Abstract
Our most important decisions often provoke the greatest anxiety, whether we seek the better of two prizes or the lesser of two evils. Yet many of our choices are more mundane, such as selecting from a slate of mediocre but acceptable restaurants. Previous research suggests that choices of decreasing value should provoke decreasing anxiety. Here we show that this is not the case. Across three behavioral studies and one fMRI study, we find that anxiety and its neural correlates demonstrate a U-shaped function of choice set value, greatest when choosing between both the highest value and lowest value sets. Intermediate (moderate-value) choice sets provoke the least anxiety, even when they are just as difficult to select between as the choice sets at the two extremes. We show that these counterintuitive findings are accounted for by decision makers perceiving low-value items as aversive (i.e., negatively motivationally salient) rather than simply unrewarding. Importantly, though, neural signatures of these anxious reactions only appear when participants are required to choose one item from a set and not when simply appraising that set’s overall value. Decision makers thus experience anxiety from competing avoidance motivations when forced to select among low-value options, comparable to the competing approach motivations they experience when choosing between high-value items. We further show that a common method of measuring subjective values (willingness to pay) can inadvertently censor a portion of this quadratic pattern, creating the misperception that anxiety simply increases linearly with set value. Collectively, these findings reveal the surprising costs of seemingly banal decisions.
Keywords: choice conflict, decision making, anxiety, reward, emotion
People often struggle with their decisions. Choosing between a set of competing options can feel effortful and anxiety provoking (Iyengar & Lepper, 2000; Patall, 2012; Schwartz, 2004), and can lead people to avoid making a decision by whatever means possible (Anderson, 2003). Our most important decisions tend to provoke the greatest anxiety, whether they are between the better of two excellent options (Shenhav & Buckner, 2014) or the lesser of two evils (Botti, Orfali, & Iyengar, 2009). Yet many of the decisions we make involve mundane options and lower stakes; for instance, when we find ourselves selecting between a slate of mediocre but acceptable restaurants for dinner. Here we investigate these seemingly trivial choices, and find that they are less trivial than may appear.
Though it might seem that low-value choices should cause little distress, there is reason to second guess that intuition. Research has shown that our responses to potential rewards are reference dependent and that they adapt to the range of options that an individual observes in a given setting (Louie & Glimcher, 2012; Louie, Khaw, & Glimcher, 2013; Rangel & Clithero, 2012; Tversky & Kahneman, 1981). In particular, people are known to treat potential rewards as aversive if social norms (Sanfey, 2009; Thaler, 1988) or other salient reference points (Coricelli et al., 2005; Coricelli & Rustichini, 2010) lead them to expect greater rewards. Recent work has extended these findings to show that the reference point for how a given item is evaluated is dynamic and influenced by the recent history of item values that the individual has observed, increasing the value of items that follow a sequence of low-value items and decreasing the value of items that follow a high-value sequence (Khaw, Glimcher, & Louie, 2017). Thus, even if a set of items is not aversive per se, it can theoretically take on a negative value when contrasted with opportunities to engage with more interesting options. This makes the intriguing prediction that as choice options become sufficiently unimportant (or low in value) they generate greater and greater avoidance motivation. The resulting avoid–avoid conflict between those options could provoke increasing anxiety (Gray & McNaughton, 2000; Miller, 1944).
At first glance, previous findings would appear to contradict this prediction. Shenhav and Buckner (2014) had participants choose between pairs of everyday items that varied in subjective value, and found that choosing between options with high subjective value increased feelings of positive affect as well as anxiety. Choosing between low-value options produced the opposite profile: low positive affect and low anxiety. Across their two fMRI studies (N = 84), choice anxiety is best explained as a linear function of the choice set value (i.e., the average value of the choice options), with no indication that anxiety increased at the lowest values. These studies also identified a set of brain regions— including dorsal anterior cingulate cortex (dACC), anterior insula (AI), and dorsomedial striatum (DMS)—that consistently tracked levels of anxiety experienced while making these choices, demonstrating less activity for low value choices. Given previous evidence that these structures are involved in evaluating the costs and benefits of potential actions and control states (Rushworth, Noonan, Boorman, Walton, & Behrens, 2011; Shenhav, Cohen, & Botvinick, 2016; Ullsperger, Danielmeier, & Jocham, 2014), these findings were interpreted as evidence that “win-win” choice conflict is evaluated as a form of response cost, in parallel with separate evaluations of the reward to be obtained (which engaged separate brain networks).
However, those specific studies suffered from a limitation common to this field of research: they excluded a large subset of items that participants had rated as “zero value” in a willingness-to-pay procedure performed prior to the main decision task. Because these lowest-value items were omitted, participants in these studies only chose between moderate- to high-value items. Given those results, which showed that lower-value choice sets generated lower anxiety (within that moderate to high value range), one might extrapolate that choice sets with even lower values should produce even less anxiety and less activity in associated brain regions. However, if the “lowest-value” items were somehow perceived to be more aversive, rather than less rewarding, then choices between them could engender avoid–avoid conflict analogous to the approach– approach conflict found in high value choices. Based on previous research examining conflicts between reliably aversive outcomes (e.g., electric shock; Botti et al., 2009; Lewin, 1935; Miller, 1944), we would then predict that these lowest-valued choices would increase feelings of anxiety and anxiety-related patterns of brain activity (cf. Blair et al., 2006).
Here we test this prediction across four studies in which participants perform choice tasks designed to include a wider array of choice sets than previously tested. We find that reported anxiety and anxiety-related neural activity were both greatest when choosing between the highest and lowest value choice sets (i.e., a U-shaped effect), independently of how difficult it was to select between those items. Building on this, we provide behavioral and neural evidence that a person’s provoked anxiety can be best explained by considering how much better or worse a choice set is than average, or by how much the choice options are wanted or unwanted, relative to the other sets they’ve encountered. These findings are consistent with the possibility that choosing between the least rewarding items can feel like choosing between the worst ones.
Our final study also provides a unique opportunity to extend our understanding of the network of brain regions associated with choice anxiety. This circuitry, including AI, dACC and DMS, corresponds closely to a network of regions that are functionally connected at rest and referred to as the salience (Seeley et al., 2007; Uddin, 2015) or cingulo-opercular (Power et al., 2011) network. In addition to their association with anxiety and other forms of affectively negative (Shackman et al., 2011) and arousing states (Touroutoglou, Hollenbeck, Dickerson, & Barrett, 2012), these areas have been previously associated with salient events or outcomes (e.g., those that are novel, surprising, or associated with high amounts of reward or loss; Bartra, McGuire, & Kable, 2013; Lindquist, Satpute, Wager, Weber, & Barrett, 2016; Uddin, 2015) and other signals that affect future adjustments of behavior or cognitive control (Shenhav, Cohen, et al., 2016; Ullsperger et al., 2014), including estimates of decision conflict (Kitayama, Chua, Tompson, & Han, 2013; Pochon, Riis, Sanfey, Nystrom, & Cohen, 2008; Shenhav & Buckner, 2014; Shenhav, Straccia, Botvinick, & Cohen, 2016). In addition to the primary goal of clarifying the conditions under which anxiety-related neural signals were observed, our study design allowed us to show that activity in this network does not simply reflect the bottom-up properties of the stimuli being presented. Instead, we find that the neural correlates of anxiety reflect an interaction between the contents of those stimulus sets and the specific task of choosing.
Study 1
In order to test whether low-value choice sets generate more or less anxiety than moderate-value choice sets, Study 1 used a procedure similar to Shenhav and Buckner’s (2014) studies. Participants made a series of choices between sets of four items that varied in subjective value, and subsequently rated the anxiety they had experienced while making those choices (Figure 1). Importantly, unlike the previous studies, Study 1 included choices between items with the lowest subjective value, and an item rating procedure that increased the granularity of ratings at this end of the scale. This allowed us to test whether anxiety continues to decrease linearly with these low values or whether it instead increases (demonstrating a U-shaped relationship with set value).
Figure 1.
