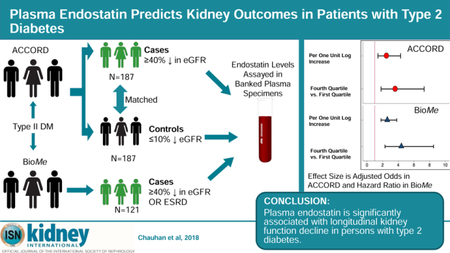
Abstract
Novel biomarkers are needed to predict kidney function decline in patients with type 2 diabetes, especially those with preserved glomerular filtration rate (GFR). There are limited data on the association of markers of endothelial dysfunction with longitudinal GFR decline. We used banked specimens from a nested case-control study in the Action to Control Cardiovascular Disease (ACCORD) trial (n=187 cases: 187 controls) and from a diverse contemporary cohort of type 2 diabetic patients from the Mount Sinai BioMe Biobank (n=871) to assess the association of plasma endostatin and kidney outcomes. We measured plasma endostatin at enrollment and examined its association with a composite kidney outcome of sustained 40% decline in estimated GFR or endstage renal disease. Baseline plasma endostatin levels were higher in participants with the composite outcome. Each log2 increment in plasma endostatin was associated with approximately 2.5-fold higher risk of the kidney outcome (adjusted OR 2.5; 95% CI 1.5 – 4.3 in ACCORD and adjusted HR 2.6; 95% CI 1.8 – 3.8 in BioMe). Participants in the highest vs. lowest quartile of plasma endostatin had approximately four-fold higher risk for the kidney outcome (adjusted OR 3.6; 95% CI 1.8 – 7.3 in ACCORD and adjusted HR 4.4; 95% CI 2.3 – 8.5 in BioMe). The AUC for the kidney outcome improved from 0.74 to 0.77 in BioMe with the addition of endostatin to a base clinical model. Plasma endostatin was strongly associated with kidney outcomes in type 2 diabetics with preserved eGFR and improved risk discrimination over traditional predictors.
Keywords: Albuminuria, Chronic Kidney Disease, Kidney Injury, Inflammation, Risk Stratification
Graphical Abstract
INTRODUCTION
Type 2 diabetes (T2D) is being recognized as an epidemic worldwide. One of the commonest complications of T2D is diabetic kidney disease (DKD) which develops in over one third of type 2 diabetics (T2D).1 DKD is the single largest cause of end stage renal disease (ESRD), accounts for 44% of incident ESRD patients and is a major independent risk factor for other complications including coronary artery disease, stroke, and retinopathy. 2,3
With growing prevalence of T2D, it is imperative to accurately discriminate patients at high risk of development and progression of DKD. This is especially important in patients who have preserved renal function at baseline so that preventative efforts can be implemented early. However, in patients with T2D and preserved renal function, eGFR and albuminuria are only modestly useful for risk prediction.4 In addition, eGFR/albuminuria predominantly assess glomerular function and there is a paucity of markers assessing the other dimensions of renal injury. One of these axes of renal injury is endothelial and vascular injury. Endothelial dysfunction has been shown to be an integral part of DKD development and progression.5 Identification of circulating biomarkers representing endothelial dysfunction could also identify a subgroup of patients that might benefit from targeted therapies. However, there are few studies on markers of vascular injury/endothelial dysfunction and DKD progression.
We sought to examine the association of endostatin, a 20-kDa C terminal fragment of collagen XVIII with kidney outcomes. Endostatin levels may be representative of several pathways involved in kidney dysfunction including endothelial dysfunction, matrix remodeling post kidney injury and angiogenesis.6–8 We utilized biospecimens linked to longitudinal clinical data from the Action to Control Cardiovascular Disease (ACCORD) trial and in a contemporary clinical cohort from the Icahn School of Medicine at Mount Sinai (ISMMS) BioMe Biobank.
RESULTS:
Characteristics of Cases and Controls
In ACCORD, 374 participants (187 matched case-control pairs) were matched appropriately with regards to demographics, baseline CKD-EPI eGFR and UACR (Table 1). Cases and controls had similar BMI, diabetes duration and HbA1c. However, participants with composite kidney outcome had more baseline cardiovascular diseases, higher baseline MAP, and a greater proportion of cases were randomized to fibrates. They also had lower eGFR than controls at the end of follow up (46 vs. 83 ml/min/1.73m2; p<0.01), but no significant differences in UACR at end of follow-up.
Table 1.
