Abstract
The insulinotropic effects of the incretin hormone, glucagon-like peptide-1 (GLP-1) are mediated via GLP-1 receptors (GLP-1R) present on pancreatic β cells. GLP-1 causes a decrease in the motility of stomach and intestine which involves both central and peripheral nervous systems. The expression and function of GLP-1R in gastrointestinal smooth muscle, however, are not clear. Muscle strips and isolated muscle cells were prepared from mouse colon and the effect of GLP-1(7–36) amide on acetylcholine (ACh)-induced contraction was measured. Muscle cells in culture were used to identify the expression of GLP-1R and the signaling pathways activated by GLP-1(7–36) amide. GLP-1R was expressed in the mucosal and non-mucosal tissue preparations derived from colon, and in smooth muscle cell cultures devoid of other cells such as enteric neurons. In colonic muscle strips, the addition of GLP-1(7–36) amide caused dose-dependent inhibition of acetylcholine-induced contractions. The effect of GLP-1(7–36) amide was partly inhibited by the neuronal blocker tetrodotoxin and nitric oxide (NO) synthase inhibitor L-NNA suggesting both NO-dependent neural and NO-independent direct effects on smooth muscle. In isolated colonic smooth muscle cells, GLP-1(7–36) amide caused an increase in Gαs activity, cAMP levels, and PKA activity, and inhibited ACh-induced contraction. The effect of GLP-1(7–36) amide on Gαs activity and cAMP levels was blocked by NF449, an inhibitor of Gαs, and the effect of GLP-1(7–36) amide on contraction was blocked by NF449 and myristoylated PKI, an inhibitor of PKA. We conclude that colonic smooth muscle cells express GLP-1R, and GLP-1(7–36) amide inhibits acetylcholine-induced contraction via GLP-1R coupled to the Gαs/cAMP/PKA pathway.
Keywords: Colon, GLP-1, GLP-1(7–36) amide, Cyclic AMP, Adenylyl cyclase, Cyclic AMP-dependent protein kinase, NF-449, Tetrodotoxin, L-NNA, l-N-nitroarginine
1. Introduction
Food intake is the primary stimulus for the release of GLP-1 from enteroendocrine L cells, localized mostly, although not exclusively, in the ileum of the small intestine [1–10]. In mammals, the preproglucagon gene is transcribed into mRNA that yields a single 180-amino acid precursor, preproglucagon, which is processed to produce GLP-1 as well as other biologically active peptides [4,6]. The preproglucagon protein contains active peptides other than GLP-1, including glucagon, GLP-2, oxyntomodulin, and other fragments with unclear roles. The various isoforms of GLP-1 are produced by additional post-translational modification, specifically by cleavage of six amino acids from the N-terminus of GLP-1(1–37) [1,2,4,6]. The products exhibit varying degrees of activity and include GLP-1(7–37) and GLP-1(7–36) in addition to the C-terminal-amidated forms, GLP-1(7–37) amide and GLP-1(7–36) amide. All isoforms mentioned are able to recognize and exert equipotent effects on pancreatic function [1,2,4,6]. GLP-1(7–36) amide is the most common and biologically active metabolite of GLP-1 in circulation that causes the increase of glucose-dependent insulin secretion [4]. GLP-1(7–36) amide is rapidly degraded by the enzyme dipeptidyl peptidase (DPP)-4 into GLP-1(9–36) amide, the primary GLP-1 metabolite without the ability to interact with its cognate receptor GLP-1R [1,2,4,6].
GLP-1R mRNA has been identified in the lung, pancreatic islets, stomach, kidney, hypothalamus, and heart, but not in adipose tissue, liver, or skeletal muscle [11–14]. In the pancreas, GLP-1R is expressed primarily in β cells and to a lesser degree in δ cells [10]. GLP-1 in pancreatic β cells has an insulinotropic effect, leading to instantaneous insulin secretion and lowered blood glucose levels [1,2]. In cardiovascular tissue, activation of GLP-1R by GLP-1 is thought to induce cardioprotective effects via activation of anti-apoptotic mechanisms in cardiomyocytes [15]. Liraglutide, a GLP-1R agonist, was shown in mice to improve cardiomyocyte survival after induced ischemia, and it also resulted in sustained improvement of cardiac function after myocardial infarction [15]. In the human and mouse CNS, GLP-1R has been identified in the arcuate and paraventricular nuclei in the hypothalamus, which are involved with regulation of appetite and satiety [16]. In humans the GLP-1R agonist liraglutide caused delayed gastric emptying and inhibited duodenal and small intestinal motility [16]. Gastric emptying was also reduced by GLP-1 and its analog, ROSE-010 [3,17]. The effect of GLP-1 on gastric and intestinal motility in various animal models and human was ascribed to activation of vagal afferents and NO release from enteric neurons [18–34]. However, the expression and function of GLP-1R in gastrointestinal smooth muscle are not clear. In the present study we show that the colonic smooth muscle cells express GLP-1R, and that GLP-1(7–36) amide inhibits acetylcholine-induced contraction via activation of Gαs and generation of cAMP.
2. Materials and methods
2.1. Drugs and animals
[32P]ATP was obtained from New England Nuclear Life Sciences (Boston, MA); NF449 (4,4′,4′’,4′”-[Carbonylbis(imino-5,1,3-benzenetriyl-bis(carbonylimino))]tetrakis-1,3-benzenedisulfonic acid), and GLP-1(7–36) amide were obtained from Tocris Bioscience (Bristol, United Kingdom); RNAqueous™ kit and TRIzol® Reagent were obtained from Ambion (Austin, TX). High Capacity cDNA Reverse Transcription Kit was obtained from Applied Biosystems (Foster City, CA); Q5® Reaction Buffer, Q5® High GC Enhancer, Deoxynucleotide (dNTP) Solution Mix, Q5® High-Fidelity DNA Polymerase were obtained from New England Biolabs (Ipswitch, MA); Western blotting materials, 4x Laemmli Sample Buffer, and DC™ Protein Assay Reagents were obtained from Bio-Rad Laboratories (Hercules, CA); SuperSignal® West Pico Chemiluminescent Substrate, Dulbecco’s Modified Eagle Medium, and Trypsin-EDTA (0.25%) were obtained from Thermo Fisher Scientific (Waltham, MA); Collagenase CLS type II and soybean trypsin inhibitor were obtained from Worthington (Freehold, NJ); T-PER® Tissue Protein Extraction Reagent was obtained from Thermo Scientific (Rockford, IL); Monoclonal anti-cAMP antibody based direct cAMP ELISA Kit and antibody to the activated GTP-bound Gαs were obtained from NewEast Biosciences (Malvern, PA); Antibody to GLP-1R (sc-390774) was obtained from Santa Cruz Biotechnology (Dallas, Tx); All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Mice (C57BL/6) were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in an animal facility directed by the Division of Animal Resources at Virginia Commonwealth University. Mice were subjected to 12-hour light and 12-hour dark cycles with food and water provided. All experiments were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
2.2. Preparation of dispersed muscle cells
Mice were euthanized by CO2 inhalation, abdominal incisions were made vertically, and the small intestine and colons were removed and placed in buffer consisting of NaCl 120 mM, KCl 4 mM, KH2PO4 2.6 mM, CaCl2 2.0 mM, MgCl2 0.6 mM, HEPES (N-2-hydroxyethylpiperazine-N’ 2-ethanesulfonic acid) 25 mM, glucose 14 mM, and basic Eagle Medium (essential amino mixture) 2.1% (pH 7.4). The mucosal layer was gently scraped from the muscle layer and smooth muscle cells were isolated by sequential enzymatic digestion of muscle strips, filtration, and centrifugation [35–38]. Strips of muscle layer were incubated at 35 °C for 20–30 minutes in HEPES medium containing 0.1% collagenase (300 U/ml) and 0.01% soybean trypsin inhibitor. During digestion the tissues were gently bubbled with 100% oxygen. Partially digested tissues were washed twice with collagenase-free medium and then the smooth muscle cells were permitted to disperse spontaneously in collagenase-free medium. Dispersed smooth muscle cells were then harvested via filtration using 500 μm Nitex and used for functional studies after suspension in HEPES medium for 30 min [35–38].
