Abstract
Social functioning is a key component of recovery after a potentially traumatic experience, and the buffering role of the social support in trauma resilience and recovery has been very well documented. Factors contributing to resilience and recovery are notable because although most people will experience a traumatic event during their lifetimes, only 6–10% are diagnosed with post-traumatic stress disorder (PTSD). The relationship between an individual and their social environment is determined both by the quality of the social environment itself, and by the individual’s perception and understanding of information conveyed by the other people around them. However, little research has considered the contribution of these internal social cognitive processes to PTSD risk or resilience. The current review draws on the existing literature on social cognitive functioning in trauma exposure and PTSD, identifying key questions and themes for future research. We utilized a meta-analytic approach to assess the evidence for alterations in social cognition in PTSD, finding a consistent large deficit in social cognitive performance in PTSD groups relative to trauma-exposed and healthy controls. We then reviewed the literature on the interaction of genes and the social environment, supporting the hypothesis that social cognitive deficits are a pre-existing risk factor for PTSD. Finally, we reviewed relevant neuroimaging findings, which suggest that alterations in social cognition affect the perception of threat cues in PTSD. Overall, research on social cognition and PTSD is still emerging, but existing findings suggest this is an important and understudied area for the understanding of PTSD.
Introduction
Alterations in social functioning have long been noted in post-traumatic stress disorder (PTSD), and are involved in the core symptom profile for PTSD diagnosis (avoiding people that remind one of the experience, negative beliefs and blaming self/others, feeling distant from others; Association, 2013). Social functioning is also likely a key component of recovery after a potentially traumatic experience, and the role of the social environment in trauma resilience and recovery has been very well documented (Hostinar, Sullivan, & Gunnar, 2014; Jennifer S. Stevens, van Rooij, & Jovanovic, 2016). Factors contributing to resilience and recovery are notable because although most people will experience a traumatic event during their lifetimes, only 6–10% are diagnosed with PTSD (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995).
For example, among former child soldiers, all with very high levels of violence and trauma exposure, those with higher levels of family acceptance and social support after reintegration show better psychosocial adjustment (Betancourt et al., 2013). Adults exposed to natural disasters have fewer symptoms of PTSD and depression if they experience or perceive positive social support (Arnberg, Hultman, Michel, & Lundin, 2012; McGuire et al., 2018). Similarly, perceived social support predicted fewer trauma-related symptoms in adults with a history of childhood maltreatment (Evans, Steel, & DiLillo, 2013).
The relationship between an individual and her social environment is determined both by the quality of the social environment itself, and by the individual’s perception and understanding of information conveyed by the other people around her. However, little research has considered the contribution of these internal social cognitive processes to PTSD risk or resilience.
The current review draws on the existing literature on social cognition in PTSD, identifying key questions and themes for future research. We assess the evidence for alterations in social cognition in PTSD, and whether such alterations are specific to the processing of social information, rather than being attributable to broader deficits in domains such as attention and memory. We also consider whether alterations in social cognition affect the perception of threat cues in PTSD. Finally, we assess whether PTSD-related differences in social cognition should be considered deficits, and the possibility that traumatic stress may have facilitating effects on social cognition.
Social cognition has been defined as including the wide variety of processes that link the perception of social information with a behavioral response (Adolphs, 2003), including perception, attention, decision-making, memory, and emotion. Although there is also a very relevant and broad literature on social attachment processes, we focus the current review on the perception and processing of social signals. Links between early attachment and social cognition in PTSD have been addressed in detail elsewhere (Sharp, Fonagy, & Allen Jon, 2012).
1. Social cognition in PTSD and control groups
We conducted a review of the literature on PTSD and social cognition for studies conducted up until May 22, 2018. The literature review focused on tasks involving perception, attention, or memory for social information. A search of Pubmed using pairing the term “PTSD” with the terms “social cognition,” “empathy,” “theory of mind,” “face processing,” “biological motion,” “gaze discrimination,” “others intentions” produced 213 individual articles. Of these, 16 studies included a comparison of performance between PTSD, trauma-exposed controls (TC), and/or healthy control (HC) groups on a social cognitive task, as summarized in Table 1. Other studies examining social function in PTSD using survey-based measures of empathic traits or social functioning more broadly are considered in this review, but were not included in Table 1 as they did not involve an experimental test of social cognition.
Table 1.
Studies of social cognition in traumatic stress: Comparisons of performance in PTSD, trauma-exposed, and healthy control groups
| Study label | Sample | Gender | Trauma type | PTSD | TC | HC | Type of cognition | Tasks | Findings | Hedge’s g* PTSD-TC |
PTSD-HC | TC-HC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schmidt et al. (2009) | Refugee | Female & Male | War-related, torture | 16 | 16 | 50 | Emotion recognition | RMET | PTSD < TC/HC | −1.69 | −1.92 | −0.25 |
| Fonzo et al. (2010) | Civilian | Female | Intimate partner violence | 12 | -- | 12 | Emotion recognition | EFMT | n.s. (anger, fear, happiness) | −0.37 | ||
| Nietlisbach et al. (2010) | Civilian | Female & Male | Variety of types | 16 | -- | 16 | Theory of mind | Faux Pas Test | n.s. | −0.67 | ||
| Empathy | Empathic resonance (laughing, yawning) | PTSD < HC | ||||||||||
| Emotion recognition | RMET | n.s. | ||||||||||
| Poljac et al. (2011) | Military | Male | Combat | 20 | 20 | -- | Emotion recognition | Dynamic facial emotion morphs | PTSD < TC (fear, sadness); n.s. (disgust, anger, happiness, surprise) | |||
| Face perception | Benton Facial Recognition Test | n.s. | ||||||||||
| Simmons et al. (2011) | Military | Male | Combat | 12 | 12 | 12 | Emotion recognition | EFMT | n.s. (anger, fear, happiness) | 0.11 | 0.28 | 0.21 |
| Mazza et al. (2012) | Military police | Male | Terrorist attack | 20 | 15 | 15 | Theory of mind | Strange Stories test | n.s. | −1.61 | −1.43 | 0.09 |
| Mentalizing | Emotion attribution task (vignettes) | PTSD < TC/HC | ||||||||||
| Emotion recognition | RMET | PTSD < TC/HC | ||||||||||
| Nazarov et al. (2014) | Civilian | Female | Childhood abuse | 31 | -- | 20 | Emotion recognition | RMET | n.s. | |||
| Inference from social interaction cues | IPT-15: Inferring kinship | PTSD < HC | ||||||||||
| Steuwe et al. (2014) | Civilian | Female | Childhood abuse | 16 | -- | 16 | Emotion recognition | Facial emotion rating for virtual characters | PTSD < HC (happiness); n.s. (anger, neutral) | −0.93 | ||
| Nazarov et al. (2015) | Civilian | Female | Childhood abuse | 29 | -- | 21 | Emotion recognition | Prosody: Recognize basic emotion from speech | n.s. | |||
| Emotion discrim. | Prosody: Discriminate emotion of 2 speech excerpts | n.s. | ||||||||||
| Fertuck et al. (2016) | Civilian | Female & Male | Variety of types | 29 | 19 | 18 | Emotion recognition | Dynamic facial emotion morphs | n.s. (fear); PTSD < TC/HC (un-trustworthiness) | −0.57 | −0.42 | 0.17 |
| Palgi et al. (2016) | Civilian & Military | Female & Male | Variety of types | 32 | -- | 30 | Mentalizing | Compassion task | PTSD < HC | |||
| Bomyea et al. (2017) | Military | Male | Military combat | 19 | 17 | -- | Emotion recognition | EFMT | n.s. (anger, fear, happiness) | |||
| Venta et al. (2017) | Pediatric | Female & Male | Variety of types | 59 | -- | 83 | Mentalizing | MASC | PTSD < HC | −0.07 | ||
| Barzilay et al. (2018) | Pediatric | Female & Male | Variety of types | -- | 4104** | 5204 | Emotion recognition | Penn-CNB: Social cognition | Trauma-exposed < HC | −0.12 | ||
| Janke et al. (2018) | Civilian | Female & Male | Variety of types | 41 | -- | 63*** | Mentalizing | TEMINT | PTSD < HC | −0.79 | ||
| Pistoia et al. (2018) | Pediatric | Female & Male | Earthquake | -- | 48 | 59 | Emotion recognition | Ekman faces | Trauma-exposed > HC | 1.14 |
Abbreviations: RMET = Reading The Mind in the Eyes Test; EFMT = Emotional Face Matching Task; TEMINT = Test of Emotional Intelligence; IPT-15 = Interpersonal Perception Task; MASC = Movie for the Assessment of Social Cognition; n.s. = no significant difference
Pooled across tasks within-study. Reported only for those studies that included either an effect size estimate or descriptive statistics. A Hedge’s g of >0.5 is considered a medium effect size, and g of >0.8 is considered a large effect size.
