Abstract
Stent infection after drug-eluting stent implantation is uncommon but is a critical event. In this study, we describe two such cases of coronary stent infection but with varied presentation. The first patient presented with recurrent stent thrombosis and acute coronary syndrome while the second patient was erroneously diagnosed as having tubercular pericarditis and was started on anti-tubercular therapy. Due to their possible fatal outcome, we herein report our experience with this uncommon clinical entity to help in rapid diagnosis and treatment.
<Learning objective: Infection involving implanted stents is rare, it can, however, occur with high morbidity and mortality. In absence of diagnostic criteria or classic signs and symptoms, one should be aware of its presence and risk factors associated with it. Certain rare conditions such as JAK2 V617F mutation significantly alter hemogram leading to changes in red blood cell, white blood cell, and platelet counts facilitating coronary thrombosis should also be kept in mind.>
Keywords: Coronary angiography, Coronary stent infection, Percutaneous coronary intervention, JAC2 V617F mutation
Introduction
Coronary stent infection is a rare complication associated with a high mortality rate. Since the introduction of coronary stents in 1987, fewer than 30 cases of stent infections have been reported in the literature [1]. Patients with stent infection often present with fever (93%), chills (41%), and chest pain (51.7%) [2] presumably related to peri-procedural bacteremia or direct septic stent implantation [3]. About 80% of coronary stent infections are also associated with pseudo-aneurysm of coronary arteries [4]. In this study, we present two unique case reports dealing with coronary stent infection.
Case report 1
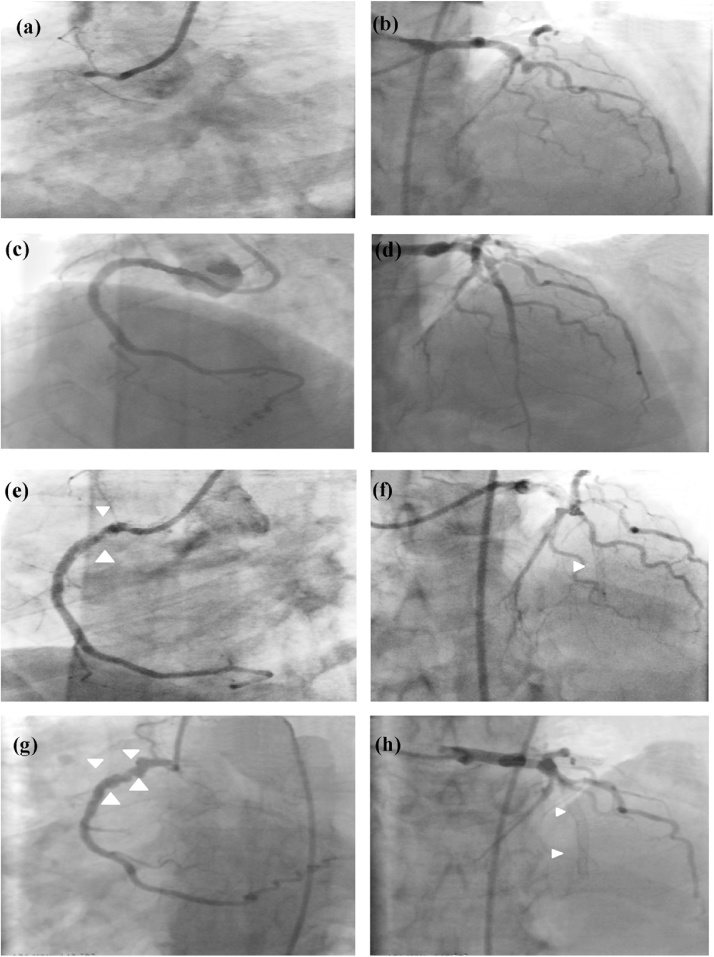
A 50-year-old male with history of hypertension and type II diabetes mellitus (DM) was admitted to a local hospital with a history of inducible ischemia. Cardiac catheterization results revealed total occlusion of proximal right coronary artery (RCA) (Fig. 1a) and left anterior descending artery (LAD) (Fig. 1b). The left circumflex artery was non-dominant and showed no significant disease.
Fig. 1.
