Abstract
Objectives:
To determine the effects of age and sex on physical activity and time budgets of Hadza children and juveniles, 5–14 years old, including both in-camp and out-of-camp activities.
Methods:
Behavioral data were derived from ~15,000 hourly in-camp scan observations of 76 individuals and 13 out-of-camp focal follows on 9 individuals. The data were used to estimate energy expended and percentage of time engaged in a variety of routine activities, including food collection, childcare, making and repairing tools, and household maintenance.
Results:
Our results suggest that 1) older children spend more time in economic activities; 2) females spend more time engaged in work-related and economic activities in camp, whereas males spend more time engaged in economic activities out of camp; and 3) foraging by both sexes tends to net caloric gains despite being energetically costly.
Conclusions:
These results show that, among the Hadza, a sexual division of labor begins to emerge in middle childhood and is well in place by adolescence. Furthermore, foraging tends to provide net caloric gains, suggesting that children are capable of reducing at least some of the energetic burden they place upon their parents or alloparents. The findings are relevant to our understanding of the ways in which young foragers allocate their time, the development of sex-specific behavior patterns, and the capacity of children’s work efforts to offset the cost of their own care in a cooperative breeding environment.
Keywords: activity budget, childhood, forager, Hadza, time budget
I. Introduction
Hadza childhood is often characterized as carefree (Konner, 2010), and children report that they have freedom to decide how to spend their day (Wells, Froehle, and Crittenden 2014). While Hadza children do spend considerable amounts of time engaged in play, they also run many errands for adults, provide significant amounts of childcare (Crittenden and Marlowe, 2008), and significantly contribute to the household foraging economy (Crittenden, Conklin-Brittain, Zes, Schoeninger, and Marlowe, 2013). In areas of low predator pressure characterized by terrain that is easily navigated, Hadza children have historically spent considerable time each day collecting food (Hawkes, O’Connell, and Blurton Jones, 1989; Crittenden et al., 2013).
Forager children are in a unique position of being both consumers and producers (Kramer, 2011). Most young foragers begin making important contributions to household foraging economy shortly after they are weaned, at age two or three, and long before they are fully independent around age 18 (Blurton Jones et al., 1989; Konner, 2016). Some Hadza children and juveniles have proven to be capable foragers by collecting the majority of their daily caloric needs, or even foraging a surplus of calories to help feed others (Crittenden et al., 2013). Some scholars, such as Kramer, have argued quite convincingly that such productivity makes sub-adult foragers important contributors to overall group economic productivity, supporting the notion that humans evolved as cooperative breeders (Kramer, 2005; 2014).
Despite the wealth of research suggesting high foraging productivity during childhood, few studies have either attempted to quantify the activity budgets of young Hadza foragers or have examined how participation in these activities changes with age and sex. Indeed, the Hadza, like other foragers, maintain a strong sexual division of labor in adulthood (Marlowe 2007; Kelly 1995), and it appears that young male and female foragers begin targeting sex specific resources (i.e., plants for females and animal products for males) by middle childhood (Crittenden 2016b; Crittenden et al., 2013). While previous cross-cultural studies on forager children’s gendered participation in play and work suggest that the Hadza, like other foragers, begin conforming to the sexual division of labor in their culture in middle childhood or adolescence (e.g., Lew-Levy, et al. 2018; Boyette 2016; Gallois et al., 2015), a recent meta review of the literature on forager children’s learning found no published studies on the development of gendered behaviors among the Hadza (Lew-Levy et al. 2018). Thus, data on foraging returns and activity budgets are critical in determining when sex specific patterns emerge and how caloric contributions relate to energy balance and age.
Here, we seek to address this gap in the literature by estimating the activity budgets of young Hadza foragers. We present observational data on in-camp scan sampling and out-of-camp focal follows, providing a breakdown of time spent on routine activities and their associated estimated energetic costs. We additionally test for the effects of age and sex to determine how time and energy allocation behaviors mature, and when the sexual division of labor emerges. In doing so, we make a contribution to the burgeoning literature on forager energetics and activity patterns which, heretofore, has exclusively focused on adults (Pontzer et al., 2012; 2015; 2016). While methodological limitations exist, these data provide an important baseline for future work objectively measuring children’s physical activity levels and energy expenditure.
2. Methods
2.1. Study Population
The Hadza of northern Tanzania reside in a 4000 km2 region around the shores of Lake Eyasi, in a savannah woodland habitat south of the Serengeti. At the time of data collection, an estimated 250 – 300 individuals, out of a total population of 1000, were practicing hunting and gathering as their primary means of subsistence (Blurton Jones, 2016). During this time the Hadza were living in mobile camps of around 30 individuals, and moved approximately every two to three months in response to the seasonal availability of food and water. Diet composition was highly variable and was composed of predominantly wild foraged foods, with less than 10% of the diet coming from domesticated cultigens (Marlowe, 2010). Hadza children and juveniles targeted a variety of plant products including baobab fruit, several types of berries, figs, drupes, legumes, and multiple species of tubers (underground storage organs) (Crittenden, 2016a). They also targeted an assortment of animal products, including honey and bee larvae, a wide array of avian species, and mammals ranging in size from small to large game animals (Marlowe, 2010).
Young Hadza foragers are in a unique position of being both consumers of food collected by adults, and producers of food they collect themselves. While children are an energetic burden on adults and require caloric investment for well beyond a decade, they do appear to attenuate the cost of their own care by provisioning themselves and others (Crittenden, 2016b; Crittenden and Zes, 2015). The economic productivity of young Hadza foragers is one of the most well-studied attributes of Hadza foraging economy (Blurton Jones, Hawkes, and Draper, 1994; Crittenden 2016b; Hawkes, O’Connell, and Blurton Jones, 1995). Activities range from food collection, where smaller children focus on more easily collected resources, such as baobab fruit or berries (Crittenden, 2016c), to the collection of water and firewood. While the gross caloric contributions made by children are well studied, there is little quantifiable information on how Hadza children spend their time and energy, both in acquiring food and in other behaviors in and out of camp.
2.2. Ethics Approval
Human Research Subjects approval was obtained from the University of California, San Diego, Human Research Subjects Institutional Review Board, and the Committee on the Use of Human Subjects at Harvard University. Informed consent was obtained from all individual participants included in the study, which included both participating children and their parents/guardians. Participant consent was given verbally with subsequent witnessed affidavits obtained. The necessary Tanzanian government agencies approved the consent procedure and all data were collected with the permission of the Tanzanian Commission for Science and Technology (COSTECH).
2.3. Data collection and funding
All data presented here were collected by A. Crittenden (ANC) and Tanzanian field assistants in 2005 as part of ANC’s doctoral dissertation research. The data included in this study only include camps where ANC was field site director. Data collection in the field was funded by the National Science Foundation (NSF) (9529278 and 0544751 to Frank W. Marlowe [FWM], retired) and the University of California, San Diego (to ANC). Additional data were collected in subsequent camps (not directed by ANC as part of her dissertation) as part of FWM’s funding in the same NSF cycle; those camps are not included in these analyses. Data analysis was funded by the National Institutes of Health (NIH) General Medical Sciences (8 P20 GM103440–11 to ANC and G.K. Wells).
