Abstract
BACKGROUND:
Tourette syndrome (TS) has a well-established genetic background, but its genetic architecture remains largely unknown. The authors investigated the role of polygenic risk scores (PRS) derived from a TS genome-wide association study (GWAS) in relation to the occurrence of tics and associated traits in a general population cohort.
METHODS:
Using the most recent TS GWAS (N cases = 4,819; N controls = 9,488) as the discovery sample, PRS were calculated in ALSPAC participants (N = 8,941). Regression analyses were used to assess whether PRS predicted the presence and chronicity of tics, and symptom severity of obsessive-compulsive (OCD), attention-deficit/hyperactivity (ADHD), and autism spectrum disorder (ASD) in ALSPAC participants.
RESULTS:
Following correction for multiple testing, the PRS significantly predicted presence (R2 = 0.48%, P empirical = 0.01, Q = 0.04), but not the chronicity of tics (R2 = 0. 16%, P empirical = 0.07, Q = 0.14) in the ALSPAC cohort, nor the severity of OCD (R2 = 0.11%, P empirical = 0.11, Q = 0.15), ADHD (R2 = 0. 09%, P empirical = 0.19, Q = 0.21), and ASD (R2 = 0. 12%, P empirical = 0.09, Q = 0.14).
CONCLUSIONS:
The authors found a significant polygenic component of tics occurring in a general population cohort based on PRS derived from a GWAS of individuals with a TS diagnosis. This supports the notion that tics along a spectrum from non-clinical to clinical symptom levels share a similar genetic background.
Keywords: Attention-deficit/hyperactivity disorder, Autism spectrum disorder, Avon Longitudinal Study of Parents and Children (ALSPAC), Obsessive-compulsive disorder, Polygenic risk score, Tourette Syndrome
Introduction
Tourette syndrome (TS) has a well-established genetic background, with twin study based heritability estimates in the range of 25–56% (1, 2). While its genetic etiology remains elusive, there is involvement of common polygenic and/or de novo variation (3, 4) The first genome-wide association study (GWAS) of TS did not identify genome-wide significant single nucleotide polymorphism (SNPs) (5). In line with other neuropsychiatric disorders such as schizophrenia (9), an increase in sample size will promote the detection of genome-wide significant SNPs; however, a single SNP only explains a small portion of the total genetic risk of complex heterogeneous disorders such as TS (6). Genome-wide Complex Trait Analysis (using data from the first TS GWAS; (5)) indicated that common variation distributed across the genome together account for a large proportion of TS heritability (6). Therefore, utilizing sub-threshold GWAS signals may offer a better approach in understanding the involvement of common genetic variation in TS.
One method that includes sub-threshold GWAS signals is a polygenic risk score (PRS) analysis in which an aggregate score derived from the risk alleles based on a TS GWAS is used to predict tic phenotypes in an independent sample. So far, two TS PRS studies have been conducted of which the first found that TS PRS (nonsignificantly) predicted 0.6% of the phenotypic variance in a TS case-control target cohort, the non-significant prediction probably due to a modest sample size (a target sample of 265 cases and 196 controls) (7). The second study found that TS PRS were significantly associated with a symmetry phenotype (R2 = 0.57%), as defined by factor analysis that included items such as evening up and ordering things (4), behaviors that are often seen in subjects with TS and obsessive-compulsive disorder (OCD).
While these studies (4, 6, 7) pointed to a polygenic component of TS in clinical samples, it is currently not known whether TS-based PRS would predict the broader spectrum of tics in the general population. The notion of a tic disorder spectrum has been supported by some studies describing a continuum along TS and chronic motor/vocal tic disorders (8)(9), also including transient tic disorder (2, 10).
The current study investigated whether PRS derived from the most recent GWAS of individuals diagnosed with TS would predict the presence and chronicity of tics in a population-based birth cohort, the Avon Longitudinal Study of Parents and Children (ALSPAC, (11, 12)). We also related the TS GWAS based PRS to symptoms of OCD, attention-deficit/hyperactivity disorder (ADHD), and autism spectrum disorder (ASD) within ALSPAC, given the high comorbidity rates between TS and these associated disorders suggesting at least a partially shared genetic etiology (4, 13–15).
Methods and Materials
Participants
ALSPAC is an ongoing population-based birth cohort study of 14,541 mothers and their children residing in the county of Avon (UK) (11, 12). Participants are assessed in regular intervals from birth, using clinical interviews, self-reported questionnaires, medical records, and physical examinations. The study website contains details of available data through a fully searchable data dictionary: http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/. Ethical approval was obtained from the ALSPAC Ethics and Law Committee. All participants provided written informed consent or assent.
