Abstract
Phytochemicals and antioxidant potential of sixteen varieties of citrus comprising mandarins, limes, sweet orange and underutilized fruits were assessed. Limonoids, ascorbic acid and carotenoids significantly varies in the varieties. The antioxidant potential adjudged by evaluation with four accepted assays ABTS, DPPH, FRAP and TPC. Among them, Kachai lemon retains high antioxidant capacity with the assays DPPH (9.38 mM L−1 Trolox) and also recorded highest TPC (13.57 mM L−1 Trolox). Pomelo has shown a tremendous potential having the highest ABTS (4.49 mM L−1 Trolox) and FRAP (1.92 mM L−1 Trolox) activity, which reflects its potential at par with the grapefruit. Significant correlation has been found between DPPH and TPC, and also FRAP with TPC. It can be assumed that among citrus cultivar, Kachai lemon and Pomelo underutilized citrus fruit are showing enhanced potential to antioxidant capacity and can be exploited in terms of energy, nutrients and health supplements.
Keywords: Citrus fruits, Antioxidant activity, Phytochemicals, Ascorbic acid, Carotenoids
Introduction
Citrus is the general term for plants belonging to the family Rutaceae (Fejzic and Cavar 2014). Citrus fruits are one considered as the most traded and important horticultural crop with an overall production of about 80 million tonnes per year worldwide. Brazil, China, United States, Mexico, India and Spain are the topmost producers (Karoui and Marzouk 2013; Marti et al. 2009). The most common citrus fruits are mandarin (C. reticulata Blanco), sweet orange (C. sinensis Osbeck), lime (C. aurantifolia Christm), lemon (C. limon L. Burn. f.), pomelo (C. grandis Osbeck), sour orange (C. aurantium L.), citron (C. medica L.), and grapefruit (C. paradisi Osbeck) (Zarina and Tan 2013). Total citrus production in India accounts for about 12053 ‘000 MT in area of 1037’000 Ha. In India, the total agricultural yield of citrus fruits consists of mandarins (38.51%), sweet orange i.e. mosambi (26.47%), lime/lemons (21.25%) and others (13.86%) (NHB 2016–17).
Citrus fruits are among one of the important nutritive fruits which are grown and consumed throughout the world. There is an increasing demand of fruits rich in bioactive compounds (Vazquez et al. 2016). Citrus fruits and juices are rich sources of bioactive compounds, like flavonoids, carotenoids, limonoids, coumarin-related compounds, folates, essential oils, pectins and vitamin C (Marti et al. 2009). Furthermore, other compounds, such as sugars, potassium and pectin are also found in citrus fruits (Caro et al. 2004). The content of bioactive compounds in citrus juices depends on various factors such as genomic differences, climatic conditions, cultural practices, harvest time, industrial extraction systems and juice processing (Marti et al. 2009).
The health and disease preventing properties are attributed to the phytonutrient rich citrus fruits (Zarina and Tan 2013). The carotenoid is responsible for color of citrus fruit and its peel. Numerous health benefits have been attributed due to the presence of Phenolic acid, vitamin C and pectin in citrus fruits. Vitamin C, known as ascorbic acid, acts as a powerful antioxidant and may reduce the risk of cardiovascular diseases, arteriosclerosis, and some forms of cancer (Wang et al. 2007). The antioxidant compounds namely; phenolics, vitamins, flavonoids, anthocyanins, carotenoids and minerals scavenge the free radicals, reduces the level of oxidative stress and prevent the oxidation of biomolecules, that would break the reaction chains of pathogenesis in the deterioration of physiological functions, which could occur in the coronary heart diseases and cancer (Almeida et al. 2011).
Several researchers have focused on the quantification of phytochemicals of citrus varieties from the world. However, no more work has been reported on the study of antioxidant capacity and bioactive compounds of juice and peel of citrus varieties grown at its origin in India. The present study was carried out to analyze the antioxidant components and evaluate the various phytochemicals of citrus varieties, which can provide noticeable benefits to humans.