Clinical And Kidney Markers Characteristics By Case-Control (ACCORD) And Composite Kidney Endpoint (Biome) Status
| ACCORD | BioMe | |||||
|---|---|---|---|---|---|---|
| Controls (n=187) | Cases (n=187) | p | Without Kidney Endpoint (n= 750) |
With Kidney Endpoint (n= 121) |
p | |
| Clinical Characteristics | ||||||
| Age in years1, Median (IQR) | 61.8 (5.4) | 62.4 (5.7) | 0.37 | 60 [53 – 66] | 61 [51 – 66] | 0.64 |
| Female1, n (%) | 92 (49.1) | 91 (49.1) | 0.88 | 434 (57.9) | 73 (60.3) | 0.62 |
| Race1 | 0.96 | 0.09 | ||||
| White | 140 (74.9) | 138 (73.8) | 59 (7.9) | 3 (2.5) | ||
| African-American | 20 (10.7) | 20 (10.7) | 290 (38.7) | 56 (46.3) | ||
| Hispanic/Latino | 13 (6.9) | 16 (8.6) | 355 (47.3) | 57 (47.1) | ||
| Other | 13 (6.9) | 13 (6.9) | 46 (6.1) | 5 (4.1) | ||
| Hypertension, n (%) | 132 (70.6) | 138 (74.2) | 0.4 | 694 (92.5) | 119 (98.4) | 0.02 |
| Cardiovascular disease, n (%) | 44 (23.8) | 76 (40.6) | <0.001 | 358 (47.7) | 81 (66.9) | <0.001 |
| Heart Failure, n (%) | 4 (2.1) | 11 (5.9) | 0.07 | 144 (19.2) | 48 (39.7) | <0.001 |
| Body Mass Index in kg/m2, Median [IQR] | 32.7 (5.5) | 33.4 (5.9) | 0.23 | 31.0 [26.9 – 36.2] | 29.7 [24.7 – 35.5] | 0.28 |
| Mean Arterial Pressure in mm Hg, Mean [SD] | 94.9 (10.3) | 96.9 (11.1) | 0.07 | 93.7 (8.9) | 97.3 (10.3) | <0.001 |
| Laboratory Characteristics | ||||||
| Baseline eGFR,1 Median [IQR} | 90.2 [78.6–95.9] | 86.9 [77.2–93.8] | 0.18 | 69.0 [56.3 – 80.3] | 62.6 [50.5 – 78.0] | 0.02 |
| Baseline Hemoglobin A1C,1 Median [IQR] | 8.4 (1.1) | 8.4 (1.01) | 0.65 | 6.9 [6.2 – 8.5] | 7.6 [6.3 – 9.7] | 0.01 |
| Baseline UACR,1 Median [IQR] | 21.3 [7.62–66.4] | 19.6 [7.7–101.8] | 0.1 | 11.0 [4.0 – 38.0] | 204.0 [25.0 – 1240.0] |
<0.001 |
| Medications | ||||||
| ACE/ARB, n (%) | 122 (65.6) | 138 (73.8) | 0.08 | 575 (76.7) | 100 (82.6) | 0.15 |
| Plasma Biomarker Concentrations | ||||||
| Endostatin, in ng/ml, Median [IQR] | 36.2 [31.0–44.2] |
41.5 [34.4–51.6] |
<0.001 | 37.8 [29.9–47.4] |
44.7 [36.5– 62.4] |
<0.001 |
Indicates variables matched in case-control; Values are presented as mean (standard deviation) or median [interquartile range] for continuous values and N (%) for categorical values
Missing in 363 (37.4%) of participants in BioMe.
In the BioMe Biobank, of 871 participants, 121 (12.5%) experienced the composite kidney outcome over a median follow-up of 4.6 (IQR 3.4–5.6) years. Participants that experienced the outcome had higher proportion of hypertension, heart failure, and higher baseline MAP. In addition, they had lower baseline eGFR (62.6 vs. 69 ml/min; p=0.02); higher hemoglobin A1C, and higher UACR (204 vs. 11 mcg/mg; p<0.01).
Differences in Plasma Endostatin and correlation with baseline characteristics
Participants that experienced the kidney outcome in ACCORD had higher baseline median endostatin compared to controls (41.5 [IQR 34.4–51.6] ng/ml vs. 36.1 [IQR 31.0–44.2] ng/ml; p<0.01). Similarly, the endostatin levels were higher in participants that reached the kidney endpoint (44.7 [IQR 36.5– 62.4] vs. 37.8 [IQR 29.9–47.4] ng/ml; p<0.01) in BioMe. The correlations of endostatin with clinical variables are listed in Table 2.
Table 2.
Partial Pearson Correlations Of Plasma Endostatin Log2 Transformed Levels With Baseline Characteristics In ACCORD And Biome Biobank
| Biomarker | Age | MAP | Baseline eGFR |
UACR | HbA1c | BMI |
|---|---|---|---|---|---|---|
| ACCORD | ||||||
| Plasma Endostatin | −0.16* | −0.06 | −0.24* | 0.07 | −0.05 | 0.10* |
| BioMe | ||||||
| Plasma Endostatin | 0.2* | 0.03 | −0.4* | 0.3* | −0.1* | −0.04 |
P<0.05
P<0.01
Log transformation was to base 2
Association of Plasma Endostatin with kidney endpoints
In ACCORD, log2 transformed endostatin levels were significantly associated with kidney function in fully adjusted models; each log2 increment was associated with 2.5-fold greater odds of outcome (aOR 2.5; 95% CI 1.4–4.3). When categorized into quartiles, there was a graded relationship between endostatin levels and the kidney outcome. The top quartile was associated with three-fold increased odds compared to the lower quintile (aOR 3.6; 95% CI 1.8–7.3; Table 3, Figure 1).
