Abstract
Background/Objective.
Recent prospective trials support the use of sentinel lymph node biopsy (SLNB) in breast cancer patients after neoadjuvant chemotherapy (NAC) with a lower false negative rate if three or more sentinel lymph nodes (SLNs) are identified. In this study, we investigated whether the pre-NAC axillary lymph node status influences the number of SLNs identified.
Methods.
Stage I–III breast cancer patients who received NAC and underwent SLNB from May 2014 to April 2016 were identified from an institutional prospective database. Clinical and pathological factors among clinically node-negative (cN−) and clinically node-positive (cN+) patients who converted to cN− post-NAC were compared. Generalized linear mixed models analyzed factors associated with the number of SLNs removed.
Results.
Among 343 patients who underwent SLNB during the study period, 335 (98%) had at least one SLN identified, and subsequently comprised the study population. The median number of SLNs identified was 4 (range 1–14), which did not differ according to pre-NAC nodal status (p = 0.15). Overall, 85% of patients had three or more SLNs identified (80% cN− group vs. 89% cN+ group; p = 0.02). On univariable analysis, age <50 years and presenting with a positive axillary node were significantly associated with identifying three or more SLNs.
Conclusions.
Our study confirms that SLNB was successfully performed in 98% of our patients after NAC, with very few failed mapping procedures. In the post-NAC setting, the median number of SLNs identified was four, and the status of the axilla prior to NAC did not negatively affect the number of SLNs identified.
INTRODUCTION
The ideal method of staging the axilla in breast cancer patients who present with node-positive disease prior to neoadjuvant chemotherapy (NAC) is unknown. Traditional staging is with axillary lymph node dissection (ALND); however, with modern chemotherapy, 40–65% of node-positive patients treated with NAC achieve a complete pathological response (pCR) in the axillary lymph nodes.1–3 Thus, performing a less-invasive staging procedure post-NAC to identify patients in whom ALND can be avoided is of current interest.
The main concern with utilizing sentinel lymph node biopsy (SLNB) after NAC is the false negative rate (FNR). In patients who are clinically node-negative (cN−) prior to NAC, the accuracy of SLNB is comparable to upfront surgery, with an FNR <10% using standard techniques;4,5 however, in patients who are cN+ prior to NAC, the FNR is higher and ranges from 12.6 to 14.2% in prospective studies.6,7 The most important determinant of FNR post-NAC is the number of sentinel lymph nodes (SLNs) retrieved. For example, in over 1100 cN+ patients undergoing SLNB and ALND in the ACOSOG Z1071 and SENTINA trials, the FNR ranged from 24 to 36% when only one SLN was identified, but improved to 7–9% when three or more SLNs were retrieved.6–8
Current National Comprehensive Cancer Network (NCCN) guidelines support the use of SLNB after NAC in patients presenting with axillary metastasis only when the axilla is restaged to node negative after NAC and when techniques to lower the FNR are practiced. Specifically, the use of dual-agent mapping, and obtaining three or more SLNs when possible, are recommended.9
However, three or more SLNs were identified only 30–55% of the time in the aforementioned studies, thus questioning whether SLNB alone is a practical staging procedure in patients who are cN+ prior to NAC. To our knowledge, this is the first study to evaluate factors associated with the number of SLNs identified after NAC. In this study, we investigated whether the pre-NAC axillary lymph node status influences the number of SLNs identified.
METHODS
Upon Institutional Review Board approval, patients with clinical stage I–III breast cancer receiving NAC at Memorial Sloan Kettering Cancer Center (MSKCC) were entered into a prospective Health Insurance Portability and Accountability Act (HIPAA)-compliant database to allow for evaluation of surgical outcomes. The surgeon’s pre- and post-NAC clinical assessment of the axilla was recorded. A clinically negative (cN−) axilla pre-NAC was defined as the absence of palpable disease in the nodal basin, whereas a clinically node-positive (cN+) axilla pre-NAC was defined as palpable disease in the nodal basin and/or biopsy-proven metastasis based on core needle biopsy or fine-needle aspiration when performed. Clips were not routinely placed in the suspicious or positive node. After completion of NAC, patients who remained cN−, or who converted to cN− based on physical examination, were eligible for SLNB. Axillary ultrasound is not routinely performed after NAC. Patients who presented with cN2/cN3 disease were considered ineligible for SLNB regardless of response to NAC.
