies treated by several approaches, including stereotactic radiosurgery.
The preferable therapy of cardiac fibroma is surgical resection, which is recommended even in asymptomatic cases.6, 7, 8 In some cases, extensive resection is viewed as a bridge to cardiac transplantation.9 The latter strategy is another therapeutic option, especially in cases with intractable ventricular arrhythmias.10 The outcome of patients with unresectable fibromas is unknown. Based on the pediatric literature, even asymptomatic tumors increase the risk of sudden death owing to malignant ventricular arrhythmias.4 Therefore, consideration of an ICD is usually recommended.11, 12 Catheter ablation has been described anecdotally in some cases of other tumors associated with arrhythmias.13
Our case highlights multiple therapeutic options for VT in a patient with nonresectable cardiac fibroma. To prevent sudden cardiac death, an ICD was implanted. During the exploratory surgery, epicardial cryoablation was performed on a beating heart with the prospect of interruption of border zones of presumed reentry circuits. The effect was transient, and incessant VT of 2 morphologies recurred despite amiodarone therapy. Subsequent endocardial ablation was able to suppress 1 of the 2 VTs, which appeared to be related to scar tissue in the basal anterolateral segment of the left ventricle. The other VT, presumably of focal origin, had an origin deep in the myocardium of the inferior wall at the tumor border. Since selective coronary instillation of cold saline did not affect the arrhythmia, we did not proceed with alcohol ablation or with coiling. Owing to incessant runs of VT of a single morphology, stereotactic radiosurgery was considered as the next step of the management, leading to the gradual abolition of residual VT. This strategy was described recently as a viable therapeutic option in patients with structural heart disease and recurrent VT refractory to catheter ablation.14, 15
Conclusion
Management of VTs associated with cardiac fibromas may be challenging. Our case documents a combination of surgical cryoablation, endocardial catheter ablation, and stereotactic radiosurgery applied to the tumor mass, which resulted in the abolition of all recurrent arrhythmias.
Footnotes
Dr Kautzner reports personal fees from Bayer, Biosense Webster, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, EPIX, Medtronic, Merck Sharp & Dohme, Liva Nova (MicroPort CRM), Mylan, and St. Jude Medical (Abbott) for participation in scientific advisory boards and/or educational events. Dr Peichl received personal fees for participation in advisory boards from Biosense Webster and Biotronik, and fees for educational events from St. Jude Medical (Abbott). All other authors have nothing to declare.
References
- 1.Burke A., Tavora F. The 2015 WHO Classification of tumors of the heart and pericardium. J Thorac Oncol. 2015;11:441–452. doi: 10.1016/j.jtho.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y., Yamabe H., Yamasaki H., Tsuda H., Nygayoshi Y., Kawano H., Kimura Y., Hokamura Y., Ogawa H. A case of reversible ventricular tachycardia and complete atrioventricular block associated with primary cardiac B-cell lymphoma. Pacing Clin Electrophysiol. 2009;32:816–819. doi: 10.1111/j.1540-8159.2009.02372.x. [DOI] [PubMed] [Google Scholar]
- 3.Krasuski R.A., Hesselson A.B., Landolfo K.P., Ellington K.J., Bashore T.M. Cardiac rhabdomyoma in an adult patient presenting with ventricular arrhythmia. Chest. 2000;118:1217–1221. doi: 10.1378/chest.118.4.1217. [DOI] [PubMed] [Google Scholar]
- 4.Miyake C.Y., Del Nido P.J., Alexander M.E., Cecchin F., Berul C.I., Triedman J.I., Geva T., Walsh E.P. Cardiac tumors and associated arrhythmias in pediatric patients, with observations on surgical therapy for ventricular tachycardia. J Am Coll Cardiol. 2011;58:1903–1909. doi: 10.1016/j.jacc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Nwachukwu H., Li A., Nair V., Nguyen E., David T.E., Butany J. Cardiac fibroma in adults. Cardiovasc Pathol. 2011;20:e146–152. doi: 10.1016/j.carpath.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Cho J.M., Danielson G.K., Puga F.J., Dearani J.A., McGregor C.G., Tazelaar H.D., Hagler D.J. Surgical resection of ventricular cardiac fibromas: early and late results. Ann Thorac Surg. 2003;76:1929–1934. doi: 10.1016/s0003-4975(03)01196-2. [DOI] [PubMed] [Google Scholar]
- 7.Elahi M., Poh C.L., Krishna A., Grant P. Surgical considerations for large asymptomatic cardiac fibromas in the context of fatal ventricular arrhythmias. Heart Lung Circ. 2012;21:750–753. doi: 10.1016/j.hlc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Nathan M., Fabozzo A., Geva T., Walsh E., del Nido P.J. Successful surgical management of ventricular fibromas in children. J Thorac Cardiovasc Surg. 2014;148:2602–2608. doi: 10.1016/j.jtcvs.2013.11.052. [DOI] [PubMed] [Google Scholar]
- 9.Waller B.R., Bradley S.M., Crumbley A.J., Wiles H.B., McQuinn T.C., Bennett A.T. Cardiac fibroma in an infant: single ventricle palliation as a bridge to heart transplantation. Ann Thorac Surg. 2003;75:1306–1308. doi: 10.1016/s0003-4975(02)04655-6. [DOI] [PubMed] [Google Scholar]
- 10.Sharma K., Rohlicek C., Cecere R., Tchervenkov C.I. Malignant arrhythmias secondary to a cardiac fibroma requiring transplantation in a teenager. J Heart Lung Transplant. 2007;26:639–641. doi: 10.1016/j.healun.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Myers K.A., Wong K.K., Tipple M., Santani S. Benign cardiac tumours, malignant arrhythmias. Can J Cardiol. 2010;26:58–61. doi: 10.1016/s0828-282x(10)70009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakim F.A., Pandit A., Mookadam F., Mamby S. Cardioverter-defibrillator implantation to treat cardiac fibroma-induced ventricular tachycardia in a 70-year-old woman. Tex Heart Inst J. 2014;41:329–331. doi: 10.14503/THIJ-13-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haydin S., Onan B., Kiplapinar N., Akdeniz C., Tuzcu V., Bakir I. Combined resection and radiofrequency ablation of rhabdomyoma in a child with sustained ventricular tachycardia. J Card Surg. 2012;27:649–652. doi: 10.1111/j.1540-8191.2012.01496.x. [DOI] [PubMed] [Google Scholar]
- 14.Cvek J., Neuwirth R., Knybel L. Cardiac radiosurgery for malignant ventricular tachycardia. Cureus. 2014;6(7):e190. [Google Scholar]
- 15.Cuculich P.S., Schill M.R., Kashani R. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377:2325–2336. doi: 10.1056/NEJMoa1613773. [DOI] [PMC free article] [PubMed] [Google Scholar]