Materials and methods
Plant materials
The fruits including six varieties of mandarins (Citrus reticulata Blanco), five varieties of limes (Citrus aurantifolia Swingle), one from sweet orange (Citrus sinensis L. Osbeck), and four uncommon citrus fruits Kachai lemon (Citrus jambhiri Lush), citron (Citrus medica Linn.), pomelo (Citrus grandis L. Osbeck) and grapefruit (Citrus paradisi Macf.) were harvested and collected from mature trees between October, 2016 to February, 2017 from the states of Maharashtra, Punjab, West Bengal, Karnataka, Rajasthan and Nagaland. These citrus fruits were washed with running water to remove the surface contamination. Citrus fruits samples were peeled and juice was extracted using screw type juice extractor in case of mandarins and by hydraulic press in case of limes and lemons.
Reagents and standards
The standards of limonin, sugars, ascorbic acid, carotenoids (β-carotene), trolox, ABTS·+ (radical cation azino-bis [3-ethylbenzthiazoline-6-sulfonic acid]), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), 2, 4, 6-Tri (2- pyridyl)- s- triazine (TPTZ), and total phenols (Gallic acid) were purchased from Sigma–Aldrich (Mumbai, India). The other chemicals used in the study were of analytical grade.
Determination of ascorbic acid (vitamin C) content
Ascorbic acid content was determined using the 2, 6 dichlorophenol-indophenol titration method (Ranganna 1986). Ascorbic acid content was expressed as mg AA/100 mL of juice.
Determination of limonin content
The limonin content of juice samples were determined as per the method described by Wilson and Crutichfield (1968) and the value were expressed in parts per million (ppm).
Determination of browning index content
The method of Meydav et al. (1977) for the analysis of browning content was used. The results were expressed in optical density (O.D).
Determination of sugars
For total and reducing sugar content examination, the method of Miller (1972) was used and expressed in percent (%).
Determination of carotenoid content
The method of Ting and Rouseff (1986) was employed to determine the carotenoid composition of citrus fruits. Total carotenoid was expressed as β-carotene equivalents and results expressed in mg/100 mL of juice.
Antioxidant activity
The assay was performed by means of an automated microplate reader Tecan Infinite M200 Pro (Tecan Group Ltd, Switzerland) with 96-well plates.
ABTS·+ radical scavenging assay
The method used was the ABTS·+ (radical cation azino-bis [3-ethylbenzthiazoline-6-sulfonic acid]) decolourisation assay according to Mena et al. (2011).The assay is based on the ability of an antioxidant compound to quench the ABTS·+ relative to that of a reference antioxidant such as trolox. Results were expressed as mmol L−1 Trolox. All samples were analyzed in triplicate.
DPPH radical scavenging assay
The DPPH free radical-scavenging activity of juices was measured using the method described by Mena et al. (2011) where 2, 2-diphenyl-1-picrylhydrazyl radical was used as a stable radical. The electron donation ability was measured by bleaching of the purple colored solution of 2, 2-diphenyl-1-picrylhydrazyl radical (DPPH). Results were expressed as mmol L−1 Trolox. All samples were analyzed in triplicate.
FRAP assay
The FRAP assay (Ferric Reducing Ability of Plasma) was performed as previously described by Benzie and Strain (1996) with some modifications. In the FRAP assay, antioxidants present in the sample extract reduce Fe(III)-tripyridyltriazine complex to the blue ferrous form, which has an absorption maxima at 593 nm. The working FRAP reagent was prepared fresh on the day of analysis by mixing acetate buffer (300 mM), TPTZ solution, and ferric chloride solutions in the ratio 10:1:1. Diluted extract (2 μL) and FRAP reagent (250 μL) were put into each well. The absorbance at time zero and after 40 min was recorded at 593 nm. The calculated difference in absorbance is proportional to the ferric reducing/antioxidant power of the extract. For quantification, a calibration curve of trolox was prepared. The final results were expressed as mmol L−1 Trolox. Tests were carried out in triplicate.