We performed a retrospective review of consecutive patients in this database who underwent NAC and were eligible for SLNB between May 2014 and April 2016. The SLNB method was standardized. All patients who were cN+ pre-NAC and who converted to cN− based on clinical examination post-NAC required dual mapping using technetium-99m sulfur colloid and isosulfan blue dye. In patients who were cN− and remained cN−, the use of a dual-agent technique was left to the surgeon’s discretion. All hot, blue, or palpably abnormal nodes were considered SLNs and were subsequently removed. A ‘hot’ node is defined as a node containing radioactivity counts of at least 10% of the hottest node. All patients with tumor found in SLNs (including micrometastases and isolated tumor cells), or who had failed SLN mapping, underwent ALND. Additionally, in patients who had presented as cN+ pre-NAC, ALND was performed if fewer than three SLNs were identified regardless of the malignant status of the retrieved SLNs.
Patient, tumor, and treatment variables were collected. Continuous variables were summarized using median (minimum, maximum), while categorical variables were summarized using frequency (%). Between-group comparisons were made using the Wilcoxon rank-sum test and Fisher’s exact test for continuous and categorical variables, respectively. We used the median number of three SLNs removed as the cut-off for analysis, relevant to current clinical guidelines.9 Generalized linear mixed models were implemented for univariable and multivariable analysis of factors associated with having three or more SLNs removed. All models incorporated a random surgeon effect to account for intersurgeon variability in the number of SLNs removed. A p-value <0.05 was considered statistically significant. All statistical analyses were conducted using R software version 3.4.1 (R Core Development Team, Vienna, Austria).
RESULTS
Overall, 348 patients with clinical stage I–III breast cancer received NAC and were subsequently eligible for SLNB at MSKCC during the study period. Five patients had bilateral disease and were excluded from the analysis. Of the remaining 343 patients, 144 were cN− and 199 were cN+ prior to NAC receipt. Dual-mapping was used in 97% of procedures. Failed mapping occurred in three cN− patients and five cN+ patients, for identification rates of 97.9% and 97.5%, respectively. The remaining 141 cN− and 194 cN+ patients had at least one SLN identified and constituted the study population.
Table 1 compares the clinical and pathological characteristics of the population overall and according to pre-NAC clinical nodal status. Median patient age was 50 years (range 27–82), and average tumor size was 4 cm (range 0.7–12). Receptor status was estrogen receptor-positive (ER+) in 191 (57%) patients, ER−/HER2+ in 40 (12%) patients, and triple-negative in 104 (31%) patients. There were no significant differences between groups.
TABLE 1.
Clinicopathological characteristics of patients overall and by pre-NAC clinical nodal status
| Characteristic | Overall [n =335] | cN− a [n=141] | cN+ a [n=194] | p-Value |
|---|---|---|---|---|
| Median age, years (range) | 50 (27–82) | 50 (28–72) | 50 (27–82) | 0.50 |
| Median BMI, kg/m2 (range) | 26 (15–55) | 25 (17–55) | 26 (15–48) | 0.53 |
| Median tumor size, cm (range) | 4 (0.7–12) | 4 (1.1–11) | 4 (0.7–12) | 0.60 |
| Nuclear grade [n (%)] | 0.62 | |||
| 1 | 2 (0.6) | 0 (0) | 2 (1) | |
| 2 | 79 (23.6) | 32 (22.7) | 47 (24.2) | |
| 3 | 242 (72.2) | 103 (73) | 139 (71.6) | |
| NA | 12 (3.6) | 6 (4.3) | 6 (3.1) | |
| Histology [n (%)] | 0.07 | |||
| Ductal | 305 (91) | 129 (91.5) | 176 (90.7) | |
| Lobular/mixed | 27 (8.1) | 12 (8.5) | 15 (7.7) | |
| Other | 3 (0.9) | 0 (0) | 3 (1.5) | |
| Receptor status [n (%)] | 0.06 | |||
| ER+/HER2− | 97 (29) | 34 (24.1) | 63 (32.5) | |
| ER+/HER2+ | 94 (28.1) | 47 (33.3) | 47 (24.2) | |
| ER−/HER2+ | 40 (11.9) | 12 (8.5) | 28 (14.4) | |
| ER−/HER2− | 104 (31) | 48 (34) | 56 (28.9) |
cN status based on pre-neoadjuvant-presenting nodal status
NAC neoadjuvant chemotherapy, cN− clinically node-negative, cN+ clinically node-positive, BMI body mass index, ER estrogen receptor, HER human epidermal growth factor receptor, NA not available
Final surgical procedure and pathological characteristics are shown in Table 2. Compared with patients with pre-NAC cN− status, patients who initially presented as cN+ prior to NAC were more likely to have a positive SLN on final pathology (41.8% vs. 13.5%; p < 0.001), and were also more likely to undergo ALND (42.3 vs. 12.1%; p < 0.001).
