Abstract
Avocado oil, which has a high content of monounsaturated fatty acid and health-beneficial phytochemicals, is consumed in salads and also can be used for cooking. Therefore, is essential to study its oxidative and photochemical stability under different temperatures. So this work aimed to evaluate the oil oxidation and the phytochemical degradation of avocado oil under three different temperatures: room, 100 °C and 180 °C. The oil oxidation was evaluated by peroxide value and specific extinction in ultraviolet. The phytochemical degradation was evaluated for phytosterol, chlorophylls, and carotenoids contents. The temperature was found to significantly influence the oil oxidation and phytochemical stability, with the oxidation/degradation rate constants increasing with temperature. At room temperature, all oxidative parameters increased linearly with time, indicating a zero-order kinetic. At 100 and 180 °C, peroxide value, K232 and K270 increased linearly at a higher rate, becoming constant or decreasing after a short reaction time. The activation energy from specific extinction at 270 nm curves was 17.74 kcal mol−1 for oil degradation. For phytochemical compounds, the mechanism of reactions depended on the temperature, in which the reaction orders increased with heating. The activation energies for carotenoids, chlorophylls and sterols degradations at high temperatures were 5.00, 6.93, and 4.48 kcal mol−1, respectively. In this way, we found that avocado oil has its stability and quality affected by temperature, and, therefore, is not indicated for use in long and/or successive heating processes.
Keywords: Persea americana, Lipid, Stability, Heating, Storage
Introduction
The world production of avocado oil is estimated at thousands of tons per year, with significant production in countries of the Americas, Asia–Pacific, and Africa. Production and marketing are only occurring in the twenty-first century, and published information about the product is still limited (Berasategi et al. 2012).
Avocado oil can be extracted from the mesocarp of avocado fruit using the cold method and has physicochemical properties similar to those of olive oil (Tango et al. 2004). Avocado oil can be used as an ingredient in functional foods because of its high content of monounsaturated fatty acid and minor components with health-beneficial and relevant physiological activities, like antioxidants and hypocholesterolemic effects (Berasategi et al. 2012).
Several health benefits are related to compounds found in avocado oil. A high concentration of oleic acid is related to a decrease in low-density lipoprotein (LDL)-cholesterol levels and cardiovascular risk without decreasing high-density lipoprotein (HDL)-cholesterol or triglycerides levels (Lottenberg 2009). Phytosterols, which are triterpene compounds with structures similar to cholesterol, have been shown to decrease LDL-cholesterol levels in addition to anticancer, anti-inflammatory, antiatherogenic, and antioxidative activities (Berger et al. 2004). Chlorophylls and carotenoids contribute to the oil color and are also related to relevant bioactivities. Carotenoids have antioxidant properties that have been associated with cell protective mechanisms and the regulation of cell growth, differentiation, and apoptosis (Unlu et al. 2005). Meanwhile, chlorophylls have been shown to have positive effects on inflammation, anemic processes, blood purification, and cancer prevention, among other bioactivities (Ínanç 2011). These pigments also play significant roles in the oxidative stability of the oil due to their antioxidant nature (Ayadi et al. 2009).
Vegetable oils, such as avocado oil, are mainly consumed raw in salads, but can also be used in cooking at high temperatures. This heating can promote the oxidation and degradation of bioactive compounds, such as unsaturated fatty acids, phytosterols, and antioxidants. The magnitude of deterioration depends on the conditions of the culinary process, including temperature, time, type of oil, and type of processed food (Kmiecik et al. 2009; Lampi et al. 2002; Rudzinska et al. 2005). A comparative study showed that the stability of avocado oil during heat treatment was similar to that of olive oil concerning the degradation of fatty acids, phytosterols, and vitamin E (Berasategi et al. 2012). Similarly, deterioration can also occur during the oil storage. Crude avocado oil is highly sensitive to oxidation when exposed to daylight, in contrast to its stability in the dark at room temperature. Furthermore, the chlorophyll content in crude avocado oil decreases rapidly with exposure to daylight but can act as an antioxidant in the dark (Werman and Neeman 1986).
Kinetic studies to understand the effects of temperature on fatty acid oxidation and the degradation of bioactive compounds of vegetable oils are fundamental. The obtained data and calculated kinetic parameters provide a better understanding of the quality of a product under storage conditions and extreme processing temperatures (Teixeira Neto et al. 2004). According to Patras et al. (2011), kinetic modeling is often used to assess food safety objectively, quickly, and economically, and can also be employed to predict the influence of processing on critical quality parameters.