Total phenols content (TPC)
The concentration of total phenols was measured by the method described by Singleton and Rossi (1965) with some modification. Total phenols content (TPC) was determined by the Folin–Ciocalteu method, adapted to a micro scale. In a 1.5 mL Eppendorf microtube, Milli-Q water (790 μL), sample (10 μL), and Folin–Ciocalteu reagent (50 μL) were added and vortexed to mix properly. After exactly 1 min, 20% solution of sodium carbonate (150 μL) were added, mixed well and again vortexed, and allowed to stand at room temp (23.5 °C) in the dark for 1 h (60 min). Absorbance was measured at 750 nm, and quantified using gallic acid as a standard. Total polyphenol was expressed as gallic acid equivalents (mg GAE L−1).
Statistical analysis
The results of this investigation are means of three replications. To verify the statistical significance of all parameters the values of mean ± standard deviation were calculated. The probability values (P) of < 0.01 and < 0.05 were adopted as statistically significant. An analysis of variance (ANOVA) and a multiple range test (Tukey’s HSD test) were carried out. Pearson correlation analysis was performed to correlate relationships between selected parameters.
Results and discussion
Sugar and browning index content
The sugar content of citrus cultivars was evaluated and is regarded as one of the major parameter in determining the fruit quality. During citrus fruit development, juice sacs obtain their sugar supply via the phloem through nonvascular cell-to-cell apoplastic transport. Sucrose is the major carbohydrate stored in the fruit (Canan et al. 2016). Among citrus cultivars, the highest content of total (7.40%), reducing (6.24%) and non-reducing (1.10%) sugar was found in Khasi mandarin and the lowest total (5.58%) and reducing (4.72%) sugar content was obtained in the underutilized citrus fruit i.e. Kachai lemon. The lowest content of non-reducing sugar of 0.38% was found in Darjeeling mandarin as depicted in Fig. 1. Kumar et al. (2013) reported that mandarins have the highest sugar content. Depending on the citrus sp., the total sugar content in juice could range from below 1% in some fruits to as high as 15% in case of oranges (Ranganna et al. 1983).
Fig. 1.
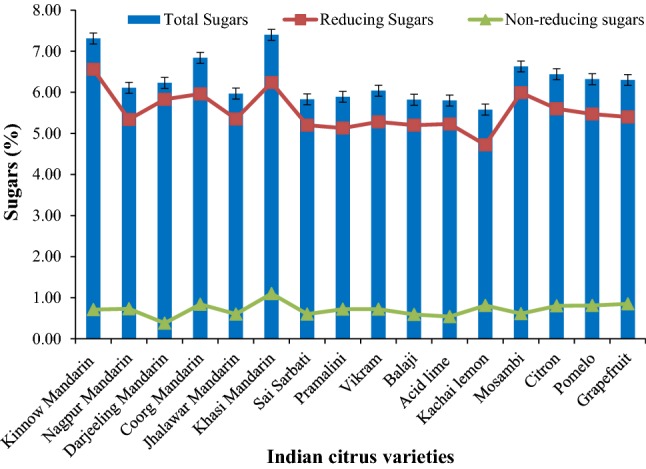
Total, reducing and non-reducing sugars in Indian citrus varieties
The browning index content is regarded as one of the detrimental chemical reaction for citrus juice quality problems (Bharate and Bharate 2014). In citrus juices evaluated, the browning index content ranged from 0.10 to 0.32 O. D (Fig. 2). Meydav et al. (1977) reported 0.08 and 0.06 O. D browning index content in orange and grapefruit juice respectively. According to Bharate and Bharate 2014, the non-enzymatic browning is caused due to reactions of sugars, amino acids and ascorbic acid.
Fig. 2.