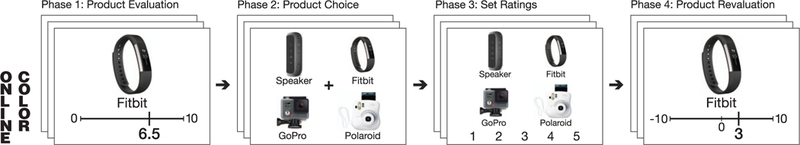
Task timeline. Participants rated products individually, then chose between sets of four products. After making these choices, participants viewed the choice sets again and (in separate blocks) rated how much they liked the set overall, how anxious they were about the choice, and how confident they were in their earlier choice. Studies 1–2 included a fourth phase, in which participants reevaluated each item using either a bipolar scale (Study 1) or a willingness-to-pay procedure (Study 2). For Study 4, Phase 2 was performed while undergoing fMRI, and interleaved with set liking trials. Product images were always labeled with 2– 4 word descriptions to clarify the product’s identity, and all phases were free response. See the online article for the color version of this figure.
Method
Participants.
Our sample was based on effect sizes observed in previous studies using a similar design (Shenhav & Buckner, 2014; see online supplemental Table S1), which suggest that an approximate sample size of N = 8 was sufficient to observe a within-subject linear effect of set value on anxiety with 90% power (α = .05). We recruited 25 individuals from Brown University and the general community. Of these, three individuals were excluded for generating item ratings with insufficient variance,1 leaving 22 participants (68.2% female, Mage = 18.7, SDage = 0.9) for our analysis. Participants in Studies 1–3 were required to be between the ages of 18 and 45 and have normal or corrected-to-normal vision, and provided informed consent in accordance with the policies of the Brown University Institutional Review Board.
Procedure.
At the start of the experiment, participants completed some or all of the following personality inventories: the Behavioral Inhibition/Activation Scales (BIS/BAS), Neuroticism subscale of the NEO Five Factor Inventory, Intolerance for Uncertainty Scale, and Need for Cognition. These are not analyzed further. The main experiment was performed in four phases occurring sequentially within the same experimental session (see Figure 1). In Phase 1, participants evaluated how much they would hypothetically like to have each of a series of individual products. They used a mouse to evaluate each product on a computerized visual analog liking scale, anchored at 0 (not at all) and 10 (a great deal) and were encouraged to distribute their ratings across the full scale. All participants evaluated the same set of 317 products. In addition, a small number of additional products were included in each participant’s product set that were more targeted by gender (males = 21 products; females = 45 products). These were items that we had determined would be significantly more familiar and/or preferred by one gender (e.g., for females this includes jewelry or a curling iron; for males, an electric shaver or aftershave lotion). Thus, participants evaluated 338–362 products in total (see https://osf.io/wna4d/). Each product rating was free response, with no time constraints, and there were no delays between item ratings.
In Phase 2, participants made hypothetical choices between sets of four products. For each choice set, they used a mouse to click on the product they preferred most. Choice sets were individually tailored based on each participant’s own Phase 1 ratings by rank ordering them and then splitting the distribution into tertiles (Low, Mid, High). Similar-value product sets were generated by selecting 60 nonoverlapping sequences of four consecutively rank-ordered products (20 from each tertile). Mixed-value sets were generated by randomly sampling four products from across the entire value distribution (without replacement). Sets were constructed such that each product would appear exactly twice (once in a similar-value set, once in a mixed-value set), but each choice set was only seen once during the choice task. Participants made a total of 120 choices. Each choice was free response and was followed by a fixed 1.5-s intertrial interval (ITI), during which participants viewed a fixation cross.
In Phase 3, participants viewed each choice set again and rated each of the following in separate blocks: (a) how stressed/anxious they had felt when facing each set, (b) how confident they were with the choice they had made, and (c) how much they liked the set as a whole. The blocks were always presented in this same order, and the choice sets were always presented in the same order they were presented during Phase 1. Each of these experiences was rated on a 5-point scale using a key press. Each rating was free response and was followed by a fixed 0.5-s ITI. Phase 4 consisted of a final individual item evaluation, similar to Phase 1, but with different instructions. Participants used a mouse to rerate each product from Phase 1 on a bipolar scale, anchored at −10 (dislike a great deal) and 10 (like a great deal). Phase 4 item evaluations followed the same timing as Phase 1. Each complete experimental session typically lasted around 90 min.
Whether appearing alone (Phases 1 and 4) or as part of a set (Phases 2–3), the individual products were always labeled with a brief (1–4 word) name or description to clarify the product’s identity. In addition, an independent control experiment confirmed that these products were highly identifiable, and showed that their identifiability did not interact with our results of interest (online supplemental Results).
Analysis.
Data were analyzed using trial-wise linear mixed-effects regressions (Matlab’s fitlme function), fitting fixed and random (subject-specific) slopes for each variable, as well as random intercepts for each participant. All models examining quadratic effects of a given variable (e.g., set value) also covaried linear effects of the same variable. We estimated degrees of freedom for our fixed effects using Satterthwaite approximation. Decision time was log transformed to reduce skew, and all variables were normalized (z-scored) prior to analysis.
Visualization.
Figures displaying projected values (Ŷ) and standard errors of a given dependent variable were generated using Matlab’s predict function, based on the relevant mixed-effects regressions. For ease of interpretation, these figures (and their associated regressions) use nonstandardized versions of each variable (e.g., anxiety, average value), unless otherwise indicated.
Results
We first replicated the finding from earlier research—shown in Figure 2 (left)—that anxiety increases linearly for moderate- to high-value choice sets (see also online supplemental Table S1). Specifically, using a trial-wise mixed effects regression, we tested whether anxiety correlated with set value (i.e., the average value of the items in a choice set), focusing only on the trials in which set value was greater than the average set observed over the course of a given participant’s session. Put another way, this analysis excluded the “lowest-value” sets. As expected, for choices in this upper range, we find a positive linear relationship between set value and anxiety (β = 0.42, t(17.9) = 4.82, p < .001). As set value increased, choice anxiety increased. However, when we perform this same regression across all choice sets, we no longer observe this linear effect of set value (β = 0.02, t(20.9) = 0.22, p > .250). Instead, a significant relationship reemerges only when adding a quadratic term to this model of our complete dataset. This analysis shows a significant quadratic (U-shaped) effect of set value on anxiety (Figure 2, right; β = 0.22, t(20.9) = 4.99, p < .001) such that anxiety was greatest for the highest and lowest set values. When accounting for this quadratic effect, there still was no linear effect of set value on anxiety (t < 1.0, p > .250; online supplemental Table S1).
Figure 2.
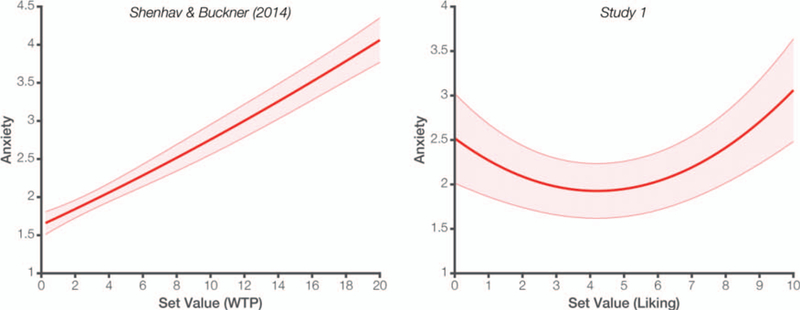
Left: In Shenhav and Buckner (2014), item values were determined by a WTP procedure and $0.00 items were excluded. Anxiety was primarily a linear function of average set value (online supplemental Table S1). Right: In Study 1, item values were determined by liking ratings and no items were excluded (see also Figure 3). Anxiety instead only exhibited a quadratic relationship with set value. Unless otherwise noted, plots reflect parameter fits based on trial-wise mixed-effects regressions. Shaded error bars reflect standard error of the mean (SEM). See the online article for the color version of this figure.
