Abstract
Objective:
Newer MRI techniques have shown promise in capturing early Parkinson’s disease (PD)-related changes in the substantia nigra pars compacta (SNc), the key pathological loci. Their translational value, however, is hindered by technical complexity and inconsistent results.
Methods:
A novel yet simple MRI contrast, the T1w/T2w ratio, was used to study 76 PD patients and 70 controls. The T1w/T2w ratio maps were analyzed using both voxel-based and region-of-interest approaches in normalized space. The sensitivity and specificity of the SN T1w/T2w ratio in discriminating between PD and controls also were assessed. In addition, its diagnostic performance was tested in a subgroup of PD patients with disease duration ≤2 years (PDE). A second independent cohort of 73 PD and 49 controls was used for validation.
Results:
Compared to controls, PD patients showed a higher T1w/T2w ratio in both the right (cluster size=164 mm3, p<0.0001) and left (cluster size=213 mm3, p<0.0001) midbrain that was located ventrolateral to the red nucleus and corresponded to the SNc. The region-of-interest approach confirmed the group difference in the SNc T1w/T2w ratio between PD and controls (p<0.0001). The SNc T1w/T2w ratio had high sensitivity (0.908) and specificity (0.80) to separate PD and controls (AUC=0.926), even for PDE patients (AUC=0.901, sensitivity=0.857, specificity=0.857). These results were validated in the second cohort.
Interpretation:
The T1w/T2w ratio can detect PD-related changes in the SN and may be used as a novel, parsimonious in vivo biomarker for the disease, particularly early stage patients, with high translational value for clinical practice and research.
Introduction
Parkinson’s disease (PD) is marked pathologically by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) of the basal ganglia.1, 2 During the past decade, many research groups including ours have endeavored to find reliable and objective biomarkers for the PD process.3, 4 These efforts have yielded significant progress,4 although important challenges remain in translating these postulated biomarkers into tools that are useful for clinical practice and translational research.
Multi-modal MRI has been demonstrated to capture PD-related pathophysiological processes.5–11 Quantitative susceptibility mapping (QSM, reflecting iron deposition)9, 12 and free-water diffusion (reflecting neuro-inflammation-related edema)10, 11 have been particularly promising as potential in vivo MRI markers of PD. The barriers in applying these advanced MRI approaches to clinical practice and research are the complexity of the MRI techniques, lack of standardized imaging procedures, and the fact that the sequences are prone to motion artifacts.13–16 In addition, the results from both iron-sensitive and diffusion MRI have not been consistent in detecting early-stage PD-related changes.5, 17, 18 There is a clear need for practical and sensitive objective markers to detect early PD-related pathophysiological changes.
Nearly all clinical and research MRI protocols obtain T1- (T1w) and T2-weighted (T2w) images for anatomical information. Originally proposed by Glasser et al.,19 a simple division of these images can yield a new quantitative contrast (T1w/T2w ratio) with high spatial resolution, test-retest reliability, and sensitivity to neurodegenerative changes demonstrated by recent studies.20–22 Its simplicity and broad availability make it a promising candidate for studying PD-related changes. To our knowledge, there is neither a study that has used the T1w/T2w ratio to study structures other than cortical regions, nor has it been applied yet to a PD population.
In the current study, we compared midbrain T1w/T2w ratio maps in PD patients and matched controls in an attempt to develop a marker with high translational potential to assist in the diagnosis of PD, even in the early stages of the disease. We tested four hypotheses: 1) T1w/T2w ratio maps capture PD nigral pathology; 2) the T1w/T2w ratio change in PD also is sensitive to early-stage changes in PD patients with ≤2 years of disease duration, 3) the T1w/T2w ratio can separate PD patients from controls with high sensitivity and specificity even in the early stages; and 4) T1w/T2w ratio may differentiate early and later stage PD patients and associate with clinical measurements.
Methods
Subjects
Seventy-six PD patients and 70 age- and sex-matched controls participating in an NIH-funded MRI biomarker study (NS060722, with baseline enrollment from 2009–2012) were included as the testing cohort (Study 1). The participants were recruited from a tertiary movement disorders clinic (see Table 1 for detailed demographic information). PD diagnosis was confirmed by a movement disorder specialist according to UK brain bank criteria.23 The enrollment criteria for PD patients included Mini-Mental State Examination (MMSE) score >25 and the lack of neurological disorders other than PD, and disease duration within 10 years. Disease duration was defined as the date since first PD diagnosis by a physician. MDS-Unified Parkinson’s Disease Rating Scale parts III scores (UPDRS-III) were obtained for each PD patient after withholding all PD medication overnight (~12 hrs). Hoehn-Yahr (HY) staging was rated in the “off” medication state. Hamilton Depression Rating Scale (HDRS), Montreal Cognition Assessment (MoCA), and levodopa equivalent daily dosage (LEDD) also were obtained. All controls were free of any neurological, psychiatric, or major medical conditions.