Image showing total occlusion of right coronary artery (RCA) (a) and coronary artery disease (CAD) (b); Percutaneous transluminal coronary angioplasty (PTCA) to RCA (c) left anterior descending artery (LAD) (d) with Drug-eluting stent (DES); thrombotic sub-total occlusion of proximal RCA stent (e) (arrows), total thrombotic occlusion of the stented segment of LAD (arrow) (f) on day 5 post PTCA, multiple aneurysms in the RCA stent (g) (arrows) and total re-occlusion of LAD stent (h) on day 10 post PTCA.
Coronary angioplasty was planned as a staged procedure under appropriate dual anti-platelet therapy consisting of aspirin and clopidogrel. The RCA lesion was first stented with drug-eluting stent (DES) and LAD (Fig. 1c,d) on the following day. All the procedures were done through the right femoral artery. The patient was discharged from hospital on the third day. However, he developed sudden onset of chest pain and breathlessness five days after hospital discharge. He was immediately re-hospitalized. Electrocardiography (ECG) showed acute anterior wall myocardial infarction (MI).
A repeat coronary angiography (CAG) was done through the right femoral artery and it showed a near total occlusion of the proximal RCA stent (Fig. 1e) with thrombolysis in myocardial infarction II flow and total thrombotic occlusion of the LAD stent (Fig. 1f). Thrombosuction of the LAD and RCA was done and showed good antegrade flow. The LAD lesion was stented with another longer DES but the RCA lesion was subjected to balloon angioplasty only. The patient was discharged on day 3 with prescription of aspirin and ticagrelor.
The patient remained asymptomatic for 7 days. Thereafter he started complaining of high-grade fever with chills, chest pain, and breathlessness. He was later referred to our hospital. ECG showed a normal sinus rhythm with evolving anterior wall ST-elevation MI. The hemoglobin (Hb) levels at presentation were 13 g/dl, leucocyte count was 22,000/cu mm, and the platelet count was 550,000/cu mm. Two-dimensional echocardiography (2D-ECHO) showed loculated pericardial effusion with thick adhesions. Hypokinesia of the anterior wall with preserved wall thickness with left ventricular ejection fraction (LVEF) of 35% was noted. No diastolic collapse of right atrium or right ventricle was observed.
CAG was performed which showed complete re-occlusion of LAD stent (Fig. 1g) and focal aneurysmal dilatation of the stented segment of the RCA (Fig. 1h) without any significant luminal stenosis. The patient was advised to undergo coronary artery bypass graft (CABG) surgery. With such a history of recurrent stent thrombosis and baseline thrombocytosis, there was a strong suspicion of underlying hypercoagulable states. To our surprise, the results came out to be positive for somatic mutations of JAK2V617F (Janus kinase transcription regulator). Hence, we investigated whether there was any relationship between JAK2V617F mutation and recurrent stent thrombosis.
Blood analysis of our patient showed increased levels of Hb (20.65 g/dl), packed cell volume (PCV) of 60%, white blood cell (30.43 × 109/l), and platelet count (3843 × 109/l). These values are significantly higher (p < 0.001) than in non-JAK2V617F mutation subjects, suggesting this mutation has great impact on hemogram variation, but not on platelet count. Excessive platelet counts may result in vascular complications, such as thrombosis, microvascular disturbances, and hemorrhage. Tests to detect other hypercoagulable disorders were negative [5].
Intraoperative findings on sternotomy showed dense pericardial adhesions to the underlying LV with exuberant granulation tissue proliferation in the stented LAD region leading to distortion of the artery. The implanted RCA stent near the right atrioventricular (AV) groove also showed signs of disease with purulent material exuding from the infected site. The RCA showed multiple aneurysms at the stented segment with flakes of necrotic tissue surrounding it. Pericardial fluid was purulent admixed with blood clots at the sites of infection. Both the infected stents were removed and distal vessels were grafted with arterial conduits from the aorta.
Histopathology of the excised stented segment predominantly showed dense neutrophilic infiltration in the walls of the lumen with areas of hemorrhage and necrosis of the intima and the media. The lumen of the vessel was occluded with fibrin-platelet plug and necrotic debris. Gram staining of the material was positive for cocci in chains, and subsequent cultures grew methicillin-resistant Staphylococcus aureus (MRSA). Blood, pericardial fluid, and coronary artery aspirated culture samples were also positive for MRSA. Antibiotics were continued for four weeks. The patient was later referred to a hematologist for subsequent treatment. He was diagnosed with essential thrombocytosis. The patient is on regular follow-up and is doing well on dual antiplatelet therapy (DAPT) (aspirin + ticagrelor) and oral hydroxyurea. This case underlines the importance of investigating the cause of thrombocytosis before any elective coronary intervention.