The aim of this work was to evaluate the changes related to quality of avocado oil during storage at room temperature (25 °C) and under heating at mild (100 °C) and drastic (180 °C) conditions considering oxidative quality indices (peroxide value and specific extinction in ultraviolet) and phytochemical contents (phytosterols, chlorophylls, and carotenoids).
Materials and methods
Samples
Avocado of the Hass cultivar, grown in the micro-region of Serra da Mantiqueira, Minas Gerais, Brazil, were used in this study. High-quality fruits (minimal levels of rots and physiological disorders) were sanitized in 2.5% chlorinated water. For complete removal of residues from the sanitization, the fruits were washed in abundance with potable water. Then, the mesocarp was separated from the skin and seed and malaxated at 34 °C for 60 min. The oil was then extracted using an Abencor® extraction system (Suárez et al. 1975), which is divided into three stages: crushing, thumping and spinning. The oil was packaging on amber glasses, refrigerated (4 °C) and protected from light before experiments. The avocado fruits and the extraction equipment used in this study are also used for commercial purposes; so the avocado oil obtained is equivalent to the commercial product sold in the market. The obtained avocado oil was limpid and greenish, with a characteristic avocado flavor, a free acidity of 0.5%, and a peroxide value of 8.9 meqO2 kg−1, which can be classified as virgin oils according to Codex Alimentarius (Codex Alimentarius 1999).
Chemicals
All reagents and solvents were purchased as analytically pure from Vetec (Sigma Aldrich) and used without further purification. The cholesterol standard was purchased from Sigma Aldrich.
Experimental conditions
The oils were subjected to three different conditions: 180 °C for 8 h (analysis interval of 2 h), 100 °C for 16 h (analysis interval of 4 h), and room temperature for 180 days (analysis interval of 45 days). Similar to other studies on oil stability under storage (Shim and Lee 2011; Mancebo-Campos et al. 2008; Gómez-Alonso et al. 2004), the samples (30.0 g) were placed in closed amber glass bottles (100 ml capacity, 45 mm diameter and 75 mm height, surface area exposed to the atmosphere of 15.9 cm2) protected from light by aluminum foil and submitted to thermal treatment in an oven at a pre-stabilized temperature. Oxidative parameters and phytochemical contents were determined in triplicate at each time interval.
Peroxide and specific extinction values
Peroxide value (PV) and specific extinction coefficients were determined according to AOAC methods (1998).
For PV determination, oil (5 g) was weighed in a 250-mL flask. Acetic acid–chloroform solution (30 mL), saturated potassium iodide (0.5 mL), and distilled water (30 mL) were then added with occasional agitation. The solution was titrated with 0.1 mol L−1 Na2S2O3, 1% starch solution (0.5 mL) was added, and titration was continued with vigorous agitation until the dark color (from iodine) disappeared. The PV was determined using the Eq. 1 above:
| 1 |
where S is the volume of Na2S2O3 solution used (mL; blank corrected), and M is the concentration of the Na2S2O3 solution (mol L−1).
The specific UV absorptivity, determined using a UV–Vis spectrophotometer by measuring the absorbance of a 1% solution in cyclohexane at 232 and 270 nm with a path length of 1 cm, was calculated using Eq. 2:
| 2 |
where Aλ is the absorption at wavelength λ, c is the oil concentration in the solution (g 100 mL−1), L is the cell length (cm), and Kλ is the specific absorptivity at wavelength λ.
Sterols
The total sterol (TS) content was determined with a UV–Vis spectrophotometer using the Lieberman–Burchard reaction (Araujo et al. 2013; Sabir et al. 2003). Standard cholesterol solutions of 1–20 mg g−1 were prepared in chloroform. For Lieberman–Burchard reagent preparation, acetic anhydride (10 mL) was placed in an ice bath for 30 min, and sulfuric acid (1 mL) was added. Reading samples were prepared by adding the Lieberman–Burchard reagent (0.8 mL) to the sample (0.1 g) or standard solution in chloroform (3.1 mL) and allowing to stand for 12 min. The solution was analyzed using a spectrophotometer at 625 nm, with chloroform used as the blank.
Chlorophylls and carotenoids
The total chlorophyll (TCh) content, determined using a UV–Vis spectrophotometer according to the method described by AOCS (1998) based on absorbances at 630, 670, and 710 nm, was calculated using the following equation:
| 3 |
where A is the absorbance of the oil at the chosen wavelength and L is the path length (cm).