Table 1.
Demographic and clinical data for PD and control subjects
| Control | PD | PDE | P values | |
|---|---|---|---|---|
| Study 1 participants (N) | 70 | 76 | 35 | |
| Age, yrs | 61.3 (6.7) | 63.3 (8.4) | 61.1 (8.7) | 0.113 |
| Female/Male (N) | 36/34 | 29/47 | 18/17 | 0.134 |
| Disease duration, yrs | - | 4.9 (5.5) | 0.8 (0.6) | - |
| Hoehn-Yahr Stage, I/II/III | - | 29/33/14 | 19/11/5 | - |
| UPDRS III | - | 21.5 (14.9) | 16.1 (9.7) | - |
| LEDD | - | 608 (466) | 292 (230) | - |
| HDRS | 3.8 (2.5) | 7.0 (4.2) | 7.0 (4.0) | <0.001 |
| MoCA | 26.1 (2.5) | 24.6 (3.6) | 25.3 (2.6) | 0.041 |
| Study 2 participants (N) | 49 | 73 | 35 | |
| Age, yrs | 68.7 (11.6) | 67.7 (10.2) | 64.6 (10.2) | 0.424 |
| Female/Male (N) | 23/26 | 32/41 | 17/18 | 0.736 |
| Disease duration, yrs | - | 5.8 (6.0) | 0.8 (0.7) | - |
| Hoehn-Yahr Stage, I/II/III/IV/V | - | 17/39/8/5/4 | 17/16/2 | - |
| UPDRS III | - | 30.1 (22.9) | 19.8 (13.2) | - |
| LEDD | - | 678 (445) | 413 (239) | - |
| HDRS | 3.2 (3.0) | 5.9 (4.4) | 4.8 (3.7) | <0.001 |
| MoCA | 25.2 (2.4) | 23.8 (4.2) | 24.8 (2.5) | 0.004 |
Date represent means with standard deviations in parentheses unless otherwise indicated. P values represent the difference between control and overall PD groups and controls. PDE (early stage PD) is defined by disease duration <2 yrs. UPDRS III: MDS-Unified Parkinson’s Disease Rating Scale III motor subscore; LEDD: Levodopa equivalent daily dosage; HDRS: Hamilton Depression Rating Scale; MoCA: Montreal Cognitive Assessment.
One-hundred-twelve PD patients and 87 controls from the NIH PD Biomarker Program (NCT01888185) were included as a validation study (Study 2) that had baseline enrollment from 2012–2015. The enrollment criteria for Study 2 were the same for Study 1 except there was no MMSE thresholding or disease duration requirement. The MDS-UPDRS and HY stage for Study 2 were obtained without withholding medication (in the “on” state). Two PD patients in Study 2 were excluded due to motion artifacts that resulted in low MRI data quality. A subgroup of early-stage PD patients (PDE) was defined as those with disease duration ≤2 years from both cohorts, with the remaining subjects categorized as later-stage PD patients (PDL). A total of seventy-five subjects overlapped between the two studies. The overlapping subjects were excluded from Study 2 for the current study. In total, 73 PD patients and 49 controls were included for the validation analyses. All subjects gave written informed consent, consistent with the Declaration of Helsinki and reviewed and approved by the Penn State Hershey Institutional Review Board.
MRI acquisition and T1w/T2w ratio maps
All subjects were scanned with a 3.0 Tesla MR scanner (Trio, Siemens Magnetom, Erlangen, Germany, with an 8-channel phase array head coil) to obtain high resolution T1w and T2w images. A magnetization-prepared rapid acquisition gradient echo sequence was used to obtain T1w images with repetition time/echo time=1,540/2.34 ms, field of view=256 × 256, slice thickness=1 mm (with no gap), and slice number=176. A 3D T2w Sampling Perfection with Application Optimized Contrast using Different Angle Evolution (SPACE) sequence was used to obtain T2w images with repetition time/echo time=2,500/316 ms and the same spatial resolution settings as T1w images. All T1w and T2w images were inspected offline and deemed free of severe motion artifacts or any major structural abnormalities (except for two subjects from Study 2 who were excluded). T1w/T2w ratio maps for each subject then were generated using a similar method proposed by Glasser et al.19 Namely, T1w and T2w images were co-registered using a rigid registration and then the T1w/T2w ratio maps were obtained by simply dividing the T1w image by the T2w image at each voxel. The T1w/T2w value at each voxel was multiplied by 100 to obtain values for robust image analysis.