Table 3.
Measures Of Association (OR/HR) And 95% Confidence Intervals (CI) For Plasma Endostatin With The Kidney Function Decline Outcome Modeled Categorically And Continuously
| ACCORD | Model 1 (95% CI) |
OR | Model 2 (95% CI) |
OR | Model 3 (95% CI) |
OR | Model 4 (95% CI) |
OR | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma Endostatin in ng/ml | |||||||||||
| OR per 1-unit log2 increase | 2.6 (1.7 – 4.4) | 2.6 (1.6 – 4.4) | 2.5 (1.5 – 4.1) | 2.5 (1.4 – 4.3) | |||||||
| <=32.3 ng/ml | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | |||||||
| 32.4–38.2 ng/ml | 1.7 (0.9 – 3.1) | 1.7 (0.9 – 3.2) | 1.7 (0.9 – 3.4) | 1.7 (0.9 – 3.4) | |||||||
| 38.3–47.6 ng/ml | 3.0 (1.6 – 5.4) | 2.8 (1.5 – 5.3) | 2.9 (1.5 – 5.7) | 3.1 (1.5 – 6.1) | |||||||
| >47.7 ng/ml | 3.6 (2.0 – 6.7) | 3.6 (1.9 – 6.8) | 3.6 (1.8 – 7.1) | 3.6 (1.8 – 7.3) | |||||||
| BioMe |
Model 1 (95% CI) |
HR |
Model 2 (95% CI) |
HR |
Model 3 (95% CI) |
HR |
Model 4 (95% CI) |
HR | |||
| Plasma Endostatin in ng/ml | |||||||||||
| HR per 1-unit log2 increase | 3.0 (2.1 – 4.1) | 2.9 (2.1 – 4.1) | 2.9 (2.1 – 4.1) | 2.6 (1.8 – 3.8) | |||||||
| ≤30.5 ng/ml | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | |||||||
| 30.6 – 38.7 ng/ml | 1.6 (0.9 – 3.1) | 1.9 (0.9 – 3.7) | 1.9 (0.9 – 3.7) | 1.8 (0.9 – 3.5) | |||||||
| 38.7 – 48.6 ng/ml | 2.1 (1.1 – 3.9) | 2.2 (1.1 – 4.3) | 2.2 (1.1 – 4.3) | 2.1 (1.0 – 4.1) | |||||||
| ≥48.7 ng/ml | 4.7 (2.7 – 8.4) | 5.1 (2.7 – 9.6) | 5.1 (2.7 – 9.6) | 4.4 (2.3 – 8.5) | |||||||
In ACCORD
Model 1: Unadjusted conditional Logistic Model; Model 2: Model 1 + Cardiovascular risk factors (HbA1c; mean arterial pressure; history of cardiovascular disease, hypertension and heart failure; BMI; intensive glycemic control arm, intensive blood pressure control arm); Model 3: Model 2 + Medications (Fibrate arm, ACE-Inhibitors/Angiotensin Receptor Blocker usage); Model 4: Model 3 + baseline eGFR and UACR.
In BioMe
Model 1: Unadjusted cox regression Model; Model 2: Model 1 + Age, sex, cardiovascular risk factors (HbA1c; mean arterial pressure; history of cardiovascular disease, hypertension and heart failure; BMI); Model 3: Model 2 + Medications (ACE-Inhibitors/Angiotensin Receptor Blocker usage); Model 4: Model 3 + baseline eGFR.
Figure 1.