2.4. In-Camp Scan Samples
In-camp daily activity budget data were collected in six Hadza bush camps in 2005 by ANC. In-camp data consisted of naturalistic behavioral observations conducted on 76 children aged 2–16 years (n1 = 33 males, n2 = 43 females), using scan sampling within camp during daylight hours. Ages of children were determined using long-term census data of FWM (1991 – 2004) and by asking parents to rank their children by relative age to assist researchers in determining a reliable estimate of age in years.
Data collection was restricted to children above the age of two years, as children younger than this cutoff were typically not very active, were still breastfeeding, and/or rarely spent significant time in camp without their mothers. Every child above the age of two years was included in the sample, yielding a total of ~15,000 direct observations. Each day for 165 days, hourly instantaneous scan observations were collected from 7:00am to 7:00pm. The following behaviors were recorded: resting, foraging, eating, walking, playing, providing care (e.g., childcare, holding infant, nurturing), processing food, making or maintaining tools, and assembling camp supplies such as water and/or firewood. The categories walking and playing are bundled in our analysis, as it was often difficult to make a distinction between the two during scan sampling.
Each activity, or combination of activities, was then also coded according to exertion level, as follows: resting, light activity, moderate activity, or heavy activity. Assignment of specific activities to exertion levels was guided in part by the opinions of Hadza parents and children, who were asked whether each activity was easy, moderate, or difficult to perform, and in part by attempting to match each activity to a corresponding metabolic equivalent of task (MET) value from the Compendium of Physical Activities (Ainsworth et al., 2011). A list of activities by exertion category can be found in Table 1.
Table 1.
Activities by exertion category*
| Light | Moderate | Heavy |
|---|---|---|
| Arrange things/straighten up hut | Make arrow (shaft, head, fletch) | Build or fix house |
| Bead | Make bow | Carry water |
| Carry berries/small fruits | Carry firewood | Collect firewood |
| Cook (various) | Crack drupe fruits | Chop tree |
| Distribute food, feed others | Cut/butcher meat | Hold another child (in early/middle childhood) |
| Dress other | Cut branches/grass for house | Hunt honey |
| Eat | Fix (arrow, bike, etc.) | Hunt (running) |
| Fix (radio, shoes, ankle bells, earrings, etc.) | Grind/pound maize | |
| Harden arrow shaft in fire | Hold another infant or child (in adolescence) | |
| Hunting (sitting in blind) | Hunt (walking) | |
| Hygiene (brush teeth, cut hair, bathe, etc.) | Make axe | |
| Make baobab juice | Make knife | |
| Make/maintain fire | Open baobab pods | |
| Make pipe | Play | |
| Make quiver/sheath | Pound baobab | |
| Make bow string | Process food (baobab, drupe fruit, maize, meat, etc.) | |
| Pour juice | Walk/play | |
| Process animal skin (rinse, dry, stretch, stake, etc.) | ||
| Rest | ||
| Roasting maize | ||
| Sew | ||
| Sharpen (knife, arrowhead, chisel, axe, etc.) | ||
| Shoot at bird | ||
| Sift maize | ||
| Sort baobab seeds | ||
| String bow | ||
| Trade arrow | ||
| Wash other | ||
| Whittle wood |
If any of the light or moderate tasks was performed while holding another child, the recorded exertion was increased by one level. For example, sorting baobab seeds while holding an infant would be recorded as moderate rather than light. Heavy tasks were almost never performed while holding another child.
The scan sampling data were then used to create daily activity budgets for each individual child based on the frequencies of observed activity types, as well as levels of exertion. In other words, of the total number of scans for each child on any particular day, the percentages of scans in which the child was observed in each type of activity and in each level of exertion were calculated. This resulted in two sets of activity budgets. The first set was specific to activity types, providing a snapshot of how children tended to divide their days into different tasks and behaviors. The second set focused on exertion categories and was used to estimate total energy expenditure associated with daily activity patterns.
All statistical analysis was performed in SAS 9.4 (Cary, NC) with statistical significance set to α = 0.05. Data analysis was limited to individuals present for at least 100 scans to remove visitor children appearing in only a few scans, who could have biased estimates of percent time engaged in any given activity. Descriptive statistics were calculated for both the activity-type and exertion-level activity budgets, split by sex and by age group. Age groups were defined as “early childhood” (2 – 6 years), “middle childhood” (7 – 10 years), and “adolescence” (11 −16 years).
For each activity type and exertion level, two-way linear mixed models were used to test for the sex*age group interaction effect. The interaction effect provides information on sex differences in the slope of age-group-related changes in activity frequencies. In the absence of a significant interaction effect, the main effects of sex and age were analyzed. The main effect of sex tests for differences between males and females, independent of age, whereas the age main effect tests for a relationship between age and activity, independent of sex. Because of small cell sizes, the test degrees of freedom were adjusted for unequal variances using a Satterthwaite approximation. Where the interaction effect was significant, post hoc between-groups tests of least squares means were run, correcting for multiple comparisons (Tukey-Kramer), to compare age group levels within each sex, and to test for sex differences within each of the age groups. Where the age main effect was significant, the same type of post hoc tests were run to locate significant differences between age group levels. Post hoc tests were not necessary for significant sex main effects, as the variable has only two levels.
2.5. Focal Follows
Additional supplemental out-of-camp focal follow data were collected on a sub-set of participants. The goal of this sub-analysis was to gauge activity patterns and economic productivity during foraging trips using energy expenditure estimates and food returns to calculate foraging efficiency. Focal individuals were selected using random sampling without replacement, as the aim was to follow every child at least once. Individual focal follows occurred in three-hour intervals outside of camp, during which the researcher recorded the focal child’s activity every five minutes. Complete data were available for 13 total follows. Seven came from four male individuals (two had one follow each, another had two follows, and the fourth had three follows), and six came from five female individuals (one had two follows, the rest had only one follow each). The age range of children with focal follow data was 5–14 years, with males averaging 11.5 ± 1.9 years of age, and females 7.8 ± 2.2 years of age.