The total carotenoid (TCa) content was determined at 445 nm using a UV–Vis spectrophotometer according to the procedure of Mba et al. (2017), wherein the oil (0.5 g) was dissolved in n-hexane (2 mL), with n-hexane used as the blank. The TCa content was calculated using Eq. 4:
| 4 |
where A is the absorbance at 445 nm, v is the solution volume (mL), and w is the sample weight (g).
Kinetics calculations
In order to standardize units and comparisons in the kinetics models, each measurement was expressed as , which regressed with time according to the models below:
| 5 |
| 6 |
| 7 |
where Eqs. 5, 6, and 7 are for zero-, first-, and second-order models, respectively; [At] is the value of evaluated parameter A at time t, [A0] is the initial value of parameter A at t = 0, and k is the rate constant of the process (Marangoni 2017).
The kinetic order for each parameter was determined by best fit according to the coefficient of determination (R2).
The activation energy (Ea) was calculated using the Arrhenius approach, in which the regression of ln(k) against 1/T resulted in the slope Ea/R, where R = 1.98 cal mol−1 K−1 and T is the average temperature (K). When the number of experimental data was reduced, integrated form of Arrhenius equation was applied.
All curve fittings were performed in SciDavis software (version 1.22) using the scaled Levenberg–Marquardt algorithm.
Results and discussion
Fatty acid oxidation
The oxidative degradation of fatty acids in vegetable oils is a complex process leading to various decomposition products. These oxidative processes, known as autoxidation, occur slowly at room temperature but are accelerated with heating (Gutiérrez et al. 2002; Choe and Min 2007). In general, autoxidation involves the initial formation of hydroperoxides from unsaturated fatty acids (induction stage), which are then decomposed to secondary oxidation products, such as aldehydes, ketones, esters, and carboxylic acids (Bendini et al. 2009). The level of oxidative degradation of fatty acids can be determined using physicochemical parameters, such as peroxide value (PV), and absorptivities at 232 nm (K232) and 270 nm (K270). The PV is related to the amount of peroxide, expressed as milliequivalents (1 milliequivalent (meq) = 0.5 mmol) of O2 per kilogram of oil, and provides initial evidence of autoxidation. K232 is related to intermediate oxidation stage, while K270 is related to carbonyl compounds formed in the secondary oxidation stage due to hydroperoxide degradation (Zaid et al. 2013).
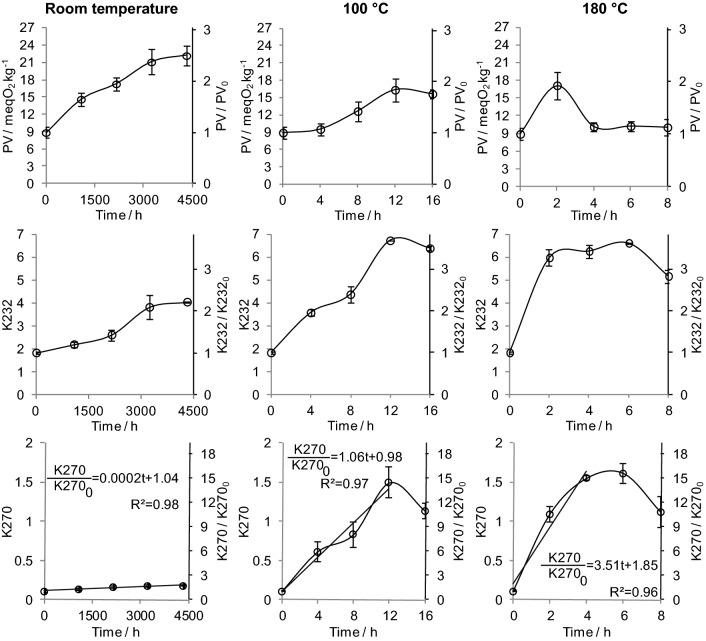
Changes in the oxidative parameters of the avocado oil at room temperature, 100, and 180 °C are shown in Fig. 1. At room temperature, all oxidative parameters increased linearly with time during a time interval of 4500 h, indicating a zero-order kinetic. At the higher temperatures, PV, K232, and K270 increased linearly at a higher rate (the slopes of the model graphs), becoming constant or decreasing before only 12 h of reaction. Interestingly, whereas the attained maximum values of K232 and K270 were higher at 100 and 180 °C than at room temperature, PV magnitudes were around 5 meqO2 kg−1 lower at the higher temperatures. These distinct behaviors of the oxidative parameters versus time curves suggested that the increase of the temperature changed the mechanisms of reaction and/or the relative magnitude of the rate constants for the steps of the oxidative degradation mechanism.
Fig. 1.