We hypothesized that this surprising pattern reflected our participants’ experience of low-valued products as aversive rather than simply unwanted. If this were the case, such patterns of choice anxiety might be better explained as a response to the overall motivational salience of the choice sets—that is, the degree to which the competing options are approach-worthy or avoidance-worthy—than as a response to set value per se. In other words, participants may have felt like they were choosing the best of four good options when it came to highest-valued sets, but the least of four “evils” when it came to lowest-valued sets.
We tested this motivational-salience hypothesis in a number of ways. First, for each participant we calculated a grand mean, or average value they assigned all of the products in Phase 1, as a way of estimating their possible “true zero value” or neutral value midpoint. As a proxy for motivational salience, we then calculated the absolute difference between that imputed neutral midpoint and the average value of the products in each choice set, such that high salience values reflected sets with much higher value or much lower value than that participant’s grand mean product rating. We then regressed trial-wise anxiety ratings on this measure of set salience, along with set value (the mean value of the four items in the set) and the spread of individual item values (e.g., max value– min value; a measure of selection difficulty). As shown in Column 1 of Table 1, the motivational salience of a set was a significant predictor of anxiety (p < .001), consistent with our hypotheses (online supplemental Figure S1). Value spread was also correlated with anxiety (negatively), such that more similarly valued choice sets caused greater anxiety (p = .003). Set value, however, was no longer a significant predictor of anxiety using this complete range of choice set values (p > .250). As further evidence that this quadratic effect is not accounted for by the difficulty of selecting between similarly valued options, we also found that this effect is present in models that separately focus on choice sets with mixed item values (β = 0.17, t(17.1) = 2.65, p = .017) and those in which all items were nearly equal in value (β = 0.17, t(20.7) = 3.38, p = .003).
Table 1.
Linear Mixed-Effects Regression Estimates Predicting Anxiety Ratings Within Each Study
| Regressor | Study 1 | Study 2 | Study 3 | Study 4 |
|---|---|---|---|---|
| Set salience | β = .16 | β = .16 | β = .11 | β = .15 |
| t = 4.06 | t = 4.22 | t = 2.93 | t = 4.83 | |
| p < .001 | p <.001 | p =.007 | p < .001 | |
| Set value | β = .02 | β = .05 | β = −.01 | β = .10 |
| t = .18 | t = .73 | t = −.08 | t = 1.25 | |
| p > .250 | p > .250 | p > .250 | p = .222 | |
| Value spread (max–min) |
β = −.09 | β = −.05 | β = −.08 | β = −.04 |
| t = −3.42 | t = −2.42 | t = −2.28 | t = −1.61 | |
| p = .003 | p = .021 | p = .031 | p = .120 | |
| R2 | .4463 | .3845 | .449 | .4520 |
| N | 2637 | 2280 | 3120 | 3581 |
Note. Set value refers to the average of Phase 1 ratings for the items in a choice set, while set salience refers to the (unsigned) difference between a given set value and a central reference point determined either by the participant-specific grand mean item value (Studies 1, 2, and 4) or the fixed midpoint on a bipolar rating scale (Study 3).
We next tested whether the costs of motivationally salient choices could be detected in the time it took participants to make a given choice. Using a similar regression model to examine response time, we found that participants took longer to decide when facing more salient choice sets (β = 0.07, t(20.8) = 2.66, p = .015), even when controlling for effects of the value spread and the average value of the set (both of which led to faster decision times; value spread: β = −0.20, t(19.9) = −8.61, p < .001, set value: β = −0.11, t(17.2) = −3.58, p = .002). Taken as a whole, these findings suggest that more salient choices were more costly both in terms of decision time and experiences of anxiety. Importantly, though, the relationship between set salience and anxiety is robust to controlling for decision time (β = 0.17, t(21.8) = 6.33, p < .001), suggesting that anxiety reflects subjective decision costs over and above deliberation time.
The set values calculated above are based on Phase 1 item ratings, in which participants rated each product on a unipolar positive scale (from 0 to 10), similar to methods used in prior research. However, our motivational salience account assumes that participants are treating the lowest value items as negative, rather than as weakly positive. To explore whether participants would explicitly label these items as negative, Phase 4 in this study had participants rerate all products using a bipolar rating scale (−10 to 10; anchored at dislike a great deal and like a great deal; average Pearson’s correlation with Phase 1 rating = 0.84). This enabled participants to rate items relative to an explicit zero (neutral) midpoint. When reanalyzing choice anxiety using these bipolar ratings, we again found that these experiences were better predicted by the salience of the set (i.e., distance from the fixed zero point on the scale; β = 0.18, t(16.0) = 5.33, p < .001) than by the set value (β = −0.03, t(19.3) = −0.34, p > .250).
A natural question is whether the U-shaped pattern we observe for anxiety is a general property of any affective reaction to choice sets. Our study design allowed us to address this question as participants also provided an appraisal of the overall attractiveness of each choice set (i.e., how much they liked the set as a whole). (Note that this is distinct from the estimate of set value used in the experimental analyses, which was derived from the average value of the items in the set rather than the participant’s overall appraisal of the set.) In stark contrast to the nonmonotonic (U-shaped) relationship we observed between set value and anxiety, the relationship between set value and attractiveness appraisals was (slightly nonlinear but) clearly monotonic (linear term: β = 0.67, t(20.5) = 12.5, p < .001, quadratic term: β = 0.08, t(18.6) = 2.93, p < .01). This dissociation between set value’s monotonic relationship with appraisal versus its nonmonotonic relationship with anxiety persisted through our remaining studies (online supplemental Figure S2; online supplemental Table S2, compare Table 1).
Discussion
Study 1 demonstrates that choice anxiety is a U-shaped function of set value, greatest for the highest and lowest value sets. This raises two further questions. First, is the difference between item rating procedures (and associated omissions of zero-valued items) sufficient to explain the discrepancy between (a) the quadratic relationship we observe between set value and anxiety and (b) previous findings of a linear relationship between these variables (compare left and right panels in Figure 2)? Second, to what extent is the significant association between anxiety and bipolar rating salience in Study 1 an artifact of participants having previously rated these products on a unipolar scale? We address these questions in Studies 2 and 3, respectively.
Study 2
Study 2 tests whether we can replicate both quadratic and linear anxiety patterns within the same participants, simply by altering how products are evaluated and incorporated into the participant’s choice set. Shenhav and Buckner’s (2014) linear effects were observed when participants performed a willingness-to-pay (WTP) procedure during Phase 1. As in other studies (e.g., Kang, Rangel, Camus, & Camerer, 2011; Krajbich, Lu, Camerer, & Rangel, 2012), this procedure resulted in a bimodal distribution of WTP ratings, with a substantial number of products being rated as having “zero value.” Those zero-value products were not used in the option sets that participants went on to choose between, preventing an examination of their influence on choice anxiety. Study 2 tests whether such exclusions, common in neuroeconomic studies, omit meaningful variance in item values that might have otherwise generated greater anxiety.
Participants in this study performed two sets of product evaluations: a “liking”-based procedure that encouraged participants to distribute their ratings across the entire scale (as in Study 1) and a standard WTP-based procedure. This enabled us to directly compare variability in set value from measures like those in Study 1 with measures like those in Shenhav and Buckner (2014). We tested whether the form of the relationship we observed between set value and anxiety would transform from quadratic to linear depending on which form of evaluation was used.
Method
Participants.
To identify the minimum sample size required for Studies 2–4, we performed a power analysis over our salience findings in Study 1 (first column of Table 1) and determined that a sample size of N = 14 was sufficient to observe a within-subject linear effect of set salience on anxiety with 90% power (α = .05). For Study 2, 22 individuals were recruited from Brown University and the general community. Of these, two others were excluded for generating item ratings with insufficient variance and one for an incomplete experimental session, leaving 19 participants (47.4% female, Mage = 19.3, SDage = 1.2) for our analysis.
Procedure.