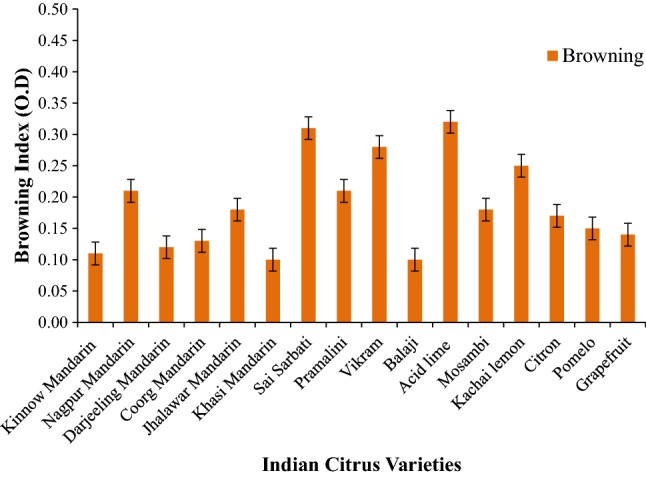
Browning index content of Indian citrus varieties
Limonin, ascorbic acid and carotenoid content of citrus varieties
Limonin which is responsible for delayed bitterness is one of the major limonoid commonly present in most citrus fruit juices (Hasegawa et al. 2000). In limonoids, the limonin content was estimated which varies from 9.08–13.81 ppm as depicted in Table 1. Ohta and Hasegawa (2006); Wattanasiritham et al. (2005) reported limonin content of 18 ppm concentration and in the range of 6.82–32.40 ppm in the juice of pomelo cultivars. Pichaiyongvongdee and Haruenkit (2009) reported the limonin content in juice ranged from 10.07–29.62 ppm among the seven pummelo cultivars studied. Considering the above results, the limonin content in pomelo was found in the same range. It is recommended that the variety having the lowest content of limonin should be used in juice processing industries.
Table 1.
Limonin, ascorbic acid and carotenoid content in citrus varieties
| Sr. no. | Samples | Limonin (ppm) | Ascorbic Acid (mg/100 mL) | Carotenoid (mg/100 mL) | |
|---|---|---|---|---|---|
| Peel | Juice | ||||
| A | Mandarin | ||||
| 1 | Kinnow Mandarin | 12.98ab ± 0.40 | 30.42f ± 0.82 | 3.28c ± 0.12 | 0.85d ± 0.06 |
| 2 | Nagpur Mandarin | 9.08f ± 0.91 | 26.78g ± 0.53 | 5.40a ± 0.29 | 1.26b ± 0.14 |
| 3 | Darjeeling Mandarin | 12.75ab ± 0.03 | 20.67h ± 0.53 | 3.27c ± 0.14 | 0.98cd ± 0.11 |
| 4 | Coorg Mandarin | 10.14ef ± 0.07 | 26.08g ± 0.30 | 5.42a ± 0.08 | 0.98cd ± 0.01 |
| 5 | Jhalawar Mandarin | 9.62f ± 0.05 | 25.41g ± 0.59 | 3.40c ± 0.04 | 1.19bc ± 0.10 |
| 6 | Khasi Mandarin | 12.36abc ± 1.47 | 25.45g ± 1.50 | 4.13b ± 0.30 | 1.58a ± 0.22 |
| B | Lime | ||||
| 7 | Sai Sarbati | 13.75a ± 0.02 | 35.64cd ± 1.62 | 0.19g ± 0.01 | 0.08h ± 0.01 |
| 8 | Pramalini | 13.75a ± 0.26 | 31.32ef ± 1.08 | 0.22g ± 0.04 | 0.07h ± 0.01 |
| 9 | Vikram | 13.76a ± 0.01 | 34.38de ± 0.82 | 0.29g ± 0.01 | 0.08h ± 0.01 |
| 10 | Balaji | 13.81a ± 0.99 | 38.70bc ± 1.12 | 0.33fg ± 0.04 | 0.09h ± 0.02 |
| 11 | Acid lime | 10.22def ± 0.02 | 37.26cd ± 0.54 | 0.33fg ± 0.03 | 0.16gh ± 0.03 |
| C | Sweet orange | ||||
| 12 | Mosambi | 10.48cdef ± 0.02 | 25.13g ± 0.79 | 2.57d ± 0.01 | 0.40fg ± 0.01 |
| D | Underutilized citrus fruits | ||||
| 13 | Kachai lemon | 11.80bcde ± 0.19 | 34.67d ± 1.02 | 0.35fg ± 0.10 | 0.31fgh ± 0.02 |
| 14 | Citron | 13.12ab ± 0.04 | 20.65h ± 0.61 | 0.28g ± 0.03 | 0.10h ± 0.01 |
| 15 | Pomelo | 10.24def ± 0.06 | 40.50b ± 0.54 | 0.73ef ± 0.10 | 0.72de ± 0.02 |
| 16 | Grapefruit | 12.08abcd ± 0.52 | 53.64a ± 0.62 | 0.79e ± 0.02 | 0.46ef ± 0.01 |
| Tukey HSD at 1% | 1.8864 | 3.1622 | 0.4203 | 0.2706 | |
Data presented are in mean ± standard deviation (n = 3)
Statistical note: Means (n = 3) within a column followed by different letters are significantly different at P < 0.01 according to the Tukey HSD multiple range test
*Means with superscripts having the same letter are not significantly different
