Abstract
A new generation of novel cannabinoid compounds have been developed as marijuana substitutes to avoid drug control laws and cannabinoid blood tests. 5F-MDMB-PINACA (also known as 5F-ADB, 5F-ADB-PINACA), MDMB-CHIMICA, MDMB-FUBINACA, ADB-FUBINACA, and AMB-FUBINACA (also known as FUB-AMB, MMB-FUBINACA) were tested for in vivo cannabinoid-like effects to assess their abuse liability. Locomotor activity in mice was tested to screen for locomotor depressant effects and to identify behaviorally-active dose ranges and times of peak effect. Discriminative stimulus effects were tested in rats trained to discriminate Δ9-tetrahydrocannabinol (3 mg/kg, 30-min pretreatment). 5F-MDMB-PINACA (ED50=1.1 mg/kg) and MDMB-CHIMICA (ED50=0.024 mg/kg) produced short-acting (30 min) depression of locomotor activity. ADB-FUBINACA (ED50=0.19 mg/kg), and AMB-FUBINACA (ED50=0.19 mg/kg) depressed locomotor activity for 60–90 min; whereas MDMB-FUBINACA (ED50=0.04 mg/kg) depressed locomotor activity for 150 min. AMB-FUBINACA produced tremors at the highest dose tested. 5F-MDMB-PINACA (ED50=0.07), MDMB-CHIMICA (ED50=0.01 mg/kg), MDMB-FUBINACA (ED50=0.051 mg/kg), ADB-FUBINACA (ED50=0.075 mg/kg) and AMB-FUBINACA (ED50=0.029) fully substituted for the discriminative stimulus effects of Δ9-THC following 15-min pretreatment. All 5 compounds decreased locomotor activity and produced discriminative stimulus effects similar to those of Δ9-THC, which suggests they may have abuse liability similar to that of Δ9-THC. AMB-FUBINACA may have an increased risk of toxicities in recreational users.
Keywords: drug discrimination, locomotor activity, rat, mouse, abuse liability
1. Introduction
There continues to be an increase in the use of synthetic designer compounds which have become widely distributed and easily available (EMCDDA, 2018). Cannabinoids have been consistently one of the most commonly used of these compounds since 2011 (UNODC, 2017). These synthetic cannabinoids act directly at cannabinoid CB1 and CB2 receptors as does Δ9-tetrahydrocannabinol (Δ9-THC) found in marijuana, but have different chemical structures unrelated to Δ9-THC, different metabolism, and often greater toxicity (Fantegrossi et al., 2014). As various synthetic cannabinoid compounds have become legally controlled, new compounds are developed and sold as legal alternatives to marijuana which are not detectable by blood tests for illicit drug use.
A major cause of concern is that some of the more recently seen synthetic cannabinoids are more likely to produce extremely toxic effects than the older synthetics (Tai and Fantegrossi, 2017). Waves of emergency room visits with severe adverse effects including death have been related to the introduction of particular compounds to geographical areas (Davidson et al., 2017; Weinstein et al., 2017), such that these compounds have been referred to as “super-strength” (Kaneko, 2017). It is not known whether the increased toxicity is due only to activation of CB1 cannabinoid receptors more strongly than Δ9-THC or whether these “super-strength” cannabinoids produce effects at other receptors. One recent study has looked at other mechanisms of action in some of the older synthetic cannabinoids and reported that some produced varying amounts of activity at sites which are related to cardiotoxicity and heart disease (Wiley et al., 2016). Whether this is also true for these newer “super-strength” cannabinoids has not been tested.
The most common adverse effects of synthetic cannabinoids are confusion, dizziness, drowsiness, agitation, irritability, nausea, vomiting, hallucinations, delusions, increased heart rate, hypertension, vertigo and chest pain (Adamozicz and Gieroń, 2016; Hasegawa et al., 2015; Hermanns-Clausen et al., 2013; Katz et al., 2016; Trecki et al., 2015; Schwartz et al., 2015). Less common central nervous system effects listed by these articles include headache, psychosis, seizures, myoclonus, catatonic stupor, cerebral ischemia, encephalopathy and coma. Less common cardiac effects include chest pain, myocardial infarction, and cardiac arrest. Acute kidney damage and even kidney failure have been reported following use of synthetic cannabinoids (Davidson, et al., 2017).
The compounds assessed in the present study, 5F-MDMB-PINACA (5F-ADB, 5F-ADB-PINACA), MDMB-CHIMICA, MDMB-FUBINACA, ADB-FUBINACA, and AMB-FUBINACA (FUB-AMB, MMB-FUBINACA) have recently been identified as potentially significant hazards by the US Drug Enforcement Agency. All of these are amino-acid derived indazole-3-carboxamides or indole-3-carboxamides, which have been associated with significant adverse effects, including lethality (Banister and Connor, 2018). 5F-MDMB-PINACA, MDMB-CHMICA and MDMB-FUBINACA, and ADB-FUBINAC have been temporarily placed into Schedule I in the United States (DEA, 2017). AMB-FUBINACA is not currently controlled despite being identified as the cause of the intoxication of 33 persons in New York City, in which the affected individuals were described as zombie-like (Adams et al., 2017). There has also been a reported case of myocardial infarction following the use of AMB-FUBINACA (Hamilton et al., 2017). There are currently no published reports on the use or adverse effects of MDMA-FUBINACA.
5F-MDMB-PINACA has been associated with reports of intoxication (Barceló et al., 2017), increased rates of traffic accidents (Kaneko, 2017) and fatalities (Angerer et al., 2017; Kusano et al., 2018). MDMB-CHIMICA intoxication resulted in numerous adverse effects including acidosis, reduced level of consciousness or agitation, mydriasis, tachycardia or bradycardia, and tonic-clonic convulsions, with recovery occurring within 24 h (Hill et al. 2016). ADB-FUBINACA was the most commonly found synthetic cannabinoid in Turkey from 2011–2015 (Göl and Çok 2017). People presenting to the emergency room following use of ADB-FUBINACA displayed agitation/delirium or confusion, chest pain, supraventricular tachycardia, ischemic stroke, and death due to coronary arterial thrombosis (Brandehoff et al., 2018; Lam et al., 2017; Moeller et al., 2017; Shanks et al., 2016). As is the case with abuse of many synthetic designer drugs, many of these individuals presenting to the emergency room had taken other illicit compounds including stimulants or hallucinogens as evidenced by drug testing.