Phases 1–3 of this study were identical to Study 1. However, the Phase 4 evaluation consisted of indicating their willingness to pay (WTP) for each item. Participants were asked to imagine that each trial was an independent event in which they had $20.00 to spend. They were asked to indicate how much of this money they would give up to obtain that trial’s product, assuming that they would keep any unspent money.2
Results
Focusing on the product liking ratings derived from Phase 1, we replicated our Study 1 finding that anxiety increases with set salience (p < .001) and not set value (p > .250; Figure 3, left; Table 1). To directly compare these data with Shenhav and Buckner (2014), we restricted our analysis to the kinds of choice sets used in that paper. This was done by excluding sets with zero-valued items, as defined by Phase 4’s WTP ratings. Consistent with past research using WTP, participants indicated $0.00 as their WTP for 29% of items. This is substantially higher than the 5% of items that received a 0 rating on the liking scale (compare histograms in Figure 3), and resulted in 53% of choice sets being excluded. As expected, regressing anxiety on set value in this zero-excluded subset yielded a linear effect of set value on anxiety for these same participants (β = 0.24; t(14.4) = 2.88, p = .012; Figure 3, right). Within this subset of choices, a linear model provided a better fit (Akaike Information Criterion [AIC] = 2,738.5) than a model that included both linear and quadratic terms (AIC = 2,744.5). These results help resolve the apparent conflict between the U-shaped relationship between anxiety and set value observed in Study 1, and the linear relationships reported in Shenhav and Buckner (2014).
Figure 3.
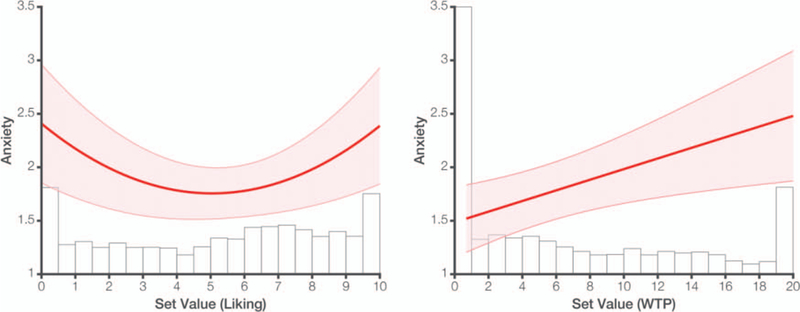
In Study 2, we again observe a quadratic relationship between anxiety and set value (left) but this becomes a positive linear relationship when we exclude choice sets that include a zero-valued bid (based on a follow-up item WTP rating; right). Projected anxiety ratings are overlaid on histograms of item ratings based on the two rating procedures, demonstrating a pronounced peak at $0.00 for the WTP procedure. Shaded error bars reflect SEM. See the online article for the color version of this figure.
Discussion
Study 2 replicates the U-shaped relationship of anxiety to set value. It further demonstrates how extrapolating from a limited range of set values (e.g., moderate-to-high value sets) can lead to mischaracterizations of this relationship. Study 3 builds on secondary evaluations in Study 1 to test more directly whether anxiety for lowest-value choices results from participants treating those items as aversive rather than simply unrewarding.
Study 3
To test whether anxiety toward low-valued choice sets is related to a desire to avoid those items, Study 3 uses a bipolar rating scale for the initial item evaluation (Phase 1) and subsequent choice set construction. These ratings provide an objective midpoint (neutral rating) for measuring set salience and its relationship with choice anxiety.
Method
Participants.
27 individuals were recruited from Brown University and the general community. One individual was excluded due to a technical issue that prevented data collection, leaving 26 participants (88.5% female, Mage = 18.5, SDage = 0.9) in our analysis.
Procedure.
Study 3’s procedure was identical to Phases 1–3 of Study 1 with one exception. In Phase 1 participants rated each product on a bipolar scale anchored at −10 (dislike a great deal) and 10 (like a great deal), rather than Study 1’s unipolar (0 to 10) scale. The choice sets used in the rest of the experiment were constructed based on these Phase 1 values. Phase 4 was omitted for this study.
Results
As in Study 1, we found that anxiety is best described as a quadratic function of set value, as defined by our bipolar rating scale (β = 0.13, t(24.3) = 4.19, p < .001; Figure 4, left). However, unlike Phase 1 ratings in Studies 1–2, the midpoint of this quadratic function had a fixed definition across subjects, corresponding to zero motivational salience (i.e., neither positive nor negative). We therefore calculated a motivational salience measure for each choice set based on this fixed zero point rather than the participant-specific grand mean (used in Studies 1–2). Based on this measure, we again find that anxiety linearly increased with the salience (p = .007) but not the average value (p > .250) of the choice set (Figure 4, right; Table 1).
Figure 4.
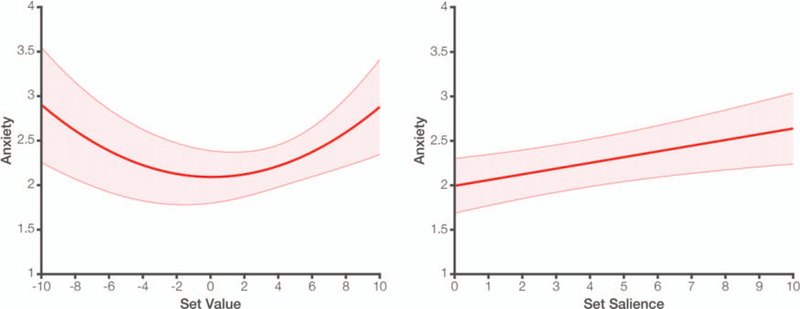
Left: In Study 3, we again observe a quadratic relationship between set value and anxiety, but in this case a bipolar rating scale was used (−10 to 10) and we see that anxiety is minimal around the zero point. Right: Anxiety increases linearly with the distance between a given set value from the fixed zero midpoint on the rating scale. Shaded error bars reflect SEM. See the online article for the color version of this figure.
Discussion
Study 3 suggests that the U-shaped anxiety pattern may be explained in terms of the strength of approach–approach or avoid– avoid conflict participants’ experience (i.e., set salience). However, Studies 1–3 rely primarily on self-report, and so do not address whether the anxiety over low-value sets and high-value sets is driven by the same underlying mechanisms. They are also unable to address whether anxiety is elicited by salient choice sets per se, or by the need to choose between salient options. To explore this more directly, Study 4 tested whether anxiety-related brain activity correlates with choice set salience specifically when a choice is required
Study 4
Study 4 tested whether the relationships observed in affective ratings would be mirrored in neural responses within an a priori defined set of anxiety-related brain regions. In particular, Shenhav and Buckner (2014) used a similar choice paradigm to identify neural correlates of choice anxiety, within a network of regions that included dACC, AI, and DMS. These regions are functionally connected at rest, forming a network often referred to as the cingulo-opercular or salience network (Power et al., 2011; Seeley et al., 2007; Uddin, 2015). Meta-analyses of the literature further show that these regions demonstrate increased activity in the presence of increasingly salient outcomes (i.e., those that are increasingly appetitive or aversive; Bartra et al., 2013; Kahnt, Park, Haynes, & Tobler, 2014; Lindquist et al., 2016; Liu, Hairston, Schrier, & Fan, 2011), particularly when those outcomes bear on one’s future actions or control states (Shackman et al., 2011; Shenhav, Cohen et al., 2016).
Examining this network allows us to extend our understanding of choice anxiety findings in multiple ways. In Studies 1–3, we demonstrate a positive relationship between anxiety and set salience. Thus first and foremost, we can test whether activity in this network continues to track choice anxiety when such anxiety is determined by set salience rather than simply set value. If this network tracks set salience then we should find that its activity increases with anxiety and set salience and is otherwise not associated with set value. We are able to test a further prediction, that these anxiety-related neural responses specifically reflect anxiety arising from the need to choose between salient options rather than a more reflexive anxiety reaction that occurs whenever an individual simply evaluates such options. To do so, we examined whether neural signals related to anxiety and salience appeared specifically when participants were instructed to choose between the options or whether they also appeared when evaluating equally salient items for a nonchoice task (i.e., appraisal of the overall value of the choice set).