Phenotypic assessment within ALSPAC
Tics.
Presence of tics was assessed during nine time-points: annually between the ages 1.5 and 7.6 years, at age 10.7, and at age 13.8. Parents received a questionnaire in which they reported whether or not their child “has a tic or twitch” (“Never” = 0, “Less than once a week”, and “once a week or more” = 1) and at age 10 whether or not their child “has any tics or twitches in the past year that he/she can’t seem to control” (“No” = 0, “Yes” = 1). At age 13, the presence of specific motor and/or vocal tics was reported by the mothers using a questionnaire containing five questions regarding the presence of repeated movements in the past year of the (1) face and head, (2) neck, shoulder, or trunk, (3) arms, hands, legs, feet; and of (4) repeated noises and sounds and (5) repeated words or phrases; (“not at all” = 0, “definitely” and “probably” = 1). The frequency of these tic symptoms was also assessed (“less than once a month” = 1; “1–3 times a month” = 2; “about once a week” = 3; “more than once a week” = 4; “every day” = 5).
Consistent with previous ALSPAC publications (16, 17), we used three diagnostic constructs to define the presence of tics (0=absent, 1=present): (1) Presence of “Tics intermediate” (approximating the ‘Tourette syndrome/chronic tic disorder intermediate’ definition by Scharf et al. (16)), which required the presence of motor and/or vocal tics occurring at least once a week at age 13 and at least one positive response to a tic-screening question between the ages of 1.5 and 7 years and at age 10 years; (2) Presence of “Tics broad” (approximating the ‘Tourette syndrome/chronic tic disorder broad’ definition Scharf et al. (16)), which only required the presence of motor and/or vocal tics occurring at least once a week at age 13; and (3) Presence of “Tics all” that only required at least one positive answer to the tic screening question between the ages 1.5 and 13. Moreover, we calculated a chronicity score (possible range 0-13), i.e., the sum score of the number of times tics were present up to age 10.7 (possible range 0-8) plus the number of different tic symptoms at 13.8 years (possible range 0-5). Consistent with a previous publication of Scharf et al. (16), we removed subjects who likely had non-tic movement such as stereotypies (e.g., only reporting repeated movements of arms, legs, or feet) or isolated echolalia. However, we did not exclude participants based on IQ. Furthermore, we did not use the diagnostic construct “TS/CT narrow” as defined by Scharf et al. (16) due to an insufficient sample size of cases (N < 100).
OCD severity.
We used the parent-on-child Development and Well-Being Assessment (DAWBA; (18)) questionnaire data as administered at ages 7.5 and 13.8 years that has shown high validity to detect obsessions and compulsions (19). A total of 10 OCD symptoms were extracted (e.g. excessive cleaning, checking things), and scored as absent (“no” = 0), “sometimes present” (=1), or “often present” (= 2) (11, 12, 20). OCD symptom severity was based on the highest sum score (i.e., either at age 7.5 or age 13.8 years, whichever was highest) of all 10 items (possible range 0-20), using prorating in case of less than 30% missing items (11, 12).
ADHD severity.
The DAWBA at age 7.5 years was also used to assess 18 ADHD symptoms reported on a 3-point scale (“no” = 0, “a little more than others” = 1, “a lot more than others” = 2) (18). ADHD symptom severity was calculated as the sum score of all 18 items again using prorating when less than 30% of the items were missing (possible range 0-36; (11, 12, 21)).
ASD severity.
The Social Communication Disorders Checklist (SCDC; (22)) was used to assess 12 ASD symptoms (“not true” = 0, “quite or sometimes true” = 1, “very often true” = 2) relating to social and emotional skills (e.g. “not aware of other people’s feelings”, “does not realize when others are upset or angry”) by parental report at age 7.5 years. The SCDC has been demonstrated to have excellent internal consistency (0.93) and test-retest reliability (0.81) making it a valid screening questionnaire for autistic traits (22). ASD symptom severity reflected the sum score of all 12 items using prorating (possible range 0-24; (11, 12, 23, 24)).