Voxel-based image data analysis
Voxel-based analysis (VBA) was performed to detect differences in the T1w/T2w ratio between PD patients and controls. First, a cohort-specific T1w/T2w ratio template was created from all subjects by using an unbiased atlas building algorithm provided in the ANTs package.24 Second, T1w/T2w ratio maps were co-registered to the template images using the SyN registration algorithm within ANTs.25 The FSL randomize tool was used to conduct voxel-wise two-sample unpaired t-tests with age and sex as nuisance variables and the TFCE option to correct for family-wise error. A p-value of 0.01 and cluster size of 100 then was used to define significant regions.
Region-of-interest (ROI)-based image data analyses
Bilateral SNc were defined on the template image generated for the VBA analysis according to previous protocols.9, 26 Namely, the SNc was defined in six slices from superior to inferior, starting from one slice lower than the middle of the red nucleus. A kidney-shaped region ventrolateral to the red nucleus and dorsomedial to the SN pars reticulata (hypo-intensity band between the cerebral peduncles and red nucleus visualized in T2w images) was identified as the SNc. The mean T1w/T2w ratios of both the left and right SNc regions from normalized T1w/T2w images were used for group and subgroup comparisons, and discriminative analysis. Detailed image processing steps, tools, and estimated processing time are provided in Supplementary Table 3.
Statistical analyses
Demographic data were compared between groups using the Chi-square exact test for sex and two-tailed Student’s t-test for age. Clinical scores and ROI-based T1w/T2w ratios between controls and PD patients, as well as early-stage PD patients, were compared using analysis of covariance with age and sex as covariates. Statistical significance was defined as p < 0.05 for these group comparisons. The ability of SNc T1w/T2w values (from the ROI-based approach) to discriminate PD (and the early PD subgroup) from controls was assessed using logistic regression (the model included the SN T1w/T2w ratio, age, and sex as covariates) and receiver operating characteristic (ROC) curve analyses. A best cutoff point was determined using the Youden method. Multiple regression analysis then was used to test the correlations between clinical measures and the SNc T1w/T2w ratio. The clinical measures were used as independent variables, and the SNc T1w/T2w ratio as the dependent variable. All statistical analyses were performed using R version 3.14.
Results
Demographic and clinical data are summarized in Table 1. There were no significant differences between PD patients and controls in age and sex for both the testing and validation studies. In both studies, PD patients showed significantly higher depression scores and lower MoCA scores compared to controls. Participants in Study 2 were overall older and had longer disease durations, more severe disease, and higher LEDD usage.
VBA analysis
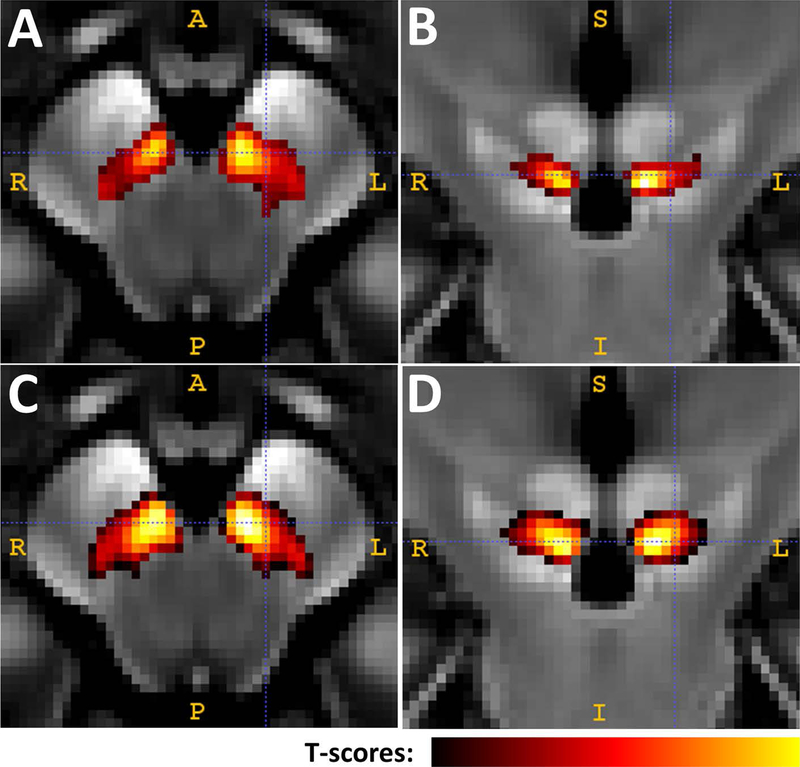
PD patients displayed a significantly higher T1w/T2w ratio only in the midbrain region (Figure 1). The total region (cluster size) of significant T1w/T2w values for Study 1 was 377 mm3, distributed in the right (164 mm3, with a peak p-value < 0.0001) and left (213 mm3, with a peak p-value < 0.0001) midbrain. The total region (cluster size) of significant T1w/T2w values for Study 2 was 654 mm3, distributed in the right (323 mm3, with a peak p-value < 0.0001) and left (331 mm3, with a peak p-value < 0.0001) midbrain. The location of the significant area was ventrolateral to the red nucleus and dorsomedial to the SN pars reticulata (within the hypo-intensity band between the red nucleus and cerebral peduncle), a region consistent with the location of the SNc.