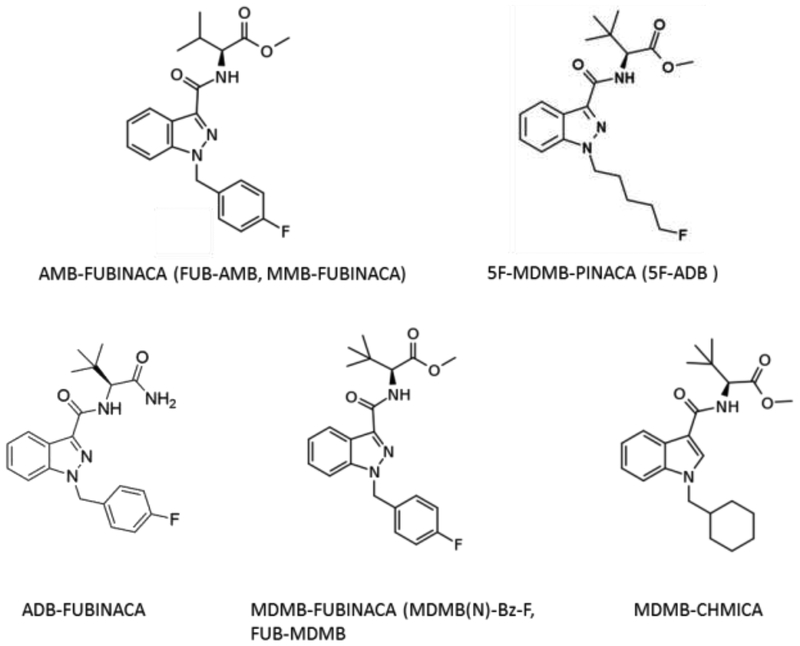
Assessment of abuse liability is based on several factors, including chemical structure, pharmacological mechanism of action, and finally, subjective and reinforcing behavioral effects (FDA, 2010; Swedberg, 2013). The chemical structures of the recent synthetic cannabinoids are unlike that of Δ9-THC, but are largely based on the structure of older synthetic cannabinoids that are known to have substantial abuse liability (Fig. 1). However, not all structural congeners act as agonists at CB1, and not all compounds with receptor activity produce behavioral effects (Wiley et al., 2012), so behavioral testing is necessary to confirm activity. The compounds tested in the present study (MDMB-PINACA, MDMB-CHMICA, MDMB-FUBINACA, ADB-FUBINACA, and AMB-FUBINACA) all act as agonists at CB1 receptors (Banister et al., 2015, 2016; Gamage et al., 2018). In addition, 5F-MDMB-PINACA activates midbrain dopamine neurons, but has no effect on serotonin neurons (Asaoka et al., 2016). In confirmation that at least some of the behavioral effects of these compounds are mediated by CB1 receptors, the bradycardia and hypothermia induced by 5F-AMB and MDMB-FUBINACA was reversed by a CB1 antagonist (Banister et al., 2016).
Figure 1.
Chemical structures of the synthetic cannabinoid compounds tested in the present study.
Drug discrimination is a well-known animal model of the subjective effects of drugs and correlates well with abuse liability (Young 2009; Horton et al. 2013). Synthetic cannabinoids have consistently been shown to produce discriminative stimulus effects similar to those of Δ9-THC (Bannister and Connor, 2018), and MDMB-FUBINACA fully substituted for Δ9-THC (Gamage et al., 2018). Assays of the reinforcing effects of drugs such as self-administration and conditioned place preference (CPP) are also important assays for assessing abuse liability; however, CPP and self-administration assays have mostly failed to show consistent reinforcing effects by Δ9-THC and/or the synthetic cannabinoids (e.g., Tanda 2016). The findings produce an apparent paradox, since CPP and self-administration predict with high reliability the likelihood that a compound will be abused by humans, and cannabinoids are well-known to produce active drug-seeking in humans. It is likely that the current animals models of self-administration are not adequate to detect Δ9-THC reinforcement. Since there is currently no robust measure of the reinforcing/rewarding effects of cannabinoids, drug discrimination is currently the best model for assessing abuse liability of cannabinoids.
The purpose of the present study was to assess the abuse liability of 5F-MDMB-PINACA, MDMB-CHIMICA, MDMB-FUBINACA, ADB-FUBINACA, and AMB-FUBINACA. The locomotor activity assay was used to identify approximate time courses and dose ranges of psychoactive effects, which is useful for identifying parameters for drug discrimination experiments and are also predictive of the time course of the psychoactive effects in human users. The other three components of the cannabinoid Tetrad Test used to characterize the behavioral effects of cannabinoids (catalepsy, hypothermia, and analgesia were not tested as they have limited relevance for determining abuse liability. Rats trained to discriminate Δ9-THC were used to identify whether 5F-MDMB-PINACA, MDMB-CHIMICA, MDMB-FUBINACA, ADB-FUBINACA, and AMB-FUBINACA produce behavioral effects similar to other abused synthetic cannabinoids; and in particular whether they produce discriminative stimulus effects similar to those of Δ9-THC.
2. Methods
2.1. Subjects
Male ND4 Swiss–Webster mice were obtained from Envigo (Houston, TX) at approximately 8 weeks of age and maintained in the University of North Texas Health Science Center (UNTHSC) animal facility for two weeks prior to testing. Mice were housed 3–4 per cage on a 12:12-h light/dark cycle (lights on at 7:00 AM) and were allowed free access to food and water except during test sessions. Twenty-four male Sprague-Dawley rats were obtained from Envigo (Houston, TX). All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00 A.M.). Body weights were maintained at 320–350 g by limiting food to 15 g/day, which included the food received during sessions. Water was continuously available in the home cage. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
2.2. Locomotor activity
Each study was conducted using 32 Digiscan locomotor activity testing chambers (40.5 × 40.5 × 30.5 cm) (Omnitech Electronics, Columbus OH) each housed within a sound-attenuating chamber that provided dim illumination. A panel of 16 infrared beams and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. Separate groups of 8 mice were injected with either vehicle (ethanol/Cremophor EL/0.9% saline 1:1:18) or a cannabinoid: Δ9-THC (2.5 – 25 mg/kg), AMB-FUBINACA (0.05–0.5 mg/kg), ADB-FUBINACA (0.05–1 mg/kg), 5F-MDMB-PINACA (0.133–1.33 mg/kg), MDMB-CHIMICA (0.01–0.05 mg/kg), and MDMB-FUBINACA (0.01–0.1), immediately prior to locomotor activity testing. Each dose range included doses that were without effect to those producing at least 50% depression compared to vehicle control. In all studies, horizontal activity (interruption of photocell beams) was measured for 8 hours within 10-min periods, beginning at 8:00 AM (1 h after lights on).