TABLE 2.
Final surgical procedure and pathological characteristics in patients overall and by pre-NAC clinical nodal status
| Characteristic | Overall [n=335] | cN0 [n=141] | cN1 [n = 194] | p-Value |
|---|---|---|---|---|
| Surgery year | 0.23 | |||
| 2014 | 85 (25.4) | 42 (29.8) | 43 (22.2) | |
| 2015 | 202 (60.3) | 78 (55.3) | 124 (63.9) | |
| 2016 | 48 (14.3) | 21 (14.9) | 27 (13.9) | |
| Breast surgery | 0.10 | |||
| BCS | 155 (46.3) | 73 (51.8) | 82 (42.3) | |
| Mastectomy | 180 (53.7) | 68 (48.2) | 112 (57.7) | |
| Axillary surgery | <0.001 | |||
| SLNB alone | 236 (70.4) | 124 (87.9) | 112 (57.7) | |
| SLNB + ALND | 99 (29.6) | 17 (12.1) | 82 (42.3) | |
| pCRa | 0.02 | |||
| No | 226 (67.5) | 85 (60.3) | 141 (72.7) | |
| Yes | 109 (32.5) | 56 (39.7) | 53 (27.3) | |
| SLN statusb | <0.001 | |||
| SLN− | 235 (70.1) | 122 (86.5) | 113 (58.2) | |
| SLN+ | 100 (29.9) | 19 (13.5) | 81 (41.8) |
Data are expressed as n (%)
NAC neoadjuvant chemotherapy, BCS breast-conserving surgery, SLNB sentinel lymph node biopsy, ALND axillary lymph node dissection, pCR pathological complete response, SLN sentinel lymph node, DCIS ductal carcinoma in situ, cN0 clinically node-negative, cN1 metastasis to one or more moveable ipsilateral lymph nodes
No invasive or DCIS in the breast and axilla
Any isolated tumor cell, micrometastasis (<2 mm) or macrometastasis (>2 mm) based on final pathological analysis. Immunohistochemistry not routinely performed
Table 3 compares the number of SLNs identified according to pre-NAC clinical nodal status. The median number of SLNs identified was 4 (range 1–14) overall and did not differ between groups (p = 0.15). The NCCN recommendation of identifying three or more SLNs was met in 85% of patients and occurred more often in patients who were cN+ prior to NAC (89.2% vs. 79.4%; p = 0.02). Figure 1 demonstrates the management of the pre-NAC cN+ patients in whom only one to two SLNs were retrieved; 21 patients had one to two SLNs retrieved. In 12/21 patients, at least one SLN was positive for cancer cells, and, apart from two patients in the Alliance A011202 study who were randomized to the no-ALND arm, all underwent ALND. In the remaining nine patients, SLNs were negative for cancer cells. Two patients did not have completion ALND (cALND) based on patient or surgeon preference, while the other seven patients underwent cALND. In six patients, no positive nodes were found at ALND; however, in one patient, macrometastatic disease was detected in 2/16 nodes despite no cancer cells being found in the SLNs. Thus, among the 13 patients with positive final axillary status and fewer than three SLNs retrieved, there was one false-negative event (FNR 7.7%).