N=996 of this group met criteria for PTSD, but were not analyzed separately from others with trauma exposure.
N=8 of this group reported prior trauma exposure, but were not analyzed separately from the unexposed controls.
Overall, those with PTSD had lower performance compared to controls across a variety of different demographic groups and trauma exposure types. For example, military police officers with PTSD related to a terrorist attack showed social cognition deficits relative to exposed police officers with no PTSD, and healthy controls (Mazza et al., 2012). The specific deficits included poorer performance in emotion recognition on a task involving labeling others’ emotional states from facial expressions, and in mentalizing on a task involving attributing emotional states to a protagonist in situations depicted in short vignettes. The PTSD group also reported lower empathy on a self-report of empathic traits. Similar findings have been reported in other populations with PTSD. In studies of civilian participants with PTSD related to a wide variety of trauma types, several groups reported lower performance in comparable mentalizing, emotion recognition, and empathy tasks, relative to healthy controls (Janke et al., 2018; Nietlisbach et al., 2010; Parlar et al., 2014). Although the pool of studies investigating this domain of cognition in PTSD is relatively small, a previous meta-analysis of the existing literature confirmed that deficits in emotion recognition and mentalizing have been consistently observed across studies of PTSD (N=6 studies included, with effect sizes of d = −1.6 and d = −1.1 respectively), and that these deficits are unique to PTSD versus other types of anxiety disorders (Plana, Lavoie, Battaglia, & Achim, 2014).
To quantify the support for potential differences in social cognition in PTSD versus control groups, we conducted a focused meta-analysis of the studies listed in Table 1. Five of these studies were excluded from analysis (Bomyea et al., 2017; Nazarov et al., 2015; Nazarov et al., 2014; Palgi et al., 2016; Poljac et al., 2011) because they lacked effect size estimates or the descriptive statistics needed to calculate an effect size. For the 11 studies included in the meta-analysis, Hedge’s g was used for sample-size-weighted estimates of effect size assessing group differences between PTSD, TC, and HC groups. For the majority of studies, it was possible to quantify performance on social cognition tasks in terms of “accuracy” by calculating the M and SD for the proportion of items correctly endorsed. For studies that employed an inversely-poled measure (where higher scores reflected greater inaccuracy or poorer performance), the native scale was used to calculate Hedge’s g, and the sign was then inverted. To avoid overly weighting the findings toward any particular single study, effect sizes were pooled within-study when multiple experimental conditions or multiple domains of social cognition were reported. Much like Cohen’s d, A Hedge’s g of >0.5 is considered a medium effect size, and g of >0.8 is considered a large effect size. Findings are illustrated in Figure 1.
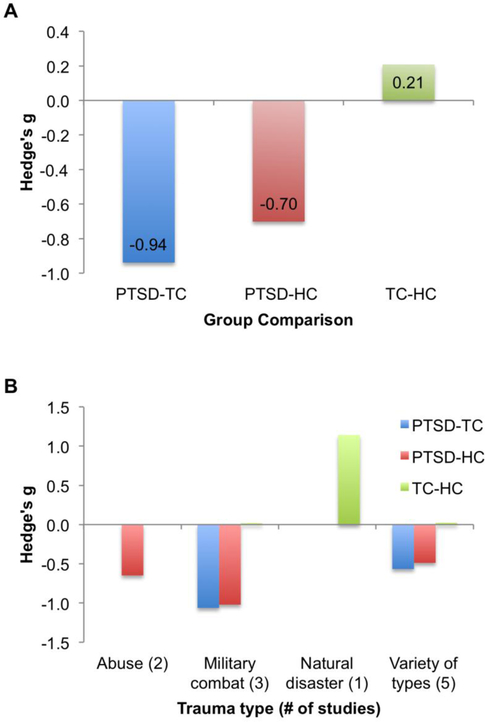
Figure 1.
Summary of effect sizes for group differences in performance on social cognitive tasks, comparing PTSD, trauma-exposed control (TC), and healthy control (HC) groups. Panel A shows the summary across all studies, and Panel B shows the effect sizes broken down by type of trauma exposure for the populations examined in each study. Because only a few studies examined specific trauma types, the results of Panel B are illustrative only, and a statistical comparison of these groups was not performed.
Overall, we found support in the literature for consistent deficits in social cognitive tasks in PTSD versus both TC and HC groups, with pooled effect sizes in the moderate to large range. These findings suggest that deficits in social cognition were not related to trauma exposure per se, but rather to the presence of PTSD. In fact, the largest effect size was for the comparison of PTSD and trauma-exposed control groups. Furthermore, the meta-analysis identified a novel finding that was not hypothesized: studies comparing TC and HC groups found a small effect in favor of the TC participants. Taken together, the findings are consistent with a model in which social cognitive deficits are associated with PTSD risk, and that social cognitive ability may on the other hand predict resilience to traumatic stress. The advantage in social cognition was driven primarily by a large study of adolescents who were exposed to a devastating earthquake, in comparison with a matched unexposed control group (Fig. 1B), whereas studies of other types of trauma found no notable differences in TC and HC groups’ performance.