Genotyping
Within ALSPAC, genotype data was available for 9,915 children out of the total of 14,541 ALSPAC participants. Genotyping was done using the Illumina HumanHap550 quad genome-wide SNP genotyping platform. Standard quality controls were applied including exclusion of individuals with excessive missingness (i.e., >3%), gender mismatch, excessive or minimal heterozygosity, cryptic relatedness as measured by identity by descent (genome-wide identity by descent (IBD)>10%), duplicate samples, and non-European ancestry as defined by multidimensional scaling modeling using the HapMap Phase II (release 22) reference populations. SNPs that showed excessive missingness (i.e., call rate less than 95%), minor allele frequency less than 1%, or a departure from the Hardy-Weinberg equilibrium (P value < 5 × 10−7) were excluded. The dataset contained 500,527 SNPs after quality control. To increase the coverage of SNPs, genotype imputation was carried out with Impute2 (v2.2.2) (25) and phasing by ShapeIt (v2.r727) (26) using the 1000 genomes phase 1 version 3 (release date 21/05/2011) as a reference dataset . Final quality control checks were performed on the imputed dataset; any SNPs with MAF less than 1%, excessive missing rate (call rate less than 95%), Impute2 information quality metric of < 0.8, and not confirming to Hardy-Weinberg equilibrium (P < 5 × 10−7) were removed. After data cleaning, a total of 8,941 individuals (4,580 males, 4,361 females) and 6,976,085 SNPs remained in the ALSPAC dataset. The final number of SNPs eligible for analyses (SNPs present in both the discovery and target dataset) was 6,425,471.
PRS calculations
PRS were based on summary statistics of a GWAS of 4,819 TS cases (75% male) and 9,488 controls (43% male; Yu et al., submitted). Cases for the GWAS study had mostly been recruited from TS specialty clinics. The calculation, application, and evaluation of the PRS was carried out with PRSice (2.1.2.beta ; github.com/choishingwan/PRSice/) (28). PRSice relies on PLINK to carry out necessary cleaning steps prior to PRS calculation (27, 28). SNPs that were present exclusively in the second GWAS dataset or in the ALSPAC dataset, as well as strand-ambiguous SNPs were removed prior to the risk scoring. Clumping was applied to thin SNPs according to linkage disequilibrium and P-value: the SNP with the smallest P-value in each 250 kilobase window was retained and all those in linkage disequilibrium (r2 > 0.1) with this SNP were removed. The Major histocompatibility complex (MHC) locus was also removed due to its complex linkage disequilibrium structure.
The PRS was calculated for each individual as a sum of the risk alleles they carried, weighted by the odds ratio estimated by the TS GWAS study. This calculation was carried out at different (GWAS) P-value thresholds of the risk alleles (between P = 0.0001 and P = 0.9999). The strength of the polygenic signal was evaluated across all P-value thresholds (Figures S1-S2).
Statistical analysis
Logistic regression analyses were applied to predict all phenotypic binary outcome measures (i.e., presence of “Tics intermediate”, “Tics broad”, and “Tics all”) and linear regression analyses to predict the continuous outcome measures (chronicity score, OCD, ADHD, and ASD symptom severity). Considering that all continuous outcome measures were highly positively skewed (Figure S3), as a sensitivity analysis, we also estimated the regression parameters using a negative binomial regression (Table S1). Sex was included as a covariate in all regression models to correct for sex differences. We did not include ancestry-informative dimensions as covariates in the regression models as we did not find evidence for bias due to population stratification (for results see Supplemental figures S4-6 and Supplemental tables S2-3).
We first predicted the presence of tics (according to the “Tics intermediate”, “Tics broad”, and “Tics all” definitions) and the chronicity score using the TS PRS. We then predicted OCD, ADHD, and ASD symptom severity using TS PRS. Note that all analyses included the total ALSPAC sample, irrespective of comorbidity (tics, OCD, ADHD, ASD). We report the PRS model with the most predictive P-value thresholds as measured by Nagelkerke’s R2; model fits at different P-value thresholds can be found in supplementary figures S1-2. Furthermore, to determine if removal of the MHC locus significantly impacted our analyses we also carried out the analyses including the MHC locus (Table S4).
Considering that the PRS were calculated at a large number of different P-value thresholds, we adjusted for multiple testing and over-fitting using the permutation option in PRSice (28); empirical P-values were obtained by comparing the uncorrected P-values from the regression models with P-values obtained from a null distribution generated by permuting the phenotypes 11,000 times. The empirical P-values were corrected for multiple testing for the number of P-value thresholds tested, and subsequently corrected for the number of phenotypes (N = 7) applying the Benjamini-Hochberg false discovery rate (FDR) method. The significance threshold was met if the FDR adjusted empirical P value (i.e. Q) was < 0.05; we present both the empirical and the FDR adjusted empirical P values (Table 2).
