Abstract
The innate immune element, cathelicidin antimicrobial peptide (CAMP), is vital in the formation of the antimicrobial barrier in skin. CAMP production is increased during epidermal differentiation and enriched in the stratum corneum. We recently identified an endoplasmic reticulum (ER) stress-mediated sphingosine-1-phosphate (S1P)- dependent mechanism of CAMP synthesis. Interestingly, in this study, we found that S1P synthesized by an isoform of sphingosine kinase (SPHK), SPHK1, serves as a signal for CAMP synthesis; and conversely, another isoform SPHK2 likely has a suppressor role or no role in CAMP production. Pertinently, prior studies showed that physiological ER stress is essential for normal epidermal differentiation. We here demonstrate that: increased ER stress is evident in differentiated cultured keratinocytes (KC); 2) increases in both CAMP and S1P production depend upon differentiation level of KC (proliferated<early-<late-stage of differentiated KC); 3) expression of SPHK1 and SPHK2 is increased and decreased, respectively, during KC differentiation; and 4) dihydroS1P that is preferentially synthesized by SPHK2 does not increase CAMP production. Finally, overexpression of wild type, but not dominant negative SPHK2, suppresses CAMP production in both proliferated and differentiated KC. Our current study suggests that alterations of both SPHK1 and SPHK2 levels coordinately increase CAMP production during epidermal differentiation.
Keywords: cathelicidin antimicrobial peptide, ER stress, keratinocyte differentiation, sphingosine kinase, sphingosine-1-phosphate
LETTER TO THE EDITOR
The innate immune element, cathelicidin antimicrobial peptide (CAMP), is a key epidermal antimicrobial peptide that protects host cells/tissues from microbial pathogens, including Staphylococcus aureus. CAMP synthesis is regulated by activation of a vitamin D receptor (VDR), and vitamin D deficiency correlates with lower levels of CAMP in plasma of subjects (Gombart, 2009). Yet, VDR binding sequences are absent on the promoter region of murine CAMP (CRAMP) (Gombart, 2009). Therefore, CAMP synthesis is also regulated by vitamin D receptor-independent regulatory mechanism(s). We identified a vitamin D receptor-independent regulatory mechanism of CAMP production initiated by physiological levels of endoplasmic reticulum (ER) stress-mediated NF-κB activation followed by activation of C/EBPα (Park et al., 2011). This pathway is important to maintain and/or to enhance antimicrobial defense, particularly in stressed cells (Park et al., 2011). We further elucidated a modulator lipid, sphingosine-1-phosphate (S1P), as a key upstream mechanism for NF-κB activation of CAMP regulation in response to ER stress (Park et al., 2013, Park et al., 2016).
Pertinently, some proteins, such as ABCA12, C/EBPβ and Kruppel-like factor 4 (KLF4), that are required for normal epidermal differentiation, are regulated by ER stress-induced proteins, suggesting that ER stress is required for normal epidermal differentiation (Sugiura et al., 2009). Additionally, we found that CAMP production is increased in differentiated keratinocytes (Park et al., 2011). Moreover, we found that S1P synthesized by sphingosine kinase (SPHK) 1 activates NF-κB, leading to stimulation of CAMP production; while silencing of another isoform of SPHK, SPHK2, further stimulated ER stress-mediated increases in CAMP production (Park et al., 2013). These results suggest that SPHK2 does not contribute to S1P-mediated CAMP synthesis and/or has a negative role in CAMP production. Hence, we hypothesize that activation of the ER stress-induced S1P signaling mechanism accompanied with coordinate regulation by SPHK1 and SPHK2 is involved in increases in CAMP production during epidermal differentiation.
We previously established a method to obtain proliferated cultured normal human KC and two stages (early and late) of differentiated KC (Uchida et al., 2001, Uchida et al., 2007). Changes in ceramide metabolic enzymes, and generation of heterogeneous ceramide species, lamellar bodies, external lamellar membrane structures, corneocyte lipid envelopes and cornified envelopes become evident, depending upon which stage of differentiation KC are reproduced in these cultured cells (Hamanaka et al., 2005, Uchida et al., 2001, Uchida et al., 2007). We further validated our established cultures by assessing cell morphological changes, and protein expression levels of both early-stage (keratin 10) and late-stage (loricrin) of KC differentiation. While this protein production is not evident in proliferated KC, keratin 10 and loricrin are synthesized in early-stage and late-stage differentiated KC, respectively ( Suppl. Fig. 1A and B). In addition, ER stress assessed by mRNA and protein expression of an ER stress marker, CHOP, already occur in early-stage differentiated KC and is maintained in late-stage differentiated cells, albeit ER stress level is lower than early-stage differentiated cells (Suppl. Fig. 1C-D). Taken together with our prior studies, these results justify utilization of our cell culture models of proliferated KC and two stages of differentiated KC to investigate whether the S1P-dependent CAMP mechanism is responsible for increased CAMP production during KC differentiation.