Variation in oxidation parameters for avocado oil at room temperature, 100 °C, and 180 °C
The formation of carbonyl compounds in secondary oxidation stage, indicated by the increase of K270 values, comes from complex consecutive reactions that involve as precursors the fatty acids. In the intermediary steps, fatty acids yield peroxides and unstable hydroperoxides as primary products. Because of this, the change of PV and K232 values with time reflect a combined rate of formation and consume of intermediary oxidation products, making the kinetic of avocado oil oxidation very complex. Thus, the application of kinetic models to the PV and K232 curves could only provide apparent rate constants. Additionally, the apparent rate constants for the formation of intermediaries (peroxide and hydroperoxides) depend on the order of reactions in the steps of the mechanism, which is unknown. Therefore the zero-, first-, and second-order models cannot be applied.
Although the complexity of the kinetics of formation and consumption of the intermediary products, analysis of the evolution of the PV and K232 with time at the different temperatures can provide relevant information about the oil degradation. At room temperature, these values suggested that avocado oil remained in the induction stage for 180 days. The lower PV level at 100 °C compared with that at room temperature can be attributed to increased hydroperoxide degradation at 100 °C, as indicated by K270 increase, which also explained the slight decrease in K232 at 16 h. A similar result has been reported by Allouche et al. (2007) that observed lower PV in olive oils heated at 180 °C than in unheated oils due to hydroperoxide degradation.
Faster evolution of oxidation was observed at 180 °C, as indicated by the large PV and K232 values at 2 h (large slopes in the curves when compared with the smaller temperatures). The PV decreased after 2 h, while K232 decreased after 6 h, coinciding with a large K270, which indicated a large formation of hydroperoxide degradation products after 2 h at 180 °C. A slight decrease in K270 was observed at the end of heat treatment, probably due to the degradation or evaporative loss of secondary oxidation products, as expected for oils heated at elevated temperatures for long periods (Choe and Min 2007). Similar K232 and K270 behaviors with time have been reported by Allouche et al. (2007) for olive oils heated at 180 °C. Ayadi et al. (2009) also reported a faster increase in K270 for olive oils at elevated temperatures due to the formation of large amounts of hydroperoxide degradation products.
The temperature effect on the oxidation rate has been reported for avocado oil, with PV increasing from approximately 4–10 meqO2 kg−1 in 100 days at room temperature and less than 10 days at 60 °C (Werman and Neeman 1986). The PV, K232, and K270 have been shown to increase faster at 130 °C than at 60 °C in olive oils flavored with aromatic plants (Ayadi et al. 2009). Berasategi et al. (2012) also noted extensive oxidation of avocado and olive oils at 180 °C, reporting high levels of products from hydroperoxide oxidation after 6 h of heating.
Regarding the K270 versus time curves, in which the oxidation parameter reflected the formation of the final oxidation products, we can evaluate the kinetic parameters by applying the zero-order model. The apparent rate constants were obtained for all temperatures, and the Arrhenius model was used to estimate the activation energies (Ea) for the complete oxidation of the oil. It should be highlighted that at 100 and 180 °C, the zero-order model was applied from 0 to 12 and 4 h, respectively. For these times, the degradation or evaporative loss of secondary oxidation products were not high enough to determine the K270 values evolution.
The rate constants obtained from K270 curves (slopes of equations in Fig. 1) increased 5300 and 3.3-fold when the temperature was increased from room temperature to 100 °C, and from 100 to 180 °C, respectively, indicating an increase of the level of formation of hydroperoxide degradation products. The Ea value was 17.74 kcal mol−1 (R2 = 0.91), suggesting that the oil was slightly susceptible to thermal deterioration. Onoji et al. (2016), for instance, found activation energy of only 13.07 kJ mol−1 (3.12 kcal mol−1) for degradation of Hevea brasiliensis (rubber seed) Oil.
Phytochemical degradation
The contents of bioactive compounds can vary according to cultivar, season, and processing conditions (Cercaci et al. 2007; Li et al. 2007). The initial total contents of sterols (6.4 mg g−1), chlorophylls (16.7 mg kg−1), and carotenoids (4.9 mg kg−1) were in accordance with values reported in the literature for avocado, olive, and other vegetable oils (Ashton et al. 2006; Aparicio-Ruiz and Gandul-Rojas 2014; Dutta and Appelqvist 1996; Berasategi et al. 2012; Ayadi et al. 2009).