These findings suggest that further research on the associations between trauma exposure, PTSD, and social cognition is warranted. Longitudinal research is needed to test the hypothesis that social cognition may contribute to resilience or risk following a trauma. In addition, the existing cross-sectional literature is primarily composed of small to medium sample sizes with N=30 or less in the PTSD group, which may be underpowered to detect reliable between-group differences in social cognitive performance, particularly given the heterogeneous symptom structure of PTSD (Galatzer-Levy & Bryant, 2013). The largest study to date was not conducted in adults with PTSD, but was a developmental study of children and young adults from the Philadelphia Neurodevelopmental Cohort (N=9000+), among whom trauma load—the number of traumatic events experienced—was associated with lower social cognitive performance (Barzilay et al., 2018). This study was well-powered for sensitivity analyses addressing the extent to which effects of trauma load might be explained by co-variates such as age, gender, and socioeconomic status. In fact, the association between trauma load and social cognitive task performance was no longer significant after accounting for these factors. Furthermore, trauma load was also associated with lower performance in the domains of executive function and complex reasoning, both of which may influence task performance on computerized neurocognitive batteries and may influence performance in the social cognition tasks. This study did not directly address whether PTSD diagnosis was associated with different performance in any neurocognitive domain, however, the findings suggest that larger sample sizes may be needed in order to draw strong conclusions about potential deficits in social cognition related to PTSD. It must be noted that several adult PTSD studies did include executive function or intelligence tests, and found PTSD-related deficits in empathy or emotion recognition in the absence of executive deficits (Nazarov et al., 2014; Nietlisbach et al., 2010), providing promising initial evidence that PTSD may have a specific effect on perception and processing of social information. Future investigation in a well-powered adult sample with PTSD, including a validated battery of neurocognitive tasks across several domains, would be highly valuable for moving this literature forward. In addition, studies that separately examine females and males, and those that probe social cognitive development and its interaction with trauma exposure timing, would be particularly informative.
2. How might deficits in social cognition contribute to PTSD symptoms?
Several theoretical accounts have been developed to explain how social deficits may be a major underlying contributor to PTSD risk. These primarily focus on the recovery period after trauma exposure. Any understanding of the risk factors for PTSD must incorporate the recovery period, because this disorder begins with a discrete event, in response to which almost everyone has high initial symptom levels. The pathology of the disorder rests within a failure to recover from the intrusive memories of the trauma, and high levels of fear and anxiety elicited by trauma reminders. This has been very clearly illustrated in prospective longitudinal studies modeling the course of recovery over the first weeks and months after trauma, showing heightened PTSD symptoms in all participants in the first weeks. Whereas a majority of people show natural recovery by 3–4 months, a subset of 11–17% maintain high symptom levels after a year or more (Andersen, Karstoft, Bertelsen, & Madsen, 2014; Bryant et al., 2015; Galatzer-Levy et al., 2013; Galatzer‐Levy, Madan, Neylan, Henn‐Haase, & Marmar, 2011). The chronic PTSD diagnosis then focuses on the group of individuals who do not recover naturally in the first few months.
Theoretical accounts of social contributions to this failure to recover posit that interactions between the self and others can change the structure of the trauma memory to influence symptoms (Maercker & Horn Andrea, 2012). This model is consistent with the premise of exposure-based behavioral therapies for PTSD, an extremely well-validated treatment for PTSD that requires the patient to remember aspects of the trauma by describing the event to the therapist over repeated occasions, over which the negative emotional impact of the memory is reduced or extinguished. Similar phenomena may be recapitulated in daily life, such that through supportive conversations with others, the fear responses associated with the trauma memory may be reduced. On the other hand, social interactions may also worsen symptoms, such as in the case of children whose disclosure of sexual trauma is rejected or belittled by their primary caregivers, which has been associated with poor mental health outcomes (Roesler, 1994). More recently, Sharp, Fonagy and Allen (2012) incorporated the role of early caregiver attachment in promoting resilience or risk for later PTSD. They pointed to findings showing that secure early attachment promotes the development of mentalizing, the capacity to take the perspective of another person, and that this capacity allows individuals to take advantage of supportive elements of their social environment. Finally, DePrince and colleagues focused on evidence relating to re-victimization risk (DePrince, 2005), positing that chronic trauma exposure produces deficits in social cognition that put individuals in dangerous situations, increasing risk for additional trauma.
These theories are all well-supported by the existing literature showing a key role for the social environment in PTSD risk and resilience. However, there is as yet only a shallow understanding of the effects of traumatic stress on how social stimuli are perceived, understood, and remembered, and the specific components of social cognition that might contribute to individual differences in PTSD risk or resilience. One possibility is that social cognitive deficits may be particularly linked with certain aspects of the PTSD symptom profile. Alterations in social functioning have long been noted in PTSD, and are involved in the core symptom profile for PTSD diagnosis. Specifically, social function directly contributes to symptoms in cluster D – “Negative Cognitions” - of the DSM-5 PTSD diagnostic criteria (and cluster C – “Avoidance and Numbing” -- of DSM-IV-TR) including avoidance of social reminders of trauma, negative beliefs about others, and feeling distant from others. However, social cognitive alterations may contribute indirectly to symptoms in other clusters. Examination of relations between social cognitive task performance and specific symptom types supports this possibility. Numbing symptoms, in particular, have been consistently associated with deficits in social cognition, even in the absence of differences between PTSD and control groups. Numbing symptoms (including feeling distant from others) were the strongest predictor of deficits in emotion recognition tasks in military police officers in Iraq (Mazza et al., 2012), and in refugees (Schmidt & Zachariae, 2009), and of compassion deficits in PTSD related to combat and motor vehicle accidents (Palgi et al., 2016). Numbing symptoms often co-occur with dissociative symptoms and childhood trauma history, which have also been associated with lower performance on emotion recognition tasks involving facial expressions (Nazarov et al., 2014) and emotional prosody (Nazarov et al., 2015). However, socially-related symptoms of PTSD are not the only aspect of PTSD symptomatology that appear to be associated with performance on social cognition tasks. In the majority of these studies, performance on emotion recognition and compassion tasks was also negatively associated with the other symptoms of hyper-arousal and intrusion, consistent with the fact that social function is not an explicit component of these symptom types. Social cognitive function may therefore have a broad influence on PTSD pathology.