Table 2:
Number of SNPs contributing to the prediction of each phenotype at the P-value threshold of the best-fitted polygenic risk score model
| Phenotypea | Best fitting PT |
N SNPs at PT |
β | Nagelkerke’s Pseudo R2 |
P
empiricalb |
FDR
Q-valuec |
|---|---|---|---|---|---|---|
| Tics intermediate | 0.0001 | 162 | 17.3 | 0.0013 | 0.95 | 0.95 |
| Tics broad | 0.0022 | 2386 | 111.2 | 0.0048 | 0.01 | 0.04 |
| Tics all | 0.0022 | 8448 | 98.2 | 0.0046 | 0.002 | 0.01 |
| Chronicity score | 0.0724 | 40120 | 152.4 | 0.0016 | 0.07 | 0.14 |
| OCD symptom severity | 0.003 | 470 | 22.7 | 0.0011 | 0.11 | 0.15 |
| ADHD symptom severity | 0.00015 | 262 | 85.9 | 0.0009 | 0.19 | 0.21 |
| ASD symptom severity | 0.0016 | 1969 | 169 | 0.0012 | 0.09 | 0.14 |
SNP, single nucleotide polymorphism; OCD, Obsessive-compulsive disorder; ADHD, Attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; PT, P-value threshold; FDR, false discovery rate.
For a definition of the outcome measures see Table 1.
Empirical P-value corrected for the number of P-value threshold tested by permuting the phenotype 11,000 times.
Empirical P-value corrected for the number of phenotypes tested using the Benjamini-Hochberg false discovery rate (36).
Results
Sample description
Table 1 presents the clinical characteristics of participants from the discovery and target samples of whom both genotype and phenotype data were available. Within the ALSPAC cohort, the prevalence rates of “Tics intermediate”, “Tics broad”, and “Tics all” were 1.3% (101 / 8,941), 6.8% (612 / 8,941), and 11.7% (1,043 / 8,941) (Table 1).
Table 1:
Clinical characteristics of study subjects in the discovery sample (TS GWAS 2) and target sample (ALSPAC).
| Cases | Controls | |||||
|---|---|---|---|---|---|---|
| Phenotype | N |
N Male |
% Male | N | N Male | % Male |
| TS GWAS 2 | 14,307 | |||||
| TS | 4,819 | 3627 | 75 | 9,488 | 4073 | 43 |
| ALSPAC | 8,941 | |||||
| Tics intermediatea | 101 | 74 | 73 | 4,712 | 2,325 | 49 |
| Tics broada | 612 | 395 | 65 | 4,201 | 2,004 | 48 |
| Tics alla | 1,043 | 641 | 61 | 3,770 | 1,758 | 47 |
| ALSPAC | N |
N Male |
% Male | Mean | SD | Observed range |
| Chronicity scoreb | 4,813 | 2,399 | 50 | 0.45 | 1.06 | 0-9 |
| OCD symptom severityc | 6,006 | 3,085 | 51 | 0.45 | 1.15 | 0-16 |
| ADHD symptom severityd | 6,046 | 3,096 | 51 | 4.88 | 6.75 | 0-36 |
| ASD symptom severitye | 6,019 | 3,088 | 51 | 2.82 | 3.71 | 0-24 |
TS, Tourette syndrome; GWAS, genome-wide association study (Yu et al., in prep); ALSPAC, Avon Longitudinal Study of Parents and Children (11,12); OCD, Obsessive-compulsive disorder; ADHD, Attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder.
The presence of tics was defined by three different categorizations: “Tics intermediate” (approximating the ‘TS/chronic tic disorder (CT) intermediate’ by Scharf et al. (16)), which included individuals that experienced motor and/or vocal tics at least once a week at age 13 years and experienced at least one tic symptom between the ages of 1.5 and 10 years; “Tics broad” (approximating the ‘TS/CT broad’ by Scharf et al. (16)), which included individuals that have experienced motor and/or vocal tics occurring at least once a week at age 13; and “Tics all”, which included individuals that reported at least one positive answer to the tic screening question between the ages 1.5 and 13.
The chronicity score is the sum of the number of times tics were present up to age 10.7 plus the number of different tic symptoms at 13.8 years (11,12).
Worst ever OCD symptom severity based on the Development and Well-Being Assessment (DAWBA; (18)) at age 7 and at age 13 years using 10 items.
Based on the DAWBA (18) using 18 items.
Based on the Social Communication Disorder Checklist (SCDC; (22)) using 12 items.
PRS analyses
Presence of tics.