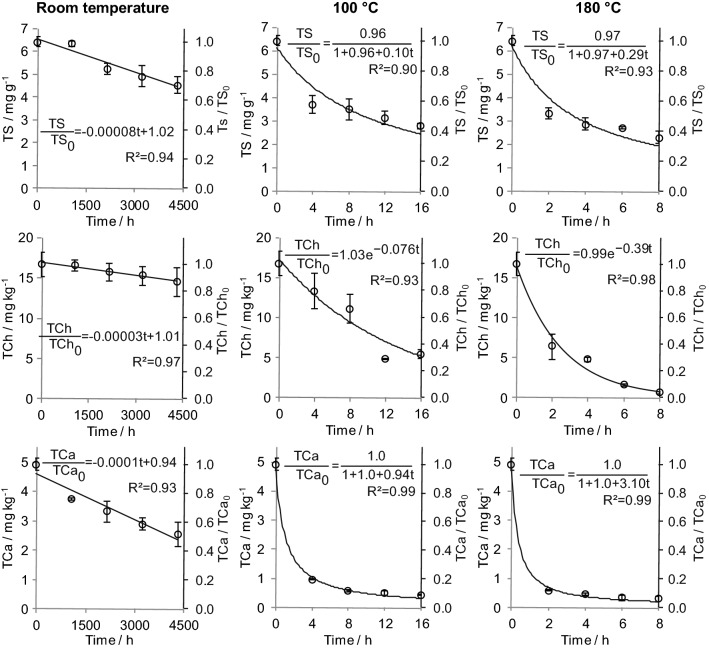
Changes in sterols, chlorophylls, and carotenoids contents in avocado oil with time at room temperature, 100 °C, and 180 °C are shown in Fig. 2. The contents of all the compounds decreased with time, and the profile of the curves was dependent on temperature. A linear decrease in the concentration of all bioactive compounds was observed at room temperature, suggesting kinetics of zero-order for the consumption of them. However, the degradation processes under heating were concentration-dependent, with sterols and carotenoids exhibiting second-order degradation at 100 °C and 180 °C, while chlorophylls degraded according to a first-order model. The values of the rate constants obtained for each condition evaluated can be accessed from Fig. 2. However, because the change in the order of the reactions with heating indicates that the temperature increase modified the mechanism of the reactions, the magnitudes of the constants cannot be compared to each other for the same bioactive compound, excepted for those reactions presenting the same order, i.e., at 100 and 180 °C. In this way, the rate constants increased 2.9-fold for sterol degradation, 5.1-fold for chlorophylls, and 3.3-fold for carotenoids when the temperature changed from 100 to 180 °C.
Fig. 2.
Curves for the degradation of bioactive compounds in avocado oil at room temperature, 100 °C, and 180 °C. TS, total sterols; TCh, total chlorophylls; TCa, total carotenoids
Similar to fatty acids, the oxidative degradation of sterols usually follows a free radical mechanism, and free radicals from these reactions may stimulate oxidative degradation (Rudzinska et al. 2014; Lengyel et al. 2012). However, natural antioxidants, such as carotenoids, can be preferentially oxidized over sterols, increasing their stability and contributing to a low level of degradation, even under severe conditions (Winkler et al. 2007). In contrast, higher carotenoid sensitivity has been observed (Ayadi et al. 2009), in which the presence of oxygen is a crucial factor in their degradation, even at low oxygen concentrations (Gross 1991). Furthermore, the presence of free radicals might also accelerate the carotenoid degradation rate. Therefore, the oxidation of carotenoids depends on the simultaneous oxidation of unsaturated fatty acids, which are oxidized during the initial stages, with their oxidation products contributing to carotenoid oxidation (Criado et al. 2008). Thus, the presence of oxygen and free radicals, especially from fatty acid oxidation, could explain the sharp decrease in carotenoid contents after a short heating period.
Regarding the susceptibility of the different bioactive compounds to degradation, carotenoids were more sensitive than chlorophylls and sterols at room temperature, as indicated by the higher rate constant. This behavior was also reported by Ayadi et al. (2009). At 100 and 180 °C, because of the different order of the reaction involving the degradation processes of the bioactive compounds, the comparison between the rate constants cannot indicate which of them degraded faster. However, as the carotenoids degradation followed a second-order model, its content had almost reached almost zero after 8 h at 100 °C and 2 h at 180 °C, indicating a faster degradation of this compound at these temperatures. This phenomenon has also been reported for avocado oil heated at 180 °C for 3 h and 9 h (Berasategi et al. 2012), and for different vegetable oils during frying (Dutta and Appelqvist 1996).
