Abstract
Kounis syndrome refers to an acute coronary syndrome, consequent to an allergic reaction. It results from mast cell degranulation with subsequent release of numerous inflammatory mediators, leading to coronary vasospasm, atheromatous plaque rupture, or stent thrombosis. Here, we describe the case of a 47-year-old Caucasian man with acute stent thrombosis, as a consequence of allergic reaction to contrast media.
<Learning objective: Kounis syndrome is an acute coronary syndrome, consequent to an allergic reaction. Cytokine release might precipitate coronary spasm, plaque rupture, or stent thrombosis. Stent thrombosis is a dramatic complication of coronary stenting, presenting as sudden death or acute myocardial infarction. Our case serves as an example for clinicians to consider the possibility of Kounis syndrome in patients with acute coronary syndrome and anaphylaxis in order to ensure appropriate treatment.>
Keywords: Stent thrombosis, Acute coronary syndrome, Kounis syndrome
Introduction
The term Kounis syndrome (KS) refers to an acute coronary syndrome, resulting from an anaphylactic or allergic reaction [1]. The pathophysiological soil of KS involves coronary artery spasm (type 1), atheromatous plaque erosion or rupture (type 2), or stent thrombosis (type 3) during an allergic reaction [2], [3]. Although, the exact mechanisms of this phenomenon have not been fully elucidated, it has been hypothesized that mast cells, platelet activation, and cytokine release, might precipitate coronary spasm, plaque rupture, or stent thrombosis.
Here, we report a case of acute stent thrombosis following an allergic reaction just after coronary angiography.
Case report
A 47-year-old Caucasian man was admitted to our hospital for appearance of dyspnea and oppressive chest pain during moderate physical activity. Clinical history was positive for: hypertension, diabetes, hyperlipidemia. Familial history was positive for cardiovascular disease (father and sister with previous myocardial infarction). Of note, clinical history did not reveal allergic reactions to drugs, environmental factors, or food. He was under treatment with metformin 1000 mg twice daily, atorvastatin 20 mg, and ramipril 5 mg. Clinical examination revealed severe obesity (body mass index = 46.8 kg/m2), blood pressure = 140/80 mmHg, whereas laboratory analyses did not show significant alterations (Table 1). Electrocardiogram (ECG) showed sinus rhythm with a heart rate of 69 beats for minutes, flattening of T wave in precordial derivation. Transthoracic echocardiography revealed an ejection fraction of 50% and mild septal hypokinesia. Chest radiography did not show alterations.
Table 1.
Patient’s clinical, laboratory, and instrumental parameters.
| Clinical data | Results |
| Gender | Male |
| Age (years) | 47 |
| Height (cm) | 176 |
| Weight (kg) | 145 |
| Body mass index (kg/m2) | 46.8 |
| Systolic blood pressure (mmHg) | 140 |
| Diastolic blood pressure (mmHg) | 80 |
| Risk factors | Hypertension, diabetes, hyperlipidemia |
| Laboratory data | |
| Glucose (mg/dL) | 203 (nv: 60–110) |
| Urea (mg/dL) | 28 (nv: 10–50) |
| Creatinine (mg/dL) | 0.83 (nv: 0.8–1.4) |
| Total-cholesterol (mg/dL) | 154 (nv: <200) |
| HDL-cholesterol (mg/dL) | 33 (nv: >45) |
| LDL-cholesterol (mg/dL) | 99 (nv: <130) |
| Triglycerides (mg/dL) | 111 (nv: <150) |
| Serum iron (mg/dL) | 89 (nv: 50–170) |
| ALT (UI/L) | 61 (nv: <50) |
| AST (UI/L) | 50 (nv: <50) |
| CPK (UI/L) | 112 (nv: 0–173) |
| CPK-MB (UI/L) | 11 (nv: 7–25) |
| LDH (UI/L) | 242 (nv: 135–460) |
| Sodium (mmol/L) | 138 (nv: 136–146) |
| Potassium (mmol/L) | 4.0 (nv: 3.5–5.1) |
| Calcium (mg/dL) | 9.6 (nv: 8.4–10.2) |
| White blood cells count (x103/uL) | 11.9 (nv: 5.2–12.4) |
| Red blood cells count (x103/uL) | 5.40 (nv: 4.2–5.2) |
| Hemoglobin (g/dL) | 15.8 (nv: 12–15) |
| Hematocrit (%) | 47 (nv: 37–47) |
| Platelet count (x103/uL) | 219 (nv: 130–400) |
| Instrumental data | |
| Left ejection fraction (%) | 50 |
| Wall motion abnormalities | Mild septal hypokinesia |
| Number of stents | 2 |
| Stents size (diameter x length mm) | 3.0 × 20 and 3.0 × 12 |
| Type of stents | Platinum–chromium everolimus-eluting |
| Minimum lumen area (mm2) | 7.5 |
| Minimum lumen diameter (mm) | 3.0 × 3.2 |
| Diameter stenosis (%) | 30 |
nv, normal values; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ALT, alanine transaminase; AST, aspartate transaminase; CPK, creatine phosphokinase; LDH, lactate dehydrogenase.