Dispersed muscle cells were washed twice before being prepared for culture in Dulbecco Modified Eagle Medium (DMEM) containing penicillin (200 U/ml), streptomycin (200 μg/ml), gentamycin (100 μg/ml), amphotericin B (2.5 μg/ml), and 10% fetal bovine serum (DMEM-10). Using sterile protocol, cells were plated at a concentration of approximately 5 × 105 cells/ml and cultured at 37 °C in an incubator with 10% CO2. DMEM-10 was replaced every 1–3 days as needed until 80–90% confluence was reached. Experiments were performed on cells in the first and second passage. Previous studies have shown that cultured smooth muscle cells in the first passage are devoid of neurons, interstitial cells of Cajal and endothelial cells [37]. When placed in culture, the muscle cells grow rapidly and regain their smooth muscle phenotype upon reaching confluence as indicated by the following criteria: confluent cells assume a typical elongated spindle-shape morphology and express γ-actin characteristic of visceral smooth muscle; they do not express neural markers (neuronal nitric oxide synthase), the interstitial cells of Cajal marker (c-kit), or the endothelial cell marker (vascular endothelial growth factor receptor) [37]. In addition, cultured muscle cells express all the components involved in signaling for contraction or relaxation found in freshly dispersed muscle cells [35–38].
2.3. RT-PCR analysis
TRIzol (0.5 ml) was added to smooth muscle cells for 5 min followed by 0.2 ml chloroform. The mixture was incubated at room temperature for 2–3 min and then centrifuged at 12,000 g at 4 °C for 15 min. The upper aqueous phase was transferred to a new tube, and 0.2 ml of isopropanol was added. The mixture was incubated and centrifuged at 12,000 g at 4 °C for 15 min. The supernatant was discarded and 1 ml of cold ethanol (75%) was used to resuspend the pellet. The mixture was again centrifuged at 12,000 g for 15 min at 4 °C. The supernatant was aspirated and allowed to air dry at room temperature for 15 min. Total RNA was then dissolved into 0.1% diethylpyrocarbonate (DEPC)-treated water, and concentration determined via spectrophotometry.
Specific primers for GLP-1R were designed to span across exons of sequences homologous in mice and humans. The primer sequences for mouse GLP-1R were as follows: Forward 5′−CCC GCC CTC AGG GTA CCA CGG T-3′ and Reverse 5′-TCA GGA AAG TTT CTC TCC CCT C-3′, yielding a fragment of 346 bp (obtained from Invitrogen, Carlsbad, CA). Sequences for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a housekeeping gene with known expression in smooth muscle cells, primers were as follows: Forward 5′-AGA AAC CTG CCA AGT ATG ATG-3′ and Reverse 5′-GGA GTT GCT GTT GAA GTC G-3′. The High Capacity cDNA Reverse Transcription Kit was used to synthesize cDNA. The reaction mixture was prepared as described by the given protocol using 1 μg of RNA. Controls were created by substituting the reverse transcriptase enzyme with an equal volume of DNase/RNase-free water. An Eppendorf Vapo Protect Mastercycler was used for thermal cycling with the following conditions: 10 min at 25 °C, 120 min at 37 °C, 5 min at 85 °C, and a hold at 4 °C as needed.
PCR products were generated using primers specific for GLP-1R and GAPDH as previously described [37]. The PCR reaction mixture was prepared as follows: 5 μL of 5x Q5 Reaction buffer, 0.5 μl of 10 mM dNTP, 1.25 μl of 10 μM forward primer, 1.25 μl of 10 μM reverse primer, 0.25 μl of Q5 DNA polymerase, 2 μl of cDNA, and 9.75 μl of DNase/RNase-free water. Due to the GC-rich content of the forward and reverse GLP-1R primers, 5 μl of 5x Q5 GC Enhancer was also added for a total reaction volume of 25 μl. Thermal cycling conditions were determined empirically, with the following conditions used to generate GLP-1R PCR products: initial denaturation step at 95 °C for 3 min; 37 cycles of 95 °C for 30 s, 55 °C for 45 s, and 72 °C for 1 min; final extension step at 72 °C for 10 min; hold at 4 °C as needed. The following conditions were used to generate GAPDH PCR products: initial denaturation step at 95 °C for 3 min; 30 cycles of 95 °C for 30 s, 55 °C for 45 s, and 72 °C for 1 min; final extension step at 72 °C for 10 min; hold at 4 °C as needed. The PCR products were separated by electrophoresis in a 1.2% agarose gel at 100 V in the presence of ethidium bromide. Bands were visualized by ultraviolet fluorescence and recorded with a Bio-Rad Gel Doc EZ Imager.
2.4. Western blot analysis
Western blot analysis of GLP-1R was performed as previously described [38]. Briefly, smooth muscle cells isolated from colon were solubilized in Triton X-100-based lysis buffer plus protease and phosphatase inhibitors. Lysates were centrifuged (20,000 g for 10 min at 4 °C) and protein concentrations of the supernatant determined with the DC Protein Assay kit. Proteins were fractionated by SDS-PAGE and transferred to PVDF membranes. The blots were incubated for 12 h at 4 °C with antibody to GLP-1R (sc-390774, 1:1000) and then for 1 h with secondary antibody conjugated with horseradish peroxidase. The protein bands were visualized by enhanced chemiluminescence.
2.5. Gαs activation assay
Activation of Gs in response to GLP-1(7–36) amide was analyzed by Western blot using an antibody that specifically recognizes the activated (GTP-bound) Gαs subunit (NewEast Biosciences; Malvern PA). Muscle cells in culture were treated with GLP-1(7–36) amide (1 μM) for 5 min in the presence or absence of Gαs activation inhibitor, NF449 (10 μM). In some experiments, muscle cells were treated with 1 μM vasoactive intestinal peptide (VIP), a known ligand for a Gαs-coupled VPAC2 receptor, for 5 min [36]. At the end of 5-minute incubation, medium was removed and muscle cells were solubilized on ice for one hour in medium containing 20 mM Tri-HCl (pH 8.0), 1 mM 1,4-ditiothreitol, 100 mM NaCl, 0.5% sodium dodecyl sulfate, 0.75% deoxycholate, 1 mM phenylmethylsulfonyl floride, 10 μg/ml of leupeptin and 100 μg/ml of aprotinin. The proteins in the lysate were resolved by SDS/PAGE and electrophoretically transferred onto nitrocellulose membranes. The membranes were incubated for 12 h with the anti-Gαs-GTP antibody (1:1500) and then for 1 h with horseradish peroxidase-conjugated secondary antibody (1:5000). The protein bands were identified by enhanced chemiluminescence reagent.