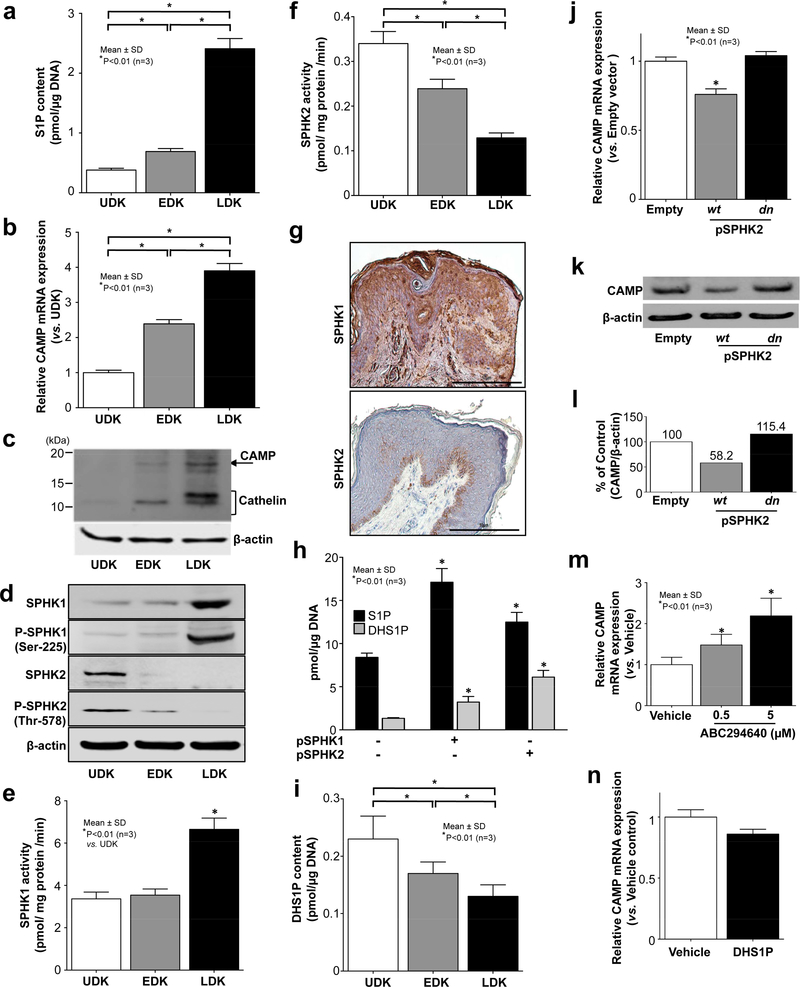
We first assessed S1P content, and CAMP mRNA and protein levels in proliferated, early and late stages of differentiated keratinocytes. S1P, CAMP mRNA and protein levels are significantly increased during KC differentiation (Fig. 1A-C).
Figure 1: CAMP expression is increased by the actions of keratinocyte of SPHK1 and SPHK2 during keratinocyte differentiation.
S1P (A, H) and DHS1P levels (H, N), CAMP mRNA (B, J, M) and protein (C, K) were assessed by LC-ESI-MS/MS, qRT-PCR and Western blot analysis, respectively. Both intact and/or phosphorylated form of SPHK1 and SPHK2 in cells or human skin tissues were assessed by Western blot (D) or immunohistochemistry (G) analyses. SPHK1 (E) and SPHK2 (F) activities were determined by LC-ESI-MS/MS. KC were overexpressed with pSPHK1-Myc-wild type (wt), pSPHK2-HA-wt and pSPHK2-HA-dominant negative (dn) (H, J-L). The intensity of CAMP protein from experiment of (K) was quantified and the integrated areas were normalized to the corresponding value of β-actin (L). KC were incubated with ABC294640 (0.5 −5 μM), SPHK2 specific inhibitor, for 24 hrs (M). UDK, undifferentiated KC; EDK, early stage of differentiated KC; and LDK, late stage of differentiated KC. Scale bar=10 μm.