2.3. Discrimination procedures
Standard two-lever behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC-compatible computers via Med Associates interfaces (East Fairfield, VT). Response levers were positioned to the left and right of the food hopper. A houselight was centered over the hopper close to the ceiling and was illuminated only when the levers were active. The computers were programmed in Med-PC for Windows, version IV (Med Associates, East Fairfield, VT) for the operation of the chambers and collection of data. Rats were first trained to discriminate Δ9-THC (3 mg/kg) from vehicle (ethanol/ Cremophor EL/0.9 % saline in a ratio of 1:1:18) using a two-lever choice methodology. Thirty minutes prior to the training sessions, rats received an injection of either vehicle or Δ9-THC and were subsequently placed in the behavior-testing chambers, where food (45-mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer for every ten responses (FR10) on a designated injection appropriate lever. Each training session lasted a maximum of 10 min, and the rats could earn up to 20 food pellets. Rats were used in tests of substitution of the experimental compounds once they had achieved nine of ten sessions at 85% or greater injection-appropriate responding for both the first reinforcer and the total session, which occurred after approximately 60 training sessions. The training sessions occurred on separate days in a double-alternating fashion (drug-drug-vehicle-vehicle-drug etc.) until the training phase was complete, after which substitution tests were introduced into the training schedule such that at least one vehicle and one drug session occurred between each test (drug-vehicle-test-vehicle-drug-test-drug etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions. During test sessions, both levers were active, such that ten consecutive responses on either lever led to reinforcement. For dose-effect experiments, sessions lasted until 20 reinforcers were obtained or for a maximum of 20 min. Each compound was tested in a group of at least six rats using a repeated-measure design such that each rat was tested at all doses of a given drug. Vehicle (1 ml/kg) and Δ9-THC (3 mg/kg) controls were tested before the start of each compound evaluation. Doses of ADB-FUBINACA (0.025–0.25 mg/kg), AMB-FUBINACA (0.01–0.1 mg/kg), MDMB-FUBINACA (0.005–0.1 mg/kg), MDMB-CHIMICA (0.005–0.025 mg/kg), and 5F-MDMB-PINACA (0.025–0.1 mg/kg) were tested. A dose range was tested from no effect (< 20 % Δ9-THC-appropriate responding) to full effect (≥ 80 % Δ9-THC-appropriate responding or suppression of responding to less than 20% of vehicle control). Doses were tested in no particular order. Pretreatment times were based on the time of peak depression for each compound in the previous locomotor activity testing. All compounds were tested using a 15-min pretreatment.
2.4. Drugs
Δ9-Tetrahydrocannabinol, 5F-MDMB-PINACA (methyl 2-(1-(5-fluoropentyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate), MDMB-CHMICA (methyl 2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate), MDMB-FUBINACA (methyl 2-(1-(4-fluorobenzyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate), ADB-FUBINACA (N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide), and AMB-FUBINACA (methyl 2-(1-(4-fluorobenzyl)-1H-indazole-3-carboxamido)-3-methylbutanoate) were provided by the National Institute on Drug Abuse Drug Supply Program. All drugs were dissolved in ethanol/Cremophor EL/0.9 % saline (in a ratio of 1:1:18) and were administered i.p. in a volume of 1 ml/kg. Cremophor EL was obtained from Sigma Aldrich (St. Louis, MO).
2.5. Data analysis
Locomotor activity data were expressed as the mean number of photocell counts in the horizontal plane (ambulation counts) during each 10-min period of testing. A 30-min period, beginning when maximal depression of locomotor activity first appeared as a function of dose, was used for analysis of dose-response data and calculation of ED50 values. OriginGraph (OriginLab Corporation, Northampton, MA) was used to estimate the maximal depression induced by each cannabinoid. The ED50 values were calculated by estimating the dose producing ½ of maximal depression (0 photocell counts) from the descending linear portion of the dose response curve. A two-way analysis of variance, with dose as a between groups factor and time as a within subject factor, was conducted on horizontal activity counts/10 min interval. Subsequently, a one-way analysis of variance was conducted on horizontal activity counts for the 30-min period of maximal effect, and planned comparisons were conducted for each dose against the vehicle control using single degree-of-freedom F tests.
Drug discrimination data were expressed as the mean percentage (± standard error) of drug-appropriate responses occurring in each test period. The rate of responding was calculated by dividing the total number of responses for each rat tested by the session time. Response rate data are expressed as the mean (± standard error) of all rats tested. Because response suppression may compromise stimulus control, rats failing to complete at least ten responses during the test session were excluded from the analysis of the discriminative stimulus effects of that dose of test compound. If three or more of the rats did not complete the first reinforcer at a given dose, the discrimination data for that dose is not shown. Graphs for percent drug-appropriate responding and response rate were plotted as a function of the dose of the test compound (log scale). Percent drug-appropriate responding was shown only if at least three rats completed the first fixed ratio, whereas all rats are shown for the response rate data. Full substitution was defined as ≥ 80 % drug-appropriate responding and not statistically different from the training drug. The potencies of 5F-MDMB-PINACA, MDMB-CHIMICA, MDMB-FUBINACA, ADB-FUBINACA, and AMB-FUBINACA were calculated by fitting straight lines to the dose-response data for each compound by means of OriginGraph (OriginLab Corporation, Northampton, MA). Straight lines were fitted to the linear portion of dose-effect curves, including not more than one dose producing < 20 % of the maximal effect and not more than one dose producing > 80 % of the maximal effect. Other doses were excluded from the analyses. Response-rate data were analyzed by one-way repeated-measure analysis of variance. Effects of individual doses were compared to the vehicle control value using a priori contrasts. The criterion for significance was set a priori at p < 0.05.