Using the five-minute blocks, total time spent in each type of activity for the duration of the focal follow was calculated. Activities were then placed into exertion categories as described above, and the total time spent at rest, and in light, moderate, and heavy activities was calculated. To convert activity times into energy expenditure estimates, each child’s basal metabolic rate (BMR) was first estimated from sex, age, body mass (measured on a scale in camp), and the local mean annual temperature using equation (2) from Froehle (2008). Energy expenditure above the basal level was then calculated using the factorial method. Minutes spent in each exertion category were multiplied by BMR in kcal/min and also by a corresponding metabolic equivalent of task (MET) value, which expresses exertion as multiples of BMR. We assigned MET values as follows: resting/eating = 1.1; light activity = 2; moderate activity = 4; and heavy activity = 6. These general values were arrived upon by attempting to match Hadza activities to those contained in the Compendium of Physical Activities (Ainsworth et al., 2011) and using feedback from Hadza adults and children on exertion levels. Additionally, handheld global positioning system (GPS) devices were used to derive the focal child’s rate of walking speed and distance traveled while out of camp. Walking distance, time, and speed were then used to calculate walking energy expenditure during the foraging trips. Total energy expenditure (TEE) during each foraging trip was calculated as the sum of resting, light, moderate, heavy, and walking energy expenditure, and was expressed as raw kcal as well as kcal per kg body mass. Activity energy expenditure (AEE) values were calculated and expressed the same way, except that they excluded energy expenditure during rest.
The weight of all foods brought back to camp was recorded (wet weight kg values) using an OHAUS stainless steel hanging spring scale. In addition, while on focal follows, the weight of all foods consumed was estimated using similar methods to Rothman, Chapman, and Van Soest (2012). Using this procedure, the researcher visually estimated volume or weight of each food type on the basis of previously collected field estimates of the specific food item. This method has also been used for consumption patterns of Hadza adults (see Berbesque, Wood, Crittenden, Mabulla, and Marlowe, 2016; Wood and Marlowe, 2014). Energy values for all foods were calculated by taking the raw-as-eaten wet weight of the food and then subtracting the percentage of water and inedible material (e.g., seeds, fibrous husk) to obtain a kilogram dry weight, which was then multiplied by the appropriate kilocalorie value for each food type (Crittenden, 2009; Crittenden et al., 2013). Energy expenditure and foraging returns were used to calculate net calories (surplus or deficit) as: calories brought back to camp – TEE. We calculated foraging efficiency in two ways: net calories / foraging time; and calories brought back to camp / TEE. Because the sample of focal individuals was small, we present only descriptive summary statistics on these data.
3. Results
3.1. In-Camp Scan Samples
Age- and sex-specific in-camp activity frequencies are reported in Table 2, both as activity types and exertion levels, and are displayed visually in Fig. 1. The analysis of activity type frequencies showed that approximately 50–60% of in-camp time was spent resting or eating, regardless of sex or age. The other primary activity type observed was walking, running, and playing, which accounted for 23–43% of in-camp time, with considerable age-related variation. In-camp time spent on food processing and tool making was fairly low, but showed interesting trends with age and sex. Females were more frequently observed engaged in food processing at any age compared to males, and the reverse was true for tool making, which was more common among males. Both behaviors increased in frequency with age in both sexes, with a maximum of 14% of scans for food processing in females, and a maximum of 7% of scans for tool making in males. Finally, providing direct childcare and collecting food in camp (e.g., plant foods directly adjacent to camp or small game/birds) were both quite uncommon, with females exhibiting the highest rate of in-camp time for both: 5% for providing care and 6% for food collection.
Table 2.
Scan sample descriptive statistics by age group and sex
| Variable | Males | Females | ||||
|---|---|---|---|---|---|---|
| Early childhood | Middle childhood | Adolescence | Early childhood | Middle childhood | Adolescence | |
| (N = 16) | (N = 7) | (N = 10) | (N = 21) | (N = 14) | (N = 8) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Age (years) | 3.9 ± 1.6 | 9.1 ± 1.2 | 13.2 ± 2.4 | 4.5 ± 1.1 | 8.0 ± 1.0 | 13.6 ± 1.8 |
| Time in camp (hours) | ||||||
| Frequency of activity type | ||||||
| Resting/eating | 0.57 ± 0.17 | 0.54 ± 0.11 | 0.58 ± 0.18 | 0.51 ± 0.10 | 0.52 ± 0.11 | 0.49 ± 0.15 |
| Providing care | 0.00 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.03 | 0.03 ± 0.04 | 0.05 ± 0.03 |
| Food collection | 0.01 ± 0.01 | 0.04 ± 0.08 | 0.01 ± 0.01 | 0.01 ± 0.02 | 0.02 ± 0.03 | 0.06 ± 0.06 |
| Food processing | 0.01 ± 0.01 | 0.04 ± 0.03 | 0.03 ± 0.03 | 0.02 ± 0.03 | 0.05 ± 0.04 | 0.14 ± 0.05 |
| Tool making | 0.01 ± 0.01 | 0.03 ± 0.02 | 0.07 ± 0.07 | 0.00 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.02 |
| Walking/running/playing | 0.40 ± 0.16 | 0.35 ± 0.05 | 0.29 ± 0.12 | 0.43 ± 0.09 | 0.36 ± 0.10 | 0.23 ± 0.08 |
| Frequency of exertion level | ||||||
| Resting/eating | 0.57 ± 0.17 | 0.54 ± 0.11 | 0.58 ± 0.18 | 0.51 ± 0.10 | 0.52 ± 0.11 | 0.49 ± 0.15 |
| Light | 0.40 ± 0.16 | 0.35 ± 0.05 | 0.30 ± 0.12 | 0.43 ± 0.08 | 0.37 ± 0.09 | 0.24 ± 0.07 |
| Moderate | 0.02 ± 0.01 | 0.07 ± 0.08 | 0.05 ± 0.03 | 0.03 ± 0.03 | 0.06 ± 0.05 | 0.15 ± 0.08 |
| Heavy | 0.01 ± 0.02 | 0.05 ± 0.02 | 0.07 ± 0.07 | 0.02 ± 0.02 | 0.05 ± 0.04 | 0.13 ± 0.05 |
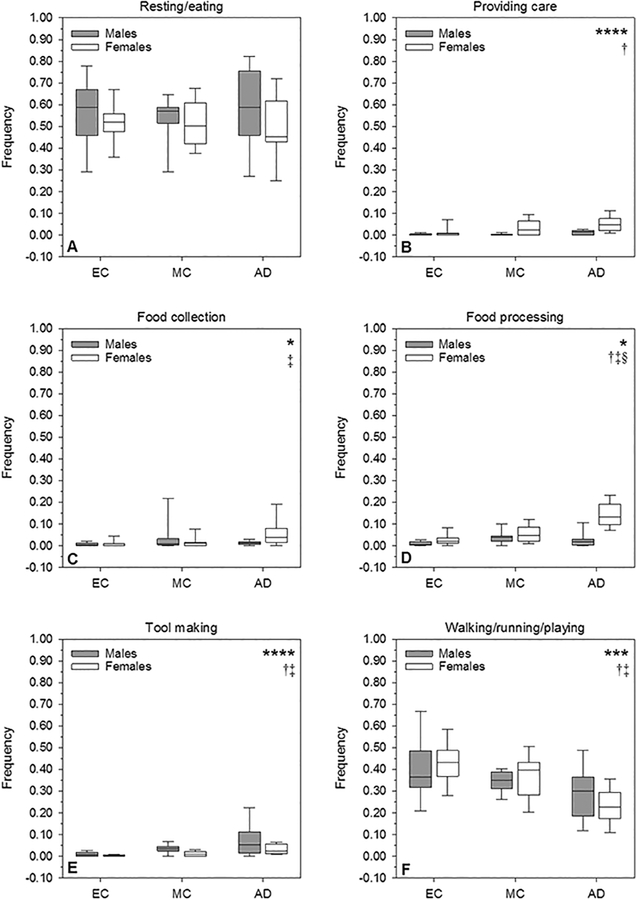
Fig. 1.