Ascorbic acid is the water-soluble vitamin. Among the sixteen selected citrus juices, the Grapefruit (53.64 mg/100 mL) and Pomelo (40.50 mg/100 mL) had the highest ascorbic acid content. The mandarins are ranged as follows: Kinnow mandarin > Nagpur mandarin > Coorg mandarin > Khasi mandarin > Jhalawar mandarin > Darjeeling mandarin. In case of limes, ascorbic acid content was in the range of 31.32–38.70 mg/100 mL (Table 1). The results are in accordance with Marti et al. (2009), grapefruits, mandarin/lemons and pomelo is having 25–60 mg/100 mL, 20–60 mg/100 mL, and 30–47 mg/100 mL respectively. Different parameters like cultural practice, variety, climatic and processing factors, maturity stage of the fruit, etc. influence the vitamin C content of oranges and its derivatives (Nagy 1980).
The levels of carotenoid content were estimated and the results are reflected in Table 1, the carotenoid content in juice ranged from 0.07 to 1.58 mg/100 mL and was found to be highest in mandarins ranging 0.85–1.58 mg/100 mL comparatively to other varieties. The lowest carotenoid content was recorded in lime variety viz. Pramalini (0.07 mg/100 mL) and in Kachai lemon (0.31 mg/100 mL). In Mosambi and Grapefruit, it recorded as 0.40 mg/100 mL and 0.46 mg/100 mL respectively. In peels, the carotenoid content was found higher than juice and it ranged from 0.19–5.42 mg/100 mL and is all over again found higher in mandarins as compared to oranges and other citrus varieties. It was reported that mandarin fruits had much higher content of beta-cryptoxanthin and vitamin A than oranges (Xu et al. 2008; Abeysinghe et al. 2007). Similar results were reported by Fanciullino et al. (2006) who observed higher contents of carotenoids in mandarin citrus group than pomelo or orange types of citrus varieties. Factors such as the geographical origin, growing conditions, the maturity of the fruits and especially the varietal factor are responsible for influencing the carotenoids content of the orange juices (Louaileche et al. 2015).
Antioxidant activity assays
The antioxidant capacity carried out by foodstuff is determined by combining more than one method in vitro. Among the most accepted assays, four different antioxidant assays were employed in the present work. The assays of ABTS and DPPH are typically based on the scavenging of radical thereby converting it to a colorless product. The degree of this discoloration affects the quantity of ABTS or DPPH that has been scavenged from the sample (Almeida et al. 2011). Results of antioxidant activity assays using ABTS, DPPH, FRAP and TPC, are presented in Table 2. The potential as measured by the ABTS assay ranged from 2.54–4.64 mM L−1 Trolox and by DPPH assays ranged from 3.35–9.38 mM L−1 Trolox. The lowest DPPH values was observed for Kinnow mandarin (3.35 mM L−1 Trolox) and highest for Kachai lemon (9.38 mM L−1 Trolox). It was observed that for all the fruits analyzed for antioxidant activity, the DPPH values were found higher than those obtained for ABTS assay.