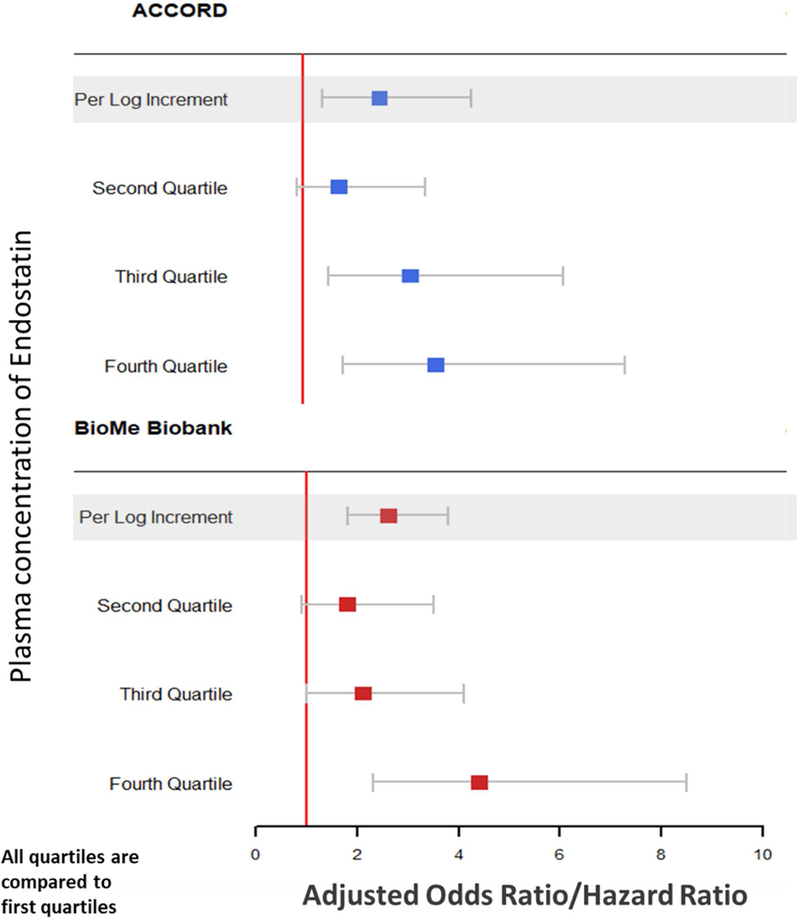
Adjusted Point Estimates And Confidence Intervals Of Plasma Endostatin For The Kidney Outcome In ACCORD And BioMe Biobank
In ACCORD: 1st Quartile was <32.3 ng/ml; 2nd quartile was 32.4–38.2 ng/ml; 3rd quartile was 38.3–47.6 ng/ml and 4th quartile was >47.7 ng/ml
In BioMe Biobank: 1st Quartile was <30.5 ng/ml; 2nd quartile was 30.6–38.7 ng/ml; 3rd quartile was 38.7–48.6 ng/ml and 4th quartile was >48.7 ng/ml
Odds ratios were used in ACCORD (case-control design) and hazards ratios in BioMe (cohort study).
In the BioMe cohort, there was also a similar and significant association with the composite kidney outcome with endostatin levels (2.6-fold HR for each log2 increment in plasma endostatin). Similar to ACCORD, the top quartile of plasma endostatin was significantly associated with four-fold increased hazards of the composite kidney outcome (aHR 4.4; 95% CI 2.3–8.5; Table 3, Figure 1). These associations were consistent in the subset of 608 BioMe participants with existing proteinuria/albuminuria at baseline: per log2 increment the aHR was 2.7 (95% CI 1.7–4.3) and the aHR for the top quartile vs. bottom quartile was 5.1 (95% CI 2.2–11.7; Supplementary Table 1).
Change in risk discrimination for kidney function decline with addition of plasma endostatin to traditional predictors and clinical utility of the prediction model in BioMe cohort
An addition of plasma endostatin alone to a baseline model consisting of traditional clinical predictors of kidney function decline improved the C-statistic in BioMe from 0.74 to 0.77 (p= 0.01). The performance of the Model 4 (Table 3, BioMe cohort) to predict the composite kidney outcome was evaluated for a range of predicted risk thresholds to potentially inform use in future studies (Supplementary Table 2). For example, using a predicted probability threshold of 15% for the development of the outcome, 33% of all the patients screened with the similar model could be excluded from the study enrollment. From the remaining 67% of the patients, 27% would eventually develop sustained 40% decline in eGFR or ESRD. In BioMe, at a cutoff value of 45 ng/mL (roughly the value for the 4th quartile for both ACCORD and BioMe), sensitivity and specificity were 50% and 71%, respectively, and positive predictive value was 21%, with negative predictive value of 90%.
DISCUSSION
This analysis evaluated plasma endostatin as a prognostic biomarker for kidney function decline in two cohorts, from a clinical trial cohort as well as from a large multiethnic cohort of patients from a quaternary care center. We demonstrated that plasma endostatin is significantly associated with the development of a significant kidney function decline, both categorically and continuously, after adjusting for traditional predictors and confounders. In addition, endostatin improved risk discrimination for the composite kidney outcome when added to either traditional predictors.