Figure 1.
Voxel-wise analysis of T1w/T2w ratio maps in the midbrain area A is an axial view and B is a coronal view of the midbrain change from participants in Study 1. C and D are the midbrain change from participants in Study 2. The significantly colored areas were derived from a voxel-based analysis using the FSL randomize tool with p=0.01 and cluster size=100.
ROI-based group comparisons
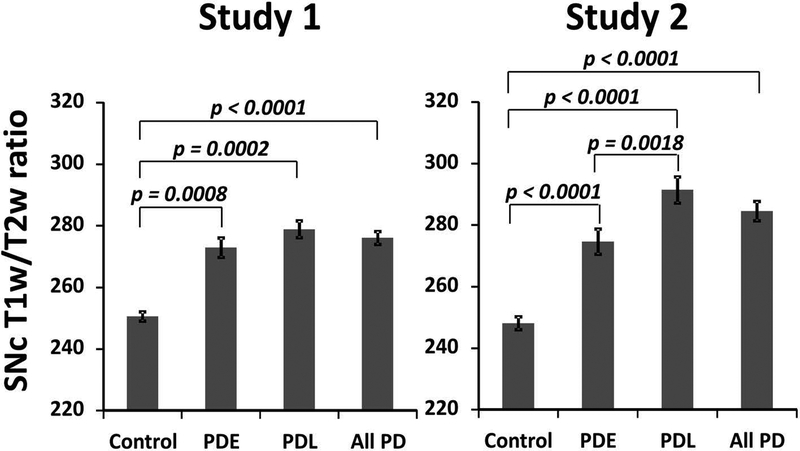
Compared to controls [250.6 (95% CI: 246.7 – 254.5)], PD patients in Study 1 showed a higher T1w/T2w ratio value in the SNc [276.1 (95% CI: 271.6 – 280.6); the age and sex corrected difference between PD and controls is 24.9 (95% CI: 19.7 – 30.1) and p < 0.0001; as shown in Figure 2A]. PDE [272.9 (95% CI: 267.5 – 278.2), with a difference of 22.4 (95% CI: 16.3 – 28.4) and p = 0.0008] and PDL [278.9 (95% CI: 274.3 – 283.5), with a difference of 26.9 (95% CI: 20.9 – 33.0) and p = 0.0002] patients in Study 1 also had significantly higher T1w/T2w values compared to controls. There was no significant difference, however, between PDE and PDL subgroups in Study 1. Study 2 showed similar, significant differences between total PD, PDE, and PDL groups when each was compared to controls (Figure 2B). In addition, PDL patients in Study 2 had T1w/T2w values that were significantly higher than the PDE group [274.7 (95% CI: 267.7 – 281.6) for PDE; 291.5 (95% CI: 284.3 – 298.7) for PDL; with a difference of 16.9 (95% CI: 4.5 – 29.2) and p = 0.0018]. The raw means and variations are presented in Supplementary Table 1. The original model included age and gender as covariates, and adding MoCA and/or HDRS into the model did not affect the results significantly.
Figure 2.
Group comparison between controls, early-stage, and later-stage PD subgroups P values are from comparisons between controls, early-stage PD, and later-stage PD subgroups. Error bars are standard error of means.