Method
Participants.
We performed a power analysis over anxiety-related fMRI findings reported in Shenhav and Buckner (2014)3 and determined that a sample size of N = 28 was sufficient to observe blood oxygen level dependent (BOLD) correlates of choice anxiety within our regions of interest, with 90% power (α = .05). Thirty-one individuals from Harvard University and the general community completed this study.4 Of these, one was excluded for excessive head motion (between-volume rotational movement two orders of magnitude greater than the group SD), leaving 30 participants (56.7% female, Mage = 24.9, SDage = 4.5) in the final analysis. Participants in this study were all required to be between the ages of 18 and 35, right-handed, fluent English speakers, have normal or corrected-to-normal vision, and not indicate any standard MRI safety-related contraindications (e.g., pregnancy, metal in the body). All participants provided informed consent in accordance with the policies of the Harvard University Institutional Review Board.
Procedure.
Phase 1 was conducted similarly to Studies 1–2, using a product set that was tailored toward this community (males = 310 products; females = 328 products; 288 products overlapping). Phase 2 was performed while undergoing fMRI (see details below). On each trial, participants viewed choice sets that were constructed as in Studies 1–3 (Low, Mid, High; Mixed) and were prompted to perform one of two tasks: (a) select the product they most prefer (Choose trials) or (b) evaluate how much they like the set as a whole, on a 4-point scale (Like trials).5 To ensure that all products were identified before the explicit evaluation period, the set appeared for 3 s at the start of each trial, after which a LIKE or CHOOSE cue appeared to indicate the task on the current trial. To separate evaluation and response selection periods, after the cue appeared participants were instructed to press a key only to indicate once they had completed their evaluation and made their choice (without a deadline). After pressing a key to end this Evaluation period, participants viewed a fixation cross for a variable ITI (2–7 s) and then the four options reappeared in a random horizontal arrangement, in parallel with either the numbers 1– 4 (on Like trials) or pound (#) symbols (on Choose trials). Participants used the keypad to move a cursor left (right index finger) or right (right ring finger) before submitting their response (right middle finger). Participants were required to provide an answer in the Selection period within 5 s, encouraging them to determine their response prior to this period (during the Evaluation period).
While in the scanner, participants viewed each choice set once and performed the Choose task for half of the trials and the Like task for the other half (with the distribution of choice set values matched across the two tasks). This was counterbalanced outside of the scanner; participants once again viewed all of the choice sets, and performed the opposite task for each set (i.e., Like ratings for sets that were previously in the Choose condition, and vice versa). This procedure ensured that each participant made an appraisal and choice for every set. Participants then completed Phase 3, involving anxiety and confidence ratings as in the previous behavioral studies. This study omitted Phase 4.
Imaging parameters.
Scans were acquired on a Siemens Trio 3T scanner with a 12-channel phase-arrayed head coil, using the following gradient-echo planar imaging (EPI) sequence parameters: repetition time (TR) = 2,500 ms; echo time (TE) = 30 ms; flip angle (FA) = 90°; 2.5-mm voxels; 0.50-mm gap between slices; field of view (FOV): 210 × 210; interleaved acquisition; 37 slices. To reduce signal dropout in regions of interest, we used a rotated slice prescription (30° relative to AC/PC) and modified z-shim prepulse sequence. The slice prescription encompassed all ventral cortical structures but limited regions of dorsal posterior parietal cortex. Structural data were collected with T1-weighted multiecho magnetization prepared rapid acquisition gradient echo image (MEMPRAGE) sequences using the following parameters: TR = 2,200 ms; TE = 1.54 ms; FA = 7°; 1.2-mm isotropic voxels; FOV = 192 × 192. Head motion was restricted with a pillow and padded head clamps. Stimuli were generated using Matlab’s Psychophysics Toolbox and were viewed through a mirror mounted on the head coil. Participants used their right hand to respond with an MR-safe response keypad.
fMRI analysis.
fMRI data were analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK). Preprocessing consisted of realigning volumes within participant, resampling to 2-mm isotropic voxels, nonlinear transformation to align with a canonical T2-weighted template, and spatial smoothing with a 6-mm full-width at half-max (FWHM) Gaussian kernel.
Preprocessed data were submitted to a linear mixed-effects analyses, using a two-step procedure. First, we generated BOLD signal change estimates for each trial using a first-level general linear models (GLM) in SPM. This GLM separately modeled stick functions onsetting at the Evaluation and Selection period of each trial. We compared two versions of this GLM: one version that locked the onset of the Evaluation period around the appearance of the task prompt, and another version that locked the Evaluation onset to the time when the participant indicated that they completed their evaluation. Trials were concatenated across the two task blocks, and additional regressors were included to model the mean of each block and within-block linear trends. When relevant, we included additional event regressors to model the Evaluation and Selection periods for missed trials (trials on which the participant failed to respond within the 5-s Selection deadline). These occurred rarely (0.4% of all trials) and were nonexistent for a majority (24/30) of our participants. No additional nuisance regressors were included in the GLM. Finally, the GLM was estimated using a reweighted least squares approach (RobustWLS Toolbox; Diedrichsen & Shadmehr, 2005) in order to minimize the influence of outlier time points (e.g., due to head motion). After estimating this first-level GLM, we extracted average beta estimates for each trial from our primary region of interest (ROI; see below), transformed these beta estimates with the hyperbolic arcsine function (to achieve normality), and then analyzed trial-to-trial variability in these BOLD estimates with linear mixed-effects regressions (using Matlab’s lmefit function).
We drew our a priori ROI for regions associated with choice anxiety from the results of two fMRI experiments reported in Shenhav and Buckner (2014). Specifically, we generated a binary mask that reflected the conjunction of the regions that reliably tracked choice anxiety in those two studies (with both input maps individually thresholded at a voxel-wise p < .001 and cluster threshold of 50 voxels). The resulting whole-brain mask consisted of regions of dACC (468 voxels), AI (201 voxels, combining 101 in L-AI and 100 in R-AI), and right dorsomedial striatum (R-DMS; 48 voxels). While this conjunction was generated to perform inference regarding an independent dataset and therefore doesn’t meet standard criteria for whole-brain multiple-comparisons correction, we note for reference that the conjunction map we generated has an effective voxel-wise p < .000015 (t > 5.12), calculated with Fisher’s (1925) test for combining multiple hypotheses (Zaki, Davis, & Ochsner, 2012). At this height threshold, all of the clusters exceed the extent threshold required for cluster-level correction (k ≥ 15 voxels), calculated with John Ashburner’s Corr-ClusttTh script (http://www.sph.umich.edu/~nichols/JohnsGems2.html).
Results
Study 4 again replicates the positive correlation between anxiety ratings and set salience (see Table 1). We next tested for neural correlates of these anxiety ratings in an a priori network previously implicated in choice anxiety (comprising regions of dACC, AI, and R-DMS; Figure 5 inset). Activity in these regions during the choice period was positively correlated with anxiety. This correlation held irrespective of whether we analyzed activity in this ROI early in the trial (β = 0.08, t(875.3) = 3.04, p = .002) or closer to when the participant responded (β = 0.14, t(137.9) = 5.09, p < .001). Since the latter effect was substantially stronger, we locked our remaining analyses to the time of response.6 The relationship between neural activity and anxiety during this period was robust to controlling for reaction time (RT; β = 0.12, t(109.8) = 4.43, p < .001). Critically, consistent with the patterns we observe underlying anxiety reports, we find that activity in anxiety-related regions was significantly correlated with the salience of the choice set (β = 0.07, t(1542.2) = 2.81, p = .005) but not with the average value of that set (β = 0.01, t(761.9) = 0.34, p > .250).
Figure 5.