3. Genetic contributors to social cognition, and links with PTSD
In addition to the main effects of increasing recovery and resilience, social environment can also interact with genotype (G x SE). Some studies have evaluated this G x SE interaction on a macro level, using geocoding to examine neighborhood effects, and found that single nucleotide polymorphisms (SNPs) coding for serotonin transporter (5-HTTLPR) impact PTSD symptoms in high crime neighborhoods (Koenen et al., 2009), while SNPs in the gene coding for pituitary adenylate polypeptide activating protein receptor (ADCYAP1R1) are associated with high depression in high trauma neighborhoods (Lowe et al., 2015). On the individual level, hurricane survivors with the short/short form of the 5-HTTLPR gene were less likely to develop PTSD if they had high levels of social support (Kilpatrick et al., 2007). The candidate gene that has received the most attention with respect to social support is the oxytocin receptor gene (OXTR), with most studies reporting on SNP at locus rs53576 (Bradley, Davis, Wingo, Mercer, & Ressler, 2013; Sippel et al., 2017). Studies in military cohorts have found that individuals with the minor allele of rs53576 and insecure attachment style had the greatest odds of having PTSD (Sippel et al., 2017), while civilian trauma populations show a similar interaction with childhood family environment (Bradley et al., 2013). The significance of the oxytocin system in ameliorating PTSD symptoms is also indicated by recent studies showing that administration of exogenous oxytocin can reduce symptoms (Flanagan, Sippel, Wahlquist, Moran-Santa Maria, & Back, 2018) or even prevent the disorder (van Zuiden et al., 2017). However, other studies have also found that oxytocin genes can increase vulnerability in some populations by increasing social sensitivity (McQuaid, McInnis, Matheson, & Anisman, 2016). Therefore, there may be caveats to the G x SE interactions, and the specific nature of the social environment needs to be considered. Further, social cognition may provide more insight into the impact of social environment on risk and resilience (McGuire et al., 2018).
When looking at the brain-related intermediate phenotypes that have been identified in PTSD, such as activation to face stimuli, several studies have found SNPs that are associated with higher amygdala reactivity (Morey et al., 2011; J.S. Stevens et al., 2014). Morey and colleagues found that 5-HTTLPR was associated with greater amygdala response to trauma-related distractor images presented during a face memory task in combat-related PTSD (Morey et al., 2011), while Stevens and colleagues found that the risk allele in ADCYAP1R1 gene was associated with more amygdala reactivity to fearful faces in women with civilian PTSD (J.S. Stevens et al., 2014). While these candidate gene studies support the G x SE research above, it should be noted that imaging genetics studies focusing on amygdala reactivity have not shown good replicability in the literature (Avinun, Nevo, Knodt, Elliott, & Hariri, 2018). However, genome-wide studies have also identified genes associated with PTSD that show increased amygdala activity, as well as thalamus and fusiform gyrus, in response to face stimuli (Kilaru et al., 2016). Most imaging genetics studies have not specifically examined brain areas related to social cognition—in the next section we describe these areas in more detail.
4. Neuroimaging studies of social cognition in PTSD
Studies probing the neurobiology underlying social cognitive processes can provide a more detailed picture of links between traumatic experiences, the processing of social cues, and symptoms of PTSD. Neuroimaging studies in healthy participants have identified a set of “core” brain regions involved in social cognition (Figure 2). Regions along the ventral and dorsal visual streams are implicated in specialized perceptual processing for stimuli involving the human body and biological motion. These include the fusiform “face area” in face perception, the extrastriate “body area” in perceiving bodies and biological or intentional motion, and posterior aspects of the temporoparietal junction (TPJ) in a host of mentalizing tasks including thinking about the perspective of someone else, making judgments about others’ traits, recognizing emotions from facial expressions, and perceiving motivated behaviors (Carter & Huettel, 2013; Schurz, Radua, Aichhorn, Richlan, & Perner, 2014). Similar to the TPJ, the dorsomedial prefrontal cortex (dmPFC) is consistently activated across a number of different task demands involving social information, but appears closely linked with the capacity to make inferences about stable traits in others, whereas the TPJ is more involved in making inferences about transient social information (Schurz et al., 2014; Van Overwalle, 2009). Finally, more focused meta-analysis of responses to others’ emotional states suggests that tasks involving passive viewing (dubbed “affective-perceptual empathy”) activate the bilateral insula, whereas tasks involving assessments of others’ emotional states (“cognitive empathy”) activate the mid-cingulate cortex (Fan, Duncan, de Greck, & Northoff, 2011). With the large number of neuroimaging studies and detailed modeling of social cognition in healthy participants, we are able to draw inferences about specific components of social perception and inference that may be altered with traumatic stress.
Figure 2.
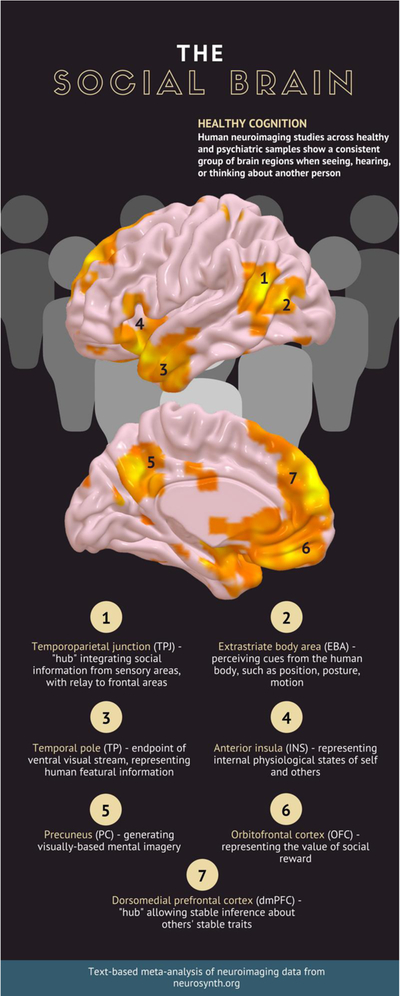
An extensive human neuroimaging literature has demonstrated a set of brain regions consistently engaged by social stimuli. To provide a schematic example of key brain regions involved in social cognition, we created a brain map using the text-based meta-analytic tool Neurosynth. The search term “social” aggregated findings from the 1000 published papers in the database containing the term, and compared the results against findings of all papers not containing the word “social.” Thresholded maps for this analysis are shown above (p<.01, FDR-corrected).
Only a small number of neuroimaging studies have directly investigated social processing in PTSD. An interesting series of studies conducted by Steuwe, Lanius, and colleagues (2014; 2015) used a social threat paradigm, in which participants viewed dynamic video stimuli of virtual characters who entered the visual field with an emotional facial expression, and directed their gaze directly at the participant, or averted their gaze. 16 adult women with a history of childhood abuse and current PTSD were compared with a control group of healthy participants without a history of trauma. Relative to controls, the PTSD group showed less engagement of brain regions typically involved in perception of facial and bodily motion, and mentalizing, including the left TPJ and right temporal pole. The PTSD group also showed greater activation in brain regions in the descending sympathetic arousal pathway including the left superior colliculus/periaqueductal gray, and the locus coeruleus (Steuwe et al., 2014). The superior colliculus and locus coeruleus also showed greater functional connectivity with a broad network of regions involved in emotion regulation in the PTSD versus control groups, including the anterior cingulate cortex, amygdala, insula, thalamus, caudate, and putamen (Steuwe et al., 2015). These findings suggest that women with PTSD mobilize a broad network of regions involved in responding to threat, when confronted with a social stimulus that is not overtly threatening. Furthermore, women with PTSD did not activate the same set of mentalizing-related brain regions engaged by controls (TPJ, temporal pole).