Predictive analysis based on ROI-based results
Using SNc T1w/T2w ratio values derived from the ROI-based approach with age and sex, logistic regression analysis showed that the SNc T1w/T2w ratio is a significant predictor for separating PD patients from controls (p < 0.0001) in Study 1. The area-under-the-curve (AUC) statistic was 0.926 (95% CI: 0.883 – 0.968; see Figure 3A), with high sensitivity [0.908 (95% CI: 0.763 – 0.974)] and specificity [0.80 (95% CI: 0.614 – 0.886)] using the Youden’s index criteria. For PDE patients, the AUC statistic was 0.901 (95% CI: 0.833 – 0.970; Figure 3B), with high sensitivity [0.857 (95% CI: 0.614 – 0.943)] and specificity [0.857 (95% CI: 0.6 – 0.943)]. The SNc T1w/T2w values also showed modest but significant discriminability between PDE and PDL. The AUC for PDE vs. PDL was 0.744 (95% CI: 0.632 – 0.855; see Figure 3C). Values from Study 2 showed a similar predictive performance (see Figure 3, D, E, and F for the overall PD, PDE, and PDE vs. PDL, respectively). The detailed statistics from the predictive analyses are presented in Supplementary Table 2.
Figure 3.
ROC curve analysis of the T1w/T2w ratio indicating area under the curve (AUC), sensitivity (Sen), and specificity (Spec). A, B, and C are the ROC curves separating all PD (A) and PDE (B) patients from controls, and PDE from PDL (C) in Study 1. D, E, and F are the ROC curves separating all PD (C) and PDE (D) subjects from controls, and PDE from PDL (C) in Study 2.
Correlation analysis between T1w/T2w ratio and clinical measure
The T1w/T2w value in the SNc was strongly correlated with MoCA scores in Study 1 (p = 0.0004). This finding was not, however, replicated in Study 2. The T1w/T2w value in the SNc was correlated with disease duration in Study 2 (p = 0.005). No other correlations between the clinical measures and the SNc T1w/T2w ratio reached significance (see Table 2).
Table 2.
Correlations between the T1w/T2w ratio in the SNc and clinical measures.
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| p-value | β (95% CI) | p-value | β (95% CI) | |
| Duration | 0.41 | 0.53 (−0.02 – 1.79) | 0.005 | 2.3 (0.70 – 3.87) |
| LEDD | 0.86 | −0.001 (−0.02 – 0.01) | 0.89 | 0.001 (−0.02 – 0.02) |
| UPDRS III | 0.07 | 0.36 (−0.03 – 0.75) | 0.07 | −0.51 (−1.08 – 0.05) |
| HDRS | 0.85 | −0.11 (−1.27 – 1.04) | 0.87 | 0.16 (−1.73 – 2.05) |
| MoCA | 0.0004 | 3.4 (1.58 – 5.14) | 0.74 | 0.38 (−1.88 – 2.64) |
Multiple regression analysis was used to test the correlation between clinical measures and T1w/T2w ratio values in the SNc. The clinical measures were independent variables, and the T1w/T2w was the dependent variable. Data are presented as p-values, β (95% CI).
Discussion
In the current study, we demonstrated for the first time that PD patients have a significantly higher T1w/T2w ratio in the midbrain compared to controls using a voxel-based analysis, and the significant region is consistent with the anatomical location of the SNc, which is the region associated with hallmark PD pathology. This finding was confirmed using an ROI approach. Moreover, the T1w/T2w ratio in the SNc was significantly higher in patients early in their disease (≤2 years of disease), suggesting that it may be an early-stage marker for PD. The SNc T1w/T2w ratio also had high sensitivity and specificity to discriminate PD patients and controls, a finding that also upheld to very early PD patients (≤2 years of disease). In addition, we validated these findings using an independent cohort. Together, these findings suggest that the T1w/T2w ratio can detect PD-related changes in the SNc. Because of its simple implementation, high resolution, and wide availability, these findings may have high translational value for clinical practice and research.