Table 2.
Antioxidant capacity of citrus varieties
| Sr. no. | Samples | Antioxidants | |||
|---|---|---|---|---|---|
| ABTS (mM L−1 Trolox) | DPPH (mM L−1 Trolox) | FRAP (mM L−1 Trolox) | TPC (mg GAE L−1) | ||
| A | Mandarin | ||||
| 1 | Kinnow Mandarin | 2.54g ± 0.33 | 3.35j ± 0.28 | 1.80abc ± 0.10 | 10.61cde ± 0.19 |
| 2 | Nagpur Mandarin | 2.95defg ± 0.08 | 3.93ij ± 0.34 | 1.33efg ± 0.07 | 8.30kl ± 0.34 |
| 3 | Darjeeling Mandarin | 2.69fg ± 0.18 | 7.26bc ± 0.20 | 1.95a ± 0.03 | 11.98b ± 0.11 |
| 4 | Coorg Mandarin | 3.26cdefg ± 0.20 | 6.16ef ± 0.27 | 1.54cde ± 0.08 | 9.64efgh ± 0.25 |
| 5 | Jhalawar Mandarin | 2.84efg ± 0.15 | 5.23fgh ± 0.44 | 1.24fg ± 0.09 | 9.08hijk ± 0.12 |
| 6 | Khasi Mandarin | 3.43bcde ± 0.35 | 5.53efg ± 0.30 | 1.64bcd ± 0.01 | 8.63ijkl ± 0.24 |
| B | Lime/lemons | ||||
| 7 | Sai Sarbati | 3.61bcd ± 0.10 | 6.28de ± 0.42 | 1.19fg ± 0.05 | 9.29ghij ± 0.16 |
| 8 | Pramalini | 2.97cdefg ± 0.06 | 5.60efg ± 0.39 | 1.41def ± 0.03 | 9.43ghi ± 0.16 |
| 9 | Vikram | 3.29cdef ± 0.22 | 5.74efg ± 0.14 | 1.29efg ± 0.07 | 9.57fghi ± 0.45 |
| 10 | Balaji | 4.53a ± 0.06 | 7.14bcd ± 0.17 | 1.36ef ± 0.05 | 8.44jkl ± 0.20 |
| 11 | Acid lime | 3.14cdefg ± 0.15 | 5.04gh ± 0.15 | 1.08g ± 0.07 | 10.21defg ± 0.22 |
| C | Sweet orange | ||||
| 12 | Mosambi | 3.11cdefg ± 0.11 | 7.61b ± 0.16 | 1.92a ± 0.01 | 10.54cdef ± 0.39 |
| D | Underutilized citrus fruits | ||||
| 13 | Kachai lemon | 4.14ab ± 0.39 | 9.38a ± 0.31 | 1.85ab ± 0.11 | 13.57a ± 0.31 |
| 14 | Citron | 3.70bc ± 0.26 | 4.44hi ± 0.17 | 1.18fg ± 0.10 | 7.84l ± 0.36 |
| 15 | Pomelo | 4.49a ± 0.09 | 7.92b ± 0.04 | 1.92a ± 0.03 | 10.74cd ± 0.32 |
| 16 | Grapefruit | 4.64a ± 0.14 | 6.33cde ± 0.23 | 1.71abc ± 0.15 | 11.36bc ± 0.28 |
| Tukey HSD at 1% | 0.7321 | 0.9679 | 0.2627 | 0.972 | |
Data presented are in mean ± standard deviation (n = 3)
Statistical note: means (n = 3) within a column followed by different letters are significantly different at P < 0.01 according to the Tukey HSD multiple range test
*Means with superscripts having the same letter are not significantly different
The FRAP values varied from 1.08 mM L−1 Trolox (Acid lime) to 1.95 mM L−1 Trolox (Darjeeling mandarin). As depicted in the Table 2, the TPC (Total phenol content) ranges from 7.84–13.57 mg GAE L−1. Among all the citrus varieties, the Citron showed the lowest amount of phenolics content while Kachai lemon showed the highest (13.57 mg GAE L−1) amount. Total phenolic content is one of the indicators showing the bioactive compounds enriched components. The difference in the results of phenolic content are may be due to several environmental related factors like climate, fertility, maturity period, location, diseases, pest exposure, temperature, and part tested. In addition to all these factors, rainfall is also reported to affect the phenolic content (Rajurkar and Hande 2011).