One possible interpretation is that activation of midbrain descending arousal pathways may reflect a panic response which occurs at the expense of potential perspective-taking, and may partly explain the mentalizing and emotion recognition deficits often observed in individuals with PTSD. Similarly, Mazza and colleagues (2015) asked survivors of the 2009 earthquake in L’Aquila, Italy to participate in fMRI using naturalistic scene stimuli depicting people with emotional and neutral facial expressions, and to rate their own and the others’ feelings. Participants with PTSD reported feeling less emotion than earthquake survivors with no PTSD when confronted with others’ emotional expressions, but the groups did not differ in their subjective responses to the neutral stimuli. In addition, the PTSD group showed greater activation of subcortical emotional brain regions including the anterior insula and ventral striatum in a comparison of own > other emotion ratings (Mazza et al., 2015). Further examination of the ventral striatum, a region involved in reward and motivation, showed that both groups engaged this region more for self- than other-focused emotion rating (the PTSD group to a greater extent). For the anterior insula, a region involved in representing one’s own and others internal physiological states, the control group showed more activation for other- than self-focused emotion rating, and the PTSD group did not. Taken together, these initial neuroimaging studies of social cognition in PTSD suggest that deficits may not be produced by alterations in the core set of brain regions involved in mentalizing or emotion recognition (e.g., TPJ, dmPFC), but may instead be related to heightened emotional responses to social signals conveying information about threat, which interfere with the ability to reason about others’ internal states. While responses to social threat cues appear heightened in PTSD, in contrast, fMRI responses to social reward may be impaired. A recent within-subjects placebo-controlled study of intranasal oxytocin administration showed that PTSD diagnosis was associated with lower anterior insula activation in a social reward task (Nawijn et al., 2017), and that oxytocin administration increased anterior insula activation in the PTSD group.
Although neuroimaging-based efforts to investigate social function in PTSD are just beginning, a large number of studies of PTSD have investigated arousal- or vigilance-related brain activation in response to stimuli that conveyed emotional information using social cues. One of the most prolific paradigms in the field of PTSD has been to use symptom provocation by presenting threatening and non-threatening social cues in the form of fearful or angry facial expressions, and neutral or happy facial expressions. Some of these tasks included an explicit emotion-recognition component, whereas others presented emotional faces but did not require the performance of any social cognitive task. The great majority of these studies find that individuals with PTSD show amygdala hyper-reactivity to threatening (fearful/angry) versus non-threatening stimuli (neutral/happy/non-face comparison) (e.g., Fonzo et al., 2010; Rauch et al., 2000; Shin et al., 2005; Simmons et al., 2011; Jennifer S. Stevens et al., 2013). Such findings have provided the central narrative for neuroimaging research on PTSD, and are thought to reflect heightened fear or vigilance responses to threatening environmental cues. Consistent with this idea, amygdala hyper-reactivity in PTSD is correlated with the severity of hyper-arousal symptoms, but not re-experiencing or avoidance/numbing symptoms (Lieberman, Gorka, DiGangi, Frederick, & Phan, 2017; Jennifer S. Stevens et al., 2013).
Importantly, a heightened amygdala response to negative affective face stimuli is not just related to current PTSD symptoms, but also appears to be a risk factor associated with vulnerability to future symptoms. In a longitudinal study of young adults who participated in fMRI at a baseline visit, amygdala reactivity prior to exposure to stressful life events was a significant predictor of subsequent anxiety and depression symptoms in individuals with high levels of stress exposure (Swartz, Knodt, Radtke, & Hariri, 2015). Similarly, in studies of military personnel and adolescents exposed to a terrorist attack, using fMRI with tasks that included implicitly social content (e.g., portraits of military personnel or scenes depicting interpersonal violence), amygdala reactivity prior to the traumatic event predicted subsequent PTSD symptom severity (Admon et al., 2009; McLaughlin et al., 2014). Parallel findings also extend to the peri-traumatic period – amygdala reactivity to negative affective face stimuli shortly after an Emergency Department trauma has been associated with the maintenance of high PTSD symptom levels over the next year (Jennifer S. Stevens et al., 2017). Interestingly, the PTSD-linked profile of heightened amygdala reactivity to negative face stimuli has also been linked with oxytocin. In a placebo-controlled study of police officers with PTSD, those with the highest amygdala reactivity to emotional faces under placebo had the largest reduction in reactivity with oxytocin, further highlighting that neuropeptides central to social processing may be tightly linked with amygdala hyper-reactivity in PTSD (Koch et al., 2015). Taken together, the neuroimaging literature suggests that the processing of emotional information from social cues may be an important contributor to later risk and resilience to trauma or other stressful life events.
5. Are hyper-arousal responses in PTSD greater for social than non-social cues?
Interestingly, PTSD-related amygdala hyper-reactivity to threatening or negatively valenced stimuli is observed less consistently for classes of stimuli other than faces. For example, both Phan (2006), and later Van Rooij (2015), found that war veterans with PTSD did not show amygdala hyper-reactivity to negatively-valenced complex scene stimuli. In fact, the PET study conducted by Phan and colleagues showed less left amygdala activation than healthy or combat control groups in response to negative scenes, whereas Van Rooij and colleagues showed no difference between the three groups in their fMRI study. This is also the case for negative emotional words (e.g., Thomaes et al., 2009), and for narrative scripts prompting participants to recall a trauma during fMRI (Lanius et al., 2001; Shin et al., 1999). In response to these stimuli, PTSD groups also do not typically show amygdala hyper-reactivity relative to controls.
A meta-analysis of neuroimaging studies comparing different task types partially confirmed a dissociation between different types of tasks involving negative stimuli (Hayes, Hayes, & Mikedis, 2012). A subset of studies involving symptom provocation through trauma reminders did not show consistent amygdala hyper-reactivity associated with PTSD. In contrast, the meta-analysis aggregating cognitive-emotional studies, which used stimuli that were negative, but not trauma-related, showed amygdala hyper-reactivity for PTSD versus control participants (Hayes et al., 2012). This second analysis included emotional faces, but also tasks involving complex scene stimuli, negative words, and other non-social stimuli. A similar large-scale comparison of social and non-social stimuli has not been performed, and would be useful for testing the hypothesis that individuals with PTSD may preferentially engage the amygdala for threat cues involving social stimuli, in contrast with non-social stimuli.
If confirmed, this would entail a significant narrowing of the interpretation of amygdala hyper-reactivity in PTSD. Amygdala reactivity in this case would not reflect a broad increase in threat-related responding or vigilance to environmental threat, but would be specifically linked with the processing of social cues. This may be reasonable, as evidence from amygdala lesion studies indicates that this region plays a role in face processing which is not specific to the emotional content of the faces, as deficits have been observed in perceiving eye gaze direction and the ability to learn to recognize new faces post-lesion (Young et al., 1995). Furthermore, neurons in the medial amygdala of macaques have been shown to be selective for either faces (Leonard, Rolls, Wilson, & Baylis, 1985) or other social stimuli such as movies of conspecific behavior (Brothers, Ring, & Kling, 1990), and not responsive to negative affective stimuli. Therefore, some aspects of amygdala function may be specialized for the processing of social stimuli in particular.