Average in-camp time allocation patterns as percent of total time spent in camp, by sex and age group (EC = early childhood; MC = middle childhood; AD = adolescence). Time allocation frequencies were quantified by type of activity (Fig. 1A: males; Fig. 1B females), as well as by level of exertion (Fig. 1C: males; Fig. 1D: females).
Linear mixed models analysis found several of the above observed trends to be statistically significant (see Table 3 and Figs. 2 and 3). The sex*age group interaction effects for food collection and food processing were both significant (P = 0.022 and P < 0.001, respectively). For food collection, this amounted to significantly more food collection behavior in adolescence vs. early childhood within females (P = 0.030), whereas males did not exhibit a similar increase (comparisons of age groups within males: for each, P ≥ 0.283). In terms of food processing, adolescent females engaged in this behavior significantly more frequently than females in early or middle childhood (for each, P < 0.001), a trend which, again, was not observed within males (for each age group comparison, P ≥ 0.227). Adolescent female foragers also spent significantly more time in food processing than their adolescent male counterparts (P < 0.001), a sex difference which was not statistically significant at earlier ages (for each, P ≥ 0.108).
Table 3.
Scan sample two-way mixed models effect F-statistics and P-values
| Dependent variable | Sex*age interaction | Main effects | ||||
|---|---|---|---|---|---|---|
| Sex | Age | |||||
| F | P-value | F | P-value | F | P-value | |
| Frequency of activity type | ||||||
| Resting/eating | 0.38 | 0.684 | 2.79 | 0.080 | 0.09 | 0.966 |
| Providing care | 2.03 | 0.139 | 19.17 | <0.001 | 5.38 | 0.007 |
| Food collection | 4.05 | 0.022 | --- | --- | --- | --- |
| Food processing | 17.28 | <0.001 | --- | --- | --- | --- |
| Tool making | 2.63 | 0.080 | 10.94 | 0.008 | 14.61 | <0.001 |
| Walking/running/playing | 1.11 | 0.336 | 0.04 | 0.875 | 11.03 | <0.001 |
| Frequency of exertion level | ||||||
| Resting/eating | 0.38 | 0.684 | 2.79 | 0.080 | 0.09 | 0.966 |
| Light | 1.13 | 0.329 | 0.00 | 0.768 | 11.49 | <0.001 |
| Moderate | 7.53 | 0.001 | --- | --- | --- | --- |
| Heavy | 2.60 | 0.081 | 5.07 | 0.027 | 28.47 | <0.001 |
Fig. 2.
Box and whisker plots showing distributions of in-camp time allocation to each activity type within age categories for each sex. Age groups are abbreviated as follows: EC = early childhood, MC = middle childhood, AD = adolescence. See Table 3 for interaction and main effects p-values from linear mixed models analysis. Significant effects (P < 0.05) are indicated in the top right corner of each plot as follows: *sex-by-age-group interaction; **sex main effect only; ***age group main effect only; ****sex and age main effects. Where the interaction effect was significant, significant post hoc between-groups differences are indicated as follows: †AD males differed from AD females; ‡EC females differed from AD females; §MC females differed from AD females. Where the age group main effect was significant, significant post hoc between-groups differences are indicated as follows: †EC differed from AD; ‡MC differed from AD; §all groups differed. Any other post hoc comparison not specifically indicated above was not statistically significant. Post hoc tests were not necessary for the sex main effect since the variable has only two levels.
Fig. 3.
Box and whisker plots showing distributions of in-camp exertion levels within age categories for each sex. Age groups are abbreviated as follows: EC = early childhood, MC = middle childhood, AD = adolescence. See Table 3 for interaction and main effects p-values from linear mixed models analysis. Significant effects (P < 0.05) are indicated in the top right corner of each plot as follows: *sex-by-age-group interaction; **sex main effect only; ***age group main effect only; ****sex and age main effects. Where the interaction effect was significant, significant post hoc between-groups differences are indicated as follows: †AD males differed from AD females; ‡EC females differed from AD females; §MC females differed from AD females. Where the age group main effect was significant, significant post hoc between-groups differences are indicated as follows: †EC differed from AD; ‡MC differed from AD; §all groups differed. Any other post hoc comparison not specifically indicated above was not statistically significant. Post hoc tests were not necessary for the sex main effect since the variable has only two levels.
There were no significant sex*age interaction effects for providing childcare (P = 0.139) or for tool making (P = 0.080). There were, however, significant main effects of sex and age for both variables (for each, P ≤ 0.008 see Table 3). Collectively, these results mean that for any age, males and females differed in the observed frequencies of these behaviors. Additionally, in both sexes the frequencies of these behaviors changed with age. However, the slope or rate of that change with age was not different between males and females. Females provided childcare significantly more often than males at all ages (P < 0.001). Across sexes, the frequency of providing care was significantly greater in adolescence compared to early childhood (P = 0.006), but not significantly different from middle childhood (P= 0.111). Similarly, males were observed making or maintaining tools significantly more often than females at any age (P = 0.008), and adolescents of both sexes made tools significantly more frequently than children of either age group (early: P < 0.001; middle: P = 0.006).
The interaction and sex main effects for walking, running, and playing were not significant (interaction: P = 0.336; sex: P = 0.875), but the main effect of age was significant (P <0.001). Across sexes the behaviors walking, running, and playing were significantly less frequent in adolescence than in early (P < 0.001) or middle childhood (P = 0.041). Finally, there were no statistically significant effects of sex, age, or their interaction on the frequency of observed resting behavior (sex: P = 0.080; age: P = 0.966; interaction: P = 0.684).
Within exertion levels, the only significant interaction effect was for moderate activity (P = 0.001). The pattern for moderate activity was similar to that for food processing, in that adolescent females engaged significantly more frequently in moderate exertion level behaviors than did females in early or middle childhood (for each, P < 0.001), but there was no difference in moderate activity between early and middle childhood (P = 0.373). Meanwhile, none of the male age groups differed significantly for moderate exertion levels (for each, P ≥ 0.104). Adolescent females also engaged in significantly more behaviors with a moderate exertion level than did adolescent males (P < 0.001), but these sex differences were not observed in the younger age groups (for each, P ≥ 0.894).