Case report 2
A 52-year-old male with a history of hypertension and type II DM was diagnosed with acute pericarditis with pericardial effusion by symptoms, ECG, and 2D-ECHO at a local hospital and started on rifampicin, isoniazid, pyrazinamide, and ethambutol therapy for tuberculosis, for the previous 15 days. However, he continued to be symptomatic, developed drug-induced hepatitis, and was referred to our hospital. The patient had undergone coronary angioplasty one month previously for acute inferior wall myocardial infarction (MI) and a DES was implanted in the RCA at a resource-poor hospital. He developed low-grade fever from the 5th day of the procedure and was treated with oral anti-pyretics. The fever did not subside and he was later started on anti-tubercular medication. However, he continued to be symptomatic and developed severe drug-induced hepatitis.
ECG showed 1st degree AV block, sinus rhythm with ST-T changes in the inferior leads. Chest X-ray was normal. Blood count revealed Hb of 13.4 g/dl, leucocyte count of 22,000/dl, platelet count of 150,000/ml erythrocyte sedimentation rate (ESR) 60 mm/hr and high sensitivity C-reactive protein of 5.6 mg/dl. Serology for human immunodeficiency virus, hepatitis B and C viruses and other viral markers were negative. 2D-ECHO showed thickening of pericardium with loculated hypodense shadow in the right AV groove with moderate pericardial effusion and a mild diastolic collapse of right atrium (RA) and right ventricular (RV) chambers. There was also hypokinesia but preserved thickness of inferior wall of the myocardium. LVEF was 35%. Blood cultures showed positive for MRSA. The patient was treated with intravenous tiecoplanin for 4 weeks as advised by infectious disease specialist.
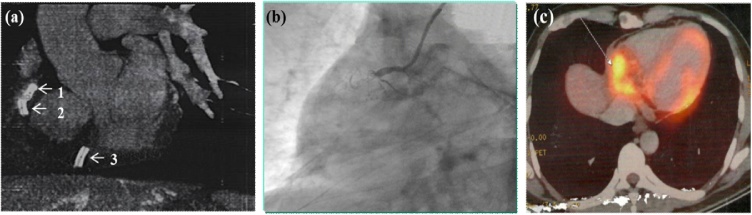
Computed tomography (CT) scan of thorax with CAG showed a large multi-loculated, hypodense, fusiform collection at the right AV groove involving the myocardium of the RV with complete fracture of RCA stent into three fragments (Fig. 2a). The proximal two stent fragments were embedded in the superior aspect of the AV groove, and the distal fragment was found floating free in the pericardial space. There was a total occlusion of RCA stent with pseudo-aneurysm formation (Fig. 2b). Positron emission tomography scan showed increased fluorodeoxyglucose uptake in right AV groove (Fig. 2c). RA and RV had exuberant granulation tissue proliferation in the right AV groove.
Fig. 2.
Computed tomography (CT) image showing fracture of right coronary artery (RCA) stent into three fragments (arrows 1–3) with distal fragment floating (arrow 3) in the pericardial space (a) and coronary angiography (CAG) showing total occlusion of RCA stent (b). Positron emission tomography (PET) image showing increased fluorodeoxyglucose (FDG) uptake in right atrioventricular (AV) groove (c).
Examination of surgical material revealed dense pericardial adhesions to underlying RA and RV with exuberant granulation tissue proliferation in the right AV groove with total destruction and displacement of the RCA stent. Histo-pathology from the excised stented segment showed predominant neutrophilic infiltration in the walls of the lumen with areas of hemorrhage and necrosis. Gram staining of the material showed Gram-positive cocci in chains and subsequent culture from pericardial fluid grew MRSA.
Discussion
Studies have shown that incidence of stent thrombosis is higher in patient with thrombocytosis and hypercoagulable conditions [5]. Our patient (Case 1) is positive for somatic mutations of JAK2V617F (Janus kinase transcription regulator).