6. Conclusions
PTSD patients consistently show deficits in social cognitive task performance, particularly for emotion recognition and mentalizing (perspective-taking) tasks. Given the consistency of these findings, and the clear role of interpersonal relationships in shaping responses to trauma, additional resources should be devoted to understanding the role of social cognition in PTSD risk and resilience. In particular, further investigation is needed to determine the timecourse of social cognitive deficits – whether they exist prior to trauma and contribute to a weaker social network less likely to enhance recovery, or whether the deficits are a product of the stress reaction to the trauma. Initial evidence from the findings of the meta-analysis also suggests that trauma-exposed individuals without PTSD may have an advantage in social cognition, and longitudinal research can help determine the extent to which this reflects a trait-like resilience factor, or whether the trauma exposure itself can increase social cognition in a subset of individuals.
Neuroimaging studies of PTSD suggest that hyper-reactivity of limbic and midbrain arousal pathways to even low-level social threat cues may be a primary driver of deficits in emotion recognition and mentalizing, interfering with the ability to make inferences about the internal states of others. Additionally, findings implicating the amygdala in PTSD symptoms of hyper-reactivity to threat may be limited to social stimuli, suggesting that reactivity to incoming social cues may partly explain hyper-arousal symptoms in PTSD. However, only a limited number of neuroimaging studies has directly compared social and non-social stimuli, or incorporated tasks designed to probe different aspects of social cognitive processing. Further research in this area is needed. Taken together, the findings point to an important role of social cognition in PTSD, and suggest that interventions designed to increase social cognitive functioning may represent promising new approaches to improving trauma-related outcomes.
Acknowledgements
Dr. Stevens has support from National Institutes of Health for a BIRCWH K12HD085850. Dr. Jovanovic has grant support from National Institutes of Health, MH092576, MH098212, MH100122, MH111682, and Brain and Behavior Research Foundation (NARSAD).
Footnotes
Conflict of Interest
The authors report no conflict of interest.
References
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, & Hendler T (2009). Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences, 106(33), 14120–14125. doi: 10.1073/pnas.0903183106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R (2003). Cognitive neuroscience of human social behaviour. Nat Rev Neurosci, 4(3), 165–178. [DOI] [PubMed] [Google Scholar]
- Andersen SB, Karstoft K-I, Bertelsen M, & Madsen T (2014). Latent trajectories of trauma symptoms and resilience: the 3-year longitudinal prospective USPER study of Danish veterans deployed in Afghanistan. J Clin Psychiatry, 75(9), 1001–1008. [DOI] [PubMed] [Google Scholar]
- Arnberg FK, Hultman CM, Michel P, & Lundin T (2012). Social Support Moderates Posttraumatic Stress and General Distress After Disaster. Journal of Traumatic Stress, 25(6), 721–727. doi:doi: 10.1002/jts.21758 [DOI] [PubMed] [Google Scholar]
- Association, A. P. (2013). Diagnostic and statistical manual of mental disorders, (DSM-5®). Washington, D.C.: American Psychiatric Pub. [Google Scholar]
- Avinun R, Nevo A, Knodt AR, Elliott ML, & Hariri AR (2018). Replication in Imaging Genetics: The Case of Threat-Related Amygdala Reactivity. Biological Psychiatry, 84(2), 148–159. doi: 10.1016/j.biopsych.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay R, Calkins ME, Moore TM, Wolf DH, Satterthwaite TD, Cobb Scott J, . . . Gur RE (2018). Association between traumatic stress load, psychopathology, and cognition in the Philadelphia Neurodevelopmental Cohort. Psychol Med, 1–10. doi: 10.1017/S0033291718000880 [DOI] [PubMed] [Google Scholar]
- Betancourt TS, Borisova I, Williams TP, Meyers‐Ohki SE, Rubin‐Smith JE, Annan J, & Kohrt BA (2013). Research Review: Psychosocial adjustment and mental health in former child soldiers – a systematic review of the literature and recommendations for future research. Journal of Child Psychology and Psychiatry, 54(1), 17–36. doi:doi: 10.1111/j.1469-7610.2012.02620.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomyea J, Matthews SC, Buchsbaum MS, Spadoni AD, Strigo IA, & Simmons AN (2017). Neural differences underlying face processing in veterans with TBI and co-occurring TBI and PTSD. J Affect Disord, 223, 130–138. doi: 10.1016/j.jad.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Bradley B, Davis TA, Wingo AP, Mercer KB, & Ressler KJ (2013). Family environment and adult resilience: contributions of positive parenting and the oxytocin receptor gene. European Journal of Psychotraumatology, 4, 10.3402/ejpt.v3404i3400.21659. doi: 10.3402/ejpt.v4i0.21659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers L, Ring B, & Kling A (1990). Response of neurons in the macaque amygdala to complex social stimuli. Behavioural Brain Research, 41(3), 199–213. doi: 10.1016/0166-4328(90)90108-Q [DOI] [PubMed] [Google Scholar]
- Bryant RA, Nickerson A, Creamer M, O’Donnell M, Forbes D, Galatzer-Levy I, . . . Silove D (2015). Trajectory of post-traumatic stress following traumatic injury: 6-year follow-up. British journal of psychiatry, 206(5), 417–423. [DOI] [PubMed] [Google Scholar]
- Carter RM, & Huettel SA (2013). A nexus model of the temporal–parietal junction. Trends Cogn Sci, 17(7), 328–336. doi: 10.1016/j.tics.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SE, Steel AL, & DiLillo D (2013). Child maltreatment severity and adult trauma symptoms: Does perceived social support play a buffering role? Child Abuse & Neglect, 37(11), 934–943. doi: 10.1016/j.chiabu.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, & Northoff G (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience & Biobehavioral Reviews, 35(3), 903–911. doi: 10.1016/j.neubiorev.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Fertuck EA, Tsoi F, Grinband J, Ruglass L, Melara R, & Hien DA (2016). Facial trustworthiness perception bias elevated in individuals with PTSD compared to trauma exposed controls. Psychiatry Research, 237, 43–48. doi: 10.1016/j.psychres.2016.01.056 [DOI] [PubMed] [Google Scholar]
- Flanagan JC, Sippel LM, Wahlquist A, Moran-Santa Maria MM, & Back SE (2018). Augmenting Prolonged Exposure therapy for PTSD with intranasal oxytocin: A randomized, placebo-controlled pilot trial. Journal of Psychiatric Research, 98, 64–69. doi: 10.1016/j.jpsychires.2017.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, & Stein MB (2010). Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry, 68(5), 433–441. doi: 10.1016/J.Biopsych.2010.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Ankri Y, Freedman S, Israeli-Shalev Y, Roitman P, Gilad M, & Shalev AY (2013). Early PTSD symptom trajectories: persistence, recovery, and response to treatment: results from the Jerusalem Trauma Outreach and Prevention Study (J-TOPS). PLoS One, 8(8), e70084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, & Bryant RA (2013). 636,120 ways to have posttraumatic stress disorder. Perspectives on Psychological Science, 8(6), 651–662. [DOI] [PubMed] [Google Scholar]
- Galatzer‐Levy IR, Madan A, Neylan TC, Henn‐Haase C, & Marmar CR (2011). Peritraumatic and trait dissociation differentiate police officers with resilient versus symptomatic trajectories of posttraumatic stress symptoms. J Trauma Stress, 24(5), 557–565. doi:doi: 10.1002/jts.20684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, & Mikedis AM (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord, 2(1), 9. doi: 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, & Gunnar MR (2014). Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin, 140(1), 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke K, Driessen M, Behnia B, Wingenfeld K, & Roepke S (2018). Emotional intelligence in patients with posttraumatic stress disorder, borderline personality disorder and healthy controls. Psychiatry Research, 264, 290–296. doi: 10.1016/j.psychres.2018.03.078 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, & Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry, 52(12), 1048–1060. doi: 10.1001/archpsyc.1995.03950240066012 [DOI] [PubMed] [Google Scholar]
- Kilaru V, Iyer SV, Almli LM, Stevens JS, Lori A, Jovanovic T, . . . Ressler KJ (2016). Genome-wide gene-based analysis suggests an association between Neuroligin 1 (NLGN1) and post-traumatic stress disorder. Translational Psychiatry, 6(5), e820. doi: 10.1038/tp.2016.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, . . . Gelernter J (2007). The Serotonin Transporter Genotype and Social Support and Moderation of Posttraumatic Stress Disorder and Depression in Hurricane-Exposed Adults. Am J Psychiatry, 164(11), 1693–1699. doi: 10.1176/appi.ajp.2007.06122007 [DOI] [PubMed] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, & Olff M (2015). Intranasal Oxytocin Administration Dampens Amygdala Reactivity towards Emotional Faces in Male and Female PTSD Patients. Neuropsychopharmacology, 41, 1495. doi: 10.1038/npp.2015.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.nature.com/articles/npp2015299#supplementary-information.