T1w/T2w ratio as a parsimonious MRI marker
T1w and T2w sequences are two of the most mature MRI techniques that have been improved consistently since their first use in clinical MRI. Within a total scan duration of 7 minutes (in our study), we were able to obtain high spatial resolution (1×1×1 mm3) images for both T1w and T2w sequences. The signal-to-noise ratio and contrast to noise ratio for both of these sequences are superior compared to other quantitative MRI contrasts in terms of common gray/white matter contrasts. Using these two sequences, simple division, and a few standard image processing steps (registrations) produces the T1w/T2w ratio image, a high quality quantitative MRI contrast. Previous studies have shown that it not only has superior sensitivity to myelin compared to diffusion MRI, but also superior test-retest reliability compared to other myelin imaging techniques.20
Previous studies have used the T1w/T2w ratio to detect cortical changes in patients with multiple sclerosis.22, 27 To our knowledge, the current study is the first to utilize this quantitative MRI contrast to study a subcortical structure and PD patients. Voxel-based analyses demonstrated that the T1w/T2w ratio clearly can delineate changes in the SNc, the location of hallmark PD pathology in early-stage PD patients. The ROI-based approach confirmed this finding and supported that the T1w/T2w ratio may capture SNc changes even in subjects with early-stage PD (namely, disease duration ≤2 years). T1w and T2w images routinely are acquired as a structural reference in clinical scan protocols and most large-scale MRI studies [e.g., the Parkinson’s Progression Marker Initiative (PPMI) and PD Biomarker Program (PDBP) studies]. The validation of our results in these cohorts will have important implications in both clinical and research settings.
In addition, our study also suggested that the SNc T1w/T2w ratio may be an early-stage marker for PD by showing a significant difference between early-stage PD and controls. The ability of T1w/T2w ratio to gauge disease severity and its association with clinical outcomes, however, is uncertain. Namely, whereas Cohort 2 showed significant differences between PD patients with disease duration less and more than 2 years, this result was not found in Study 1. Similarly, the significant association between T1w/T2w ratio and MoCA scores in Study 1 was not replicated in Study 2. The inclusion of more advanced stage PD patients in Study 2 with significant cognitive impairment may contribute to the discrepancies between the two studies. Future studies including PD patients with different durations, stages, clinical subtypes, and longitudinal follow-up are needed to understand fully the value and limitations of the T1w/T2w ratio as a marker for PD clinical stage, severity, and/or progression.
The discriminability of the T1w/T2w ratio between PD and controls
Although the clinical criteria for diagnosing PD has evolved over two decades, recent studies have raised concerns about the accuracy of PD diagnoses, especially in the early stages of the disease. A meta-analysis reported a diagnostic accuracy of 80.6% on average from both general neurologists and movement disorder specialists.28 Adler et al., using neuropathological diagnosis as the gold standard, reported that the accuracy of a fellowship-trained movement disorders specialist was 26% for early untreated PD cases, 53% for early levodopa-responsive cases, and 83% for later-stage PD cases.29 In a recent autopsy cohort of 14 subjects, we found non-movement disorder specialists can predict pathology in less than 50% of cases.30 There is a clear need for an early detection marker in PD patients that has high accuracy and translational value. The current best marker is proposed by Nalls et al.,31 who used a combination of clinical and genetic information and found an AUC=0.926 for classifying PD patients from controls. Both susceptibility and diffusion MRI also have been proposed as potential early detection markers in PD.5–7 The performance of these MRI markers for early PD detection, however, is less promising according to follow-up studies.11, 17, 18, 32 For example, a meta-analysis of diffusion MRI in PD reported a rather modest effect size for FA,18 and the performance of R2* in early-stage PD patients is inconsistent between studies.5, 26
One promising MRI marker of early-stage PD is free-water diffusion MRI in the posterior SN reported by Ofori et al.11 There are no data on the discriminability of free-water diffusion in the posterior SN for early-stage PD, however. In the current study, we demonstrated an AUC=0.901 using the T1w/T2w ratio to separate early-stage PD patients from controls using a single region where the key PD pathology is located. This is remarkable and further supportive of its translational value to clinical practice and research. Future studies are needed to investigate whether adding T1w/T2w ratios from other regions, or including clinical data and/or genetic features, may further improve PD/control discriminability, and thus potentially provide a more accurate diagnostic tool for early-stage PD.
Pathological underpinning of the T1w/T2w ratio in PD
Glasser et al have linked the T1w/T2w ratio to myelin content in cortical gray matter.19 The meaning of the T1w/T2w ratio at the subcortical level, particularly in the SNc, however, is unknown. Compared to controls, the SN T1 value of PD patients is decreased (which translates to an increased T1w intensity since the T1w signal is in the opposite direction of the T1 value).33, 34 The lower T1 value is postulated to reflect gray matter integrity, microglia activation, and/or neuromelanin-neuron loss based on human and lab animal studies.33, 35, 36 The T2 value also is decreased but this represents a decrease in T2w intensity in the SN of PD patients compared with controls.5, 9, 37 A recent MRI-pathology study has linked the T1w/T2w ratio to dendrite density but not myelin content.22 Thus, the T1w/T2w ratio in the midbrain may reflect a sum of multiple PD-related changes involving neurons, dendrites, microglia, and iron content. This information might generate a more potent contrast that may be superior to other MRI sequences in capturing PD-related pathology and can be used as a biomarker for PD early detection. A future MRI-pathology correlation study is warranted to test this hypothesis.