A parameter that can indicate which bioactive compound is the most sensitive to temperature increase is the activation energy for the reaction of degradation. We cannot apply the Arrhenius equation using the rate constants obtained at all temperature to calculate the Ea because the reaction mechanism changed when temperature increased from room temperature to 100 °C. Then, we used the integrated form of the Arrhenius equation to determine the Ea associated with the reaction mechanisms occurring at high temperatures. It should be highlighted that at temperatures below 100 °C, our data could not provide a more detailed kinetic description of the degradation process of the compounds.
The activation energy (Ea) for degradation of bioactive compounds at elevated temperatures are shown in Table 1. The Ea was higher for chlorophylls than sterols and carotenoids, indicating that the degradation of this pigment was more sensitive to temperature change. Aparicio-Ruiz and Gandul-Rojas (2014), studying the discoloration kinetics of chlorophylls and carotenoids in olive oils from 60 to 120 °C, showed the same tendency for the Ea values in the degradation of the pigments. However, the discoloration processes in the olive oil were described by a first-order kinetic mechanism for both pigments, with Ea values (16.03 kcal mol−1 for chlorophylls and 15.45 kcal mol−1 for carotenoids) around three times higher than ours. These results indicated that the pigments were less susceptible to degradation thermal in the avocado oil than in the olive oil. This different kinetic behavior could be explained by the distinct temperature range evaluated and by the absence of oxygen in the studies of chlorophylls and carotenoids degradation in olive oils, which changed the determinant steps of the reaction rate and, consequently, the Ea values.
Table 1.
Activation energy based on the Arrhenius equation for the degradation of bioactive compounds from avocado oil at 100 and 180 °C
| Bioactive | Ea (kcal mol−1) |
|---|---|
| Total sterols | 4.48 |
| Total chlorophylls | 6.93 |
| Total carotenoids | 5.00 |
Conclusion
A kinetic study of avocado oil oxidation process at a temperature range from 25 to 180 °C was made from an evolution of PV, K232 and K270 parameters with time. The results revealed a complex mechanism of fatty acids oxidation and indicated that the avocado oil was slightly susceptible to thermal degradation. The kinetics of degradation of the chlorophylls, carotenoids, and sterols in the avocado oil was also evaluated, and the mechanisms of phytochemical degradation depended on temperature, as indicated by the increase in the order of the discoloration reactions during the heating. Regarding the phytochemical stability, chlorophylls were the most sensitive to temperature increase, followed by carotenoids and sterols.
Acknowledgements
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília, Brazil) by granting the scholarship and the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG, Belo Horizonte, Brazil) by financial support for this research (CAG - APQ-00638-14 and CAG - PPM-00498-16 FAPEMIG).
Contributor Information
Lívia Maria Braga Resende, Email: liviabrr@gmail.com.
Vanessa Rios de Souza, Email: vanessa.souza@dca.ufla.br.
Guilherme Max Dias Ferreira, Email: guilherme.ferreira@dqi.ufla.br.
Cleiton Antônio Nunes, Phone: +55(35)2142-2094, Email: cleiton.nunes@dca.ufla.br.
References
- Allouche Y, Jimenez A, Gaforio JJ, Uceda M, Beltran G. How heating affects extra virgin olive oil quality indexes and chemical composition. J Agric Food Chem. 2007;55:9646–9654. doi: 10.1021/jf070628u. [DOI] [PubMed] [Google Scholar]
- AOCS-The American Oil Chemists’ Society. (1998) Official methods and recommended practices of the American oil chemists’ society. (5th ed.). Press, Champaign (Cc 13i—96)
- Aparicio-Ruiz R, Gandul-Rojas B. Decoloration kinetics of chlorophylls and carotenoids in virgin olive oil by autoxidation. Food Res Int. 2014;65:199–206. doi: 10.1016/j.foodres.2014.05.046. [DOI] [Google Scholar]