Endostatin is a fragment of collagen XVIII which is highly expressed in renal glomeruli and peritubular capillaries.9,10 It is formed during extracellular matrix (ECM) remodeling and has anti-angiogenic effects that may lead to impaired kidney repair after injury. However, the exact pathways reflected in circulating levels of endostatin are currently a matter of debate. Endostatin may reflect endothelial dysregulation and indeed, immobilization of endostatin related fragments promotes endothelial cell survival in murine models.6 However, endostatin has also endogenous anti-angiogenic effects and the balance between pro and antiangiogenic factors is important in kidney function decline.7,11 Finally, circulating endostatin levels may also represent matrix remodeling secondary to fibrosis post renal injury.10,12
With regards to human studies, circulating endostatin levels are associated cross-sectionally with impaired eGFR and albuminuria, particularly in hypertensive, elderly individuals.13,14 In addition, endostatin levels are also associated with mortality in two independent cohorts of community-based individuals.15 There have been few published studies on the association between plasma endostatin levels and DKD development/progression. Carlsson et al., in an analysis of the Swedish Cardiovascular Risk Factors in Patients with Diabetes: A Prospective Study in Primary Care (CARDIPP) study, demonstrated that endostatin at baseline was significantly associated with kidney outcome (defined as a eGFR decline of ≥ 20%) and mortality over a follow-up period of 6.7 years.16 However, this study was limited since it included only white participants, had very few CKD events (only 20 over follow up), used an arbitrary renal outcome and did not assess improvement in discrimination. In contrast, we used a multiethnic cohort that is representative of the United States population, utilized robust kidney outcomes, and assessed incremental improvement when added to traditional markers of eGFR and albuminuria.
DKD is a complex disease and involves the interlinked axes of ongoing inflammation, tubular injury and endothelial/vascular dysfunction. 17,18 Thus, it is likely that a multidimensional panel assessing these interlinked axes could characterize additional kidney health dimensions and accurately sub-phenotype patients at high risk.19 In this manuscript, we demonstrated that plasma endostatin is an independent prognostic marker for kidney function decline. These findings were remarkably consistent across two cohorts which differed in key characteristics including time period, ethnic make-up and settings. The ACCORD was a clinical trial and follow up measurements were conducted as per a standard protocol, but the BioMe is a contemporary clinical cohort that represents patterns of diagnoses and treatment of “real-world” practice. The consistency of results across these two diverse cohorts indicates that plasma endostatin is a robust marker of DKD progression and that endothelial dysfunction, measured non-invasively can add significantly to risk discrimination in an unselected group of patients with T2D and preserved eGFR. In addition, there was minimal correlation with eGFR indicating that endostatin captures an axis of kidney injury that is not captured by standard clinical metrics. Finally, biomarker enhanced risk models may have practical clinical trial applications as well. Depending on the predicted probability of eGFR decline using a biomarker enhanced clinical model, the tradeoff between sensitivity and specificity can be accurately determined for optimizing enrollment numbers.
Our study has some limitations. There was storage time of up to 14 years between sample collection and biomarker assessment. Although these were all stored at −80°C, there are limited data on these analytes stability at this temperature. We cannot rule out imperfect case-control matching, although there were no significant differences between key characteristics. Randomization to fibrate intervention has shown to increase serum creatinine from baseline in a reversible manner.20 However, the estimates were robust when fibrate intervention was included in the multivariable model. eGFR values were calculated from serum creatinine measurements, and not directly measured, although direct measurement of eGFR in large populations is impractical. In the BioMe cohort, 37% lacked baseline UACR measurement in this cohort, however this is likely due to preserved kidney function at baseline. However, it should be noted that in the 608 participants with UACR available at the time of enrollment, the independent association between the biomarkers and outcomes was maintained despite adjustment for albuminuria or proteinuria. In addition, we used EMR-based lab data, thus there was potential for ascertainment bias for the kidney outcome in those with more comorbidities or worse kidney function, however, this was mitigated by linkage to USRDS. The ACCORD study was a matched 1:1 case: control study and thus is subject to limitations including selection bias, information bias and residual confounding. Additionally, we could not match more controls to cases because of sample size restrictions and lack of appropriately matched controls. However, the association between plasma endostatin and kidney outcomes was replicated in an independent cohort study and thus is likely to be robust. We only had a single measurement of endostatin at baseline and could not assess the association between longitudinal changes in endostatin and kidney outcomes.
In summary, we demonstrate that plasma endostatin is significantly associated with longitudinal kidney function decline in persons with type 2 diabetes. Further studies should be focused on further validation in cohort studies, addition of this novel biomarker in multimarker panels for kidney disease prognostication, as well as whether this early risk stratification improves clinical care in this vulnerable population.
MATERIALS AND METHODS
The Action to Control Cardiovascular Disease (ACCORD) trial
The ACCORD trial enrolled individuals with uncontrolled T2D and high risk of cardiovascular disease. The specific inclusion/exclusion criteria have been previously described.21 All 10,251 patients were randomly assigned to receive intensive or standard glycemic therapy. With a double two-by-two factorial design, 4733 patients were randomly assigned to lower their blood pressure by receiving either intensive therapy or standard therapy. Similarly, 5518 patients treated with open-label simvastatin were randomly assigned to receive fenofibrate or placebo. Total follow-up of patients was 5 years with banked plasma/urine specimens from the baseline visit.