We then investigated protein levels and activities of two isoforms of the S1P synthetic enzyme, SPHK1 and SPHK2, in KC. Phosphorylation of SPHK by ERK kinase is required for activation of their catalytic activity (Chan and Pitson, 2013). Both SPHK1 and phosphorylated SPHK1 (P-SPHK1) levels are significantly increased in late stage of differentiated KC, while SPHK2 and P-SPHK2 levels are decreased during differentiation (Fig. 1D). Consistent with SPHK protein levels, enzyme assays revealed that SPHK1 activity is significantly elevated in later stages of differentiated cells (Fig. 1E), while conversely, SPHK2 activity declines in cells as differentiation continues (Fig. 1F). Moreover, consistent with in vitro cultured KC study, immunostaining of human epidermis showed that SPHK2 is highly expressed at the basal layer, while SPHK1 expression increased in differentiated cell layers (Fig. 1G), suggesting that alterations of SPHK1 and SPHK2 expression occur in parallel with changes in CAMP production during epidermal differentiation.
Prior study demonstrated that SPHK2 preferentially catalyzes dihydrosphingosine to synthesize dihydroS1P (DHS1P) (Liu et al., 2000). We overexpressed SPHK1 and SPHK2 in KC. Both S1P and DHS1P were increased in SPHK1 and SPHK2 overexpressed cells (Fig. 1H), while increases in DHS1P were much higher than S1P in SPHK2 overexpressed cell (S1P, 1.49-fold and DHS1P, 4.57-fold vs. control) (Fig. 1H). In our prior study, we demonstrated that S1P, but not DHS1P binds to HSP90 leading to a signaling complex formation that activates NF-κB (Park et al., 2016). As shown above (Fig. 1D, F and G), SPHK2 expression/activity is decreased during KC differentiation. We confirmed DHS1P levels were decreased in differentiated KC (Fig. 1I). These results suggest that SPHK2 is unable or less able to initiate a S1P-mediated signal for CAMP production. Moreover, increases in CAMP production occur in cells in which SPHK1 and SPHK2 activity are increased and decreased, respectively.
It is possible that SPHK2 protein structure may suppress or inhibit formation of S1P-HSP 90 signaling complex or NF-κB activation (Park et al., 2016) resulting in attenuating CAMP production in undifferentiated KC. When we overexpressed dominant negative (dn) and wild type (wt) SPHK2, we found that SPHK2 mRNA and protein levels are significantly elevated in both wt and dn SPHK2 overexpressed KC, while SPHK1 levels are not altered (Suppl. Fig. 2A-B [proliferated-KC] and C [differentiated-KC]. Note: anti-SPHK2 does not distinguish wt or dn SPHK2.) Both mRNA and protein levels of CAMP were not changed in dn SPHK2 expressed cells, while CAMP levels were decreased in wt SPHK2 expressed cells (Fig. 1J-L [proliferated-KC], Suppl. Fig. 2D [differentiated-KC]). These results exclude the possibility that SPHK2 protein structure did not affect S1P-mediated increases in CAMP production.
Lastly, a specific pharmacological inhibitor of SPHK2 increased CAMP mRNA expression in undifferentiated KC (Fig. 1M), while exogenous DHS1P did not change CAMP mRNA levels (Fig. 1N). Thus, DHS1P could not directly inhibit CAMP production. Instead, SPHK2 that preferentially synthesizes DHS1P could decrease sphingoid base pool, which is utilized by S1P synthesis that stimulates CAMP synthesis.
Taken together with our prior study, i.e., ER stress increases ceramide levels followed by increasing S1P production (Park et al., 2013, Park et al., 2016), our current studies demonstrated that increased SPHK1 expression and S1P production, and conversely decreases in SPHK2 expression and DHS1P production, are likely responsible for increased CAMP levels in differentiated KC in parallel with ER stress.
Since VDR activation is important for normal keratinocyte differentiation (Bikle, 2011), both ER stress-mediated S1P and VDR mechanisms should be responsible for increases in CAMP production in differentiated KC. However, it has not been clarified whether VDR activation or S1P signaling plays a major role in increased CAMP production during differentiation. Yet, ER stress-mediated SPHK1- dependent S1P signaling mechanism for CAMP upregulation should be important in vitamin D-deficient subjects, who may live in certain areas or during certain seasons where there is insufficient ultraviolet irradiation to produce vitamin D from 7-dehydrocholesterol (Bikle, 2011).