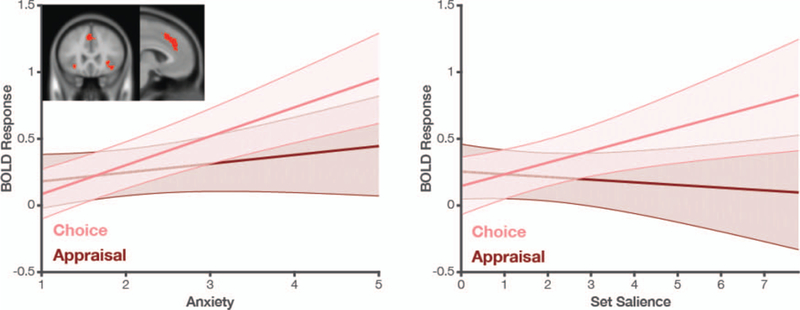
BOLD activity in anxiety-related regions correlates with anxiety (left) and set salience (right) when participants are making a choice (pink) but not when they are just “browsing” the set (i.e., appraising the overall value of the set; burgundy). The a priori mask (defined by previous anxiety-related activations) is shown in the left inset. Shaded error bars reflect SEM.
An important aspect of the study design allowed us to compare neural data from participants both when they were making choices (when we expect them to experience choice anxiety) and when they were merely appraising the value of sets (when we do not expect them to experience such anxiety). Unlike the correlation observed during choice, during appraisal activity in our ROI was not significantly correlated with anxiety (β = 0.03, t(26.5) = 0.93, p > .250) nor set salience (β = −0.01, t(659.2) = −0.60 p > .250). Consequently, we found that activity in these regions exhibited a significant interaction between task type (choice vs. appraisal) and anxiety (β = 0.09, t(29.2) = 2.05, p = .049). We observed a similar interaction between task type and set salience (β = 0.08, t(85.5) = 2.36, p = .021; see Figure 5). Thus these neural responses arise in situations involving a choice between salient items, but not when those same items are evaluated for a nonchoice task (e.g., set appraisal).
For completeness, we performed a set of analyses to examine whether the patterns of neural activity we observed for our anxiety network differed across its constituent regions (dACC, AI, and R-DMS). We did not find that region modulated the interactions between task and anxiety (three-way interaction F(2, 112.0) = 2.05, p = .133) or the interaction between task and salience, (F(2, 190.4) = 1.50, p = .227). Thus, these more spatially specific analyses find that the patterns of anxiety and salience described at the network level do not vary substantially by region. With that said, a more constrained analysis using only the Choice trials, did reveal a Region × Anxiety interaction, F(2, 216.3) = 4.18, p = .0166. This interaction was driven by stronger anxiety-related signals in dACC and AI (βs = 0.11, ps < 0.001) than in DMS (β = 0.04, p = .141; online supplemental Table S3). Of note, we still do not find a significant region x salience interaction during these same (Choice) trials, F(2, 159.0) = 1.32, p > .250.
Confirmation of Anxiety’s U-Shaped Relationship and Reference Dependence
Across Studies 1– 4 we have shown that there is a significant quadratic relationship between set value and anxiety. However, in order to show that this relationship reflects a “U” shape it is important to also demonstrate that anxiety ratings exhibit a significant negative slope at the lower end of set value and a significant positive slope at the higher end, within the range of values tested in our study (Simonsohn, 2017). We therefore separately tested the two ends of our quadratic function to confirm that it meets these criteria. Collapsing across Studies 1, 2, and 4, which used the same Phase 1 rating scales,7 we find that anxiety significantly decreases with set value for lower-valued choice sets (set values below a given participant’s grand mean set value; β = −0.26, t(62.5) = −4.49, p < .001). We also find that anxiety significantly increases with set value with higher-valued choice sets (β = 0.35, t(66.2) = 6.85, p < .001; Figure 6, left).
Figure 6.
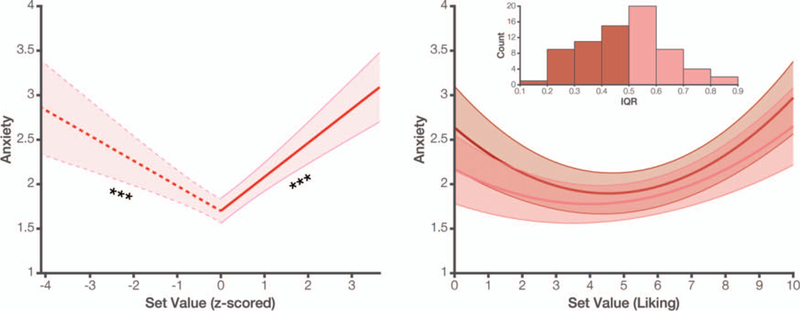
Left: Collapsing across Studies 1, 2, and 4, we find a negative association between set value and anxiety for lower-value choice sets (those below a participant’s mean set value; dashed line) and a positive association for higher-value choice sets (solid line), confirming that our data exhibit a true U-shaped effect. Right: Across these same studies, we find that the quadratic relationship between set value and anxiety is invariant to within-participant variability in item ratings. This quadratic curve did not differ significantly between participants who used a wider range of the Phase 1 rating scale (light red) and those who used a more limited range (dark red), suggesting that choice anxiety reflected an adaptation to the range of items observed by a given participant. Inset: distribution of interquartile ranges (IQRs) for Phase 1 ratings across subjects. For display purposes, we divide these based on a median split of IQR values, but analyses in the main text analyze this as a continuous variable. Shaded error bars reflect SEM. ***p < .001. See the online article for the color version of this figure.
We have suggested that our participants’ negative reactions to low set values may have resulted from a form of individual reference dependence (Tversky & Kahneman, 1981), possibly in combination with range adaptation (cf. Khaw et al., 2017; Louie et al., 2013) whereby they come to view their own lowest-valued items as aversive. An alternative hypothesis is that, being all based on the same scale, value ratings are treated in a fixed manner, such that an option rated 3 by Participant A would evoke the same response from A as an option rated 3 by Participant B would evoke from B, regardless of the larger distribution pattern of A and B’s ratings. In other words, whereas a fixed account assumes that anxiety is a U-shaped function of the absolute ratings given for that set, a reference-dependent account assumes that this U-shape shifts and scales with the range of ratings the participant gave in Phase 1. We tested two key predictions that help adjudicate between these two accounts.
First, a reference-dependent account predicts that our measure of set salience, which adjusts to each participant’s own mean Phase 1 rating, should outperform a measure that assumes a fixed mid-point (the center of the rating scale). Collapsing across the studies that used the same methods for ratings (Studies 1, 2, and 4), we find that a fixed-midpoint salience measure has a weaker correlation with anxiety than our participant-specific (dynamic-midpoint) salience measure (fixed: β = 0.13, t(68.4) = 4.44, dynamic: β = 0.18, t(67.8) = 10.20, ps < 0.001). When both salience measures are entered into the same regression, we find that only the dynamic measure continues to predict anxiety (β = 0.23, t(34.1) = 2.22, ps = 0.034), whereas the fixed measure does not (β = −0.05, t(35.8) = −0.47, p = .642).
Second, a fixed-reference account predicts that the shape of the quadratic function should vary with the distribution of Phase 1 ratings (e.g., whether they spanned only low to moderate vs. low to high [absolute] ratings). In contrast, a reference-dependent account predicts that the quadratic function should be relatively stable across different rating distributions. Consistent with the reference-dependent prediction, we found that the quadratic relationship between set value and anxiety did not vary with the variability in a given participant’s Phase 1 ratings, whether measured as the standard deviation (β = −0.07, t(65.8) = −1.03, p = .309) or the interquartile range (β = −0.06, t(65.3) = −0.96, p = .342) of that distribution (Figure 6, right).
General Discussion
When making choices, people commonly find that the more valuable their options are, the more difficult and anxiety-provoking the decision becomes (Chernev & Hamilton, 2009; Shenhav & Buckner, 2014). Across four studies we demonstrate that this anxiety can also arise from choices between items that have little to no value for the decision maker. We provide behavioral and neural evidence that anxiety is a U-shaped function of choice set value; holding constant the similarity of item values within a set, people are most anxious for the sets with the highest and lowest overall value. Our neuroimaging results further demonstrate that this anxiety arises from an interaction between the choice set’s value and the specific task of comparing the options to select just one of them.