3. Results
3.1. Locomotor Activity
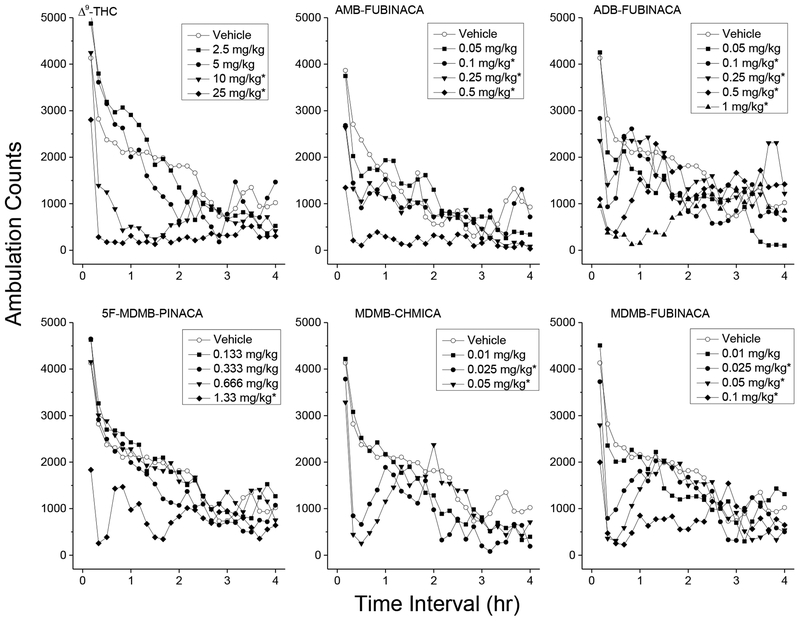
3.1.1. Δ9-THC.
Figure 1 shows average horizontal activity counts/10 min as a function of time (0–4 h) and dose of Δ9-THC. Treatment with Δ9-THC resulted in time- and dose-dependent depression of locomotor activity following 10 and 25 mg/kg (ED50 = 7.9±0.053 mg/kg). Depressant effects of 10 and 25 mg/kg occurred within 10–20 minutes following injection and lasted 120–340 minutes. A two-way analysis of variance conducted on horizontal activity counts/10 min indicated significant effects of Treatment F(4,35)=12.1, p<.001, of 10-Minute Periods F(47,1645)=18.1, p<.001, and the interaction of Periods and Treatment F(188,1645)=2.2, p<.001. A one-way analysis of variance conducted on horizontal activity counts for the 30–60 min time period indicated a significant effect of Treatment F(4,35)=18.02, p<.001, and planned comparisons (a priori contrast) against the vehicle group showed a significant depressant effect for 10 and 25 mg/kg.
3.1.2. AMB-FUBINACA.
Treatment with AMB-FUBINACA resulted in time- and dose-dependent depression of locomotor activity following 0.1 to 0.5 mg/kg (ED50 = 0.19±0.091 mg/kg). Substantial depressant effects were observed within the first 10 min, and maximal depression was observed between 10–40 min and lasted up to 2.5 to 3 h at the highest dose tested (0.5 mg/kg). A two-way analysis of variance conducted on horizontal activity counts/10 min indicate a significant effect of Treatment F(4,35)=4.68, p=0.004, a significant effect for 10-Minute Periods F(47,1645)=17.191, p<.001, and a significant interaction of Periods and Treatment F(188,1645)=1.835, p<.001. A one-way analysis of variance conducted on horizontal activity counts for the 10–40 min time period (maximal depressant effect) indicated a significant effect of Treatment F(4,356)=6.887, p<.001, and planned comparisons (a priori contrast) against the vehicle group showed a significant depressant effect for 0.1, 0.25, and 0.5 mg/kg. Tremors were observed 30 minutes following 1 mg/kg AMB-FUBINACA in 3 of 8 mice (data not shown).
3.1.3. ADB-FUBINACA.
Treatment with ADB-FUBINACA resulted in time- and dose-dependent depression of locomotor activity following 0.1 to 1 mg/kg (ED50 = 0.19±0.13 mg/kg). Substantial depressant effects were observed within the first 10 min, and maximal depression was observed between 0–30 min following administration. Duration of the locomotor depression increased over dose from 30 min following 0.1 mg/kg to 2.5 h following 1 mg/kg. A two-way analysis of variance conducted on horizontal activity counts/10 min did not indicate a significant effect of Treatment F(5,42)=20.78, p=.087, but indicated a significant effect for 10-Minute Periods F(47,1974)=7.664, p<.001, and a significant interaction of Periods and Treatment F(47,1974)=1.941, p<.001. A one-way analysis of variance conducted on horizontal activity counts for the 0–30 min time period (maximal depressant effect) indicated a significant effect of Treatment F(5,42)=8.2, p<.001, and planned comparisons (a priori contrast) against the vehicle group showed a significant depressant effect for 0.1, 0.25, 0.5, and 1 mg/kg.
3.1.4. 5F-MDMB-PINACA.
Treatment with 5F-MDMB-PINACA resulted in time- and dose-dependent depression of locomotor activity following 1.33 mg/kg (ED50=1.05±0.085). Depressant effects of 1.33 mg/kg were observed within 10 min following administration and peak depressant effects were observed between 0–30 min. Locomotor activity returned to baseline within 50 min following administration. A two-way analysis of variance conducted on horizontal activity counts/10 min failed to indicate a significant effect of Treatment F(4,35)=2.472, p=.062, but yielded a significant effect for 10-Minute Periods F(47,1645)=30.431, p<.001, and a significant interaction of Periods and Treatment F(188,1645)=1.858, p<.001. A one-way analysis of variance conducted on horizontal activity counts for the 0–30 min time period (maximal depressant effect) indicated a significant effect of Treatment F(7,56)=9.52, p<.001, and planned comparisons (a priori contrast) against the vehicle group showed a significant depressant effect for 1.33 mg/kg. Note: An apparent small stimulant effect 1–2 h following 0.33 and 1.33 mg/kg 5F-MDMB-PINACA was not statistically significant.