The sex*age group interactions were not significant for light or heavy exertion levels (light: P = 0.329; heavy: P = 0.081). The age main effect was significant for light exertion (P < 0.001), but the sex main effect was not (P = 0.768). For both males and females, significantly less time was spent in light activity by adolescents than by children in early (P < 0.001) or middle childhood (P = 0.031). The age main effect for heavy exertion was also significant (P < 0.001). Across sexes, behaviors requiring heavy exertion levels were more frequent in middle childhood than in early childhood (P = 0.021), and again more frequent in adolescence than in middle childhood (P < 0.001). The sex main effect for heavy exertion approached significance (P = 0.052), with a strong trend toward females engaging in more frequent heavy exertion level behaviors than males. Finally, because rest was coded the same way as an activity and as an exertion level, the mixed models results for this category are the same for both sub-analyses.
3.2. Focal Follows
Durations of foraging trips, as well as the total energy expended on foraging trips, varied considerably within and between the sexes (see Table 4 and Fig. 4). In males, mean total foraging energy expenditure (TFEE) was 431 ± 365 kcal, ranging from 135–1159 kcal and with a coefficient of variation (CV) of 85%. Time spent foraging explained much of the variation in TFEE, the two variables being closely correlated (r = 0.94; P = 0.002). Therefore, when TFEE was normalized to foraging time (TFEE/duration) it was considerably less variable, with a mean of 144 ± 41 kcal/h and a CV of 29%. There was less variability in TFEE among females, with an average of 284 ± 155 kcal (CV = 54%), and even more consistency when adjusted for duration of foraging trips, with a mean of 128 ± 9 kcal/h (CV = 7%). On average, males expended roughly 50% more energy than females on foraging trips, but this appeared to be largely related to longer foraging trips among males, since their time-adjusted TFEE was only 13% greater than that of females, on average. Body size additionally played a role, since males were heavier than females (25.4 ± 3.8 kg vs. 17.9 ± 4.1 kg, respectively) and thus required more energy for both tissue maintenance and activity. When TFEE values were adjusted for both foraging time and body mass (by dividing TFEE by body mass and foraging time) females expended 7.7 ± 1.4 kcal/kg/hr compared to 6.1 ± 2.1 kcal/kg/hr in males, representing a roughly 25% higher rate of energy expenditure in females vs. males.
Table 4.
Focal follow energy expenditure data
| ID | Age (y) | Follow | Duration (h) | Energy expenditure (kcal) | Food returns to camp (kcal) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Resting | Light | Moderate | Heavy | Walking | TFEE* | ||||||
| Males | M1 | 14 | 1 | 8.4 | 407 | 8 | 366 | 0 | 378 | 1159 | 6256 |
| M2 | 12 | 1 | 1.1 | 26 | 0 | 80 | 0 | 28 | 135 | 3560 | |
| M3 | 10 | 1 | 0.8 | 0 | 0 | 45 | 0 | 100 | 145 | 295 | |
| 2 | 2.6 | 25 | 0 | 30 | 112 | 264 | 431 | 4253 | |||
| 3 | 3.9 | 41 | 7 | 299 | 134 | 150 | 631 | 3419 | |||
| M4 | 10 | 1 | 1.6 | 0 | 0 | 224 | 0 | 55 | 279 | 400 | |
| 2 | 3.8 | 169 | 0 | 0 | 0 | 67 | 236 | 1934 | |||
| Females | F1 | 10 | 1 | 3.9 | 13 | 0 | 344 | 0 | 157 | 514 | 1828 |
| 2 | 2.8 | 0 | 41 | 213 | 0 | 103 | 357 | 645 | |||
| F2 | 9 | 1 | 3.3 | 26 | 18 | 305 | 0 | 24 | 373 | 2509 | |
| F3 | 9 | 1 | 1.0 | 7 | 0 | 101 | 0 | 31 | 139 | 316 | |
| F4 | 6 | 1 | 1.0 | 0 | 6 | 89 | 0 | 29 | 124 | 371 | |
| F5 | 5 | 1 | 1.5 | 3 | 6 | 127 | 0 | 63 | 199 | 114 | |
TFEE stands for “total follow energy expenditure”, and includes the energetic costs of rest (BMR * 1.1) plus exertion above resting levels.
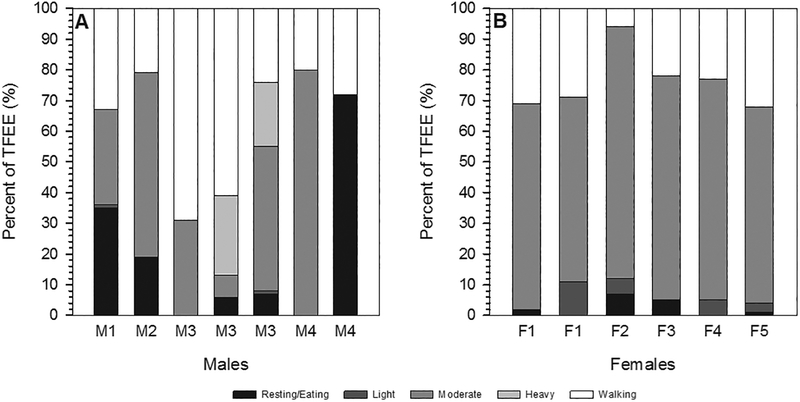
Fig. 4.
Total foraging energy expenditure (TFEE) for individual foraging trip focal follows. Bars are color coded and labeled by the individual forager.
As percentages of overall TFEE (see Fig. 5), walking and moderate activity were equal contributors in males (37 ± 19% of TFEE and 37 ± 26% of TFEE, respectively), with rest contributing 20 ± 24% and heavy activity accounting for 7 ± 11% of total TFEE. Contributions from light activity were negligible among males. Among females, moderate activity made up the bulk of TFEE at 60 ± 25%, with walking accounting for the second highest proportion of calories expended (20 ± 11%). Resting and light activity contributed 7 ± 12% and 13 ± 21% of TFEE, respectively, with no heavy foraging activity recorded among females.
Fig. 5.
Energy allocation to different exertion levels as a percent of TFEE for each focal follow. Bars are labeled by the individual forager.