After careful clinical and instrumental examination, he underwent coronary angiography. Antiplatelet therapy was administered before the intervention. Angiographic images showed critical stenosis of left anterior descending artery (LAD) treated with two platinum chromium everolimus-eluting stents of 3.0 mm × 20 mm and 3.0 × 12 mm deployed at 14 atm (Synergy; Boston Scientific, Marlborough, MA, USA) and post dilated with a non-compliant 3.25 × 12 mm balloon (NC Sprinter RX; Medtronic, Minneapolis, MN, USA) at 18 atm. The ECG after coronarography was unchanged with respect to the basal.
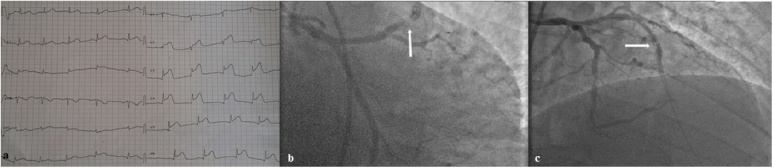
After 30 min from the end of angiography, the patient experienced nausea, sweating, chest pain, generalized itch, and appearance of cutaneous erythema. Clinical parameters showed severe hypotension (blood pressure = 80/60 mmHg) and ECG revealed significant ST segment elevation in V2–V5 (Fig. 1a).
Fig. 1.
(a) Electrocardiogram showing ST elevation in precordial derivations. (b and c) Angiographic appearance of stent thrombosis following allergic reaction.
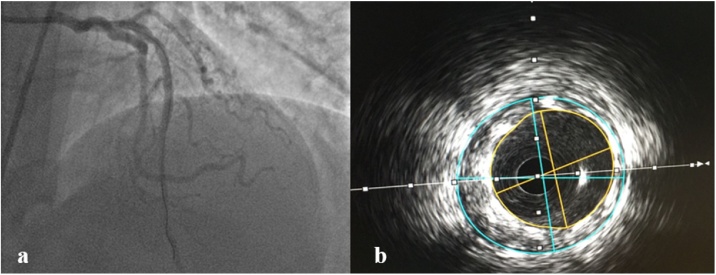
In the light of these findings, the diagnosis of ST segment elevation myocardial infarction concomitant with allergic reaction was strongly suspected. The patient was treated with, hydrocortisone, chlorpheniramine, oxygen, and saline infusion. Immediate coronary angiography was performed showing complete stent thrombosis (Fig. 1b and c). After intracoronary administration of a glycoprotein IIb/IIIa receptor blocker (eptifibatide), thrombosis was successfully treated with a 3 mm × 20 mm balloon angioplasty (Emerge; Boston Scientific) at 6 atm with restoration of thrombolysis in myocardial infarction-3 flow (Fig. 2a). Furthermore, intracoronary ultrasound (ICUS) (Opticross; Boston Scientific) was performed to detect possible under-expansion, malapposition, or stent fracture. ICUS images showed correct stents expansion and apposition over the full length with minimum lumen diameter (MLD) of 3.0 × 3.2 mm, minimal lumen area (MLA) of 7.5 mm2 (Fig. 2b) and 30% of residual stenosis. Glycoprotein IIb/IIIa receptor antagonist eptifibatide was delivered as intravenous continuous infusion for about 24 h. After the procedure, clinical parameters were ameliorated and the patient was asymptomatic. The patient was discharged three days after in good condition and under optimal medical treatment including dual antiplatelet therapy, statin, beta blocker, and angiotensin-converting enzyme inhibitor.
Fig. 2.
(a) Final angiographic appearance after thrombus removal. (b) Intracoronary ultrasound appearance of stents.
Discussion
Reports of cardiovascular symptoms and signs associated with allergic, hypersensitivity, anaphylactic, or anaphylactoid reactions started to appear in medical literature many decades ago. However, only in 1991 did Kounis and Zavras describe the “allergic angina syndrome” as coronary spasm progressed to acute myocardial infarction [4]. The ischemia in allergic reaction is secondary to the release of inflammatory mediators, including histamine, tryptase, chymase, platelet-activating factor, cytokines, and prostaglandins, and leukotriene synthesis, which leads to coronary vasospasm and plaque rupture [3].
Currently, three different Kounis syndrome subtypes are described. Type I, accounts for 72.6% of KS cases; in this variant, the release of inflammatory mediators induces coronary artery spasm with or without increase of cardiac enzymes. In type II variant (22.3%), the release of inflammatory mediators induces coronary artery spasm together with plaque erosion or rupture. Type III variant accounts for only 5.1% of KS cases and it includes patients with coronary artery stent thrombosis as a result of an allergic reaction, with histologically demonstrated presence of mast cells and eosinophils in the context of aspirated thrombus [5], [6].