Finally, it is notable that CAMP overproduction and insufficiency have been shown as factors in cutaneous inflammation; i.e., rosacea (Yamasaki et al., 2007), contact dermatitis and psoriasis (Frohm et al., 1997) and atopic dermatitis (Ong et al., 2002), respectively. CAMP overproduction and deficiency should be due to changes in SPHK1 and SPHK2 expression by abnormal differentiation. Hence, pharmacological modulation of both SPHK1 and SPHK2 expression could normalize abnormal CAMP and reduce inflammatory responses while enhancing antimicrobial defense.
Materials and Methods
Reagents, antibodies, expression vectors, detailed methods for cell culture and transfection, RNA isolation and quantitative RT-PCR, Western blot analysis, histological study, S1P quantification, enzyme activity assays, and statistical analysis used in this study can be found in supplemental material.
Supplementary Material
Acknowledgements
We gratefully thank Drs. Taro Okada and Shunichi Nakamura (Department of Biochemistry and Molecular Biology, Kobe University Graduate School of Medicine, Kobe, Japan) for providing plasmids for SPHK1 and SPHK2. We also thank Ms. Joan Wakefield for superb editorial assistance (Northern California Institute for Research and Education). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B07050504), by Hallym University Research Fund, 2017 (HRF-201703–004), by the Korea Institute for Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA)(117063031SB101)(to KP), by Grant-in-Aid for Scientific Research (C) 25440036 from the Japan Society for the Promotion of Science (JSPS), the Japan Foundation for Applied Enzymology and from Tokyo Institute of Food Technology (to H.I.) and by the National Institutes of Health Grant AR062025 (to YU).
Footnotes
Conflict of Interest
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol 2011;347:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H, Pitson SM. Post-translational regulation of sphingosine kinases. Biochimica et biophysica acta 2013;1831:147–56. [DOI] [PubMed] [Google Scholar]
- Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 1997;272:15258–63. [DOI] [PubMed] [Google Scholar]
- Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol 2009;4:1151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka S, Nakazawa S, Yamanaka M, Uchida Y, Otsuka F. Glucosylceramide accumulates preferentially in lamellar bodies in differentiated keratinocytes. Br J Dermatol 2005;152:426–34. [DOI] [PubMed] [Google Scholar]
- Hatano Y, Man M-Q, Uchida Y, Crumrine D, Scharschmidt TC, Kim EG, et al. Maintenance of an Acidic Stratum Corneum Prevents Emergence of Murine Atopic Dermatitis. Journal Invest Dermatol 2009;129:1824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem 2000;275:19513–20. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002;347:1151–60. [DOI] [PubMed] [Google Scholar]
- Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, et al. Regulation of Cathelicidin Antimicrobial Peptide Expression by an Endoplasmic Reticulum (ER) Stress Signaling, Vitamin D Receptor-independent Pathway. J Biol Chem 2011;286:34121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Elias PM, Shin KO, Lee YM, Hupe M, Borkowski AW, et al. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol Cell Biol 2013;33:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Ikushiro H, Seo HS, Shin KO, Kim YI, Kim JY, et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc Natl Acad Sci 2016;113:E1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman MR, Pham DH, Pitson SM. Isoform-selective assays for sphingosine kinase activity. Methods Mol Biol 2012;874:21–31. [DOI] [PubMed] [Google Scholar]
- Seo EY, Park GT, Lee KM, Kim JA, Lee JH, Yang JM. Identification of the target genes of atopic dermatitis by real-time PCR. J Invest Dermatol 2006;126:1187–9. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Muro Y, Futamura K, Matsumoto K, Hashimoto N, Nishizawa Y, et al. The Unfolded Protein Response Is Activated in Differentiating Epidermal Keratinocytes. J Invest Dermatol 2009;129:2126–35. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Behne M, Quiec D, Elias PM, Holleran WM. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J Invest Dermatol 2001;117:1307–13. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Hama H, Alderson NL, Douangpanya S, Wang Y, Crumrine DA, et al. Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation. J Biol Chem 2007;282:13211–9. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med 2007;13:975–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.