- Koenen KC, Aiello AE, Bakshis E, Amstadter AB, Ruggiero KJ, Acierno R, . . . Galea S (2009). Modification of the Association Between Serotonin Transporter Genotype and Risk of Posttraumatic Stress Disorder in Adults by County-Level Social Environment. American Journal of Epidemiology, 169(6), 704–711. doi: 10.1093/aje/kwn397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore PC, Boksman K, Gupta MA, Neufeld RW, . . . Menon RS (2001). Neural Correlates of Traumatic Memories in Posttraumatic Stress Disorder: A Functional MRI Investigation. American Journal of Psychiatry, 158(11), 1920–1922. doi: 10.1176/appi.ajp.158.11.1920 [DOI] [PubMed] [Google Scholar]
- Leonard CM, Rolls ET, Wilson FAW, & Baylis GC (1985). Neurons in the amygdala of the monkey with responses selective for faces. Behavioural Brain Research, 15(2), 159–176. doi: 10.1016/0166-4328(85)90062-2 [DOI] [PubMed] [Google Scholar]
- Lieberman L, Gorka SM, DiGangi JA, Frederick A, & Phan KL (2017). Impact of posttraumatic stress symptom dimensions on amygdala reactivity to emotional faces. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 79, 401–407. doi: 10.1016/j.pnpbp.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SR, Pothen J, Quinn JW, Rundle A, Bradley B, Galea S, . . . Koenen KC (2015). Gene-by-social-environment interaction (GxSE) between ADCYAP1R1 genotype and neighborhood crime predicts major depression symptoms in trauma-exposed women. Journal of Affective Disorders, 187, 147–150. doi: 10.1016/j.jad.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maercker A, & Horn Andrea B (2012). A Socio‐interpersonal Perspective on PTSD: The Case for Environments and Interpersonal Processes. Clinical Psychology & Psychotherapy, 20(6), 465–481. doi: 10.1002/cpp.1805 [DOI] [PubMed] [Google Scholar]
- Mazza M, Giusti L, Albanese A, Mariano M, Pino MC, & Roncone R (2012). Social cognition disorders in military police officers affected by posttraumatic stress disorder after the attack of An-Nasiriyah in Iraq 2006. Psychiatry Research, 198(2), 248–252. doi: 10.1016/j.psychres.2011.11.027 [DOI] [PubMed] [Google Scholar]
- Mazza M, Tempesta D, Pino MC, Nigri A, Catalucci A, Guadagni V, . . . Ferrara M (2015). Neural activity related to cognitive and emotional empathy in post-traumatic stress disorder. Behavioural Brain Research, 282, 37–45. doi: 10.1016/j.bbr.2014.12.049 [DOI] [PubMed] [Google Scholar]
- McGuire AP, Gauthier JM, Anderson LM, Hollingsworth DW, Tracy M, Galea S, & Coffey SF (2018). Social Support Moderates Effects of Natural Disaster Exposure on Depression and Posttraumatic Stress Disorder Symptoms: Effects for Displaced and Nondisplaced Residents. Journal of Traumatic Stress, 31(2), 223–233. doi:doi: 10.1002/jts.22270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, & Sheridan MA (2014). Amygdala Response to Negative Stimuli Predicts PTSD Symptom Onset Following a Terrorist Attack. Depression and Anxiety, 31(10), 834–842. doi: 10.1002/da.22284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Matheson K, & Anisman H (2016). Oxytocin and Social Sensitivity: Gene Polymorphisms in Relation to Depressive Symptoms and Suicidal Ideation. Frontiers in Human Neuroscience, 10(358). doi: 10.3389/fnhum.2016.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Hariri AR, Gold AL, Hauser MA, Munger HJ, Dolcos F, & McCarthy G (2011). Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC Psychiatry, 11, 76–76. doi: 10.1186/1471-244X-11-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawijn L, van Zuiden M, Koch SBJ, Frijling JL, Veltman DJ, & Olff M (2017). Intranasal oxytocin increases neural responses to social reward in post-traumatic stress disorder. Social Cognitive and Affective Neuroscience, 12(2), 212–223. doi: 10.1093/scan/nsw123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarov A, Frewen P, Oremus C, Schellenberg EG, McKinnon MC, & Lanius R (2015). Comprehension of affective prosody in women with post‐traumatic stress disorder related to childhood abuse. Acta Psychiatrica Scandinavica, 131(5), 342–349. doi:doi: 10.1111/acps.12364 [DOI] [PubMed] [Google Scholar]
- Nazarov A, Frewen P, Parlar M, Oremus C, MacQueen G, McKinnon M, & Lanius R (2014). Theory of mind performance in women with posttraumatic stress disorder related to childhood abuse. Acta Psychiatrica Scandinavica, 129(3), 193–201. doi:doi: 10.1111/acps.12142 [DOI] [PubMed] [Google Scholar]
- Nietlisbach G, Maercker A, Rösler W, & Haker H (2010). Are Empathic Abilities Impaired in Posttraumatic Stress Disorder? Psychol Rep, 106(3), 832–844. doi: 10.2466/pr0.106.3.832-844 [DOI] [PubMed] [Google Scholar]
- Palgi S, Klein E, & Shamay-Tsoory SG (2016). Oxytocin improves compassion toward women among patients with PTSD. Psychoneuroendocrinology, 64, 143–149. doi: 10.1016/j.psyneuen.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Parlar M, Frewen P, Nazarov A, Oremus C, MacQueen G, Lanius R, & McKinnon MC (2014). Alterations in empathic responding among women with posttraumatic stress disorder associated with childhood trauma. Brain and Behavior, 4(3), 381–389. doi:doi: 10.1002/brb3.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K, Britton JC, Taylor SF, Fig LM, & Liberzon I (2006). Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry, 63(2), 184–192. doi: 10.1001/archpsyc.63.2.184 [DOI] [PubMed] [Google Scholar]
- Pistoia F, Conson M, Carolei A, Dema MG, Splendiani A, Curcio G, & Sacco S (2018). Post-earthquake Distress and Development of Emotional Expertise in Young Adults. Front Behav Neurosci, 12(91). doi: 10.3389/fnbeh.2018.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plana I, Lavoie M-A, Battaglia M, & Achim AM (2014). A meta-analysis and scoping review of social cognition performance in social phobia, posttraumatic stress disorder and other anxiety disorders. J Anxiety Disord, 28(2), 169–177. doi: 10.1016/j.janxdis.2013.09.005 [DOI] [PubMed] [Google Scholar]