Foraging returns (see Fig. 6) recorded as kcal brought back to camp were also highly variable, with a mean of 2874 ± 2151 kcal in males (CV = 75%), and a mean of 964 ± 973 kcal in females (CV = 101%). When expressed as foraging efficiency (kcal returns / foraging duration), variability remained high, with males collecting on average 1089 ± 1051 kcal/h (CV = 97%) and females collecting a mean of 370 ± 233 kcal/h (CV = 63%). Compared to TFEE, however, there was consistency in that foraging returns almost universally resulted in a caloric surplus over the energy expended during foraging. In males, the average caloric surplus was 2443 ± 1881 kcal, with a range of 121–5097 kcal and no instances of males incurring a caloric deficit. In females, the average caloric surplus was 680 ± 860 kcal, ranging from an 85 kcal caloric deficit, to a 2136 kcal surplus. The −85 kcal difference between foraging returns and TFEE in females was the only recorded caloric deficit, and was observed in the youngest individual in the focal follows with an age of 5 years. Similarly, that same individual was the only subject observed to have a foraging return TFEE ratio less than 1.0 (at 0.6). The remainder of the focal follows resulted in ratios of between 1.4–26.4 times more calories brought back to camp than expended. In males, the mean ratio was 8.4 ± 8.5, and in females the mean ratio was 3.0 ± 2.1. The male value was likely inflated by one very high return ratio (26.4), which was the product of exploiting a honey resource close to camp, resulting in low energy expenditure and high caloric return. When this value was removed, the return ratios became more similar in males and females, at 5.4 ± 3.3 and 3.0 ± 2.1, respectively. Still, even this additional analysis shows young males producing almost twice as large a return on energy invested in foraging compared to young females.
Fig. 6.
A: Comparisons of mean TFEE, mean foraging returns, and mean caloric surpluses in males (gray bars) and females (white bars). B: Comparison of mean foraging efficiency (kcal/h) between males and females. C: Comparison of Caloric return/TFEE ratio between males and females. Error bars in all plots are standard deviations.
Also important to consider are the number of days spent foraging vs. in camp. Of 60 total days observed for each child with focal follows, the number of days on which they left camp to forage varied from eight to 32, or 13% to 53% of all observed days. This means that even the most active forager among the subset observed still spent almost half of their days without leaving camp. Percentages of days with a foraging trip were very similar between the sexes, with males averaging a foraging trip on 32 ± 14% of observed days, vs. 33 ± 19% among females. There also appeared to be an age component to the pattern of leaving camp, at least within females: the two individuals who were five and six years of age foraged on just eight and nine days, respectively. In contrast, the remaining three females who were at least nine years of age all foraged on at least 22 days (≥ 37% of observed days). No similar trend emerged among males, perhaps in part because none was younger than 10 years of age.
4. Discussion
It has been well documented that Hadza children are active foragers (Crittenden and Zes, 2015; Crittenden et al., 2013; Crittenden, 2016; Hawkes et al., 1995; Blurton Jones et al., 1994; Blurton Jones et al., 1989). To date, however, few studies have attempted to quantify the ways in which forager children expend their energy, and whether or not their foraging activities yield net caloric gains. This study represents the first to quantify energy expenditure among Hadza children and juveniles. The data presented here allow us to make several conclusions. First, as Hadza children mature, they contribute more to work related activities in and around camp, thus potentially offsetting the cost of their own care. Second, while the Hadza are an egalitarian society, energetic contributions to various tasks begin to show strong sex differences at an early age. Third, while caloric gains and expenditures vary widely across forays, young foragers generally yield net caloric gains – collecting 10.5 kcal for every 1 kcal expended during foraging activities. Given the age distribution of the children studied in the focal follows (see Table 4), it appears that net positive caloric returns from foraging emerge during middle childhood.
The most interesting findings from the analysis of both the in- and out-of-camp activity budgets were the sex differences that appeared in work related activities. As females matured, they spent more of their time in camp participating in caregiving and food processing. As males matured, they spent more of their time in camp engaged in tool making or maintenance activities. Males participated in little in-camp work at all ages except for tool making behavior, which included making bows and arrows, as well as sharpening knives and axes. By adolescence, significant sex differences emerged for every type of in-camp work. This trend is similar to other small-scale societies, including Mayan agriculturalists (Kramer, 2005), Mikea foragers (Tucker and Young, 2005), Aka foragers, Ngandu farmers (Lew-Levy and Boyette, 2018), and among foragers more generally (Lew-Levy et al., 2018). The increase in females’ time spent in food processing (and corresponding lack of change in time spent in food processing for males) corresponds directly to their patterns of moderate levels of exertion, as more than 50% of moderate activities are related to food processing.
Furthermore, by adolescence sex differences become more pronounced, with minimal overlap in what work males and females do in camp. This could be explained by sex differences in how in-camp vs. out-of-camp work is allocated. Indeed, males spent more energy on foraging trips than females, appeared to collect more kilocalories, and foraged out of camp for longer than females. Hawkes, O’Connell, and Blurton Jones (1995) noted sex differences between Hadza adult males’ and adult females’ time spent away from camp, with males spending 50% more time outside of camp than females. Our analysis here suggests that these trends are also apparent in childhood. Furthermore, females displayed less variability in their foraging returns than males. These differences may be attributed to sex differences in risk-taking behavior; indeed, Apicella and colleagues (2017) found that Hadza adult males were more likely to take risks for a higher payoff than adult females during experimental economic games. Their data also suggests that young male foragers exhibited greater variance in naturalistic foraging returns than their female counterparts. Although the present dataset is small, a trend consistent with these observed patterns is evident among our sample of young Hadza foragers.
Berbesque and colleagues (2016) also recently showed that Hadza adult males consume more calories while out foraging than their female counterparts, similar to what we might be witnessing among young foragers, and what has been reported for out-of-camp consumption patterns among Hadza children and juveniles previously (Crittenden et al., 2013). Future research will need to quantify if younger males are primarily spending their time out of camp on self-provisioning, or on food collection as common goods (i.e., collecting food to be brought back to camp and shared). Such data will be useful in understanding how sex-differences in the ontogeny of resource acquisition may be reflective of adult risk-taking behaviors.
There is no appreciable difference between males’ and females’ time spent resting or playing, but Hadza children do devote considerable amounts of time to leisure, resting, and playing. While it is less than (but approaching) the amount of time spent in leisure activities for the Aka foragers and Ngandu farmers of the Central African Republic (Boyette 2016), it is greater than that of both Maya agriculturalists and Mikea foragers (Kramer, 2005a; Tucker & Young, 2005). Explanations for these differences in time allocation may be associated with (1) variation in caloric return rates for resources in the Hadza environment compared to other ecosystems (perhaps higher than the Mikea but lower than the Aka), (2) difficulty in food processing tasks (perhaps less for the Hadza than for the Mikea, for example) or (3) population variation where children in some foraging populations are simply expected to spend more or less time engaged in work related activities than children in other populations. More cross-cultural data are needed to determine what drives these behavioral differences, and whether or not they are tethered to ecological variation.