3.1.5. MDMB-CHMICA.
Treatment with MDMB-CHMICA resulted in time- and dose-dependent depression of locomotor activity following 0.025 to 0.1 mg/kg (ED50=0.024±0.050). Peak depressant effects were observed between 10–40 min. Locomotor activity returned to baseline within 80–110 min following administration. A two-way analysis of variance conducted on horizontal activity counts/10 min indicated a significant effect of Treatment F(3,28)=5.085, p=.006, a significant effect for 10-Minute Periods F(47,1316)=17.342, p<.001, and a significant interaction of Periods and Treatment F(141,1316)=1.786, p<.001. A one-way analysis of variance conducted on horizontal activity counts for the 10–40 min time period (maximal depressant effect) indicated a significant effect of Treatment F(3,28)=20.538, p<.001, and planned comparisons (a priori contrast) against the vehicle group showed a significant depressant effect for 0.025 and 0.05 mg/kg.
3.1.6. MDMB-FUBINACA.
Treatment with MDMB-FUBINACA resulted in time- and dose-dependent depression of locomotor activity following 0.025 to 0.1 mg/kg (ED50=0.040±0.075). Depressant effects of 0.025 to 0.5 mg/kg occurred within 10–20 minutes following injection and lasted 30–60 minutes. A two-way analysis of variance conducted on horizontal activity counts/10 min indicated a significant effect of Treatment F(4,35)=2.75, p=.044, a significant effect for 10-Minute Periods F(47,1645)=15.80, p<.001, and a significant interaction of Periods and Treatment F(235,1645)=2.11, p<.001. A one-way analysis of variance conducted on horizontal activity counts for the 0–30 min time period (maximal depressant effect) indicated a significant effect of Treatment F(4,35)=11.8, p<.001, and planned comparisons (a priori contrast) against the vehicle group showed a significant depressant effect for 0.025, 0.05, and 0.1 mg/kg (ps<.05 denoted on Figure 2 with an asterisk).
Figure 2.
Locomotor activity. Average horizontal activity counts/10 min as a function of time (10 min bins) and dose. Only data from the first four hours are shown. Data are from independent groups of 8 mice per dose. Asterisks indicate doses significantly different from vehicle during the time of peak effect (p<0.05).
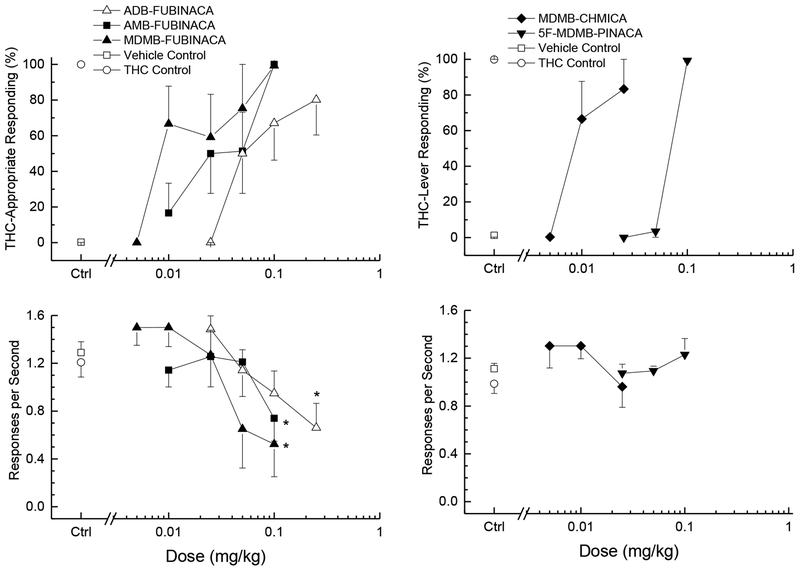
3.2. Drug Discrimination
Δ9-THC (ED50=0.44±0.14 mg/kg) dose-dependently increased drug-appropriate responding to a peak of 99% following the training dose of 3 mg/kg (data not shown). The synthetic cannabinoids AMB-FUBINACA (ED50=0.029±0.12 mg/kg), ADB-FUBINACA (ED50=0.075±0.012 mg/kg), 5F-MDMB-PINACA (ED50=0.070±0.0055 mg/kg), MDMB-CHMICA (ED50=0.010±0.0084 mg/kg), and MDMB-FUBINACA (ED50=0.051±0.013 mg/kg) each fully substituted for the discriminative stimulus effects of Δ9-THC as shown in the upper panels of Figure 3. Relative potency was MDMB-CHMICA=MDMB-FUBINACA>AMB-FUBINACA>5F-MDMB-PINACA=ADB-FUBINACA>Δ9-THC.
Figure 3.
Substitution for the discriminative stimulus effects of Δ9-tetrahydrocannabinol: Top panels show percentage of total responses made on the drug-appropriate lever. Bottom panels show rate of responding in responses per second (r/s). All of the cathinones fully substituted for the discriminative stimulus effects of Δ9-tetrahydrocannabinol (≥80% drug-appropriate responding). n=6 unless otherwise shown. Ctrl indicates vehicle and Δ9-THC control values. * indicates response rate different from vehicle control (p < 0.05).
Response rate was decreased following AMB-FUBINACA [F(5,25)=3.80, p=.011], ADB-FUBINACA [F(4,20)=4.91, p=.006], and MDMB-FUBINACA [F(5,25)=3.47, p=.016]. Response rate showed no change from vehicle control following 5F-MDMB-PINACA [F(5,25)=1.4, p=.258], and MDMB-CHMICA [F(4,20)=2.46, p=.079)]. Response rates are depicted in the lower panels of Figure 3.
4. Discussion
All of the compounds tested in the present study depressed locomotor activity as is typical for other synthetic cannabinoids (see review by Wiley et al., 2017). All of the test compounds were more potent than Δ9-THC. Relative potency was MDMB-CHMICA=MDMB-FUBINACA>AMB-FUBINACA=ADB-FUBINACA>5F-MDMB-PINACA>Δ9-THC. There seems to be a trend of newer synthetic cannabinoids being more potent than earlier compounds. A set of early cannabinoids tested for locomotor depression had an average potency of 4.43±1.47 mg/kg (Gatch et al., 2014). A year later, another set had an average potency of 3.71±1.34 mg/kg (Gatch et al., 2015). The average potency of the following set was 1.47±0.69 mg/kg (Gatch et al., 2016), and the average of the current set is 0.31±0.17 mg/kg.