- Poljac E, Montagne B, & de Haan EHF (2011). Reduced recognition of fear and sadness in post-traumatic stress disorder. Cortex, 47(8), 974–980. doi: 10.1016/j.cortex.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, . . . Pitman RK (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biol Psychiatry, 47(9), 769–776. [DOI] [PubMed] [Google Scholar]
- Roesler TA (1994). Reactions to disclosure of childhood sexual abuse: The effect on adult symptoms. Journal of Nervous and Mental Disease. [DOI] [PubMed] [Google Scholar]
- Schmidt JZ, & Zachariae R (2009). PTSD and Impaired Eye Expression Recognition: A Preliminary Study. Journal of Loss and Trauma, 14(1), 46–56. doi: 10.1080/15325020802537096 [DOI] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, & Perner J (2014). Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neuroscience & Biobehavioral Reviews, 42, 9–34. doi: 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Sharp C, Fonagy P, & Allen Jon G (2012). Posttraumatic Stress Disorder: A Social‐Cognitive Perspective. Clinical Psychology: Science and Practice, 19(3), 229–240. doi: 10.1111/cpsp.12002 [DOI] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, . . . Pitman RK (1999). Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. American Journal of Psychiatry, 156(4), 575–584. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, . . . Rauch SL (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry, 62(3), 273–281. doi: 10.1001/archpsyc.62.3.273 [DOI] [PubMed] [Google Scholar]
- Simmons AN, Matthews SC, Strigo IA, Baker DG, Donovan HK, Motezadi A, . . . Paulus MP (2011). Altered amygdala activation during face processing in Iraqi and Afghanistani war veterans. Biol Mood Anxiety Disord, 1(1), 6. doi: 10.1186/2045-5380-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel LM, Han S, Watkins LE, Harpaz-Rotem I, Southwick SM, Krystal JH, . . . Pietrzak RH (2017). Oxytocin receptor gene polymorphisms, attachment, and PTSD: Results from the National Health and Resilience in Veterans Study. Journal of Psychiatric Research, 94, 139–147. doi: 10.1016/j.jpsychires.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C, Daniels JK, Frewen PA, Densmore M, Pannasch S, Beblo T, . . . Lanius RA (2014). Effect of direct eye contact in PTSD related to interpersonal trauma: an fMRI study of activation of an innate alarm system. Social Cognitive and Affective Neuroscience, 9(1), 88–97. doi: 10.1093/scan/nss105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C, Daniels JK, Frewen PA, Densmore M, Theberge J, & Lanius RA (2015). Effect of direct eye contact in women with PTSD related to interpersonal trauma: Psychophysiological interaction analysis of connectivity of an innate alarm system. Psychiatry Research: Neuroimaging, 232(2), 162–167. doi: 10.1016/j.pscychresns.2015.02.010 [DOI] [PubMed] [Google Scholar]
- Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, . . . Ressler KJ (2014). PACAP Receptor Gene Polymorphism Impacts Fear Responses in the Amygdala and Hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 111(8), 3158–3163. doi: 10.1073/pnas.1318954111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, & Ressler KJ (2013). Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research, 47(10), 1469–1478. doi: 10.1016/j.jpsychires.2013.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, . . . Ressler KJ (2017). Amygdala reactivity and anterior cingulate habituation predict PTSD symptom maintenance after acute civilian trauma. Biol Psychiatry, 81(12), 1023–1029. doi: 10.1016/j.biopsych.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, van Rooij SJH, & Jovanovic T (2016). Developmental Contributors to Trauma Response: The Importance of Sensitive Periods, Early Environment, and Sex Differences. Current topics in behavioral neurosciences, 101007/7854_2016_1038. doi: 10.1007/7854_2016_38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz Johnna R., Knodt Annchen R., Radtke Spenser R., & Hariri Ahmad R (2015). A Neural Biomarker of Psychological Vulnerability to Future Life Stress. Neuron, 85(3), 505–511. doi: 10.1016/j.neuron.2014.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer NPJ, de Ruiter MB, Elzinga BM, van Balkom AJ, . . . Veltman DJ. (2009). Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: A pilot study. Psychiatry Research: Neuroimaging, 171(1), 44–53. doi: 10.1016/j.pscychresns.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Van Overwalle F (2009). Social cognition and the brain: a meta‐analysis. Human Brain Mapping, 30(3), 829–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooij S, Rademaker A, Kennis M, Vink M, Kahn R, & Geuze E (2015). Neural correlates of trauma-unrelated emotional processing in war veterans with PTSD. Psychol Med, 45(3), 575–587. [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Frijling JL, Nawijn L, Koch SBJ, Goslings JC, Luitse JS, . . . Olff M (2017). Intranasal Oxytocin to Prevent Posttraumatic Stress Disorder Symptoms: A Randomized Controlled Trial in Emergency Department Patients. Biological Psychiatry, 81(12), 1030–1040. doi: 10.1016/j.biopsych.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Venta A, Hatkevich C, Mellick W, Vanwoerden S, & Sharp C (2017). Social cognition mediates the relation between attachment schemas and posttraumatic stress disorder. Psychological Trauma: Theory, Research, Practice, and Policy, 9(1), 88–95. doi: 10.1037/tra0000165 [DOI] [PubMed] [Google Scholar]
- Young AW, Aggleton JP, Hellawell DJ, Johnson M, Broks P, & Hanley JR (1995). Face processing impairments after amygdalotomy. Brain, 118(1), 15–24. doi: 10.1093/brain/118.1.15 [DOI] [PubMed] [Google Scholar]