The BioMe Biobank at ISMMS
The BioMe Biobank is an Institutional Review Board (IRB)-approved, a consented electronic medical record (EMR)-linked medical care setting biorepository in an ancestrally diverse local community of upper Manhattan.22,23 BioMe operations initiated in 2007 and are fully integrated in clinical care processes, including direct recruitment from over 30 broadly selected clinical sites’ waiting areas and phlebotomy stations by dedicated recruiters. For the purpose of this study, BioMe participants with type 2 diabetes, an eGFR between 45 and 90 ml/min/1.73 m2 at the time of BioMe enrollment, and at least 3 years of follow up data in the EHR were analyzed.
Selection of Cases and Controls in ACCORD
The primary outcome was a sustained (on two or more visits 3 months apart) decline in eGFR of 40% or higher from baseline during the five-year follow up period. This endpoint independently associated with mortality and ESRD in previous studies.24 We utilized a nested case-control approach to select cases and controls. Of 10,251 ACCORD participants, 3270 had both plasma and urine available at baseline. There were no significant differences between the participants with banked biospecimens and those from the overall cohort (Supplementary Table 3). Among participants with available biospecimens, we selected cases with outcome and individually matched them to controls with ≤ 10% eGFR decline over the 5 years of follow-up in ACCORD in 1:1 fashion on key characteristics (age within 5 years, sex, race, baseline albumin/creatinine within 20 mg/g, and baseline eGFR within 10 mL/min/1.73m2). Three participants with sustained eGFR decline did not have plasma samples at baseline and were excluded from analysis with corresponding controls. Thus, final sample size was 187 cases and 187 controls (Figure 2).
Figure 2.
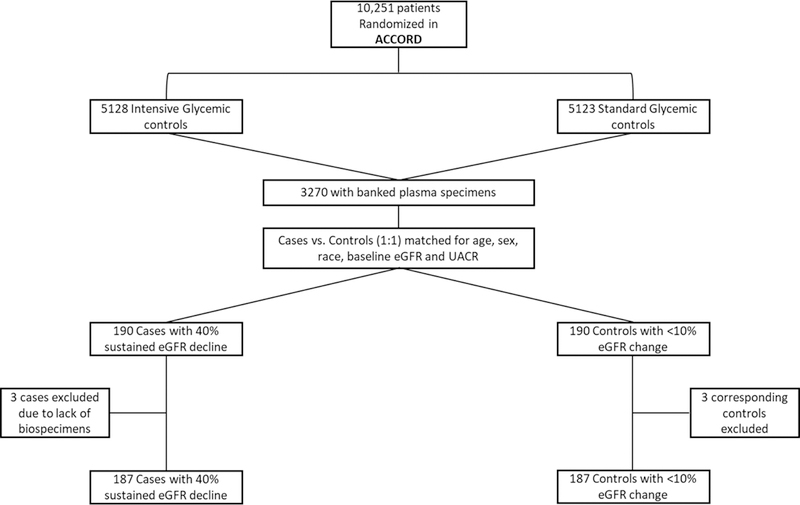

Selection of Cases and Controls in ACCORD (panel A) and Study Cohort of BioMe Biobank (Panel B)
Panel A. This figure shows the selection of cases and controls in the ACCORD clinical trial; Panel B. This figure shows the derivation of the final cohort in the BioMe Biobank
Definition of kidney outcome in BioMe Biobank
We determined eGFR using the CKD-EPI creatinine equation,25 calculated median values per 3 month period of follow up and utilized these for outcome ascertainment. We defined the primary outcome as a composite of ESRD or a sustained 40% decline in eGFR from baseline (BioMe enrollment) over the follow up period (median 4.6 years). We defined sustained 40% decline as a decrease in eGFR by 40% from baseline on two or more separate 3-month intervals and ESRD status as requirement for dialysis or transplant ascertained by linkage to the United States Renal Data System (USRDS). (Figure 2)
Exposure Ascertainment
We defined the primary and secondary exposures of interest as the baseline plasma endostatin concentrations in ng/ml, assessed log2 (continuous) and by quartiles (categorical).
Assessment of covariates in ACCORD
BMI was defined as weight divided by the square of height (kg/m2). Blood pressure was based on the average of three measurements using an automated device (Omron 907) after 5 minutes rest with the participant seated in a chair. Mean arterial pressure (MAP) was calculated with a standardized formula. Hemoglobin A1c (HbA1c) was measured by HPLC. Serum creatinine was determined using the Roche Creatinine Plus enzymatic assay with spectrometric analysis on a Roche Double Modular P Analytics analyzer (Roche Diagnostics, Indianapolis, IN, USA). The results are traceable to the isotope dilution mass spectrometry reference method.20 eGFR was calculated from the measured serum creatinine by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.25,26 Urine albumin was determined by immunonephelometry on a Siemens BN II nephelometer (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) and urinary creatinine (Cr) by a modified Jaffé reaction.20 Urinary albumin excretion was estimated from a single spot urine collection by computing the albumin-to-creatinine ratio (Alb/Cr) in units of mg/g. All laboratory and vital measurements were conducted as part of the ACCORD parent study. Medication use, cardiovascular disease and smoking history were all self-reported by participants.