2.6. Measurement of cAMP
Cyclic AMP was measured by a competitive ELISA using a monoclonal anti-cAMP antibody-based direct cAMP assay kit following the protocol provided by NewEast Biosciences (Malvern PA). Muscle cells in culture were treated with GLP-1(7–36) amide (1 μM) for 5 min in the presence or absence of Gαs activation inhibitor, NF449 (10 μM). In some experiments, muscle cells were treated with 1 μM VIP for 5 min. Isobutyl methyl xanthine (IBMX, 100 μM) was used in the incubation to prevent cAMP hydrolysis. Briefly, a 96-well plate was coated with goat-anti-mouse serum. Cyclic AMP from colonic smooth muscle extracts was competitively bound to the monoclonal anti-cAMP antibody in the presence of known amounts of cAMP-conjugated horseradish peroxidase, which were used to generate a standard curve. The plate was read on a Victor2 1420 Multi-Level microplate reader at 450 nm. Optical density readings were used to calculate the concentration of cAMP in samples based on the standard curve. Results were expressed as cAMP in pmol/mg protein [36].
2.7. Measurement of cAMP-dependent protein kinase activity
Cyclic AMP-dependent protein kinase (PKA) activity was measured in an in vitro kinase assay using PKA-selective substrate peptide kemptide and [32P]ATP. Muscle cells in culture were treated with GLP-1(7–36) amide (1 μM) for 5 min in the presence or absence of NF449 (10 μM). In some experiments, muscle cells were treated with 1 μM VIP for 5 min. After a 5-minute incubation period, cells in the plate were rinsed with a medium containing 50 mM Tris-HCl (pH 7.4), 10 mM EDTA, 0.5 mM IBMX, 10 mM 2-mercaptoethanol, and 100 mM NaCl, and were then homogenized in 0.5 ml of ice-cold medium. The mixture was separated by centrifugation at 48,000 g for 15 min. The supernatant was transferred to a new tube and used as a source for PKA. 20 μl of supernatant, containing 50 μg of protein, was used to initiate an assay in a medium containing 50 mM Tris, 20 mM magnesium acetate, 200 μM [32P]ATP, 100 μM IBMX, 150 μM kemptide [LRRASG] and 0.25 mg/ml bovine serum albumin in the presence and absence of 5 μM cAMP. The reaction mixtures were incubated at 37 °C for 30 min and the reaction was stopped by spotting the reaction mixture onto a phosphocellulose filter disc. The filters were washed 3–4 times with 75 mM H3PO4 and then dried before transferring to vials for radioactive counting using a beta counter. Radioactivity in counts per minute (CPM) per mg protein associated with kemptide adsorbed onto phosphocellulose discs were calculated in the presence or absence of cAMP and the results are expressed as ratio (-cAMP/+cAMP) of CPM [35,36].
2.8. Effect of GLP-1(7–36) amide on acetylcholine-induced contractions in muscle strips
Mice were euthanized as previously described, and colons were removed and immediately washed and flushed with Krebs solution containing 118 mM NaCl, 4.8 mM KCl, 1 mM MgSO4, 1.15 mM NaH2PO4, 15 mM NaHCO3, 10.5 mM glucose and 2.5 mM CaCl2 (95% O2/5% CO2, pH 7.4). Colons were cut into segments approximately 1 cm in length and the segments were tied at each end with silk thread using care not to obstruct the lumen. The tissues were mounted between a glass rod and FT-03C isometric force-displacement transducer (Grass Technologies, East Warwick, RI). One end was oriented near the bottom of the 5 ml organ bath maintained at 37 °C (Radnoti, Monrovia, CA) to ensure constant submersion in Krebs buffer. The other end of the segment was attached to the transducer by silk thread. Signals from the transducer were amplified by an Octal Bridge Amplifier (AD Instruments, Colorado Springs, CO), transmitted to a Powerlab 8/35 with Grass Adaptor unit, and recorded with ADInstruments LabChart Pro7 software. Force was recorded in grams [39,40].
Colonic segments were stretched to 1 g of tension and were then allowed to equilibrate in Krebs buffer for 45 min. The effect of GLP-1(7–36) amide at different concentrations (1 nM to 1 μM) was measured on spontaneous contractile activity and on acetylcholine (ACh, 10 μM)-induced contraction. The effect of GLP-1(7–36) amide on ACh-induced contraction was also measured in the presence of a voltage-dependent Na+ channel blocker tetrodotoxin (TTx, 10 μM) and an inhibitor of neuronal NO synthase L-N-nitroarginine (L-NNA, 100 μM).
2.9. Effect of GLP-1(7–36) amide on acetylcholine-induced contractions in isolated muscle cells
Relaxation of muscle cells was measured as a decrease in maximal cell contraction induced by ACh and expressed as percent inhibition of contraction. An aliquot of muscle cells (0.5 ml cell suspension) was added to 0.2 ml of HEPES medium containing ACh (1 μM) alone or ACh after pretreatment with different concentration of GLP-1(7–36) amide for 5 min. In some experiments cells were incubated for 15 min with 10 μM NF449 or 1 μM myristoylated PKI before the addition of GLP-1(7–36) amide. The reaction was stopped after 30 s with 1% acrolein. The mean length of 50 muscle cells was measured by scanning micrometry and was compared with the length of untreated muscle cells. The contractile response was expressed as decrease in the mean cell length from control cell length and the relaxation was expressed as percent inhibition of contraction [36].
2.10. Statistical analysis
Results were expressed as means ± standard error of the mean of n experiments. Experiments were designed to compare treatment to control conditions and each experiment was performed on tissues obtained from different animals. Statistically significant difference was tested by one-way ANOVA followed by Student’s t-test using GraphPad Software Prism 6 (La Jolla, CA). p < 0.05 was accepted as a statistically significant difference.
3. Results
3.1. Expression of GLP-1R in mucosa and muscle tissue and isolated muscle cells
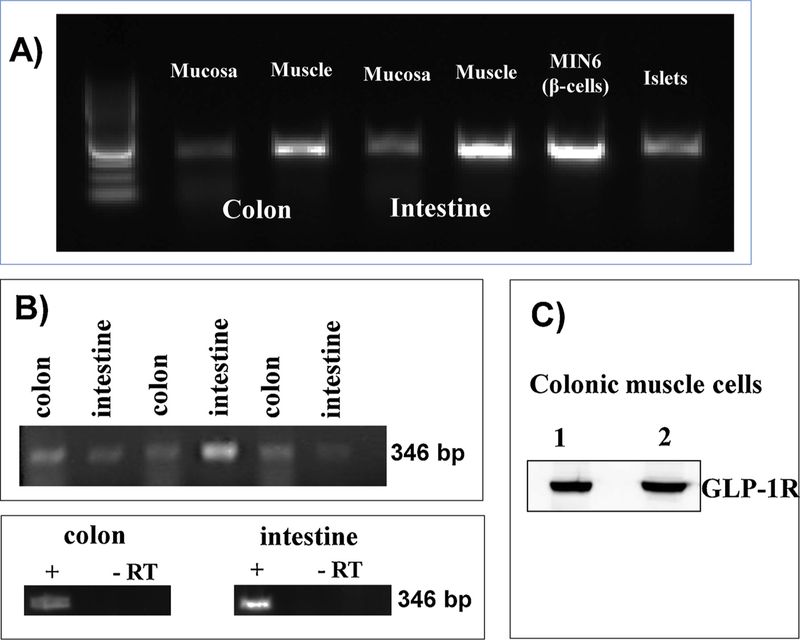
GLP-1 receptors (GLP-1R) were detected by RT-PCR in RNA extracted from mucosal and non-mucosal muscle strips of intestine and colon. A PCR product of expected size (346 bp) was obtained using specific primers for GLP-1R in RNA samples from both mucosa and non-mucosal tissue suggesting expression of GLP-1R in mucosal cells and non-mucosal cells of the intestine and colon (Fig. 1A). Pancreatic β cells (MIN6 cell line) and mouse islets of Langerhans were used as positive controls. GLP-1R mRNA expression was confirmed on RNA extracted from MIN-6 cells and mouse islets (Fig. 1A). Expression of GLP-1R mRNA was also detected in RNA isolated from cultured smooth muscle cells (Fig. 1B). A parallel control without reverse transcriptase was processed in the presence of an equal volume of distilled H2O and absence of amplification product without reverse transcriptase shows the specificity of the primers and lack of genomic DNA contamination (Fig. 1B). Western blot analysis demonstrated expression of GLP-1R in muscle cells isolated from the colon (Fig. 1C).