MDMB-CHMICA and 5F-MDMB-PINACA were short-acting locomotor depressants, lasting only 1 h. The effects of AMB-FUBINACA, ADB-FUBINACA and MDMB-FUBINACA lasted 2 to 4 hours at the peak dose, similar to Δ9-THC in the present and previous studies (Gatch et al., 2014; 2015; 2016; 2018). The duration of action of the synthetic cannabinoids tested using the 8-h protocol have varied widely, with some producing a duration of action no longer than 1 h, others producing a duration of action between 1–2 h, and others lasting more than 2 h. Early compounds, such as the JWH series, mostly produced effects in the 1–2 h range, although a few (JWH-018, JWH-122, JWH-210) produced effects lasting greater than 2 h. The short-duration of action compounds are typically newer compounds, including two compounds tested in the present study. Both the short- and long-acting compounds have potential risks. Short-onset, short-acting compounds have a greater abuse liability, and long-acting compounds pose problems of long-acting adverse effects and interactions with other drugs. It should be noted that most of the cannabinoids tested using this protocol had onsets within 10 min. BB-22, FUB-PB-22, MAB-CHMINACA, NM-2201 and RCS-4 had onsets between 10–20 min, and JWH-018, JWH-210 and JWH-122 had onsets 30 min after injection (Gatch et al., 2014; 2015; 2016; 2018).
All of the synthetic cannabinoids tested in the present study fully substituted for the discriminative stimulus effects of Δ9-THC. Relative DD potency was MDMB-CHMICA=MDMB-FUBINACA>AMB-FUBINACA>5F-MDMB-PINACA=ADB-FUBINACA>Δ9-THC. All of the test compounds in the present study were more potent than Δ9-THC. A trend of decreasing potency similar to that of the locomotor depressant data can also been seen. Average potency of the discriminative stimulus effects of early compounds was 0.81±0.17 mg/kg (Gatch et al., 2014), whereas the potency of a recent set was 0.09±0.03 mg/kg (Gatch et al., 2018), and the potency of the current set is 0.05±0.01 mg/kg. All five of the compounds in the present study fully substituted with a pretreatment time of 15 min, suggesting a rapid onset of the discriminative stimulus effects. In prior studies, some compounds had very rapid onset of the discriminative stimulus effects, others were delayed, and yet others were masked by profound rate decreasing effects, such that drug-appropriate responding did not occur until 30–60 min following administration of the test compounds (Gatch et al., 2014; 2015; 2016; 2018). As mentioned previously, short-onset compounds have a greater abuse liability; further, compounds that have fewer adverse effects while they are active are likely to be preferred. Pretreatment times and dose ranges for the drug discrimination assay were selected based on the time of peak depression in the locomotor activity assay in mice.
These findings are in agreement with earlier studies showing the synthetic cannabinoids substitute for the discriminative stimulus effects of Δ9-THC (see review by Wiley et al., 2017). All of the compounds identified as available on the recreational market and submitted to our laboratory by the US Drug Enforcement Agency for testing have fully substituted at some dose (Gatch and Forster 2014, 2015, 2016, 2018); however; it is important to note that not all structural congeners are active (Wiley et al., 2012). Following that line of reasoning, it should also be noted that some of the more recent compounds produced non-linear dose-effect curves and one compound produced an inverted U-shaped dose-effect, such that intermediate dose fully substituted, but higher doses did not (Gatch and Forster, 2018). These findings suggest that there may be limits to structural substitutions that can be made without losing efficacy.
Tremors were observed in mice 30 minutes following 1 mg/kg AMB-FUBINACA in the present study. AMB-FUBINACA has been implicated in severe adverse effects in recreational users (Adams et al., 2017; Hamilton et al., 2017), which suggests that the range between behaviorally active and toxic doses of AMB-FUBINACA is narrow. Tremors were not observed following AMB-FUBINACA during the drug discrimination study, but the maximum dose tested was only 0.1 mg/kg, which is 10-fold lower than the dose that produced tremors in the mice. In general, the locomotor depressant and discriminative stimulus effects have been observed at doses that do not produce adverse effects, although tremors were observed upon handling in mice that received JWH-210 (Gatch et al., 2016), and 5F-AMB produced sustained vocalization and convulsions in rats (Gatch et al., 2018). Since these compounds are being sold to the recreational market without testing of any kind, users may increasingly find themselves using compounds with a decreasing likelihood of marijuana-like subjective effects and increasing risk of adverse effects.
As previously mentioned, all of the compounds tested in the present study (MDMB-PINACA, MDMB-CHMICA, MDMB-FUBINACA, ADB-FUBINACA, and AMB-FUBINACA) act as agonists at CB1 receptors (Banister et al., 2015, 2016; Gamage et al., 2018), which suggests these compounds will produce Δ9-THC-like effects, including abuse liability. Little is known about non-cannabinoid receptor-mediated effects on the human body. There is indication that at least some of the first-generation synthetic cannabinoids act at receptors other than cannabinoid CB1 and CB2 (Wiley et al., 2016), and a compound from the present study, 5F-MDMB-PINACA, was found to activate midbrain dopamine neurons, but not serotonin neurons (Asaoka et al., 2016). These findings imply that at least some of the adverse effects targeting non-CNS organ systems such as heart, liver and kidney may be mediated through non-cannabinoid mechanisms, although the bradycardia induced by 5F-AMB and MDMB-FUBINACA was reversed by a CB1 antagonist (Banister et al., 2016).
There have been increasing numbers of clinical case reports of severe and even life-threatening adverse effects associated with the use of these compounds, including waves of emergency room visits when a new, and more toxic compound is introduced to regions (Davidson et al., 2017; Weinstein et al., 2017). Much of the in vivo testing of the synthetic cannabinoid compounds have been pre-clinical studies focused on their cannabinoid-like effects or like the present study, focused on their abuse liability. Toxicological testing of these compounds is increasingly warranted due to the increase in number and severity of adverse effects reported by recreational users.