Correlation coefficients of antioxidants and ascorbic acid
The Pearson’s correlation coefficients, between antioxidant activities on the basis of FRAP, DPPH, TPC, and ABTS, Ascorbic acid were carried out. The antioxidant activity from ABTS assays (r = 0.698 at 1% level of significance), was correlated with the ascorbic acid contents. Ascorbic acid played a major role for the antioxidant capacity of citrus juices (Xu et al. 2008) and is the powerful antioxidant found in fruits and vegetables (Almeida et al. 2011).
In the present study of the sixteen citrus varieties, the positive correlation between ABTS and DPPH assays were also observed (r = 0.543 at 5% level of significance), indicating the antioxidant potential of citrus fruits. Similar findings were also observed in the study carried out by Gardner et al. (2000). It was also observed that TPC showed strong correlation with DPPH and FRAP. The correlation coefficient was 0.631 and 0.690 at 1% level of significance. These results are in accordance with that reported by Rajurkar and Hande (2011). Similar findings were also reported by Mena et al. (2011). The significant correlation was also seen between DPPH and FRAP with correlation coefficient of 0.528 with 5% level of significance. Total phenolic content have very strong correlation with the DPPH assay for the antioxidant potential measured with DPPH. Hence it reflects that DPPH assay can be a major assay to assess the antioxidant potential of Indian citrus varieties.
Conclusion
The phytochemical profiling of citrus fruits comprising mandarin, sweet orange, limes, underutilized citrus fruits presented in this study revealed a range of bioactive compounds with antioxidant capacity. Consumption of these fruits may deliver greater health benefits through the supply of natural antioxidants. Ascorbic acid showed correlation with the investigated antioxidant capacity (ABTS). The antioxidant methods employed in the present study can be recommended as useful tools for the estimation of antioxidant capacity of citrus fruits. Among the sixteen varieties, most probable varieties with lesser known, underutilized Kachai lemon and Pomelo can be exploited in terms of nutrients and health supplements for their potential use in food industry.
References
- Abeysinghe DC, Li X, Sun CD, Zhang WS, Zhou CH, Chen KS. Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 2007;104:1338–1344. doi: 10.1016/j.foodchem.2007.01.047. [DOI] [Google Scholar]
- Almeida MMB, De Sousa PHM, Arriaga AMC, Do Prado GM, De Carvalho Magalhaes CE, Maia GA, De Lemos TLG. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res Int. 2011;44:2155–2159. doi: 10.1016/j.foodres.2011.03.051. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bharate SS, Bharate SB. Non-enzymatic browning in citrus juice: chemical markers, their detection and ways to improve product quality. J Food Sci Technol. 2014;51(10):2271–2288. doi: 10.1007/s13197-012-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canan I, Gundogdu M, Seday U, Oluk CA, Karasahin Z, Eroglu EC, Yazici E, Unlu M. Determination of antioxidant, total phenolic, lycopene, ascorbic acid, and sugar contents of Citrus species and mandarin hybrids. Turk J Agric For. 2016;40:894–899. doi: 10.3906/tar-1606-83. [DOI] [Google Scholar]
- Caro AD, Piga A, Vacca V, Agabbio M. Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chem. 2004;84:99–105. doi: 10.1016/S0308-8146(03)00180-8. [DOI] [Google Scholar]