Fig. 1.
Expression of GLP-1R. A) Total RNA isolated from the mucosa and muscle tissue of mouse colon was reverse transcribed, and cDNA was amplified with specific GLP-1R primers. Total RNA was also isolated from MIN-6 cells and mouse islets. Experiments were done in the presence or absence of reverse transcriptase (RT). B) Total RNA isolated from cultured (first passage) colonic and intestinal muscle cells was reverse transcribed, and cDNA was amplified with specific GLP-1R primers. PCR products with predicted size (346 bp) were obtained in the presence of reverse transcriptase. C) Lysates prepared from isolated colonic muscle cells were homogenized and protein (lane 1: 20 μg; lane 2: 30 μg) in the supernatant was fractionated by SDS-PAGE followed by western blot analysis using GLP-1R antibody. Bands corresponding to GLP-1R (53 kDa) were visualized by chemiluminescence.
3.2. Identification of signaling pathway activated by GLP-1R in smooth muscle cells
3.2.1. Activation of Gαs by GLP-1(7–36) amide
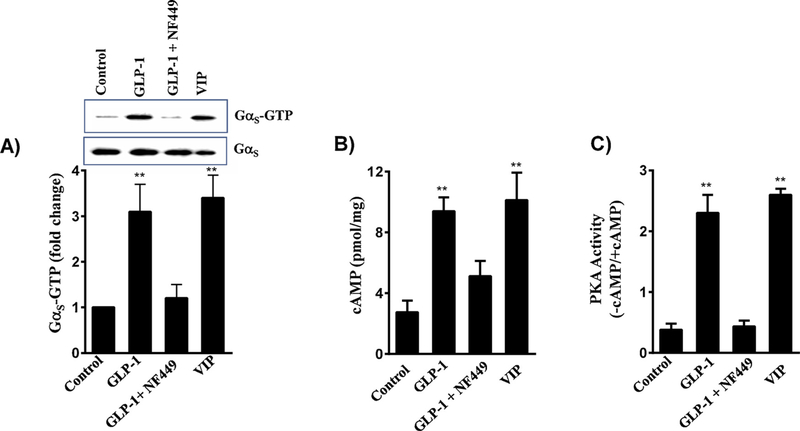
Previous studies have shown that GLP-1Rs are coupled to activation of Gαs proteins in pancreatic β cells [1,2]. We examined whether Gαs proteins are activated in muscle cells in response to GLP-1(7–36) amide using an antibody that specifically binds activated (GTP-bound) Gαs subunit by Western blot. The density of bands was determined by image analysis. Addition of 1 μM GLP-1(7–36) amide caused a 3-fold increase in the binding of GTP to Gαs suggesting that GLP-1R in smooth muscle are coupled to activation of Gαs (Fig. 2A). Activation of Gαs by GLP-1(7–36) amide was corroborated by the use of NF449, a selective inhibitor of Gαs activation. The effect of GLP-1(7–36) amide on Gαs activation was blocked in the presence of 10 μM NF449 (Fig. 2A). Vasoactive intestinal peptide, a ligand known to activate Gαs -coupled VPAC2 receptors, was used in control studies [41–44]. The results showed that VIP also caused a 3.5-fold increase in the binding of GTP to Gαs suggesting activation of Gαs by VIP (Fig. 2A). The extent of Gαs stimulation by GLP-1(7–36) amide and VIP are similar. These results suggest that in smooth muscle cells, GLP-1R are coupled to Gαs proteins similar to GLP-1R in pancreatic β cells and VPAC2 receptor in gastrointestinal smooth muscle cells [1,2,41–44].
Fig. 2.
Stimulation of Gαs/cAMP/PKA activity by GLP-1(7–36) amide in smooth muscle cells. A) Colonic smooth muscle cells in culture were treated with 1 μM GLP-1(7–36) amide in the presence or absence of Gαs inhibitor, NF449 (10 μM) for 5 min. In some experiments, cells were treated with 1 μM of VIP. Activation of Gαs was measured in a Western blot analysis using an antibody selective for GTP-bound Gαs. The density of bands was calculated by image analysis. A representative image of 3 separate experiments is shown in the figure. ** significant (p < 0.01) stimulation in Gαs activity compared to control. B) Colonic smooth muscle cells in culture were treated with 1 μM GLP-1(7–36) amide in the presence or absence of 10 μM NF449. In some experiments cells were treated with 1 μM VIP. cAMP was measured by ELISA as described in the Methods. Results are expressed as pmol of cAMP/mg protein. Values are mean ± SEM of 4 separate experiments. ** significant (p < 0.01) increase in cAMP levels compared to control. C) Colonic smooth muscle cells in culture were treated with GLP-1(7–36) amide in the presence or absence of 10 μM NF449. In some experiments cells were treated with 1 μM VIP. PKA activity was measured in the lysates using kemptide and [32P]ATP in the presence or absence of cAMP as described in the Methods. Results are expressed as the ratio of PKA activity in counts per minute in the absence or presence of cAMP (-cAMP/+cAMP). Values are mean ± SEM of 4 separate experiments. **significant (p < 0.01) stimulation of PKA activity compared to control.
3.2.2. Increase in cAMP levels by GLP-1(7–36) amide
Consistent with activation of Gαs, treatment of cells with GLP-1(7–36) amide significantly increased cAMP levels (9.4 ± 0.9 pmoles/ mg protein compared to the basal levels of 2.7 ± 0.7 pmoles/mg protein) (Fig. 2B). The increase in cAMP levels by GLP-1(7–36) amide was attenuated (65 ± 7% inhibition) in the presence of 10 μM NF449. The effect of NF449 on GLP-1(7–36) amide-induced cAMP levels is consistent with its inhibitory effect on GLP-1(7–36) amide-induced Gαs activity. VIP also caused an increase in cAMP levels (10.1 ± 1.8 pmoles/mg protein compared to the basal levels of 2.7 ± 0.7 pmoles/ mg protein) and the results are consistent with its effect on Gαs-coupled VPAC2 receptors in smooth muscle cells [36,44].