- Araújo LBDC, Silva SL, Galvão MAM, Ferreira MRA, Araújo EL, Randau KP, Soares LAL. Total phytosterol content in drug materials and extracts from roots of Acanthospermum hispidum by UV–VIS spectrophotometry. Braz J Pharmacogn. 2013;23:736–742. doi: 10.1590/S0102-695X2013000500004. [DOI] [Google Scholar]
- Ashton OBO, Wong M, McGhie TK, Vather R, Wang Y, Requejo-Jackman C, Ramankutty P, Woolf AB. Pigments in avocado tissue and oil. J Agric Food Chem. 2006;54:10151–10158. doi: 10.1021/jf061809j. [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists-AOAC . Official methods of analysis. 16. Washington: AOAC; 1998. [Google Scholar]
- Ayadi MA, Grati-Kamoun N, Attia H. Physico-chemical change and heat stability of extra virgin olive oils flavoured by selected Tunisian aromatic plants. Food Chem Toxicol. 2009;47:2613–2619. doi: 10.1016/j.fct.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Bendini A, Valli E, Cerretani L, Chiavaro E, Lercker G. Study on the effects of heating of virgin olive oil blended with mildly deodorized olive oil: focus on the hydrolytic and oxidative state. J Agric Food Chem. 2009;57:10055–10062. doi: 10.1021/jf901813s. [DOI] [PubMed] [Google Scholar]
- Berasategi I, Barriuso B, Ansorena D, Astiasarán I. Stability of avocado oil during heating: comparative study to olive oil. Food Chem. 2012;132:394–446. doi: 10.1016/j.foodchem.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Berger A, Jones P, Abumweis S. Plant sterols: factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis. 2004;3(1):1–19. doi: 10.1186/1476-511X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cercaci L, Passalacqua G, Poerio A, Rodriguez-Estrada MT, Lercker G. Composition of total sterols (4-desmethyl-sterols) in extravirgin olive oils obtained with different extraction technologies and their influence on the oil oxidative stability. Food Chem. 2007;102(1):66–76. doi: 10.1016/j.foodchem.2006.01.062. [DOI] [Google Scholar]
- Choe E, Min DB. Chemistry of deep-fat frying oils. J Food Sci. 2007;72(5):R77–R86. doi: 10.1111/j.1750-3841.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- Codex Alimentarus (1999) Codex standard for edible fats and oils not covered by individual standards. CODEX STAN 19-1981, Rev. 2-1999. Codex Alimentarius, Rome, Italy
- Criado MN, Romero MP, Casanovas M, Motilva MJ. Pigment profile and colour of monovarietal virgin olive oils from Arbequina cultivar obtained during two consecutive crop seasons. Food Chem. 2008;110:873–880. doi: 10.1016/j.foodchem.2008.02.075. [DOI] [PubMed] [Google Scholar]
- Dutta PC, Appelqvist LA. Sterols and sterol oxides in the potato products, and sterols in the vegetable oils used for industrial frying operations. Grasas Aceites. 1996;47:38–47. doi: 10.3989/gya.1996.v47.i1-2.841. [DOI] [Google Scholar]
- Gómez-Alonso S, Mancebo-Campos V, Salvador MD, Fregapane G. Oxidation kinetics in olive oil triacylglycerols under accelerated shelf-life testing (25–75 C) Eur J Lipid Sci Technol. 2004;106(6):369–375. doi: 10.1002/ejlt.200300921. [DOI] [Google Scholar]
- Gross J. Pigments in vegetables: chlorophylls and carotenoids. 1. New York: Van Nostrand Reinhold; 1991. [Google Scholar]
- Gutiérrez F, Villafranca MJ, Castellano JM. Changes in the main components and quality indices of virgin olive oil during oxidation. J Am Oil Chem Soc. 2002;79(7):669–676. doi: 10.1007/s11746-002-0541-3. [DOI] [Google Scholar]
- Ínanç AL. Chlorophyll: structural properties, health benefits and its occurrence in virgin olive oils. Akad Gida Acad Food J. 2011;9(2):26–32. [Google Scholar]
- Kmiecik D, Korczak J, Rudzinska M, Gramza-Michalowska A, Hes M. Stabilization of phytosterols in rapeseed oil by natural antioxidants during heating. Eur J Lipid Sci Technol. 2009;111(11):1124–1132. doi: 10.1002/ejlt.200800304. [DOI] [Google Scholar]
- Lampi AM, Juntunen L, Toivo J, Piironen V. Determination of thermo-oxidation products of plant sterols. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:83–92. doi: 10.1016/S1570-0232(02)00094-6. [DOI] [PubMed] [Google Scholar]