In summary, these 5F-MDMB-PINACA, MDMB-CHIMICA, MDMB-FUBINACA, ADB-FUBINACA, and AMB-FUBINACA have similar abuse liability as Δ9-tetrahydrocannabinol and should be controlled in a similar fashion. Previous studies have demonstrated that these compounds have chemical structures similar to synthetic cannabinoids known to have substantial abuse liability and act at the CB1 receptor. The current study indicates that the test compounds produce locomotor depression similar to that of Δ9-THC, and fully substitute for the discriminative stimulus effects of Δ9-THC. There may be differences in the recreational uses of these compounds and their incidence of adverse effects as compared to marijuana. Short-acting compounds with rapid onset and short duration such as 5F-MDMB-PINACA, MDMB-CHIMICA may be more likely to be used compulsively, unlike the long-acting Δ9-THC. Slow onset compounds may elicit repeat dosing in users impatient for a “high”, leading to longer duration and greater likelihood of adverse events. Long-acting compounds may also increase risk of adverse effects or at least distress. AMB-FUBINACA produced tremors and may be of increased risk in human recreational users.
Highlights.
Highlights are mandatory for this journal. They consist of a short collection of bullet points that convey the core findings of the article and should be submitted in a separate editable file in the online submission system. Please use ‘Highlights’ in the file name and include 3 to 5 bullet points.
Five recent synthetic cannabinoids found on the street act at cannabinoid receptors
Each drug produces depression of locomotor activity
Each drug produces Δ9-tetrahydrocannabinol-like discriminative stimulus effects
These drugs have substantial abuse liability and other toxicities
Acknowledgments
Funding
This work was supported by the National Institutes of Health N01DA-13–8908.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no conflict of interest.
References
- Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, Gerona R. “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N Engl J Med. 2017. January 19;376(3):235–242. [DOI] [PubMed] [Google Scholar]
- Adamowicz P, Gieroń J Acute intoxication of four individuals following use of the synthetic cannabinoid MAB-CHMINACA. Clin Toxicol (Phila) 2016. 54(8):650–654. 10.1080/15563650.2016.1190016 [DOI] [PubMed] [Google Scholar]
- Angerer V, Jacobi S, Franz F, Auwärter V, Pietsch J. Three fatalities associated with the synthetic cannabinoids 5F-ADB, 5F-PB-22, and AB-CHMINACA. Forensic Sci Int. 2017. December;281:e9–e15. [DOI] [PubMed] [Google Scholar]
- Asaoka N, Kawai H, Nishitani N, Kinoshita H, Shibui N, Nagayasu K, Shirakawa H, Kaneko S. A new designer drug 5F-ADB activates midbrain dopaminergic neurons but not serotonergic neurons. J Toxicol Sci. 2016;41(6):813–816. [DOI] [PubMed] [Google Scholar]
- Banister SD, Connor M. The chemistry and pharmacology of synthetic cannabinoid receptor agonist new psychoactive substances: Evolution. Handb Exp Pharmacol. 2018. August 14. doi: 10.1007/164_2018_144. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Banister SD, Longworth M, Kevin R, Sachdev S, Santiago M, Stuart J, Mack JB, Glass M, McGregor IS, Connor M, Kassiou M. Pharmacology of Valinate and tert-Leucinate Synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and Their Analogues. ACS Chem Neurosci. 2016. September 21;7(9):1241–54. [DOI] [PubMed] [Google Scholar]
- Banister SD, Moir M, Stuart J, Kevin RC, Wood KE, Longworth M, Wilkinson SM, Beinat C, Buchanan AS, Glass M, Connor M, McGregor IS, Kassiou M. Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem Neurosci. 2015. September 16;6(9):1546–59. [DOI] [PubMed] [Google Scholar]
- Barceló B, Pichini S, López-Corominas V, Gomila I, Yates C, Busardò FP, Pellegrini M. Acute intoxication caused by synthetic cannabinoids 5F-ADB and MMB-2201: A case series. Forensic Sci Int. 2017. April;273:e10–e14. [DOI] [PubMed] [Google Scholar]
- Brandehoff N, Adams A, McDaniel K, Banister SD, Gerona R, Monte AA. Synthetic cannabinoid “Black Mamba” infidelity in patients presenting for emergency stabilization in Colorado: a P SCAN Cohort. Clin Toxicol (Phila). 2018. March;56(3):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C, Opacka-Juffry J, Arevalo-Martin A, Garcia-Ovejero D, Molina-Holgado E, Molina-Holgado F. Spicing up pharmacology: A review of synthetic cannabinoids from structure to adverse eEvents In: Kendall David and Alexander Stephen P.H., editors, Advances in Pharmacology, Vol. 80, Burlington: Academic Press, 2017, pp. 135–168. 10.1016/bs.apha.2017.05.001 [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration, Department of Justice. Schedules of Controlled Substances: Temporary Placement of Six Synthetic Cannabinoids (5F-ADB, 5F-AMB, 5F-APINACA, ADB-FUBINACA, MDMB-CHMICA and MDMB-FUBINACA) into Schedule I. Temporary Scheduling Order. Fed Regist. 2017. April 10;82(67):17119–24. [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2018: Trends and Developments, Publications Office of the European Union, Luxembourg: 2018. 10.2810/800331 TD-AT-18–001-EN-N [DOI] [Google Scholar]
- Fantegrossi WE, Moran JH, Radominska-Pandya A and Prather PL. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ(9)-THC: mechanism underlying greater toxicity? Life Sci. 2014, 97:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. Guidance for Industry, Assessment of Abuse Potential of Drugs, Draft guidance, US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); January 2010. [Google Scholar]