Assessment of covariates of BioMe Biobank
Age, gender, and AA race were obtained from an enrollment questionnaire administered to BioMe participants. We extracted clinical data for all continuous variables (eGFR, hemoglobin A1c, urine protein or albumin to creatinine ratios) at baseline from the EHR with concurrent time stamps. We defined the baseline period as 1 year before the BioMe enrollment date. Body mass indices (BMI) were calculated as the ratio between weight and the square of height in kg/m2. Hypertension and type 2 diabetes status at baseline were determined using the Electronic Medical Records and Genomics (eMERGE) Network phenotyping algorithms.27 Cardiovascular disease and heart failure were determined by a validated algorithm and ICD-9/10 codes respectively. We considered a participant to be on an angiotensin converting enzyme-inhibitor (ACE-i) or angiotensin receptor blocker (ARB) if they had a concurrent prescription during the BioMe enrollment. We calculated follow up time from BioMe enrollment date to latest visit in the EHR.
Biospecimens Storage and Analytes Measurement
The plasma samples were stored at −80°C for both ACCORD and BioMe Biobank. Biomarkers were measured using an assay on the Mesoscale platform (Meso Scale diagnostics, Gaithersburg, Maryland, USA), which employs proprietary electrochemiluminescence detection methods combined with patterned arrays. ACCORD samples were measured in batch and BioMe samples were measured using a separate lot. The average intra-assay coefficient of variation (CV) was less than 10% for the calibrators as well as for the QC sample. The inter-assay CV for endostatin was 17% (ACCORD) and 14% (BioMe). The average lower limit of detection obtained from multiple runs was 4.05 pg/ml in ACCORD and 4.47 pg/ml in BioMe.
Statistical Analysis
We expressed descriptive results for the participants’ baseline characteristics and biomarkers via means and standard deviations, or for skewed variables, medians and interquartile ranges. We made statistical comparisons between groups by paired t-tests for data that were normally distributed, Wilcoxon tests for skewed continuous data, and McNemar’s test for categorical data. Since the distribution of concentrations of urinary biomarker/creatinine ratios was skewed to the right, we used each marker as a log2-transformed continuous variable. We also categorized each marker into quartiles based on the biomarker distributions. Using multivariable conditional logistic regression (ACCORD case-control study) and Cox regression (BioMe Biobank cohort study), we evaluated the associations of endostatin with kidney endpoint. We present results for the biomarkers both as per log2 increment and for each of the top three quartiles compared to the bottom quartile. We also adjusted for unmatched risk factors (hemoglobin A1C, cardiovascular disease history, hypertension, heart failure, ACEI/ARB use, assignment to intensive glycemic control arm, intensive blood pressure control arm or fibrate arm) and additionally adjusted for baseline UACR and baseline eGFR (to account for imperfect matching) in a series of staged models in ACCORD. We attempted to harmonize covariate structure for both cohorts. Since BioMe Biobank was an EMR based cohort, only 60% had albuminuria/proteinuria measurements at baseline. Thus, we conducted a sensitivity analysis, in the subset of BioMe participants with existing values at baseline (n=608). For the BioMe cohort, we assessed discrimination and improvement through the C-statistic that was bootstrapped with 1000 iterations with resampling to adjust for optimism bias. We also identified a threshold score for the Model 4 (Table 3, BioMe cohort) providing PPV, NPV, sensitivity and specificity for the composite outcome, which might provide clinically useful information. 28 We conducted all analyses using STATA SE, Version 12, College Station, TX, USA and SAS 9.3 (SAS Institute Inc. Cary, North Carolina).
Supplementary Material
ACKNOWLEGEMENTS
This research was directly supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grant no. R01DK096549 to S.G.C.). G.N.N. is supported by a career development award from the National Institutes of Health (NIH) (K23DK107908) and is also supported by R01DK108803, U01HG007278, U01HG009610, and 1U01DK116100-01. C.R.P. is supported by the NIH (K24-DK090203) and P30-DK079310-07 O’Brien Center grant. S.G.C., G.N.N., and C.R.P. are members and are supported in part by the Chronic Kidney Disease Biomarker Consortium (U01DK106962). S.G.C is also supported by the following grants: R01DK106085, R01HL85757, R01DK112258, and U01OH011326. L.C is supported in part by the NIH (5T32DK007757 – 18) and the recipient of a research grant from the Renal Research Institute.
G.N.N and S.G.C are co-founders of RenalytixAI and G.N.N., C.R.P. and S.G.C. are members of the advisory board of RenalytixAI and own equity in the same. G.N.N has received operational funding from Goldfinch Bio. S.G.C. has received consulting fees from Goldfinch Bio, CHF Solutions, Quark Biopharma, Janssen Pharmaceuticals, and Takeda Pharmaceuticals. G.N.N. and S.G.C. are on the advisory board for pulseData and have received consulting fees and equity in return.