TABLE 3.
Number of SLNs identified by pre-NAC cN status and univariable analysis of factors associated with <3 vs. ≥3 SLNs
| cN− [n = 141] | cN+ [n = 194] | p-Value | |
|---|---|---|---|
| SLN mean | |||
| SLN median (range) | 4 (1–14) | 4(1–14) | 0.15 |
| Excised SLNs [n (%)] | 0.02 | ||
| 1–2 | 29 (20.6) | 21 (10.8) | |
| ≥3 | 112 (79.4) | 173 (89.2) | |
| Factor | OR (95% CI) | ||
| Age <50 vs. ≥50 years | 2.00 (1.06–3.75) | 0.03 | |
| BMI | 1.00 (0.95–1.06) | 0.96 | |
| Clinical tumor size | 1.07 (0.92–1.25) | 0.39 | |
| Pre-NAC cN+ vs. cN− | 2.12 (1.15–3.90) | 0.02 | |
| Nuclear grade | 0.80 (0.38–1.70) | 0.57 | |
| pCRa | 0.62 (0.34–1.15) | 0.13 | |
SLN sentinel lymph node, OR odds ratio, CI confidence interval, BMI body mass index, NAC neoadjuvant chemotherapy, cN+ clinically node-positive, cN− clinically node-negative, pCR pathological complete response
FIG. 1.
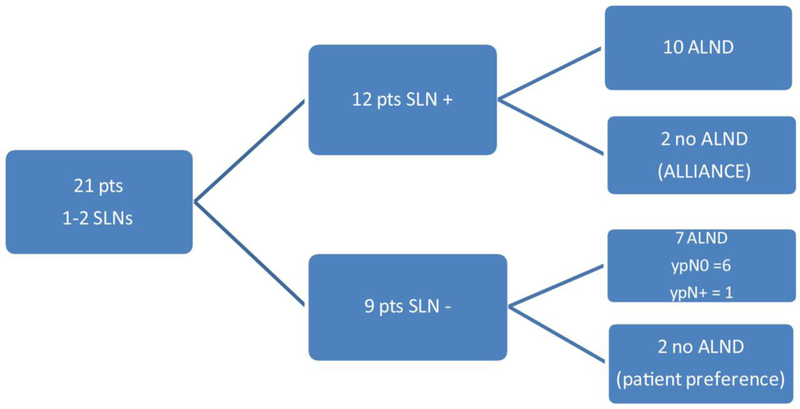
Management of pre-NAC cN+ patients. NAC neoadjuvant chemotherapy, cN+ clinically node-positive, pts patients, SLN sentinel lymph node, ALND axillary lymph node dissection, ypN0 node negative on final surgical pathology, ypN+ node positive on final surgical pathology
The number of SLNs identified by year of surgery and cN status is shown in Fig. 2. A consistent median of four SLNs was identified in the pre-NAC cN+ patients throughout the study period; however, there was a significant trend towards identifying fewer SLNs in the pre-NAC cN− patients over time, with a median of 4.5, 4, and 3 SLNs identified in this group when surgery was performed in 2014, 2015, and 2016, respectively (p-value for trend = 0.04).
FIG. 2.