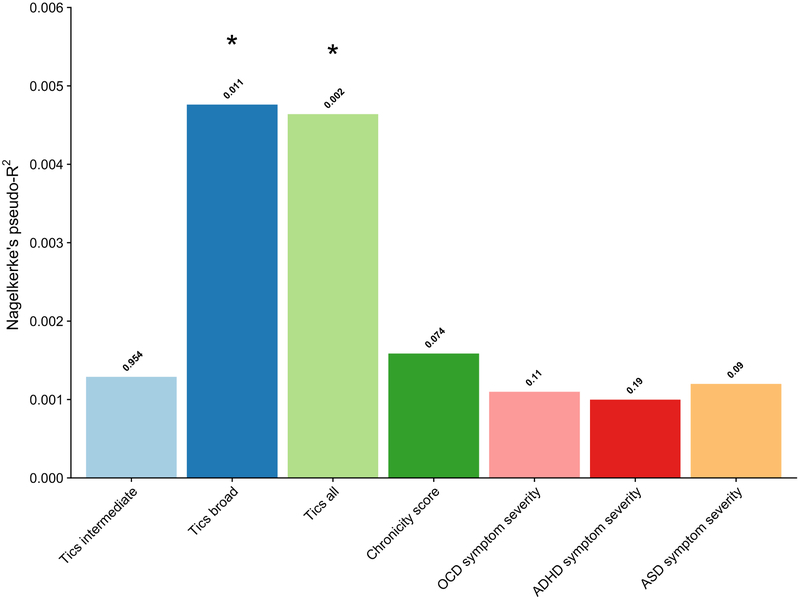
Following correction for multiple testing for the number of P-value thresholds and for the number of phenotypes tested, PRS based on the TS GWAS predicted the presence of tics in the ALSPAC population cohort as defined by the “Tics broad” criteria (R2 = 0.48; P = empirical 0.01, Q = 0.04) and “Tics all” definition (R2 = 0.46%; P empirical = 0.002, Q = 0.01), but did not predict tics per the “intermediate” definition (P empirical = 0.95, Q = 0.95; See Figure 1 and Table 2).
Figure 1:
Explained variance by the polygenic risk score for each tested phenotype in the Avon Longitudinal Study of Parents and Children (ALSPAC) target sample (7, 8), based on the second Tourette Syndrome genome-wide association study (TS GWAS 2, Yu et al., in prep) as discovery sample. The presence of tics was defined by three different categorizations: “Tics intermediate” (approximating ‘TS/chronic tic disorder (CT) intermediate’ by Scharf et al. (16)), which included individuals that experienced motor and/or vocal tics at least once a week at age 13 years and experienced at least one tic symptom between the ages of 1.5 and 10 years; “Tics broad”, (approximating ‘TS/CT broad’ by Scharf et al. (12)), which included individuals that have experienced motor and/or vocal tics occurring at least once a week at age 13; and “Tics all”, which included individuals that reported at least one positive answer to the tic screening question between the ages 1.5 and 13. The chronicity score is the sum of the number of times tics were present up to age 10.7 plus the number of different tic symptoms at 13.8 years (11,12). The risk scores were calculated at different P-value thresholds (P < 0.0001 with increments of 0.00005 to P < 1) and the model that explained the most variance is reported.
* Significance after correction for multiple testing of the different P-value thresholds and for the number of phenotypes tested using the false discovery rate (Q < 0.05).
Chronicity score.
There was no significant association between TS PRS and the chronicity score (R2 = 0.16%, P empirical = 0.07, Q = 0.14; Figure 1 and Table 2). Considering that the chronicity score was highly positively skewed (Figure S3), we also carried out negative binomial regressions and found similar results (Table S1).
OCD, ADHD, and ASD symptom severity.
The TS-derived PRS was not able to predict OCD symptom severity (R2 = 0.11%; P empirical = 0.11, Q = 0.15), ADHD symptom severity (R2 = 0.1%; P empirical = 0.19, Q = 0.21), and ASD symptom severity (R2 = 0.12%; P empirical = 0.09, Q = 0.14) in the ALSPAC cohort (See Figure and Table 2). As sensitivity analysis, we also carried out negative binomial regressions and found similar results (Table S1).
Finally, we found no significant differences in above mentioned results when the MHC locus was included in the calculation of the PRS (Table S4).
Discussion
This study suggests that polygenic scores derived from a large GWAS of individuals with TS predicts the broader spectrum of tic phenotypes in a population cohort (11, 12)). Our findings support the involvement of multiple common variants in tic phenotypes in the general population and validate the relevance of the most recent TS GWAS. A similar conclusion was reached in an ADHD PRS study (24) that found a polygenic component for ADHD traits in the ALSPAC sample. Our tic phenotypes ranged from a lenient definition that captured children who experienced at least one (transient) tic symptom to a more narrowly defined phenotype that more closely resembles a diagnosis of a chronic tic disorder. This was done to reflect the notion that individuals with a TS diagnosis represent the extreme end of a spectrum of tic disorders (29). Results were not driven by the subsample containing exclusively the most narrowly defined tic phenotype, underscoring the relationship of the broader tic phenotype with TS based PRS.