JAK2V617F is a point mutation that occurs in the tyrosine kinase gene JAK2 was discovered in 2005 in patients affected with myeloproliferative disorder (MPD). This mutation is a base replacement (1849G.T) in the Jak homology domain 2 (JH2) of JAK2 and leads to a valine to phenylalanine substitution at codon 617 (V617F or JAK2 V617F) mutation. JAK2V617F mutation has emerged as a central feature in the pathogenesis of polycythemia vera (PV) with an increased risk of thrombotic events [5]. This mutation has great impact on hemogram variation (red blood cell counts, white blood cell counts, platelet parameters, but not platelet count).
With the popular use of DES, the reports of stent infection have increased in recent years due to their immunomodulatory properties [2]. It has been reported that, since the first coronary stent infection described in 1993 [6], of the 77 cases of stent infections (including coronary and peripheral stents) 29 were coronary stent infection [7]. In addition to patient- and stent-related risks, other risk factors include inadequate sterility and repeated use of local site (commonly groin area), repeated re-use of hardware such as balloons, catheters (common in resource-poor hospitals) and multiple guidewire manipulations, prolonged indwelling catheterization also play an important role in initiating stent infections.
There is no single modality confirming diagnosis of stent infection. Early surgery with stent removal, evacuation of purulent pericardial fluid, excision of epicardial granulomatous tissue, and pericardiectomy with CABG are the main surgical interventions. The importance of recognizing the underlying thrombocytosis even before any coronary intervention is the key in these patients.
The most common organisms implicated in stent infection are S. aureus (80%) followed by Pseudomonas aeruginosa (20%) [6]. Most cases described involved early infection that can occur any time from 2 days to 4 weeks after the percutaneous coronary intervention (PCI) [7]. In a retrospective study, patients showing positive blood cultures following PCI, Samore et al. [8] reported that frequency of PCI-related bacteremia was 0.64%. The pathogenic organism we identified in our patient blood samples was MRSA. So growth of this bacterium supports the diagnosis of stent infection in our patient.
Conclusions
1) Prolonged febrile illness soon after coronary stenting and thrombocytosis before coronary intervention should not be overlooked. 2) Avoid repeated same site groin punctures. 3) Ensure proper aseptic care at the access site. 4) Avoid reuse of inadequately sterilized catheters, guide wires, and balloons. 5) Strict glycemic control before intervention can minimize the incidence of stent infection.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Elbadawi A., Saad M., Elgendy I.Y., Zafar A., Chow M.Y. Multiple myocardial abscesses secondary to late stent infection. Cardiovasc Pathol. 2017;28:1–2. doi: 10.1016/j.carpath.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Bosman W.M., Borger van der Burg B.L., Schuttevaer H.M., Thoma S., Hedeman Joosten P.P. Infections of intravascular bare metal stents: a case report and review of literature. Eur J Vasc Endovasc Surg. 2014;47:87–89. doi: 10.1016/j.ejvs.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Le M.Q., Narins C.R. Mycotic pseudoaneurysm of the left circumflex coronary artery: a fatal complication following drug-eluting stent implantation. Catheter Cardiovasc Interv. 2007;69:508–512. doi: 10.1002/ccd.21014. [DOI] [PubMed] [Google Scholar]
- 4.Roubelakis A., Rawlins J., Baliulis G., Olsen S., Corbett S., Kaarne M. Coronary artery rupture caused by stent infection: a rare complication. Circulation. 2015;131:1302–1303. doi: 10.1161/CIRCULATIONAHA.114.014328. [DOI] [PubMed] [Google Scholar]
- 5.Inami T., Okabe M., Matsushita M., Kobayashi N., Inokuchi K., Hata N. JAK2 mutation and acute coronary syndrome complicated with stent thrombosis. Heart Vessels. 2016;31:1714–1716. doi: 10.1007/s00380-016-0798-x. [DOI] [PubMed] [Google Scholar]
- 6.Günther H.U., Strupp G., Volmar J., von Korn H., Bonzel T., Stegmann T. Coronary stent implantation: infection and abscess with fatal outcome. Z Kardiol. 1993;82:521–525. [PubMed] [Google Scholar]
- 7.Gautam S.R., Mishra D., Goyal B.K. Mechanical interventionand coronary artery aneurysm. J Invasive Cardiol. 2010;22:e213–e215. [PubMed] [Google Scholar]
- 8.Samore M.H., Wessolossky M.A., Lewis S.M., Shubrooks S.J., Jr., Karchmer A.W. Frequency, risk factors, and outcome for bacteremia after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1997;79:873–877. doi: 10.1016/s0002-9149(97)00006-4. [DOI] [PubMed] [Google Scholar]