Stent thrombosis is a dramatic complication of coronary stenting, presenting as sudden death or acute myocardial infarction. Based on the elapsed time since stent implantation, stent thrombosis can be classified as: early (0–30 days post stent implantation); late (>30 days); very late (>12 months). Early stent thrombosis is further subdivided into acute (<24 h) and subacute (1–30 days) [7].
To date, multiple cases of KS have been reported in the literature. Most implied allergens able to trigger KS include antibiotics or anti-inflammatory drugs, but also environmental factors or food [8]. Recently, Tzanis et al. described a curious case of early stent thrombosis (4 days) following allergic reaction due to food consumption in a 70-year-old man [9]. Similarly, Michas et al. reported a case of ST elevation myocardial infarction due to stent thrombosis in the setting of an allergic reaction associated with mushroom consumption [10].
To the best of our knowledge, we describe the first case of acute stent thrombosis (<24 h) due to an allergic reaction. In all likelihood, the agent triggering the reaction was the contrast media employed during coronary angiography.
As known, the causes of acute stent thrombosis can be local or systemic. The first group include stent under-expansion, malapposition, and vessel tortuosity. These eventualities were excluded with ICUS that showed correct stent apposition and expansion. Systemic factors include hypercoagulable state, cancer, or use of chemotherapeutic agents. However, none of these was present in our patient, and for this reason we concluded that acute stent thrombosis was a consequence of the allergic reaction.
Unfortunately, histological examination (to detect eosinophil infiltrates) of thrombus was not available, and it is difficult to give a sure diagnosis. However, in our opinion, the close temporal correlation between allergic reaction, sudden stent thrombosis, and angiography images strongly indicate type 3 KS with a high degree of certainty.
In conclusion, as KS is often an under-diagnosed disease in daily clinical practice, our experience may help clinicians to maintain a high index of suspicion of Kounis syndrome in patients with acute coronary syndrome accompanied by symptoms of anaphylaxis.
Acknowledgments
None.
Acknowledgments
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Abdelghany M., Subedi R., Shah S., Kozman H. Kounis syndrome: a review article on epidemiology, diagnostic findings, management and complications of allergic acute coronary syndrome. Int J Cardiol. 2017;232:1–4. doi: 10.1016/j.ijcard.2017.01.124. [DOI] [PubMed] [Google Scholar]
- 2.Fassio F., Losappio L., Antolin-Amerigo D., Peveri S., Pala G., Preziosi D. Kounis syndrome: a concise review with focus on management. Eur J Intern Med. 2016;30:7–10. doi: 10.1016/j.ejim.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Biteker M. Current understanding of Kounis syndrome. Expert Rev Clin Immunol. 2010;6:777–788. doi: 10.1586/eci.10.47. [DOI] [PubMed] [Google Scholar]
- 4.Kounis N.G. Kounis syndrome: an update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin Chem Lab Med. 2016;54:1545–1559. doi: 10.1515/cclm-2016-0010. [DOI] [PubMed] [Google Scholar]
- 5.Sciatti E., Vizzardi E., Cani D.S., Castiello A., Bonadei I., Savoldi D. Kounis syndrome, a disease to know: case report and review of the literature. Monaldi Arch Chest Dis. 2018;88:898. doi: 10.4081/monaldi.2018.898. [DOI] [PubMed] [Google Scholar]
- 6.Kounis N.G. Coronary hypersensitivity disorder: the Kounis syndrome. Clin Ther. 2013;35:563–571. doi: 10.1016/j.clinthera.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Claessen B.E., Henriques J.P., Jaffer F.A., Mehran R., Piek J.J., Dangas G.D. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv. 2014;7:1081–1092. doi: 10.1016/j.jcin.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Kounis N.G., Giannopoulos S., Soufras G.D., Kounis G.N., Goudevenos J. Foods, drugs and environmental factors: Novel Kounis syndrome offenders. Intern Med. 2015;54:1577–1582. doi: 10.2169/internalmedicine.54.3684. [DOI] [PubMed] [Google Scholar]
- 9.Tzanis G., Bonou M., Mikos N., Biliou S., Koniari I., Kounis N.G. Early stent thrombosis secondary to food allergic reaction: Kounis syndrome following rice pudding ingestion. World J Cardiol. 2017;9:283–288. doi: 10.4330/wjc.v9.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michas G., Stougiannos P., Thomopoulos T., Grigoriou K., Blazakis G., Kaplanis I. Acute anterior myocardial infarction due to stent thrombosis after mushroom consumption: a case of Kounis type III syndrome. Hellenic J Cardiol. 2017;58:378–380. doi: 10.1016/j.hjc.2016.12.007. [DOI] [PubMed] [Google Scholar]