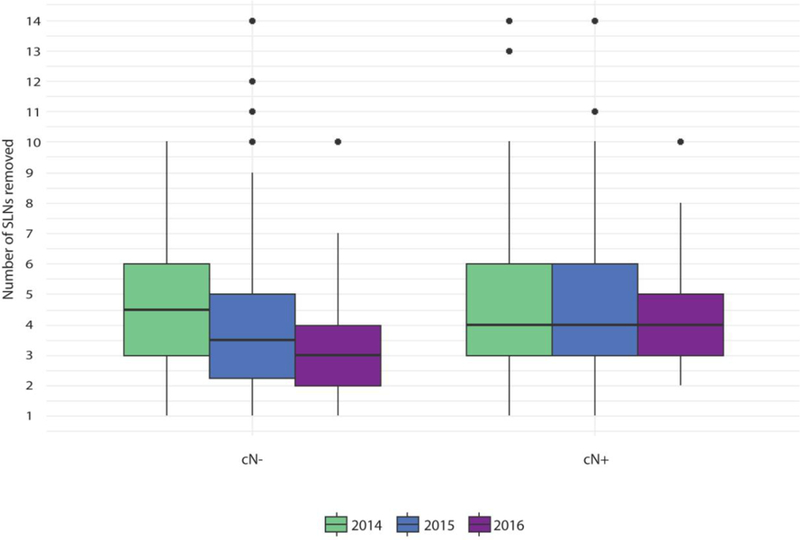
Number of SLNs identified by year according to pre-NAC cN status. SLN sentinel lymph node, NAC neoadjuvant chemotherapy, cN clinical node
The univariable analyses of factors associated with identifying three or more SLNs are shown in Table 3. Patients with age at surgery <50 years versus ≥50 years (p = 0.03) and pre-NAC cN+ versus cN− status were more likely to have three or more nodes removed (odds ratio 2.12, 98% confidence interval 1.15–3.90; p = 0.016). No other patient/tumor factors were significantly associated with identifying three or more SLNs.
DISCUSSION
Staging the axilla in patients who present with axillary metastasis and convert to cN− after NAC is controversial. Early theoretical concerns cautioned the use of SLNB in patients after NAC.10–12 Consequently, NAC was a consistent exclusion criterion in the primary SLNB clinical trials, and patients were obligated to staging with ALND. However, increased nodal pCR rates with modern chemotherapy prompted recent clinical trials to investigate SLNB performance in cN+ patients in the post-NAC setting. The results of the two largest trials found the FNR of SLNB was above the 10% threshold predetermined to be clinically acceptable.6,7 Thus, current efforts are directed toward improving technical approaches to less-invasive staging alternatives to ALND in this population. Several techniques have been proposed, including marking and retrieving the clinically positive nodes,13–15 and optimizing SLNB with dual-agent mapping and retrieval of three or more SLNs.6,7
Clinical trials support that an acceptable FNR (7–9%) can be obtained with SLNB alone, provided that three or more SLNs are obtained.6–8 However, in ACOSOG Z1071, among the 651 patients with biopsy-proven nodal metastasis prior to NAC (12% with remaining palpable disease at surgery), 279/651 (43%) had fewer than three SLNs retrieved. Likewise, in the SENTINA trial, 391/592 (66%) patients who were cN+ at presentation and converted to node-negative after NAC (arm C) had fewer than three SLNs identified at surgery. In contrast, we found the majority of the cN+ patients during our study period (173/194, 89.2%) had three or more SLNs retrieved.
The most significant factor associated with the retrieval of more SLNs in the present study was pre-NAC clinical nodal status. Despite theoretical concerns that treatment-related changes (i.e. tumor plugging or fibrosis) might impede the technical success of SLNB in patients presenting with nodal disease burden,11,12 we found this not to be the case. In fact, identification rates were equivalent between cN+ and cN− patients, and cN+ patients were more likely to have three or more SLNs removed (89% of cN+ prior to NAC vs. 80% cN−; p = 0.02). There were no differences in SLNB technique between our cN+ and cN− patients to explain this difference in node retrieval. However, the management strategy differed slightly. In cN+ patients, identifying three or more SLNs was mandatory; otherwise, staging was completed with ALND and, in cN− patients, this was not a requirement. Thus, it is possible there was surgeon bias to look harder for additional SLNs in the cN+ patients to avoid more morbid axillary surgery. Surgeon bias has been shown to be a factor in the retrieval of more SLNs in the primary setting in patients who surgeons think have worse disease or a higher risk of node positivity.16
Age was also a factor affecting the number of SLNs identified, such that patients <50 years of age had more nodes retrieved. This phenomenon is also seen in primary surgery16,17 and is not completely understood, but may be age-related changes in the lymphatic system. Interestingly, when we analyzed the factors associated with identifying fewer than four versus four or more SLNs, nodal status was no longer significant and age was the only contributing factor (data not shown). This suggests surgeon bias may be working at the critical level of identifying three SLNs, but once this critical number of nodes is met, the same factors seen with primary SLNB are associated with greater node retrieval.