3.2.3. Activation of PKA by GLP-1(7–36) amide
Stimulation of Gαs and increase in cAMP leads to stimulation of PKA activity. Treatment of cells with GLP-1(7–36) amide caused a significant 5-fold increase in PKA activity and this stimulation was blocked in the presence of NF449 (Fig. 2C). The effect of NF449 is consistent with its inhibitory effect on Gαs activation and cAMP generation. VIP also caused a 5.5-fold increase in PKA activity (Fig. 2C). These results suggest that in smooth muscle cells, activation of GLP-1R by GLP-1(7–36) amide leads to activation of Gαs /cAMP/PKA pathway, similar to that of activation of VPAC2 receptors by VIP.
3.3. Inhibition of acetylcholine-induced contraction by GLP-1(7–36) amide in colon strips
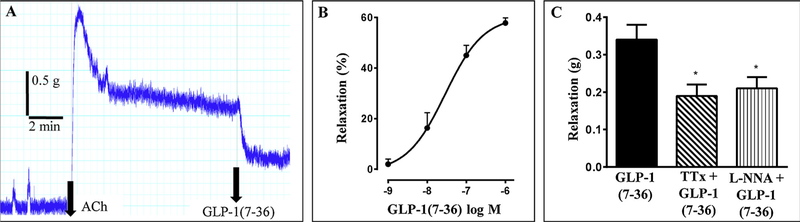
To test the effect of GLP-1(7–36) amide on agonist-induced contraction, muscle strips were treated with ACh (10 μM) to raise the tension. After the tension plateaued, GLP-1(7–36) amide was added in the range of 1 nM to 1 μM. GLP-1(7–36) amide caused a decrease in the ACh-induced tone (i.e. relaxation) in a concentration-dependent fashion (Fig. 3). The EC50 for GLP-1(7–36) amide induced relaxation was 30 nM and the maximal relaxation of 58 ± 3 percent decrease in tone was achieved at 1 μM. Although the strips were usually quiescent in the basal state, some demonstrated small phasic contractions. If present, these were also strongly inhibited or abolished by 1 μM GLP-1(7–36) amide (data not shown).
Fig. 3.
Relaxation by GLP-1(7–36) amide in colonic muscle strips. A) Representative tracings showing the inhibition of acetylcholine (ACh)-induced tone by 1 μM GLP-1(7–36) amide in colonic muscle strips. B) Concentration-dependent inhibition of ACh-induced tone by GLP-1(7–36) amide in colonic muscle strips. Results are expressed as percent inhibition of ACh-induced tone. Values are mean ± SEM of 3–27 separate experiments. C) Partial Inhibition of 1 μM GLP-1(7–36) amide-induced relaxation by tetrodotoxin (TTx, 10 μM) and L-N-nitroarginine (L-NNA, 100 μM). Relaxation was represented as inhibition of ACh-induced contraction in grams. * significant (p < 0.05) inhibition of relaxation by TTx or L-NNA induced by GLP-1(7–36) amide.
The mechanism of action of GLP-1(7–36) amide has been reported to be neurally-mediated and/or NO-mediated in other tissues [26–29]. We tested this notion in colonic strips by using the neural toxin, tetrodotoxin, and the NO synthase (NOS) inhibitor l-NNA. TTx caused a partial 44 ± 4% inhibition of the relaxation induced by 1 μM GLP-1(7–36) amide (0.34 ± 0.04 g relaxation in the absence and 0.19 ± 0.03 g relaxation in the presence of TTx; p < 0.02, n = 25). Similarly, L-NNA caused a partial 33 ± 14% inhibition of the relaxation induced by GLP-1(7–36) amide (0.34 ± 0.07 g relaxation in the absence and 0.21 ± 0.03 g relaxation in the presence of L-NNA; p < 0.02, N = 11) (Fig. 3C). This suggests that GLP-1(7–36) amide causes relaxation partly by a neural NO-mediated action and partly by a non-neuronal, presumably direct smooth muscle effect.
3.4. Inhibition of acetylcholine-induced contraction by GLP-1(7–36) amide in isolated colonic smooth muscle cells
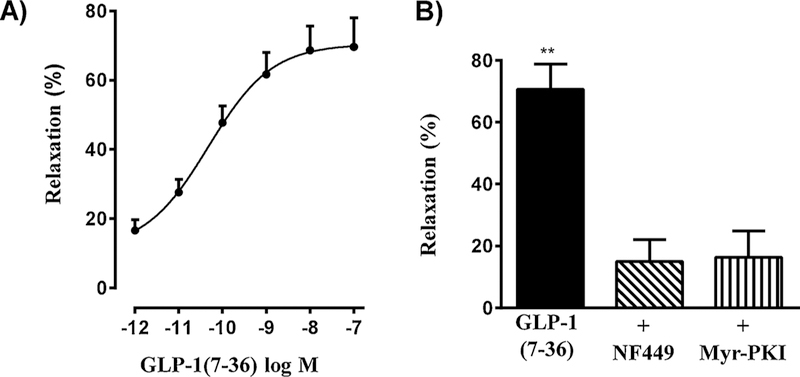
Previous studies have shown that agonists such as VIP increase cAMP levels and stimulate PKA activity to induce direct relaxation of gastrointestinal smooth muscle [36,44]. The functional significance of GLP-1R-mediated stimulation of the Gαs /cAMP/PKA pathway and the effects in colonic muscle strips in the presence of TTx and L-NNA suggest that GLP-1(7–36) amide acts on smooth muscle cells to activate cAMP pathways and cause direct relaxation. This notion was examined by measurements of muscle relaxation in isolated muscle cells. Relaxation was measured as inhibition of ACh-induced contraction. Contraction was measured as a decrease in muscle cell length in response to 1 μM ACh compared to control cell length. Basal cell length of colonic smooth muscle cells was 98 ± 6 μm and in the presence of ACh was 68 ± 4 μm (31 ± 4% contraction). Pretreatment of cells with GLP-1(7–36) amide at different concentrations inhibited ACh-induced contraction in a concentration-dependent fashion (Fig. 4A). Inhibition of contraction represents relaxation which was calculated as percent inhibition of contraction. Maximal relaxation (69 ± 5%) was obtained with 100 nM GLP-1(7–36) amide with an EC50 of 56 ± 16 pM. The extent of muscle relaxation induced by GLP-1(7–36) amide is similar to that obtained with other relaxant agents such as VIP, PACAP and isoproterenol (62 ± 3%–75 ± 6%) in smooth muscle cells [35,36]. Relaxation (70 ± 5%) induced by 100 nM GLP-1(7–36) amide was inhibited by NF449 (15 ± 4% relaxation) and by the PKA inhibitor myristoylated PKI (16 ± 5% relaxation) and this is consistent with inhibition of GLP-1(7–36) amide-induced Gαs activity and cAMP formation by NF449 and PKA activity by myristoylated PKI (Fig. 4B).
Fig. 4.
Relaxation by GLP-1(7–36) amide via cAMP/ PKA pathway in isolated muscle cells. A) Dispersed muscle cells from mouse colon were treated with acetylcholine (1 μM) for 30 s in the presence or absence of pretreatment with different concentrations of GLP-1(7–36) amide. Relaxation of muscle cells was measured as a decrease in maximal cell contraction induced by ACh and expressed as percent inhibition of contraction. Mean control cell length was 98 ± 6 μm and in the presence of 1 μM ACh cell length was 68 ± 4 μm. Values are means ± SEM of 4–5 experiments. B) Dispersed muscle cells from mouse colon were treated with acetylcholine (1 μM) for 30 s in the presence or absence of pretreatment with 0. 1 μM GLP-1(7–36) amide. In some experiments cells were incubated for 15 min with NF449 (10 μM) or myristoylated PKI (1 μM). Relaxation of muscle cells was measured as a decrease in maximal cell contraction induced by ACh and expressed as percent inhibition of contraction. Mean control cell length was 98 ± 6 μm and in the presence of 1 μM ACh cell length was 68 ± 4 μm. GLP-1(7–36) amide caused significant relaxation that was blocked by NF449 and myristoylated PKI. Values are means ± SEM of 4–5 experiments. ** significant (p < 0.01) relaxation by GLP-1(7–36) amide.