As a whole, our results were expected. Hadza children spend less time playing and more time performing in-camp chores as they mature. Starting as young as three years of age, children begin to contribute to work in camp. By middle childhood, sex differences emerge with females providing more caregiving to their siblings and other younger children in camp, and males spending more time making and maintaining tools. Additionally, as children grow older, they take on heavier burdens such as collecting water, constructing and maintaining living spaces, and participating in more difficult food processing tasks. Hadza children frequently leave camp to forage for nearby resources. These forays are valuable for developing skills necessary to their survival and future ability to support offspring of their own, as well as being immediately beneficial by producing foraging returns (Crittenden et al., 2013; Lew-Levy, Reckin, Lavi, Cristóbal-Azkarate, and Ellis-Davies, 2017). When compared to adults, children and juveniles often target food resources that are easier to attain but have lower nutritional value (Bleige-Bird & Bird, 2002; Crittenden et al., 2013). Nevertheless, despite the high energetic cost of participating in foraging activities, our results show that children are able to collect enough calories to support their foraging efforts, netting between 3.0 and 5.4 kcal for each 1 kcal spent foraging.
While these data provide insight into children’s activities, there are several limitations to the current study. First, the activity budgets were measured with time allocation and not using alternative measures such as accelerometry, as with similar studies of foraging children (Madimenos, Snodgrass, Blackwell, Liebert, and Sugiyama, 2011). Second, the current method of analysis differs from the doubly labeled water method used to investigate physical activity or total energy expenditure (TEE) among Hadza adults (Pontzer et al., 2012; Raichlen et al., 2017), making direct comparisons to adult energy expenditure challenging. Third, small sample sizes may have led to some under-powered statistical tests. Finally, the current method made distinguishing between walking and playing in camp impossible – future work will tease apart these activities to add further insight into how young foragers spend their time.
5. Conclusion
As Hadza children mature, they participate in more in-camp work and devote effort to more strenuous camp activities. By adolescence, males and females are participating in a sexual division of labor that looks quite similar to that of adults in their population; females contributing more to in-camp work than males, and males spending more time and energy out of camp engaged in economic activities. Additional research is needed to quantify the type and amount of work that both females and males may be engaging in outside of camp. Furthermore, Hadza children’s foraging efforts, while more costly than remaining in camp, often provided a net gain in kilocalories. These data support the notion that children are capable of supporting their own learning efforts, reducing at least some of the caloric burden they may place upon their parents or alloparents. This research underscores the importance of children’s work-related efforts in offsetting the cost of their own care in a cooperative breeding environment and has implications for our understanding of the unique evolutionary trajectory of human life-history.
6. Acknowledgments:
This project was supported by grants from the National Institutes of Health (NIH) General Medical Sciences (8 P20 GM103440–11 to ANC and GKW), the National Science Foundation (NSF) (9529278 and 0544751 to Frank W. Marlowe, data collected by ANC), and the University of California, San Diego. The authors would like to extend our gratitude to the Hadza for their continued friendship and research participation. We would also like to thank the Tanzanian Commission for Science and Technology (COSTECH) for research permission and Happy Msofe, Golden Ngumbuke, and Ephraim Mutukwaya for research assistance and data collection. Finally, we would like to acknowledge and thank Frank W. Marlowe, who is now retired, for his assistance with research permit approval, research funding, and mentorship.
References
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, … & Leon AS (2011). 2011 Compendium of Physical Activities: a second update of codes and MET values. Medicine & science in sports & exercise, 43(8), 1575–1581. [DOI] [PubMed] [Google Scholar]
- Apicella CL, Crittenden AN, & Tobolsky VA (2017). Hunter-gatherer males are more risk-seeking than females, even in late childhood. Evolution and Human Behavior, 38(5), 592–603. [Google Scholar]
- Bird RB, & Bird DW (2017). Martu children’s hunting strategies in the Western Desert, Australia In Hunter-gatherer childhoods (pp. 129–146). Routledge. [Google Scholar]
- Bird RB, & Bird DW (2002). Constraints of knowing or constraints of growing? Human Nature, 13(2), 239–267. [DOI] [PubMed] [Google Scholar]
- Boyette AH (2016). Children’s play and culture learning in an egalitarian foraging society. Child Development, 87(3), 759–769. [DOI] [PubMed] [Google Scholar]
- Blurton Jones NJ (2016). Demography and evolutionary ecology of Hadza hunter-gatherers. Cambridge University Press. [Google Scholar]
- Blurton Jones NG, Hawkes K, & Draper P (1994). Differences between Hadza and! Kung children’s work: Original affluence or practical reason? Key issues in hunter-gatherer research, 189–215. [Google Scholar]
- Blurton Jones NJ, Hawkes K, & O’Connell JF (1999). Some current ideas about the evolution of the human life history In Comparative primate socioecology (pp. 140–166). Cambridge University Press; Cambridge. [Google Scholar]
- Blurton Jones NJ, & Marlowe FW (2002). Selection for delayed maturity. Human Nature, 13(2), 199–238. [DOI] [PubMed] [Google Scholar]
- Blurton Jones NG, Hawkes K, & O’Connell JF (1989). Modelling and measuring costs of children in two foraging societies Comparative socioecology of humans and other mammals. London: Basil Blackwell, 367–390. [Google Scholar]
- Bock J (2002). Learning, life history, and productivity. Human Nature, 13(2), 161–197. [DOI] [PubMed] [Google Scholar]
- Bogin BA (1997). Evolutionary hypotheses for human childhood.