The U-shaped relationship between anxiety and set value may be explained by participants treating low-value sets as items that were to be avoided to a similar degree as the high-value sets were to be approached. In other words, low-value sets took on a negative but high motivational salience, generating choice conflict for the same reason that avoid–avoid conflicts between several aversive outcomes (e.g., electric shocks) would. Interestingly, though, none of the individual low-value items were intrinsically aversive, nor did they engender anxiety when participants encountered them in an appraisal task (Study 4). Rather, these sets accrued their negative salience in the context of the observed range of items. This hypothesis is supported by Study 3, in which anxiety increased with more negative ratings along a bipolar scale.
An alternative, unipolar account of these findings might suggest that low-value choice sets did not acquire negative salience but instead were merely insufficient to trigger or activate any approach-related responses. Under this account, anxiety for low-value sets is a reflection of this state of insufficient motivation (and perhaps surrounding frustrations) whereas anxiety for high-value sets may reflect a state of “over-activation” and the associated increase in conflict. This U-shaped function could therefore be related to the nonlinear functions previously reported in research on arousal and performance (e.g., Nakamura & Csikszentmihalyi, 2002; Yerkes & Dodson, 1908) rather than to low-value sets obtaining negative value. While this account is difficult to rule out with the current data, it is noteworthy that our fMRI study showed that the same regions track anxiety for both low and high value choice sets, arguing for common rather than distinct sources of anxiety experienced at the two ends of the U.
Under the assumption that participants track the difference between the overall value of the current choice set and the overall value of the average choice set, our findings also resonate with observations of unsigned prediction error (i.e., surprise) signals in our anxiety-related regions (particularly dACC and AI; Alexander & Brown, 2011; Cavanagh & Frank, 2014; Shenhav, Cohen et al., 2016; Ullsperger et al., 2014). This raises the possibility that anxiety more generally reflected surprise toward the value of the choice set. However, the data do not straightforwardly fit this interpretation. First, low-value choice sets in Shenhav and Buckner’s (2014) studies would be equally surprising under these criteria, but neural and affective responses did not engender the U-shaped pattern observed in our data. Second, and perhaps more importantly, Study 4 qualifies surprise-related interpretations by demonstrating that dACC and AI respond to these quantities in a task-specific manner. In other words, to the extent that these regions reflect the salience of the choice set, they do not do so in a “bottom-up” manner but rather only when a choice is required. This is particularly notable given that participants were paying much closer attention to overall set value when performing the alternate task in this study (set appraisal), whereas the choice task primarily drew their attention to the differences in value between the items.
Similarly, previous findings have associated the dACC and AI with salience rather than value (Bartra et al., 2013; Blair et al., 2006; Kahnt et al., 2014; Lindquist et al., 2016; Litt, Plassmann, Shiv, & Rangel, 2011), demonstrating increases in activity for both highly rewarding and highly aversive reinforcers. Our findings are consistent with these findings but also serve to qualify them in interesting ways. In addition to demonstrating the task-specificity and affective relevance of putative salience-related neural signals in our study, we also show that signals of negative salience in these regions—previously observed with aversive stimuli such as electric shock and monetary loss— can be found for nominally rewarding items that are simply considered to be low in value. With that said, we also note that on their own our findings cannot further adjudicate between proposed functions for this network or its component regions (Barrett & Simmons, 2015; Rushworth et al., 2011; Shenhav, Cohen et al., 2016; Uddin, 2015; Ullsperger et al., 2014). In particular, we cannot distinguish the degree to which the signals we observed reflect monitoring of internal states, selection of actions or control states, and/or regulation of affective experiences. Future research should seek to disentangle these interpretations using appropriate causal manipulations.
Finally, this research underscores critical and often overlooked methodological considerations when estimating an individual’s subjective values. First, our findings highlight a potential limitation of common auction-like or WTP value ratings that can engender irregular distributions of subjective values, with many items valued at zero dollars (see Figure 3; see also, e.g., Kang et al., 2011; Krajbich et al., 2012). In these cases, the interpretation or omission of zero-value items in subsequent decision tasks can be highly consequential because the zero point can be truncating or masking a range of low values. Second, our findings provide initial evidence that the overall range of items observed during the item valuation phases common in decision-making research influence the reference point by which participants evaluate later choice sets (cf. Khaw et al., 2017). This can, in turn, produce linear or quadratic patterns of anxiety and associated neural activity depending on the subset of the value range incorporated into the choice sets.
Conclusion
It is well known that anxiety increases with the importance of potential choice outcomes, whether one has to choose between the best options (e.g., dream jobs) or the worst ones (e.g., life-threatening medical procedures). We have shown that a comparable U-shaped effect can be observed with everyday decisions when they include choices between nominal gains that are simply undesirable. These findings explain why mundane decisions can be aversive, and suggest that we can be better off choosing between items we value moderately than those we have little interest in.
Supplementary Material
Acknowledgments
This work was previously presented at the 2017 Society for Affective Science and Society for Neuroeconomics Conferences, and made available as a preprint on PsyArxiv. This work was funded by a Center of Biomedical Research Excellence grant P20GM103645 from the National Institute of General Medical Sciences to Amitai Shenhav. The authors are grateful to Wasita Mahaphanit and Anna Xu for assistance in stimulus development; Michelle Basta, Elizabeth Beam, Marina Burke, Ayenna Cagaanan, Erin Guty, Tatiana Lau, Emily Levin, Wasita Mahaphanit, and Erik Nook for assistance in data collection; and Randy Buckner and Ian Krajbich for helpful discussions.
Footnotes
Our automated choice construction process relied on splitting the items into ranked tertiles with nonoverlapping value ranges, before distributing them into our designated choice types. Some participants generated rating distributions with insufficient variance to construct 120 choice sets based on this procedure. In these cases, the number of choice sets was iteratively decreased to a number that could be generated for that participant, while holding constant the proportion of choices per choice type. Because they experienced a different number of trials than the remaining participants, we have excluded such participants from our analyses, but all of our findings are robust to their inclusion.
This procedure represented a hypothetical version of the incentive compatible WTP task used in Shenhav and Buckner (2014).
This power analysis focused on Study 2 from this paper because this was the replication study used to estimate and report effect sizes for each of the variables of interest (Study 1 was used as a discovery sample). The effect sizes in this study were generated based on a comparison of the peak timepoint of interest from the BOLD timecourse (using a finite impulse response model) for trials that generated high versus low anxiety.
Three additional participants were excluded prior to entering the scanner, one due to technical difficulties and the other two due to insufficient variance in their Phase 1 ratings.
Findings pertaining directly to Study 4’s appraisal/choice manipulation are reported elsewhere (Shenhav & Karmarkar, 2017).
Note that this analysis choice could not be determined a priori based on Shehav and Buckner’s (2014) studies because those used a short response deadline and therefore could not differentiate between these two trial periods.
All regressions involving more than one study include covariates to model the means and all possible interactions with study type.