As shown before, treatment of muscle cells with VIP (1 μM) also inhibited ACh-induced contraction and the relaxation (73 ± 5%) induced by VIP was inhibited by NF449 (11 ± 6% relaxation, p < 0.001, n = 3) and by the PKA inhibitor myristoylated PKI (14 ± 3% relaxation, p < 0.001, n = 3) and this is consistent with the activation of Gα-coupled VPAC2 receptors, generation of cAMP and activation of PKA by VIP [36]. To examine the role of GLP-1(7–36) amide in intestine, the effect of GLP-1(7–36) amide on ACh-induced contraction was examined in muscle cells isolated from small intestine. Acetylcholine-induced 35 ± 5 μm decrease in cell length from the control cell length of 105 ± 6 μm. Pretreatment of cells with 10 nM GLP-1(7–36) amide inhibited ACh-induced contraction to 15 ± μm (58 ± 5% relaxation, p < 0.01, n = 3). Relaxation by GLP-1(7–36) amide in intestinal muscle cells is similar to that obtained in colonic muscle cells.
4. Discussion
The main physiological function of GLP-1 is to promote glucose homeostasis by enhancing glucose-dependent insulin release from the pancreatic β cells and suppressing glucagon secretion from the pancreatic α cells [1–9]. This postprandial regulation of glucose metabolism by GLP-1 may be complemented by its additional effects on gut motility and secretion. It causes retention of gastric contents in both proximal and distal regions of the stomach by inhibiting gastric emptying, inhibiting migratory motor complexes and prolonging the small intestine transit time [3]. The effects of GLP-1 in these organs reflect the presence of specific GLP-1 receptors (GLP-1R) which belong to the class 2 G protein-coupled receptors that are coupled to activation of adenylyl cyclase via G protein Gαs and include receptors for VIP, secretin, glucagon, glucose-dependent insulinotropic peptide (GIP), pituitary adenylate cyclase activating peptide (PACAP), growth hormone releasing hormone, calcitonin and parathyroid hormone [1,2,6].
Expression of GLP-1R is present in peripheral tissues such as pancreas, gut, heart and lung [11–14]. In the gastrointestinal (GI) tract of rat and mouse, expression of GLP-1R mRNA has been demonstrated in tissues from the gastric fundus and antrum, and small intestine [26,27,29]. In the GI tract of monkey, expression of GLP-1R protein has been demonstrated in the Brunner’s gland epithelium of the duodenum and in the myenteric nerve plexus and smooth muscle cells; staining was prominent in the epithelium whereas staining in myenteric plexus and smooth muscle was low-intensity [45]. In the guinea pig GI tract GLP-1R immunoreactivity was observed in both cholinergic and peptidergic neurons of the submucosal plexus [46]. GLP-1R immunoreactivity was also observed in lung smooth muscle cells of pulmonary artery branches implying a role of GLP-1R in the regulation of postprandial vascular tone [45].
The present study examined the expression of GLP-1R in colonic smooth muscle and characterized the signaling pathways coupled to GLP-1R using biochemical, molecular and functional approaches. The results demonstrate the expression of GLP-1R on colonic smooth muscle cells and their ability to stimulate adenylyl cyclase activity via Gαs, increase cAMP levels, stimulate PKA activity, and induce smooth muscle relaxation. The evidence for the coupling of GLP-1R to Gαs-dependent stimulation of adenylyl cyclase activity and ability to elicit muscle relaxation was based on the following results: a) expression of GLP-1R was demonstrated by RT-PCR in muscle tissues and in isolated smooth muscle cells; b) activation of Gαs in response to GLP-1(7–36) amide was demonstrated in Western blot using an antibody to Gαs that selectively binds GTP-bound (activated) Gαs and by the blockade of GLP-1(7–36) amide-induced Gαs activation in the presence of Gαs inhibitor, NF449; c) activation of the Gαs-dependent cAMP/PKA pathway was demonstrated by an increase in cAMP levels and stimulation of PKA activity in response to GLP-1(7–36) amide and by the blockade of the GLP-1(7–36) amide effect on cAMP levels and PKA activity by NF449; d) relaxation of phasic activity and ACh-induced contraction by GLP-1(7–36) amide in colonic muscle strips; and e) relaxation of ACh-induced contraction by GLP-1(7–36) amide in isolated colonic smooth muscle cells and inhibition of relaxation by NF449 as wells as by a selective PKA inhibitor, myristoylated PKI. The extent of Gαs activation, increase in cAMP and PKA activity and muscle relaxation was similar to another relaxant peptide transmitter VIP. In GI smooth muscle contraction is mediated by increase 20-kDa myosin light chain (MLC20) phosphorylation via Ca2+/calmodulin-dependent activation of MLC kinase (MLCK) and RhoA-dependent inhibition of MLC phosphatase (MLCP). Relaxation is mediated by increase in cAMP and cGMP and activation of PKA and PKG, respectively. These kinases, in turn, target different components of MLCK and MLCP signaling pathways that eventually decrease MLCK activity and/or increase MLCP activity and consequently decrease in MLC20 phosphorylation, a prerequisite for muscle relaxation. Decrease in MLCK correlates with the decrease in cytosolic Ca2+ via inhibition of inositol 1,4,5-trisphosphate (IP3) generation and IP3-dependent Ca2+ release, whereas increase in MLCP activity correlates with the inhibition on RhoA-dependent pathways via phosphorylation of RhoA and/or MLCP regulatory subunit [35,36].
Previous studies have shown that GLP-1R agonists inhibit meal-stimulated gastric emptying and the inhibitory effect is mediated by the GLP-1R present in the CNS and/or vagal sensory fibers [2,3,5]. A peripheral NO-dependent effect of GLP-1 to regulate contractility of the stomach in mice was shown [26]. In muscle strips from antrum, but not fundus of mice, GLP-1 inhibited contraction in response to the cholinergic agonist carbachol and the inhibitory effect of GLP-1 was blocked by the NO synthase inhibitor l-NNA and by the neuronal blocker tetrodotoxin suggesting activation of inhibitory myenteric neurons. The lack of effect of GLP-1 in the fundus was attributed to the lower expression of GLP-1R compared to the antrum. From a physiological point of view, GLP-1 produced by the L cells may act on different sites including enteric neurons and smooth muscle to regulate gut motility [26–29]. Our studies suggests that the effect of GLP-1(7–36) amide to regulate intestinal motility also involves activation of GLP-1R on smooth muscle cells. The direct effect of GLP-1(7–36) amide on the smooth muscle of colon to induce relaxation is in contrast to the previous reports in mice and human [26–28,31–34]. In the duodenum and colonic segments of mice, GLP-1 inhibited electrically evoked cholinergic contractions of circular muscle without affecting either spontaneous or direct contractile effects of carbachol on smooth muscle [26,27]. In contrast, GLP-1 inhibited spontaneous contractions in colonic segments of human [28]. The inhibitory effects of GLP-1 on electrically evoked contractions in mice and spontaneous contractions in human were blocked by the NO synthase inhibitor L-NNA implying that the inhibitory effect of GLP-1 was mediated via release of nitric oxide. In support of this conclusion GLP-1R immunoreactivity was demonstrated in myenteric plexus of the colon of both mice and human [28]. The inhibitory effects of low concentration of GLP-1 (up to 10 pmol/kg/min), but not high concentrations (> 10 pmol/kg/min) on migratory motor complexes in rats were blocked by L-NNA suggesting both NO-dependent and NO-independent effects of GLP-1 [29]. Our studies using muscle strips and dispersed muscle cells provide evidence that GLP-1(7–36) amide causes NO-independent muscle relaxation via activation of Gαs-coupled activation of cAMP/PKA pathway in muscle cells.