The range of SLNs retrieved was wide in our study and was similar in both the cN+ and cN− populations (range 1–14 SLNs). A wide range of node retrieval is also seen in the primary setting where the concept of ‘when to stop’ in case of multiple SLNs has been extensively studied. While a majority of patients have the first positive SLN within the first three nodes, in some patients the first positive node is found at the fourth to eighth SLN removed, thus supporting continuation of the SLNB procedure until all hot and blue nodes are removed.18,19
No other clinical or pathological factors identified in our current study predicted failure to retrieve three or more SLNs, including residual disease in the axilla/breast. Thus, we are unable to clarify why so few of our patients had fewer than three SLNs retrieved compared with other published studies. One difference is that dual mapping was performed in 97% of our patients, whereas only 79% of patients in ACOSOG Z1071 and 28% of patients in SENTINA had dual mapping.6,7 Dual mapping is known to improve identification rates post-NAC20 and is also known to increase the number of SLNs identified at primary surgery.
When fewer than three SLNs are found at SLNB in cN+ patients, SLNB has an FNR of >10%. While the clinical consequence of a false-negative event after NAC is not yet known, this threshold is considered clinically unacceptable (SENTINA). Among the 194 cN+ patients in our study, 21/194 (11%) had only one to two SLNs identified. In the 18/21 patients who ultimately had an ALND, the SLN was accurate in predicting the final axillary nodal status in 17/18 patients (94%). Among the 13 patients with residual axillary disease identified at ALND, one or more SLNs were positive in 12 patients, but falsely negative in one patient (FNR 7.7%). While this FNR appears low, a sample size of n = 13 is too few to be clinically valuable. Conversely, identifying a false-negative event in a small patient sample is more likely consistent with clinical trials stating that retrieving only one to two SLNs may be incomplete staging in these patients.
At our institution, we follow the guideline of dual-agent mapping and mandate retrieval of three or more SLNs at SLNB to stage cN+ patients after NAC. Other groups have suggested clipping and removing the clinically positive lymph nodes as well.13,15,21 The University of Texas MD Anderson Cancer Center experience demonstrates a non-statistically significant improvement with targeted ALND + SLNB compared with SLNB alone. However, it is relevant to consider that in this series, dual mapping was used in only 55% of SLNB procedures and the median number of SLNs retrieved was two. Thus, it is unclear whether there is added benefit of localizing a clipped node (in addition to SLNB) when dual-agent mapping is used and three or more SLNs are retrieved. Additionally, there are logistical problems with localizing clipped nodes as this process requires additional procedures for the patient, as well as resources and time.22
Our study emphasizes that the recommended target of obtaining three or more SLNs can be achieved in a majority of patients when dual mapping is employed, and that the presenting clinical nodal status does not lower the ability to do this.
Limitations
The main limitation to our study was limited follow-up data. Based on evidence from ACOSOG Z1071 and SENTINA, evaluating at least three SLNs in cN+ patients after NAC will result in an FNR of approximately 10%.6–8 In the primary surgery setting, despite an FNR of 10%, the risk of axillary failure after SLNB is low (<1% at 8.5 years) and is not different from patients staged with ALND.23 However, little information is available about the clinical consequences of a false-negative event after NAC.24 Thus, while we demonstrate the feasibility of optimizing SLNB performance in patients after NAC, we cannot prove SLNB alone is an equivalent alternative to ALND in this population. Long-term follow-up is needed.
CONCLUSIONS
In this single-institution study of 343 patients, we found SLNB was successfully performed in 98% of patients and the median number of SLNs retrieved was four. Clinical node status pre-NAC did not negatively affect the number of SLNs retrieved at SLNB, and more than three nodes were retrieved in 89% of patients who initially presented with node-positive breast cancer.