References
- Alexander WH, & Brown JW (2011). Medial prefrontal cortex as an action-outcome predictor. Nature Neuroscience , 14, 1338 –1344. 10.1038/nn.2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CJ (2003). The psychology of doing nothing: Forms of decision avoidance result from reason and emotion. Psychological Bulletin , 129, 139 –167. 10.1037/0033-2909.129.1.139 [DOI] [PubMed] [Google Scholar]
- Barrett LF, & Simmons WK (2015). Interoceptive predictions in the brain. Nature Reviews Neuroscience , 16, 419 – 429. 10.1038/nrn3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, & Kable JW (2013). The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage , 76, 412– 427. 10.1016/j.neuroimage.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Marsh AA, Morton J, Vythilingam M, Jones M, Mondillo K, . . . Blair JR (2006). Choosing the lesser of two evils, the better of two goods: Specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. The Journal of Neuroscience , 26, 11379 –11386. 10.1523/JNEUROSCI.1640-6.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botti S, Orfali K, & Iyengar SS (2009). Tragic choices: Autonomy and emotional responses to medical decisions. Journal of Consumer Research , 36, 337–352. 10.1086/598969 [DOI] [Google Scholar]
- Cavanagh JF, & Frank MJ (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences , 18, 414 – 421. 10.1016/j.tics.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernev A, & Hamilton R (2009). Assortment size and option attractiveness in consumer choice among retailers. Journal of Marketing Research , 46, 410 – 420. 10.1509/jmkr.46.3.410 [DOI] [Google Scholar]
- Coricelli G, Critchley HD, Joffily M, O’Doherty JP, Sirigu A, & Dolan RJ (2005). Regret and its avoidance: A neuroimaging study of choice behavior. Nature Neuroscience , 8, 1255–1262. 10.1038/nn1514 [DOI] [PubMed] [Google Scholar]
- Coricelli G, & Rustichini A (2010). Counterfactual thinking and emotions: Regret and envy learning. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences , 365, 241–247. 10.1098/rstb.2009.0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, & Shadmehr R (2005). Detecting and adjusting for artifacts in fMRI time series data. NeuroImage , 27, 624 – 634. 10.1016/j.neuroimage.2005.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA (1925). Statistical methods for research workers Edinburgh, Scotland: Oliver & Boyd. [Google Scholar]
- Gray JA, & McNaughton N (2000). The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system Oxford, England: Oxford University Press. [Google Scholar]
- Iyengar SS, & Lepper MR (2000). When choice is demotivating: Can one desire too much of a good thing? Journal of Personality and Social Psychology , 79, 995–1006. 10.1037/0022-3514.79.6.995 [DOI] [PubMed] [Google Scholar]
- Kahnt T, Park SQ, Haynes J-D, & Tobler PN (2014). Disentangling neural representations of value and salience in the human brain. Proceedings of the National Academy of Sciences of the United States of America , 111, 5000 –5005. 10.1073/pnas.1320189111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MJ, Rangel A, Camus M, & Camerer CF (2011). Hypothetical and real choice differentially activate common valuation areas. The Journal of Neuroscience , 31, 461– 468. 10.1523/JNEUROSCI.1583-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw MW, Glimcher PW, & Louie K (2017). Normalized value coding explains dynamic adaptation in the human valuation process. Proceedings of the National Academy of Sciences of the United States of America , 114, 12696 –12701. 10.1073/pnas.1715293114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S, Chua HF, Tompson S, & Han S (2013). Neural mechanisms of dissonance: An fMRI investigation of choice justification. NeuroImage , 69, 206 –212. 10.1016/j.neuroimage.2012.11.034 [DOI] [PubMed] [Google Scholar]
- Krajbich I, Lu D, Camerer C, & Rangel A (2012). The attentional drift-diffusion model extends to simple purchasing decisions. Frontiers in Psychology , 3, 193 10.3389/fpsyg.2012.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin K (1935). A dynamic theory of personality New York, NY: McGraw-Hill. [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, & Barrett LF (2016). The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex , 26, 1910 –1922. 10.1093/cercor/bhv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt A, Plassmann H, Shiv B, & Rangel A (2011). Dissociating valuation and saliency signals during decision-making. Cerebral Cortex , 21, 95–102. 10.1093/cercor/bhq065 [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, & Fan J (2011). Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews , 35, 1219 –1236. 10.1016/j.neubiorev.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, & Glimcher PW (2012). Efficient coding and the neural representation of value. Annals of the New York Academy of Sciences , 1251, 13–32. 10.1111/j.1749-6632.2012.06496.x [DOI] [PubMed] [Google Scholar]
- Louie K, Khaw MW, & Glimcher PW (2013). Normalization is a general neural mechanism for context-dependent decision making. Proceedings of the National Academy of Sciences of the United States of America , 110, 6139 – 6144. 10.1073/pnas.1217854110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NE (1944). Experimental studies of conflict. In Hunt JM (Ed.), Personality and the behavior disorders (Vol. 1, pp. 431– 465). New York, NY: Ronald Press. [Google Scholar]
- Nakamura J, & Csikszentmihalyi M (2002). The concept of flow. In Snyder CR & Lopez SL (Eds.), Handbook of positive psychology (pp. 89 –105). New York, NY: Oxford University Press. [Google Scholar]
- Patall EA (2012). The motivational complexity of choosing: A review of theory and research. In Ryan R (Ed.), The Oxford handbook of human motivation (pp. 249 –279). New York, NY: Oxford University Press; 10.1093/oxfordhb/9780195399820.013.0015 [DOI] [Google Scholar]
- Pochon J-B, Riis J, Sanfey AG, Nystrom LE, & Cohen JD (2008). Functional imaging of decision conflict. The Journal of Neuro-science , 28, 3468 –3473. 10.1523/JNEUROSCI.4195-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, . . . Petersen SE (2011). Functional network organization of the human brain. Neuron , 72, 665– 678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, & Clithero JA (2012). Value normalization in decision making: Theory and evidence. Current Opinion in Neurobiology , 22, 970 –981. 10.1016/j.conb.2012.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Noonan MP, Boorman ED, Walton ME, & Behrens TE (2011). Frontal cortex and reward-guided learning and decision-making. Neuron , 70, 1054 –1069. 10.1016/j.neuron.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Sanfey AG (2009). Expectations and social decision-making: Biasing effects of prior knowledge on Ultimatum responses. Mind & Society , 8, 93–107. 10.1007/s11299-009-0053-6 [DOI] [Google Scholar]
- Schwartz B (2004). The paradox of choice: Why more is less New York, NY: Ecco. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, . . . Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience , 27, 2349 –2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience , 12, 154 –167. 10.1038/nrn2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, & Buckner RL (2014). Neural correlates of dueling affective reactions to win-win choices. Proceedings of the National Academy of Sciences of the United States of America , 111, 10978 – 10983. 10.1073/pnas.1405725111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, & Botvinick MM (2016). Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience , 19, 1286 –1291. 10.1038/nn.4384 [DOI] [PubMed] [Google Scholar]
- Shenhav A, & Karmarkar UR (2017). Dissociable components of the reward circuit are involved in appraisal versus choice Preprint (BioRxiv 172320). 10.1101/172320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Straccia MA, Botvinick MM, & Cohen JD (2016). Dorsal anterior cingulate and ventromedial prefrontal cortex have in-verse roles in both foraging and economic choice. Cognitive, Affective & Behavioral Neuroscience , 16, 1127–1139. 10.3758/s13415-016-0458-8 [DOI] [PubMed] [Google Scholar]
- Simonsohn U (2017). Two-lines: A valid alternative to the invalid testing of U-shaped relationships with quadratic regressions Retrieved from https://ssrn.com/abstract=3021690 [Google Scholar]
- Thaler RH (1988). Anomalies: The ultimatum game. The Journal of Economic Perspectives , 2, 195–206. 10.1257/jep.2.4.195 [DOI] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, & Barrett LF (2012). Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. NeuroImage , 60, 1947–1958. 10.1016/j.neuroimage.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A, & Kahneman D (1981). The framing of decisions and the psychology of choice. Science , 211, 453– 458. 10.1126/science.7455683 [DOI] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience , 16, 55– 61. 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Danielmeier C, & Jocham G (2014). Neurophysiology of performance monitoring and adaptive behavior. Physiological Reviews , 94, 35–79. 10.1152/physrev.00041.2012 [DOI] [PubMed] [Google Scholar]
- Yerkes RM, & Dodson JD (1908). The relation of strength of stimulus to rapidity of habit-formation. The Journal of Comparative Neurology and Psychology , 18, 459 – 482. 10.1002/cne.920180503 [DOI] [Google Scholar]
- Zaki J, Davis JI, & Ochsner KN (2012). Overlapping activity in anterior insula during interoception and emotional experience. Neuro-Image , 62, 493– 499. 10.1016/j.neuroimage.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