Acknowledgements
This work was supported by grants DK34153 (JR Grider) and DK28300 and DK15564 (KS Murthy) from the National Institutes of Health.
Glossary
- Ach
acetylcholine
- ATP
adenosine triphosphate
- cAMP
cyclic adenosine 5′ monophosphate
- CNS
central nervous system
- DEPC
diethylpyrocarbonate
- DMEM
Dulbecco modified Eagle medium
- DPP
dipeptidyl peptidase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GI
gastrointestinal
- GIP
glucose-dependent insulinotropic peptide
- GLP-1
glucagon-like peptide 1
- GLP1-R
glucagon-like peptide 1 receptor
- GLP-2
glucagon-like peptide 2
- GTP
guanosine triphosphate
- HEPES
N-2-hydroxyethylpiperazine-N’ 2-ethanesulfonic acid
- IBMX
Isobutyl methyl xanthine
- L-NNA
L-N-nitroarginine
- MLC
myosin light chain
- mRNA
messenger ribonucleic acid
- NF449
4,4′,4′’,4′”-[Carbonylbis(imino-5,1,3-benzenetriyl-bis(carbonylimino))]tetrakis-1,3-benzenedisulfonic acid
- NO
nitric oxide
- NOS
nitric oxide synthase
- PACAP
pituitary adenylate cyclase activating peptide
- PC
prohormone convertase
- PKA
protein kinase A
- PKI
protein kinase inhibitor
- RT-PCR
reverse transcriptase-polymerase chain reaction
- TTx
tetrodotoxin
- VIP
vasoactive intestinal peptide
Footnotes
Declaration of interest
None.
References
- [1].Holst JJ, The physiology of glucagon-like peptide 1, Physiol. Rev 87 (2007) 1409–1439. [DOI] [PubMed] [Google Scholar]
- [2].Drucker DJ, The biology of incretin hormones, Cell Metab 3 (2006) 153–165. [DOI] [PubMed] [Google Scholar]
- [3].Hellström PM, Glucagon-like peptide-1 gastrointestinal regulatory role in metabolism and motility, Vitam. Horm 84 (2010) 319–29. [DOI] [PubMed] [Google Scholar]
- [4].Sandoval DA, D’Alessio DA, Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease, Physol. Rev 95 (2015) 513–548. [DOI] [PubMed] [Google Scholar]
- [5].Janssen P, Rotondo A, Mulé F, Tack J, Review article: a comparison of glucagon-like peptides 1 and 2, Aliment. Pharmacol. Ther 37 (2013) 18–36. [DOI] [PubMed] [Google Scholar]
- [6].Drucker DJ, Habener JF, Holst JJ, Discovery, characterization, and clinical development of the glucagon-like peptides, J. Clin. Invest 127 (2017) 4217–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Murphy KG, Bloom SR, Gut hormones and the regulation of energy homeostasis, Nature 444 (2006) 854–859. [DOI] [PubMed] [Google Scholar]
- [8].Muscogiuri G, DeFronzo RA, Gastaldelli A, Holst JJ, Glucagon-like Peptide-1 and the central/peripheral nervous system: crosstalk in diabetes, Trends Endocrinol. Metab 28 (2017) 88–103. [DOI] [PubMed] [Google Scholar]
- [9].Hjøllund KR, Deacon CF, Holst JJ, Dipeptidyl peptidase-4 inhibition increases portal concentrations of intact glucagon-like peptide-1(GLP-1) to a greater extent than peripheral concentrations in anaesthetised pigs, Diabetologia 54 (2011) 2206–2208. [DOI] [PubMed] [Google Scholar]
- [10].Kumar DP, Asgharpour A, Mirshahi F, Park SH, Liu S, Imai Y, Nadler JL, Grider JR, Murthy KS, Sanyal AJ, Activation of transmembrane bile acid receptor TGR5 modulates pancreatic islet α cells to promote glucose homeostasis, J. Biol. Chem 291 (2016) 6626–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model, Diabetes 63 (2014) 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Asmar M, Bache M, Knop FK, Madsbad S, Holst JJ, Do the actions of glucagon-like peptide-1 on gastric emptying, appetite, and food intake involve release of amylin in humans? J. Clin. Endocrinol. Metab 95 (2010) 2367–2375. [DOI] [PubMed] [Google Scholar]
- [13].Asmar M, Holst JJ, Glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide: new advances, Curr. Opin. Endocrinol. Diabetes Obes 17 (2010) 57–62. [DOI] [PubMed] [Google Scholar]
- [14].Aaboe K, Krarup T, Madsbad S, Holst JJ, GLP-1: physiological effects and potential therapeutic applications, Diabetes Obes. Metab 10 (2008) 994–1003. [DOI] [PubMed] [Google Scholar]
- [15].Noyan-Ashraf MH, Momen MA, Ban K, Sadi A, Riazi AM, Baggio LL, Drucker DJ, GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice, Diabetes 58 (2009) 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nakatani Y, Maeda M, Matsumura M, Shimizu R, Banba N, Aso Y, Yasu T, Harasawa H, Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy, Diabetes Metab 43 (2017) 430–437. [DOI] [PubMed] [Google Scholar]
- [17].Camilleri M, Vazquez-Roque M, Iturrino J, Boldingh A, Burton D, McKinzie S, Wong BS, Rao AS, Kenny E, Månsson M, Zinsmeister AR, Effect of a glucagon-like peptide 1 analog, ROSE-010, on GI motor functions in female patients with constipation-predominant irritable bowel syndrome, Am. J. Physiol. Gastrointest. Liver Physiol 303 (2012) G120–G128. [DOI] [PubMed] [Google Scholar]
- [18].Hellström PM, GLP-1: broadening the incretin concept to involve gut motility, Regul. Pept 156 (2009) 9–12. [DOI] [PubMed] [Google Scholar]
- [19].Halim MA, Degerblad M, Sundbom M, Karlbom U, Holst JJ, Webb DL, Hellström PM, Glucagon-like peptide-1 inhibits postprandial gastrointestinal motility through myenteric neuronal mechanisms in humans, J. Clin. Endocrinol. Metab 103 (2018) 575–585. [DOI] [PubMed] [Google Scholar]
- [20].Hellström PM, Smithson A, Stowell G, Greene S, Kenny E, Damico C, Leone-Bay A, Baughman R, Grant M, Richardson P, Receptor-mediated inhibition of small bowel migrating complex by GLP-1 analog ROSE-010 delivered via pulmonary and systemic routes in the conscious rat, Regul. Pept 179 (2012) 71–76. [DOI] [PubMed] [Google Scholar]
- [21].Witte AB, Grybäck P, Jacobsson H, Näslund E, Hellström PM, Holst JJ, Hilsted L, Schmidt PT, Involvement of endogenous glucagon-like peptide-1 in regulation of gastric motility and pancreatic endocrine secretion, Scand. J. Gastroenterol 46 (2011) 428–435. [DOI] [PubMed] [Google Scholar]
- [22].Edholm T, Degerblad M, Grybäck P, Hilsted L, Holst JJ, Jacobsson H, Efendic S, Schmidt PT, Hellström PM, Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis, Neurogastroenterol. Motil 22 (2010) 1191–1200. [DOI] [PubMed] [Google Scholar]