The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. ACCORD was supported by contracts N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA-Y1-HC-9035, and IAA-Y1-HC-1010 from the National Heart, Lung, and Blood Institute; by other components of the NIH, including the NIDDK, the National Institute on Aging, and the National Eye Institute; by the Centers for Disease Control and Prevention; and by General Clinical Research Centers.
Funding Support: NIH/NIDDK
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.de Boer IH, Rue TC, Hall YN et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011; 305: 2532–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet Lond. Engl 2011; 378: 31–40. [DOI] [PubMed] [Google Scholar]
- 3.Afkarian M, Sachs MC, Kestenbaum B et al. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. JASN 2013; 24: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunkler D, Gao P, Lee SF et al. Risk Prediction for Early CKD in Type 2 Diabetes. Clin. J. Am. Soc. Nephrol. CJASN 2015. [DOI] [PMC free article] [PubMed]
- 5.Malyszko J Mechanism of endothelial dysfunction in chronic kidney disease. Clin. Chim. Acta 2010; 411: 1412–1420. [DOI] [PubMed] [Google Scholar]
- 6.Bellini MH, Malpighi TF, Calvo FB et al. Immobilized kidney 28-kDa endostatin-related (KES28kDa) fragment promotes endothelial cell survival. Am. J. Nephrol 2010; 31: 255–261. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi N, Anand-Apte B, Lee M et al. Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J 1999; 18: 4414–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endostatin expression in the murine model of ischaemia/reperfusion-induced acute renal failure. - NCBI.
- 9.Saarela J, Ylikärppä R, Rehn M et al. Complete primary structure of two variant forms of human type XVIII collagen and tissue-specific differences in the expression of the corresponding transcripts. Matrix Biol. J. Int. Soc. Matrix Biol 1998; 16: 319–328. [DOI] [PubMed] [Google Scholar]
- 10.Tomono Y, Naito I, Ando K et al. Epitope-defined monoclonal antibodies against multiplexin collagens demonstrate that type XV and XVIII collagens are expressed in specialized basement membranes. Cell Struct. Funct 2002; 27: 9–20. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka S, Tanaka T, Nangaku M. Hypoxia and Dysregulated Angiogenesis in Kidney Disease. Kidney Dis. Basel Switz 2015; 1: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichinose K, Maeshima Y, Yamamoto Y et al. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes 2005; 54: 2891–2903. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Hamm LL, Kleinpeter MA et al. Elevated plasma levels of endostatin are associated with chronic kidney disease. Am. J. Nephrol 2012; 35: 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruge T, Carlsson AC, Larsson TE et al. Endostatin level is associated with kidney injury in the elderly: findings from two community-based cohorts. Am. J. Nephrol 2014; 40: 417–424. [DOI] [PubMed] [Google Scholar]
- 15.Ärnlöv J, Ruge T, Ingelsson E et al. Serum endostatin and risk of mortality in the elderly: findings from 2 community-based cohorts. Arterioscler. Thromb. Vasc. Biol 2013; 33: 2689–2695. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson AC, Östgren CJ, Länne T et al. The association between endostatin and kidney disease and mortality in patients with type 2 diabetes. Diabetes Metab [DOI] [PubMed]
- 17.Ferenbach D, Kluth DC, Hughes J. Inflammatory cells in renal injury and repair. Semin. Nephrol 2007; 27: 250–259. [DOI] [PubMed] [Google Scholar]
- 18.Kanasaki K, Taduri G, Koya D. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front. Endocrinol 2013; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weekley CC, Peralta CA. Advances in the use of multimarker panels for renal risk stratification. Curr. Opin. Nephrol. Hypertens 2012; 21: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mychaleckyj JC, Craven T, Nayak U et al. Reversibility of Fenofibrate Therapy–Induced Renal Function Impairment in ACCORD Type 2 Diabetic Participants. Diabetes Care 2012; 35: 1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tayo BO, Teil M, Tong L et al. Genetic Background of Patients from a University Medical Center in Manhattan: Implications for Personalized Medicine. PLoS ONE 2011; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadkarni GN, Galarneau G, Ellis SB et al. Apolipoprotein L1 Variants and Blood Pressure Traits in African Americans. J. Am. Coll. Cardiol 2017; 69: 1564–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coresh J, Turin TC, Matsushita K et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311: 2518–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens LA, Schmid CH, Greene T et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am. J. Kidney Dis. Off. J. Natl. Kidney Found 2010; 56: 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadkarni GN, Gottesman O, Linneman J et al. Development and validation of an electronic phenotyping algorithm for chronic kidney disease. Annu. Proc. Am. Med. Inform. Assoc In Press. [PMC free article] [PubMed]
- 28.Meisner A, Kerr KF, Thiessen-Philbrook H et al. Methodological issues in current practice may lead to bias in the development of biomarker combinations for predicting acute kidney injury. Kidney Int 2016; 89: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