The ALSPAC sample likely included less severe cases compared to the discovery sample; the discovery sample included TS cases mainly recruited through specialty clinics (Yu et al. submitted). The prevalence rates of the “Tics broad” and the “Tics all” groups were markedly higher than was reported for TS (0.3 – 5.7%; (30)) indicating that these groups likely included more subclinical individuals on the tic disorder spectrum. Despite differences in case severity and in the prevalence of co-occurring OCD and ADHD between the discovery and the target (16) datasets we were able to find a significant polygenic signal, suggesting that severe and less severe tic cases share a common genetic etiology.
We observed that the tic group that comes closest to a TS diagnosis (‘Tics intermediate”) was poorly predicted, which may be due to a low prevalence in ALSPAC. As we moved towards broader tic definitions, we saw an improvement of prediction, probably due to a higher prevalence (and therefore more statistical power) of these phenotypes (i.e., “Tics broad”, also resembling a chronic tic disorder, but not necessarily with an early onset, and “Tics all”, covering at least one tic symptom) in ALSPAC.
The TS PRS did not predict OCD, ADHD, and ASD symptom severity in the ALSPAC sample, suggesting that different genes underlie these comorbid disorders than those involved in the genetic architecture of tic disorders. It is also possible that the TS PRS derived from a fairly clinical sample is not suitable for predicting OCD, ADHD, and ASD symptom severity in a population cohort that includes less severe cases. Furthermore, the TS GWAS sample size may have been too low in relation to cross-disorder comparisons. In line with our finding, Yu et al. (7) did not find that TS based PRS predicted OCD status, perhaps again due to a small TS discovery sample (N = 776 cases) and the restriction of the polygenic risk scores to only SNPs with a minor allele frequency > 5% (7). Still one PRS study (4) and three heritability studies suggested a shared genetic background between TS and OCD (6, 14, 31). Little is known about the possible genetic overlap between TS and ADHD, with one PRS study agreeing with our finding of no overlap (4) and two heritability studies (14, 32) suggesting overlap. Lastly, consistent with our findings, Anttila et al. (14) did not find evidence for shared genetic risk factors between TS and ASD. Nevertheless, a few candidate genes studies implicated the same genes in both ASD and TS; e.g., IMMP2L and NRXN1 (33, 34). We conclude that larger samples are needed to investigate similarities and dissimilarities in the genetic architecture of tic and associated disorders.
A strength of our study was the use of a currently relatively large discovery and target sample (11, 12). Furthermore, the participants in the ALSPAC sample were all from the same geographical location, and the MDS plots indicated that they are homogenous in terms of ancestry (20). A limitation of PRS studies is that they only capture common variants of small effect sizes and miss rare genetic variants with larger effect sizes; hence, providing an incomplete picture of the full genetic architecture of neuropsychiatric disorders. Rare de novo damaging variants may contribute to the risk of TS in up to 12% of all clinical cases (3). We underscore that the explained variance found in our study was small, yet in line with what previously has been found in other neuropsychiatric disorders (between 0.1% and 0.7% explained variance) within the ALSPAC sample (21, 24, 35). Another limitation is that tics were reported by parents and not by clinicians. In conclusion, we were able to discriminate between children with and without tics in a general population cohort using PRS derived from the most recent TS GWAS. Our findings support the notion that tics along a spectrum from non-clinical to clinical levels share a similar genetic background. We did not find evidence for a shared genetic etiology of tic, OCD, ADHD, and ASD symptoms. Future PRS studies will greatly benefit from the increasing sample sizes of GWAS, as they will enhance the predictive capabilities of PRS models. Furthermore, PRS can serve as an excellent starting point for gene–environment interaction studies.
Supplementary Material
Acknowledgments
We wish to thank the TS/OCD Working Group of the PGC for providing the summary statistics of the second GWAS of Tourette syndrome. We are also extremely grateful to all the families who took part in the ALSPAC study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
This research was funded by National Institute of Mental Health (NIMH) grant R01MH092293 (to GAH and JAT) and NJCTS (New Jersey Center for Tourette Syndrome and Associated Disorders; to GAH and JAT). This work was also supported by grants from the Judah Foundation, the Tourette Association of America, National Institute of Health (NIH) Grants NS40024, NS016648, MH079489, MH073250, the American Recovery and Re-investment Act (ARRA) Grants NS040024-07S1, NS16648-29S1, NS040024-09S1, MH092289; MH092290; MH092291; MH092292; R01MH092293; MH092513; MH092516; MH092520; MH071507; MH079489; MH079487; MH079488; and MH079494. The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and Mohamed Abdulkadir will serve as guarantor for the contents of this paper.