- [23].Bucinskaite V, Tolessa T, Pedersen J, Rydqvist B, Zerihun L, Holst JJ, Hellström PM, Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat, Neurogastroenterol. Motil 21 (2009) 978–e78. [DOI] [PubMed] [Google Scholar]
- [24].Hellström PM, Näslund E, Edholm T, Schmidt PT, Kristensen J, Theodorsson E, Holst JJ, Efendic S, GLP-1 suppresses gastrointestinal motility and inhibits the migrating motor complex in healthy subjects and patients with irritable bowel syndrome, Neurogastroenterol. Motil 20 (2008) 649–659. [DOI] [PubMed] [Google Scholar]
- [25].Edholm T, Cejvan K, Abdel-Halim SM, Efendic S, Schmidt PT, Hellström PM, The incretin hormones GIP and GLP-1 in diabetic rats: effects on insulin secretion and small bowel motility, Neurogastroenterol. Motil 21 (2009) 313–321. [DOI] [PubMed] [Google Scholar]
- [26].Rotondo A, Amato A, Lentini L, Baldassano S, Mulè F, Glucagon-like peptide-1 relaxes gastric antrum through nitric oxide in mice, Peptides 32 (2011) 60–64. [DOI] [PubMed] [Google Scholar]
- [27].Amato A, Cinci L, Rotondo A, Serio R, Faussone-Pellegrini MS, Vannucchi MG, Mulè F, Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors, Neurogastroenterol. Motil 22 (2010) 664–e203. [DOI] [PubMed] [Google Scholar]
- [28].Amato A, Baldassano S, Liotta R, Serio R, Mulè F, Exogenous glucagon-like peptide 1 reduces contractions in human colon circular muscle, J. Endocrinol 221 (2014) 29–37. [DOI] [PubMed] [Google Scholar]
- [29].Tolessa T, Näslund E, Hellström PM, The inhibitory mechanism of GLP-1, but not glucagon, on fasted gut motility is dependent on the L-arginine/nitric oxide pathway, Regul. Pept 98 (2001) 33–40. [DOI] [PubMed] [Google Scholar]
- [30].Miki T, Minami K, Shinozaki H, Matsumura K, Saraya A, Ikeda H, Yamada Y, Holst JJ, Seino S, Distinct effects of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 on insulin secretion and gut motility, Diabetes 54 (2005) 1056–1063. [DOI] [PubMed] [Google Scholar]
- [31].Schirra J, Wank U, Arnold R, Göke B, Katschinski M, Effects of glucagon-like peptide-1(7–36)amide on motility and sensation of the proximal stomach in humans, Gut 50 (2002) 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schirra J, Houck P, Wank U, Arnold R, Göke B, Katschinski M, Effects of glucagon-like peptide-1(7–36)amide on antro-pyloro-duodenal motility in the inter-digestive state and with duodenal lipid perfusion in humans, Gut 46 (2000) 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Göke B, Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans, Gut 55 (2006) 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schirra J, Nicolaus M, Woerle HJ, Struckmeier C, Katschinski M, Göke B, GLP-1 regulates gastroduodenal motility involving cholinergic pathways, Neurogastroenterol. Motil 21 (2009) 609–618. [DOI] [PubMed] [Google Scholar]
- [35].Murthy KS, Makhlouf GM, Interaction of cA-kinase and cG-kinase in mediating relaxation of dispersed smooth muscle cell, Am. J. Physiol. Cell Physiol 268 (1995) C171–C180. [DOI] [PubMed] [Google Scholar]
- [36].Murthy KS, Jin J-G, Grider JR, Makhlouf GM, Characterization of PACAP receptors and signaling pathways in rabbit gastric muscle cells, Am. J. Physiol. Gastrointest. Liver Physiol 272 (1997) G1391–G1399. [DOI] [PubMed] [Google Scholar]
- [37].Teng B-Q, Murthy KS, Kuemmerle JF, Grider JR, Michel T, Makhlouf GM, Constitutive endothelial nitric oxide synthase: expression in human and rabbit gastrointestinal smooth muscle cells, Am. J. Physiol. Gastrointest. Liver Physiol 275 (1998) G342–351. [DOI] [PubMed] [Google Scholar]
- [38].Murthy KS, Zhou H, Grider JR, Makhlouf GM, Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially meditated by phosphorylation of RhoA, Am. J. Physiol. Gastrointest. Liver Physiol 284 (2003) G1006–G1016. [DOI] [PubMed] [Google Scholar]
- [39].Anderson CD Jr, Kendig DM, Al-Qudah M, Mahavadi S, Murthy KS, Grider JR, Role of various kinases in muscarinic M3 receptor-mediated contraction of longitudinal muscle of rat colon, J. Smooth Muscle Res 50 (2014) 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Al-Qudah M, Anderson CD, Mahavadi S, Bradley ZL, Akbarali HI, Murthy KS, Grider JR, Brain-derived neurotrophic factor enhances cholinergic contraction of longitudinal muscle of rabbit intestine via activation of phospholipase C, Am. J. Physiol. Gastrointest. Liver Physiol 306 (2014) G328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bitar KN, Makhlouf G, Relaxation of isolated gastric smooth muscle cells by Vasoactive Intestinal Peptide, Science 216 (1982) 531–533. [DOI] [PubMed] [Google Scholar]
- [42].Grider JR, Rivier JR, Vasoactive intestinal peptide (VIP) as transmitter of inhibitory motor neurons of the gut: evidence from the use of selective VIP antagonists and VIP antiserum, J. Pharmacol. Exp. Ther 253 (1990) 738–742. [PubMed] [Google Scholar]
- [43].Teng BQ, Grider JR, Murthy KS, Identification of a VIP-specific receptor in guinea pig tenia coli, Am. J. Physiol. Gastrointest. Liver Physiol 281 (2001) G718–25. [DOI] [PubMed] [Google Scholar]
- [44].Teng B, Murthy KS, Kuemmerle JF, Grider JR, Makhlouf GM, Selective expression of vasoactive intestinal peptide (VIP)2/pituitary adenylate cyclase-activating polypeptide (PACAP)3 receptors in rabbit and guinea pig gastric and tenia coli smooth muscle cells, Regul. Pept 77 (1998) 127–134. [DOI] [PubMed] [Google Scholar]
- [45].Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB, GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody, Endocrinology 155 (2014) 1280–1290. [DOI] [PubMed] [Google Scholar]
- [46].Baldassano S, Wang GD, Mulè F, Wood JD, Glucagon-like peptide-1 modulates neurally evoked mucosal chloride secretion in guinea pig small intestine in vitro, Am. J. Physiol. Gastrointest. Liver Physiol 302 (2012) G352–G358. [DOI] [PMC free article] [PubMed] [Google Scholar]