Strengths and limitations
One strength of this study is the relatively large PD and control participant pool that allowed us to carefully select early-stage PD patients to test the hypothesis that the T1w/T2w ratio can be a sensitive marker for early-stage disease. In addition, a validation cohort was included to replicate the current findings. Despite this strength, there are limitations. The majority of PD participants, even those in early-stage PD, already were on anti-parkinsonian medication. It is possible that the observed T1w/T2w changes in the midbrain were a consequence of medication-induced tissue alterations. Validation of these results in even larger datasets of newly diagnosed and drug naive PD patients collected by independent investigators (e.g., PPMI and PDBP), as well as longitudinal studies, would be useful. In our Study 1, PD patients with disease duration >10 y were excluded. Study 2 included more later-stage patients, but only 23 patients had disease duration >10 years. Although we detected a significant difference between PDE and PDL in Study 2, the exciting implications must be tempered by the limited sample size. A study with a larger sample size and longitudinal follow-up would be essential to verify the utility of this approach in disease staging.
The T1w/T2w ratio comparisons were conducted on spatially normalized images for both VBA and ROI-based analyses, and it is possible that volumetric or morphometric information could have contaminated the results. This seems unlikely, however, given that we did not observe significant volume differences between PD patients and controls using an atlas-based segmentation approach (the reverse of normalization, results not reported). The current data and analysis of the literature leads to the hypothesis that the T1w/T2w ratio is superior in defining early-stage PD-related changes when compared to newer diffusion and susceptibility contrasts approaches, future studies are needed to compare their performance directly. Lastly, it is believed that simple division of T1w and T2w images can cancel out most of the intensity heterogeneity, and thus result in a quantitative MRI contrast. There might be residual bias, however, that cannot be canceled out by simple division due to the complicated bias pattern from gradient distortions and an inhomogeneous B1 field. This issue is expected to be worse when data from multiple study sites with different imaging parameters are used. Future studies to test the T1w/T2w ratio from different MRI scanners with different T1w and T2w parameters are warranted. Newer simultaneous multi-compartment T1 and T2 imaging techniques (i.e. mcDEPOT38 and MR fingerprinting39) may help remedy this issue.
Supplementary Material
Acknowledgement
We express gratitude to all of the participants who volunteered for this study and study personnel who contributed to its success. This work was supported in part by the National Institute of Neurological Disorders and Stroke (NS060722 to XH) and Parkinson’s Disease Biomarker Program (NS082151 to XH), the Hershey Medical Center General Clinical Research Center (National Center for Research Resources, Grant UL1 RR033184 that is now at the National Center for Advancing Translational Sciences, Grant UL1 TR000127), and the PA Department of Health Tobacco CURE Funds, the Micheal J. Fox Foundation for Parkinson’s Research, Alzheimer’s Association, Alzheimer’s Research UK, and the Weston Brain Institute. All analyses, interpretations, and conclusions are those of the authors and not the research sponsors. We thank Dr. Richard Mailman for critically reviewing the manuscript and providing constructive comments.
Footnotes
Potential Conflicts of Interest
The image processing methods used in this manuscript have been reported to the Penn State University Office of Intellectual Technology, and a provisional patent application is pending. Drs Du, Huang, and Lewis are the listed inventors who have assigned the rights to the Penn State Research Foundation. The potential conflict-of-interest is being managed by the College of Medicine.