- Gamage TF, Farquhar CE, Lefever TW, Marusich JA, Kevin RC, McGregor IS, Wiley JL, Thomas BF. Molecular and Behavioral Pharmacological Characterization of Abused Synthetic Cannabinoids MMB- and MDMB-FUBINACA, MN-18, NNEI, CUMYL-PICA, and 5-Fluoro-CUMYL-PICA. J Pharmacol Exp Ther. 2018. May;365(2):437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ. Δ9-Tetrahydrocannabinol-like discriminative stimulus effects of five novel synthetic cannabinoids in rats. Psychopharmacology 2018. 253:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ. Δ9-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids in mice and rats. Psychopharmacology 2016. 233:1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ. Δ9-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids found on the gray market. Behavioural Pharmacology 2015. 26:460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ. Δ9-Tetrahydrocannabinol-like discriminative stimulus effects of compounds commonly found in K2/Spice. Behavioural Pharmacology 2014. 25:750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göl E, Çok İ. Assessment of types of synthetic cannabinoids in narcotic cases assessed by the Council of Forensic Medicine between 2011–2015, Ankara, Turkey. Forensic Sci Int. 2017. November;280:124–129. [DOI] [PubMed] [Google Scholar]
- Hamilton RJ, Keyfes V, Banka SS. Synthetic Cannabinoid Abuse Resulting in ST-Segment Elevation Myocardial Infarction Requiring Percutaneous Coronary Intervention. J Emerg Med. 2017. April;52(4):496–498. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Watanabe K, Suzuki O (2015) Postmortem distribution of MABCHMINACA in body fluids and solid tissues of a human cadaver. Forensic Toxicol 33(2):380–387. 10.1007/s11419-015-0272-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanns-Clausen M, Kneisel S, Szabo B, Auwärter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013. March;108(3):534–44. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- Hill SL, Najafi J, Dunn M, Acheampong P, Kamour A, Grundlingh J, Blain PG, Thomas SH. Clinical toxicity following analytically confirmed use of the synthetic cannabinoid receptor agonist MDMB-CHMICA. A report from the Identification Of Novel psychoActive substances (IONA) study. Clin Toxicol (Phila). 2016. September;54(8):638–43. [DOI] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN. A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol 2013. 24(5 and 6):410–436. 10.1097/FBP.0b013e3283644d2e [DOI] [PubMed] [Google Scholar]
- Kaneko S. Motor vehicle collisions caused by the ‘super-strength’ synthetic cannabinoids, MAM-2201, 5F-PB-22, 5F-AB-PINACA, 5F-AMB and 5F-ADB in Japan experienced from 2012 to 2014. Forensic Toxicol. 2017;35(2):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz KD, Leonetti AL, Bailey BC, Surmaitis RM, Eustice ER, Kacinko S, Wheatley SM. Case series of synthetic cannabinoid intoxication from one toxicology center. West J Emerg Med 2016. 17(3):290–294. 10.5811/westjem.2016.2.29519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano M, Zaitsu K, Taki K, Hisatsune K, Nakajima J, Moriyasu T, Asano T, Hayashi Y, Tsuchihashi H, Ishii A. Fatal intoxication by 5F-ADB and diphenidine: Detection, quantification, and investigation of their main metabolic pathways in humans by LC/MS/MS and LC/Q-TOFMS. Drug Test Anal. 2018. February;10(2):284–293. [DOI] [PubMed] [Google Scholar]
- Lam RPK, Tang MHY, Leung SC, Chong YK, Tsui MSH, Mak TWL. Supraventricular tachycardia and acute confusion following ingestion of e-cigarette fluid containing AB-FUBINACA and ADB-FUBINACA: a case report with quantitative analysis of serum drug concentrations. Clin Toxicol (Phila). 2017. August;55(7):662–667. [DOI] [PubMed] [Google Scholar]
- Moeller S, Lücke C, Struffert T, Schwarze B, Gerner ST, Schwab S, Köhrmann M, Machold K, Philipsen A, Müller HH. Ischemic stroke associated with the use of a synthetic cannabinoid (spice). Asian J Psychiatr. 2017. February;25:127–130. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals, 8th ed. 2011. The National Academies Press, Washington, D.C. [Google Scholar]
- Schwartz MD, Trecki J, Edison LA, Steck AR, Arnold JK, Gerona RR. A Common Source Outbreak of Severe Delirium Associated with Exposure to the Novel Synthetic Cannabinoid ADB-PINACA. J Emerg Med. 2015. May;48(5):573–80. doi: 10.1016/j.jemermed.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks KG, Clark W, Behonick G. Death Associated With the Use of the Synthetic Cannabinoid ADB-FUBINACA. J Anal Toxicol. 2016. April;40(3):236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedberg MDB. A proactive nonclinical drug abuse and dependence liability assessment strategy: a sponsor perspective. Behavioural Pharmacology 2013, 24:396–402. [DOI] [PubMed] [Google Scholar]
- Tai S, Fantegrossi WE. Pharmacological and toxicological effects of synthetic cannabinoids and their metabolites. Curr Top Behav Neurosci. 2017;32:249–262. doi: 10.1007/7854_2016_60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G. Preclinical studies on the reinforcing effects of cannabinoids. A tribute to the scientific research of Dr. Steve Goldberg. Psychopharmacology 2016. 233(10):1845–1866. 10.1007/s00213-016-4244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecki J, Gerona RR, Schwartz MD. Synthetic cannabinoid–related illnesses and deaths. New Eng J Med 2015. 373(2):103–107. 10.1056/NEJMp1505328 [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime, World Drug Report 2017. (ISBN: 978-92-1-148291-1, e ISBN: 978-92-1-060623-3, United Nations publication, Sales No. E.17.XI.6). [Google Scholar]
- Weinstein AM, Rosca P, Fattore L, London ED. Synthetic cathinone and cannabinoid designer drugs pose a major risk for public health. Front Psychiatry. 2017; 8: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Marusich JA, Grabenauer M, Moore KN, Huffman JW, Thomas BF. Evaluation of first generation synthetic cannabinoids on binding at non-cannabinoid receptors and in a battery of in vivo assays in mice. Neuropharmacology. 2016. November;110(Pt A):143–153. doi: 10.1016/j.neuropharm.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Martin BR, Huffman JW. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend. 2012. June 1;123(1–3):148–53. doi: 10.1016/j.drugalcdep.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Thomas BF. Combination chemistry: Structure-activity relationships of novel psychoactive cannabinoids. Curr Top Behav Neurosci. 2017;32:231–248. doi: 10.1007/7854_2016_17. [DOI] [PubMed] [Google Scholar]
- Young R. Drug Discrimination. in Methods of behavior analysis in neuroscience (2nd edition) Buccafuso Jerry J. (Ed.); 2009. CRC Press, Taylor & Francis Group LLC, Boca Raton: (http://www.ncbi.nlm.nih.gov/books/NBK5228/) [PubMed] [Google Scholar]