- Lengyel J, Rimarcík J, Vagánek A, Fedor J, Lukeš V, Klein E. Oxidation of sterols: energetics of C–H and O–H bond cleavage. Food Chem. 2012;133:1435–1440. doi: 10.1016/j.foodchem.2012.02.031. [DOI] [Google Scholar]
- Li TSC, Beveridge THJ, Drover JCG. Phytosterol content of sea buckthorn (Hippophae rhamnoides L.) seed oil: extraction and identification. Food Chem. 2007;101(4):1633–1639. doi: 10.1016/j.foodchem.2006.04.033. [DOI] [Google Scholar]
- Lottenberg AMP. Importance of the dietary fat on the prevention and control of metabolic disturbances and cardiovascular disease. Arq Bras Endocrinol Metabol. 2009;53(5):595–607. doi: 10.1590/S0004-27302009000500012. [DOI] [PubMed] [Google Scholar]
- Mancebo-Campos V, Fregapane G, Salvador MD. Kinetic study for the development of an accelerated oxidative stability test to estimate virgin olive oil potential shelf life. Eur J Lipid Sci Technol. 2008;110(10):969–976. doi: 10.1002/ejlt.200800022. [DOI] [Google Scholar]
- Marangoni AG. Kinetic analysis of food systems. 1. Cham: Springer; 2017. [Google Scholar]
- Mba OI, Dumont MJ, Ngadi M. Thermostability and degradation kinetics of tocochromanols and carotenoids in palm oil, canola oil and their blends during deep-fat frying. LWT. Food Sci Technol. 2017;82:131–138. [Google Scholar]
- Onoji SE, Iyuke SE, Igbafe AI. Hevea brasiliensis (rubber seed) oil: extraction, characterization, and kinetics of thermo-oxidative degradation using classical chemical methods. Energy Fuels. 2016;30:10555–10567. doi: 10.1021/acs.energyfuels.6b02267. [DOI] [Google Scholar]
- Patras A, Brunton NP, Tiwari K, Butler F. Stability and degradation kinetics of bioactive compounds and colour in strawberry jam during storage. Food Bioprocess Technol. 2011;4(7):1245–1252. doi: 10.1007/s11947-009-0226-7. [DOI] [Google Scholar]
- Requejo AM, Ortega RM, Robles F, Navia B, Faci M, Aparicio A. Influence of nutrition on cognitive function in a group of elderly, independently living people. Eur J Clin Nutr. 2003;57:S54–S57. doi: 10.1038/sj.ejcn.1601816. [DOI] [PubMed] [Google Scholar]
- Rudzinska M, Korczak J, Wasowicz E. Changes in phytosterols and their oxidation products during frying of French fries in rapeseed oil. Pol J Food Nutr Sci. 2005;14:381–387. [Google Scholar]
- Rudzinska M, Przybylski R, Wasowicz E. Degradation of phytosterols during storage of enriched margarines. Food Chem. 2014;142:294–298. doi: 10.1016/j.foodchem.2013.07.041. [DOI] [PubMed] [Google Scholar]
- Sabir SM, Hayat I, Gardezi SDA. Estimation of sterols in edible fats and oils. Pak J Nutr. 2003;2:178–181. doi: 10.3923/pjn.2003.178.181. [DOI] [Google Scholar]
- Shim SD, Lee SJ. Shelf-life prediction of perilla oil by considering the induction period of lipid oxidation. Eur J Lipid Sci Technol. 2011;113(7):904–909. doi: 10.1002/ejlt.201000325. [DOI] [Google Scholar]
- Suárez JMM, Aranda M, Mendoza J, Rey AL. Informe sobre utilización del analizador de rendimentos “Abencor”. Grasas Aceites. 1975;26:379–385. [Google Scholar]
- Tango JS, Carvalho CRL, Soares NB. Physical and chemical characterization of avocado fruits aiming its potencial for oil extraction. Rev Bras Frutic. 2004;26(1):17–23. doi: 10.1590/S0100-29452004000100007. [DOI] [Google Scholar]
- Teixeira Neto RO, Vitali AA, Moura SCSR. Introdução à cinética de reação em alimentos. In: Moura SCSR, editor. Reações de transformação e vida-de-prateleira de alimentos processados. Campinas: ITAL; 2004. pp. 63–83. [Google Scholar]
- Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr. 2005;135(3):431–436. doi: 10.1093/jn/135.3.431. [DOI] [PubMed] [Google Scholar]
- Werman MJ, Neeman I. Oxidative stability of Avocado Oil. J Am Oil Chem Soc. 1986;63:355–360. doi: 10.1007/BF02546046. [DOI] [Google Scholar]
- Winkler JK, Warner K, Glynn MT. Effect of deep-fat frying on phytosterol content in oils with differing fatty acid composition. J Am Oil Chem Soc. 2007;84(11):1023–1030. doi: 10.1007/s11746-007-1138-1. [DOI] [Google Scholar]
- Zaid O, Houshia OJ, Abueid M, Zaid M. Palestinian Nabali-Baladi olive oil quality: premium ultra fine extra virgin olive oil classification. USA Res J. 2013;1(2):29–34. [Google Scholar]