Reference
- 1.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 1991;114 (Pt 5):2283–2301. [DOI] [PubMed] [Google Scholar]
- 2.Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013;136:2419–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Politis M Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol 2014;10:708–722. [DOI] [PubMed] [Google Scholar]
- 4.Saeed U, Compagnone J, Aviv RI, et al. Imaging biomarkers in Parkinson’s disease and Parkinsonian syndromes: current and emerging concepts. Transl Neurodegener 2017;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin WR, Wieler M, Gee M. Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology 2008;70:1411–1417. [DOI] [PubMed] [Google Scholar]
- 6.Peran P, Cherubini A, Assogna F, et al. Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain 2010;133:3423–3433. [DOI] [PubMed] [Google Scholar]
- 7.Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 2009;72:1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du G, Lewis MM, Kanekar S, et al. Combined Diffusion Tensor Imaging and Apparent Transverse Relaxation Rate Differentiate Parkinson Disease and Atypical Parkinsonism. AJNR Am J Neuroradiol 2017;38:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du G, Liu T, Lewis MM, et al. Quantitative susceptibility mapping of the midbrain in Parkinson’s disease. Mov Disord 2016;31:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burciu RG, Ofori E, Archer DB, et al. Progression marker of Parkinson’s disease: a 4-year multi-site imaging study. Brain 2017;140:2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ofori E, Pasternak O, Planetta PJ, et al. Increased free water in the substantia nigra of Parkinson’s disease: a single-site and multi-site study. Neurobiol Aging 2015;36:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acosta-Cabronero J, Cardenas-Blanco A, Betts MJ, et al. The whole-brain pattern of magnetic susceptibility perturbations in Parkinson’s disease. Brain 2017;140:118–131. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Liu T. Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magn Reson Med 2014;73:82–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haacke EM, Liu S, Buch S, Zheng W, Wu D, Ye Y. Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging 2015;33:1–25. [DOI] [PubMed] [Google Scholar]
- 15.Hoy AR, Koay CG, Kecskemeti SR, Alexander AL. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. Neuroimage 2014;103:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine 2009;62:717–730. [DOI] [PubMed] [Google Scholar]
- 17.Du G, Lewis MM, Sica C, et al. Distinct progression pattern of susceptibility MRI in the substantia nigra of Parkinson’s patients. Mov Disord 2018;XX:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP. Diffusion tensor imaging of nigral degeneration in Parkinson’s disease: A region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. NeuroImage Clinical 2013;3:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glasser MF, Van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci 2011;31:11597–11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arshad M, Stanley JA, Raz N. Test-retest reliability and concurrent validity of in vivo myelin content indices: Myelin water fraction and calibrated T1 w/T2 w image ratio. Hum Brain Mapp 2017;38:1780–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci 2013;33:18618–18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Righart R, Biberacher V, Jonkman LE, et al. Cortical pathology in multiple sclerosis detected by the T1/T2-weighted ratio from routine magnetic resonance imaging. Ann Neurol 2017;82:519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011;54:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du G, Lewis MM, Sen S, et al. Imaging nigral pathology and clinical progression in Parkinson’s disease. Mov Disord 2012;27:1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura K, Chen JT, Ontaneda D, Fox RJ, Trapp BD. T1-/T2-weighted ratio differs in demyelinated cortex in multiple sclerosis. Ann Neurol 2017. [DOI] [PubMed] [Google Scholar]
- 28.Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Neurology 2016;86:566–576. [DOI] [PubMed] [Google Scholar]
- 29.Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology 2014;83:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis MM, Du G, Baccon J, et al. Susceptibility MRI captures nigral pathology in patients with parkinsonian syndromes. Mov Disord 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nalls MA, McLean CY, Rick J, et al. Diagnosis of Parkinson’s disease on the basis of clinical and genetic classification: a population-based modelling study. Lancet Neurol 2015;14:1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Planetta PJ, Ofori E, Pasternak O, et al. Free-water imaging in Parkinson’s disease and atypical parkinsonism. Brain 2016;139:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baudrexel S, Nurnberger L, Rub U, et al. Quantitative mapping of T1 and T2* discloses nigral and brainstem pathology in early Parkinson’s disease. Neuroimage 2010. [DOI] [PubMed] [Google Scholar]
- 34.Isaias IU, Trujillo P, Summers P, et al. Neuromelanin Imaging and Dopaminergic Loss in Parkinson’s Disease. Front Aging Neurosci 2016;8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoe H, Takeda Y, Kawahara H, Tanaka A, Morita K. Clinical significance of T1-weighted MR images following transient cerebral ischemia. J Neurol Sci 2006;241:19–24. [DOI] [PubMed] [Google Scholar]
- 36.Menke RA, Scholz J, Miller KL, et al. MRI characteristics of the substantia nigra in Parkinson’s disease: a combined quantitative T1 and DTI study. Neuroimage 2009;47:435–441. [DOI] [PubMed] [Google Scholar]
- 37.Graham JM, Paley MN, Grunewald RA, Hoggard N, Griffiths PD. Brain iron deposition in Parkinson’s disease imaged using the PRIME magnetic resonance sequence. Brain 2000;123 Pt 12:2423–2431. [DOI] [PubMed] [Google Scholar]
- 38.Deoni SC, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med 2008;60:1372–1387. [DOI] [PubMed] [Google Scholar]
- 39.Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature 2013;495:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.