- Crittenden AN (2009). Allomaternal care and juvenile foraging among the Hadza: Implications for the evolution of cooperative breeding in humans. University of California, San Diego. [Google Scholar]
- Crittenden AN (2016a). Ethnobotany in evolutionary perspective: wild plants in diet composition and daily use among Hadza hunter-gatherers. Wild harvest: plants in the hominin and pre-agrarian human worlds (eds Hardy K, Kubiak ML), 319–339. [Google Scholar]
- Crittenden AN (2016b). To share or not to share? Social processes of learning to share food among Hadza hunter-gatherer children In Social Learning and Innovation in Contemporary Hunter-Gatherers (pp. 61–70). Springer, Tokyo. [Google Scholar]
- Crittenden AN (2016c). Children’s foraging and play among the Hadza In Origins and implications of the evolution of childhood (pp. 155–172). School of Advanced Research (SAR) Series, University of New Mexico Press, Albuquerque. [Google Scholar]
- Crittenden AN, Conklin-Brittain NL, Zes DA, Schoeninger MJ, & Marlowe FW (2013). Juvenile foraging among the Hadza: Implications for human life history. Evolution and Human Behavior, 34(4), 299–304. [Google Scholar]
- Crittenden AN, & Marlowe FW (2008). Allomaternal care among the Hadza of Tanzania. Human Nature, 19(3), 249. [DOI] [PubMed] [Google Scholar]
- Crittenden AN, & Zes DA (2015). Food sharing among Hadza hunter-gatherer children. PloS one, 10(7), e0131996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper P, & Cashdan E (1988). Technological change and child behavior among the! Kung. Ethnology, 27(4), 339–365. [Google Scholar]
- Gallois S, Duda R, Hewlett BS, & Reyes-garcía V (2015). Children’s Daily Activities and Knowledge Acquisition: A case Study among the Baka from Southeastern Cameroon. Journal of Ethnobiology and Ethnomedicine, 11, 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MA, & Mace R (2005). Helpful grandmothers in rural Ethiopia: A study of the effect of kin on child survival and growth. Evolution and Human Behavior, 26(6), 469–482. [Google Scholar]
- Hawkes K, O’Connell JF, & Blurton Jones NG (1989). Hardworking hadza grandmothers. Comparative socioecology, 341–366. [Google Scholar]
- Hawkes K, O’Connell JF, & Blurton Jones NG (1997). Hadza women’s time allocation, offspring provisioning, and the evolution of long postmenopausal life spans. Current Anthropology, 38(4), 551–577. [Google Scholar]
- Hawkes K, O’Connell F, & Jones NB (1995). Hadza children’s foraging: juvenile dependency, social arrangements, and mobility among hunter-gatherers. Current Anthropology, 36(4), 688–700. [Google Scholar]
- Hrdy SB (2011). Mothers and others. Harvard University Press. [Google Scholar]
- Kaplan H, Hill K, Lancaster J, & Hurtdo AM (2000). A theory of human life history evolution: diet, intelligence, and longevity. Evolutionary Anthropology: Issues, News, and Reviews, 9(4), 156–185. [Google Scholar]
- Kelly RL (1995). The Lifeways of Hunter-Gatherers: The Foraging Spectrum. New York: Cambridge University Press. [Google Scholar]
- Kramer KL (2002). Variation in juvenile dependence. Human nature, 13(2), 299–325. [DOI] [PubMed] [Google Scholar]
- Kramer K (2005a). Maya children. Harvard University Press. [Google Scholar]
- Kramer KL (2005b). Children’s help and the pace of reproduction: cooperative breeding in humans. Evolutionary Anthropology: Issues, News, and Reviews, 14(6), 224–237. [Google Scholar]
- Kramer KL (2011). The evolution of human parental care and recruitment of juvenile help. Trends in ecology & evolution, 26(10), 533–540. [DOI] [PubMed] [Google Scholar]
- Kramer KL, & Ellison PT (2010). Pooled energy budgets: Resituating human energy‐allocation trade‐offs. Evolutionary Anthropology: Issues, News, and Reviews, 19(4), 136–147. [Google Scholar]
- Lancaster CS, & Lancaster JB (2017). The watershed: Change in parental-investment and family-formation strategies in the course of human evolution In Parenting across the life span (pp. 187–206). Routledge. [Google Scholar]
- Lee RB (1979). The! Kung San: men, women and work in a foraging society. CUP Archive. [Google Scholar]
- Leigh SR (2001). Evolution of human growth. Evolutionary Anthropology: Issues, News, and Reviews, 10(6), 223–236. [Google Scholar]
- Lew-Levy S, & Boyette AH (2017). Evidence for the adaptive learning function of work and work-themed play among Aka forager and Ngandu farmer Children from the Congo Basin. [DOI] [PMC free article] [PubMed]
- Lew-Levy S, Reckin R, Lavi N, Cristóbal-Azkarate J, & Ellis-Davies K (2017). How Do Hunter-Gatherer Children Learn Subsistence Skills?. Human Nature, 28(4), 367–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew-Levy S, Lavi N, Reckin R, Cristóbal-Azkarate J, & Ellis-Davies K (2017). How do hunter-gatherer children learn social and gender norms? A meta-ethnographic review. Cross-Cultural Research, 1069397117723552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madimenos FC, Snodgrass JJ, Blackwell AD, Liebert MA, & Sugiyama LS (2011). Physical activity in an indigenous Ecuadorian forager‐horticulturalist population as measured using accelerometry. American Journal of Human Biology, 23(4), 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlowe F (2001). Male contribution to diet and female reproductive success among foragers. Current Anthropology, 42(5), 755–759. [Google Scholar]
- Marlowe FW (2007). Hunting and Gathering: The Human Sexual Division of Foraging Labor. Cross-Cultural Research, 41(2), 170–195. [Google Scholar]
- Marlowe F (2010). The Hadza: hunter-gatherers of Tanzania. Univ of California Press. [Google Scholar]
- Marlowe FW, & Berbesque JC (2009). Tubers as fallback foods and their impact on Hadza hunter‐gatherers. American Journal of Physical Anthropology, 140(4), 751–758. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Raichlen DA, Wood BM, Mabulla AZ, Racette SB, & Marlowe FW (2012). Hunter-gatherer energetics and human obesity. PloS one, 7(7), e40503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontzer H, Raichlen DA, Wood BM, Emery Thompson M, Racette SB, Mabulla AZ, & Marlowe FW (2015). Energy expenditure and activity among Hadza hunter‐gatherers. American Journal of Human Biology, 27(5), 628–637. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Durazo-Arvizu R, Dugas LR, Plange-Rhule J, Bovet P, Forrester TE, … & Luke A (2016). Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Current Biology, 26(3), 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlen DA, Pontzer H, Harris JA, Mabulla AZ, Marlowe FW, Josh Snodgrass J, … & Wood BM (2017). Physical activity patterns and biomarkers of cardiovascular disease risk in hunter‐gatherers. American Journal of Human Biology, 29(2). [DOI] [PubMed] [Google Scholar]
- Robinson RS, Lee RD, & Kramer KL (2008). Counting women’s labour: A reanalysis of children’s net production using Cain’s data from a Bangladeshi village. Population Studies, 62(1), 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson SL, Van Schaik CP, & Hawkes K (2006). The derived features of human life history. The evolution of human life history, 17–44. [Google Scholar]
- Tucker B, & Young AG (2005). Growing up Mikea. Hunter-gatherer childhoods: Evolutionary, developmental, and cultural perspectives, 147–171. [Google Scholar]
- Turke PW (1988). Helpers at the nest: childcare networks on Ifaulk. Human reproductive behavior: A Darwinian perspective, 173–188. [Google Scholar]
- Vincent AS (1985). Plant foods in savanna environments: a preliminary report of tubers eaten by the Hadza of northern Tanzania. World Archaeology, 17(2), 131–148. [DOI] [PubMed] [Google Scholar]
- Walker R, Hill K, Burger O, & Hurtado AM (2006). Life in the slow lane revisited: Ontogenetic separation between chimpanzees and humans. American Journal of Physical Anthropology, 129(4), 577–583. [DOI] [PubMed] [Google Scholar]
- Wells GK, Froehle AW, & Crittenden AN (2014, March). Activity budgets and energy expenditure among hunter-gatherer children: results from the Hadza of northern Tanzania. In American Journal of Physical Anthropology 153: 270–270. [Google Scholar]
- Wood BM, & Marlowe FW (2014). Toward a reality-based understanding of Hadza men’s work. Human Nature, 25(4), 620–630. [DOI] [PubMed] [Google Scholar]
- Woodburn J (1968). Stability and flexibility in Hadza residential groupings In Man the Hunter (ed. Lee R and de Vore I): pp. 103–10. [Google Scholar]