Footnotes
Disclosures
Drs. Mathews and Scharf are on the scientific advisory board of the Tourette Association of America (TAA) and have received travel and grant support from the TAA. Dr. Mathews is also on the scientific advisory board of the International Obsessive Compulsive Disorder Foundation. Dr. Scharf is on the scientific advisory board of the TLC Foundation for Body-Focused Repetitive Behaviors, and has received consulting fees from Nuvelution Pharma and Abide Pharmaceuticals. The following authors reported no biomedical financial interest or potential conflict of interest: Mohamed Abdulkadir, Dongmei Yu, Jay A. Tischfield, Gary A. Heiman, Pieter J. Hoekstra, and Andrea Dietrich.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Rourke J a, Scharf JM, Yu D, Pauls DL (2009): The genetics of Tourette syndrome: a review. J Psychosom Res. 67: 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zilhão NR, Olthof MC, Smit DJA, Cath DC, Ligthart L, Mathews CA, et al. (2017): Heritability of tic disorders: A twin-family study. Psychol Med. 47: 1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willsey AJ, Fernandez T V, Yu D, King RA, Dietrich A, Xing J, et al. (2017): De Novo Coding Variants Are Strongly Associated with Tourette Disorder. Neuron. 94: 486–499.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darrow SM, Hirschtritt ME, Davis LK, Illmann C, Osiecki L, Grados M, et al. (2016): Identification of Two Heritable Cross-Disorder Endophenotypes for Tourette Syndrome. Am J Psychiatry. appi.ajp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scharf JM, Yu D, Mathews C a, Neale BM, Stewart SE, Fagerness J a, et al. (2013): Genome-wide association study of Tourette’s syndrome. Mol Psychiatry. 18: 721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis LK, Yu D, Keenan CL, Gamazon ER, Konkashbaev AI, Derks EM, et al. (2013): Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS Genet. 9: e1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu D, Mathews CA, Scharf JM, Neale BM, Davis LK, Gamazon ER, et al. (2015): Cross-Disorder Genome-Wide Analyses Suggest a Complex Genetic Relationship Between Tourette’s Syndrome and OCD. Am J Psychiatry. 172: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golden GS (1978): Tics and tourette’s: A continuum of symptoms? Ann Neurol. 4: 145–148. [DOI] [PubMed] [Google Scholar]
- 9.Cavanna AE, Critchley HD, Orth M, Stern JS, Young MB, Robertson MM (2011): Dissecting the Gilles de la Tourette spectrum: A factor analytic study on 639 patients. J Neurol Neurosurg Psychiatry. 82: 1320–1323. [DOI] [PubMed] [Google Scholar]
- 10.Kurlan R, Behr J, Medved L, Como P (1988): Transient Tic Disorder and the Spectrum of Tourette ’ s Syndrome. Am J Hum Genet. 2–3. [DOI] [PubMed] [Google Scholar]
- 11.Golding J, Pembrey M, Jones R, Team S (2001): ALSPAC ± The Avon Longitudinal Study of Parents and Children I. Study methodology. 74–87. [DOI] [PubMed] [Google Scholar]
- 12.Golding J, Team S (2004): The Avon Longitudinal Study of Parents and Children (ALSPAC) – study design and collaborative opportunities. 119–123. [DOI] [PubMed] [Google Scholar]
- 13.Robertson MM, Eapen V, Singer HS, Martino D, Scharf JM, Paschou P, et al. (2017): Gilles de la Tourette syndrome. Nat Rev Dis Prim. 3: 16097. [DOI] [PubMed] [Google Scholar]
- 14.Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, et al. (2018): Analysis of shared heritability in common disorders of the brain. Science (80-). 360: eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados M a., Illmann C, et al. (2015): Lifetime Prevalence, Age of Risk, and Genetic Relationships of Comorbid Psychiatric Disorders in Tourette Syndrome. JAMA Psychiatry. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scharf JM, Miller LL, Mathews C a., Ben-Shlomo Y (2012): Prevalence of Tourette syndrome and chronic tics in the population-based Avon Longitudinal Study of Parents and Children cohort. J Am Acad Child Adolesc Psychiatry. 51: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathews C a Scharf JM, Miller LL, Macdonald-Wallis C, Lawlor D a, Ben-Shlomo Y (2014): Association between pre- and perinatal exposures and Tourette syndrome or chronic tic disorder in the ALSPAC cohort. Br J Psychiatry. 204: 40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman R, Ford T, Richards H, Gatward R, Meltzer H (2000): The Development and Well-Being Assessment: Description and Initial Validation of an Integrated Assessment of Child and Adolescent Psychopathology. J Child Psychol Psychiatry. 41: 645–655. [PubMed] [Google Scholar]