SYNOPSIS.
We investigated whether axillary lymph node status before neoadjuvant chemotherapy (NAC) influences the number of sentinel lymph nodes (SLNs) identified, and found that the status of the axilla prior to NAC did not negatively affect the number of SLNs identified.
Acknowledgments
FUNDING The preparation of this manuscript was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Footnotes
DISCLOSURES
This study was presented in poster format at the 71st Society of Surgical Oncology Annual Cancer Symposium, 21–24 March 2018, Chicago, IL, USA. The findings presented in this study have not been published elsewhere.
REFERENCES
- 1.Barrio AV, Mamtani A, Edelweiss M, et al. How Often Is Treatment Effect Identified in Axillary Nodes with a Pathologic Complete Response After Neoadjuvant Chemotherapy? Ann Surg Oncol 2016; 23(11):3475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997; 15(7):2483–93. [DOI] [PubMed] [Google Scholar]
- 3.Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg 2014; 260(4):608–14; discussion 14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Classe JM, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol 2009; 27(5):726–32. [DOI] [PubMed] [Google Scholar]
- 5.Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2005; 23(12):2694–702. [DOI] [PubMed] [Google Scholar]
- 6.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013; 310(14):1455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013; 14(7):609–18. [DOI] [PubMed] [Google Scholar]
- 8.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 2015; 33(3):258–64. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology, Breast Cancer. Version. 1 2018.
- 10.Alvarado R, Yi M, Le-Petross H, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol 2012; 19(10):3177–84. [DOI] [PubMed] [Google Scholar]
- 11.Sharkey FE, Addington SL, Fowler LJ, Page CP, Cruz AB. Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol 1996; 9(9):893900. [PubMed] [Google Scholar]
- 12.Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer 2002; 95(4):681–95. [DOI] [PubMed] [Google Scholar]
- 13.Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg 2015; 261(2):378–82. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed M, Douek M. Targeted axillary dissection after neoadjuvant therapy in breast cancer. Br J Surg 2018; 105(4):313–4. [DOI] [PubMed] [Google Scholar]
- 15.Caudle AS, Yang WT, Krishnamurthy S, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol 2016; 34(10):1072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Port ER, Patil S, Stempel M, Morrow M, Cody HS 3rd. Number of lymph nodes removed in sentinel lymph node-negative breast cancer patients is significantly related to patient age and tumor size: a new source of bias in morbidity assessment? Cancer 2010; 116(8):1987–91. [DOI] [PubMed] [Google Scholar]
- 17.Subhedar P, Stempel M, Eaton A, Morrow M, Gemignani ML. Do the ACOSOG Z0011 Criteria Affect the Number of Sentinel Lymph Nodes Removed? Ann Surg Oncol 2015; 22 Suppl 3:S470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarter MD, Yeung H, Fey J, Borgen PI, Cody HS 3rd. The breast cancer patient with multiple sentinel nodes: when to stop? J Am Coll Surg 2001; 192(6):692–7. [DOI] [PubMed] [Google Scholar]
- 19.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 2007; 8(10):881–8. [DOI] [PubMed] [Google Scholar]
- 20.Boughey JC, Suman VJ, Mittendorf EA, et al. Factors affecting sentinel lymph node identification rate after neoadjuvant chemotherapy for breast cancer patients enrolled in ACOSOG Z1071 (Alliance). Ann Surg 2015; 261(3):547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance). Ann Surg 2016; 263(4):802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen TT, Hieken TJ, Glazebrook KN, Boughey JC. Localizing the Clipped Node in Patients with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy: Early Learning Experience and Challenges. Ann Surg Oncol 2017; 24(10):3011–6. [DOI] [PubMed] [Google Scholar]
- 23.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010; 11(10):927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galimberti V, Ribeiro Fontana SK, Maisonneuve P, et al. Sentinel node biopsy after neoadjuvant treatment in breast cancer: five-year follow-up of patients with clinically node-negative or node-positive disease before treatment. Eur J Surg Oncol 2016; 42(3):361–8. [DOI] [PubMed] [Google Scholar]