- 19.Krebs G, Liang H, Hilton K, Macdiarmid F, Heyman I (2012): Computer-assisted assessment of obsessive-compulsive disorder in young people: A preliminary evaluation of the Development and Well-Being Assessment. Child Adolesc Ment Health. 17: 246–251. [DOI] [PubMed] [Google Scholar]
- 20.Fraser A, Macdonald-wallis C, Tilling K, Boyd A, Golding J, Davey smith G, et al. (2013): Cohort profile: The avon longitudinal study of parents and children: ALSPAC mothers cohort. Int J Epidemiol. 42: 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stergiakouli E, Martin J, Hamshere ML, Langley K, Evans DM, St Pourcain B, et al. (2015): Shared genetic influences between attention-deficit/hyperactivity disorder (ADHD) traits in children and clinical ADHD. J Am Acad Child Adolesc Psychiatry. 54: 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skuse DH (2005): Measuring autistic traits: heritability, reliability and validity of the Social and Communication Disorders Checklist. Br J Psychiatry. 187: 568–572. [DOI] [PubMed] [Google Scholar]
- 23.Robinson EB, St Pourcain B, Anttila V, Kosmicki JA, Bulik-Sullivan B, Grove J, et al. (2016): Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 48: 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin J, Hamshere ML, Stergiakouli E, O’Donovan MC, Thapar A (2014): Genetic risk for attention-deficit/hyperactivity disorder contributes to neurodevelopmental traits in the general population. Biol Psychiatry. 76: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howie BN, Donnelly P, Marchini J (2009): A Flexible and Accurate Genotype Imputation Method for the Next Generation of Genome-Wide Association Studies. 5. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delaneau O, Marchini J, Zagury J-F (2012): A linear complexity phasing method for thousands of genomes. NatMeth. 9: 179–181. [DOI] [PubMed] [Google Scholar]
- 27.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. (2007): PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 81: 559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Euesden J, Lewis CM, O’Reilly PF (2015): PRSice: Polygenic Risk Score software. Bioinformatics. 31: 1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson MM, Eapen V, Singer HS, Martino D, Scharf JM, Paschou P, et al. (2017): Gilles de la Tourette syndrome. Nat Rev Dis Prim. 3: 16097. [DOI] [PubMed] [Google Scholar]
- 30.Robertson MM (2015): Series Tourette ’ s syndrome 1. A personal 35 year perspective on Gilles de la Tourette syndrome : prevalence , phenomenology , comorbidities , and coexistent psychopathologies. Lancet Psychiatry. 2: 68–87. [DOI] [PubMed] [Google Scholar]
- 31.Zilhao NR, Smit DJ, Boomsma DI, Cath DC (2016): Cross-Disorder Genetic Analysis of Tic Disorders, Obsessive–Compulsive, and Hoarding Symptoms. 7. doi: 10.3389/fpsyt.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsetsos F, Padmanabhuni SS, Alexander J, Karagiannidis I, Tsifintaris M, Topaloudi A, et al. (2016): Meta-Analysis of Tourette Syndrome and Attention Deficit Hyperactivity Disorder Provides Support for a Shared Genetic Basis. Front Genet. 10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke R a, Lee S, Eapen V (2012): Pathogenetic model for Tourette syndrome delineates overlap with related neurodevelopmental disorders including Autism. Transl Psychiatry. 2: e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertelsen B, Melchior L, Jensen LR, Groth C, Glenthøj B, Rizzo R, et al. (2014): Intragenic deletions affecting two alternative transcripts of the IMMP2L gene in patients with Tourette syndrome. Eur J Hum Genet. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones HJ, Stergiakouli E, Tansey KE, Hubbard L, Heron J, Cannon M, Holmans P (2016): Phenotypic Manifestation of Genetic Risk for Schizophrenia During Adolescence in the General Population. JAMA Psychiatry. 73: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamini Y, Hochberg Y (1995): Controlling the False Discovery Rate : a Practical and Powerful Approach to Multiple Testing. JR Stat Soc Ser B. 57: 289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.