- Fanciullino AL, Mayer CD, Luro F, Casanova J, Morillon R, Ollitrault P. Carotenoid diversity in cultivated citrus is highly influenced by genetic factors. J Agric Food Chem. 2006;54:4397–4406. doi: 10.1021/jf0526644. [DOI] [PubMed] [Google Scholar]
- Fejzić A, Ćavar S. Phenolic compounds and antioxidant activity of some citruses. Bull Chem Technol Bosnia Herzeg. 2014;42:1–4. [Google Scholar]
- Gardner PT, White TAC, McPhail DB, Duthie GG. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000;68:471–474. doi: 10.1016/S0308-8146(99)00225-3. [DOI] [Google Scholar]
- Hasegawa S, Berhow MA, Manners GD (2000) Citrus limonoids In: ACS Symposium Series; American Chemical Society: Washington, DC, pp 1–8
- Karoui IJ, Marzouk B. Characterization of bioactive compounds in tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. BioMed Res Int. 2013;2013:1–12. doi: 10.1155/2013/345415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Vijay S, Khan N. Comparative nutritional analysis and antioxidant activity of fruit juices of some citrus spp. Octa J Biosci. 2013;1(1):44–53. [Google Scholar]
- Louaileche H, Khodja YK, Bey MB. Phytochemical contents and in vitro antioxidant activity of Algerian orange juices. Int J Bioinform Biomed Eng. 2015;1(2):107–111. [Google Scholar]
- Marti N, Mena P, Canovas JA, Micol V, Saura D. Vitamin C and the role of citrus juices as functional food. Nat Prod Commun. 2009;4(5):677–700. [PubMed] [Google Scholar]
- Mena P, García-Viguera C, Navarro-Rico J, Moreno D, Bartual J, Saurab D, Mart N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J Sci Food Agric. 2011;91:1893–1906. doi: 10.1002/jsfa.4411. [DOI] [PubMed] [Google Scholar]
- Meydav S, Saguy I, Kopelman IJ. Browning determination in citrus products. J Agric Food Chem. 1977;25(3):602–604. doi: 10.1021/jf60211a030. [DOI] [Google Scholar]
- Miller GL. Use of dinitro-salicylic acid reagent for determination of sugar. Anal Chem. 1972;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Nagy S. Vitamin C contents of citrus fruit and their products. J Agric Food Chem. 1980;28:8–18. doi: 10.1021/jf60227a026. [DOI] [PubMed] [Google Scholar]
- NHB Horticulture database, 2016–17 (3rd advance estimate)
- Ohta H, Hasegawa S. Limonoids in Pummelos (Citrus grandis (L.) Osbeck) J Food Sci. 2006;60(6):1284–1285. doi: 10.1111/j.1365-2621.1995.tb04574.x. [DOI] [Google Scholar]
- Pichaiyongvongdee S, Haruenkit R. Comparative studies of limonin and naringin distribution in different parts of pummelo (Citrus grandis (L.) Osbeck) cultivars grown in Thailand. Kasetsart J Nat Sci. 2009;43(1):28–36. [Google Scholar]
- Rajurkar N, Hande SM. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J Pharm Sci. 2011;73(2):146–151. doi: 10.4103/0250-474X.91574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganna S (1986) Vitamins. In: Handbook of analysis and quality control for fruit and vegetable products, 2nd edition. Tata McGraw-Hill Publishing Company Limited, New Delhi, p 105
- Ranganna S, Govindarajan VS, Ramana KVR. Citrus fruits—varieties, chemistry, technology, and quality evaluation. Part II. Chemistry, technology, and quality evaluation. A. Chemistry. Crit Rev Food Sci Nutr. 1983;18:313–386. doi: 10.1080/10408398309527366. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Ting SV, Rouseff RL. Citrus fruits and their products, analysis and technology. New York: Marcel Dekker Inc.; 1986. [Google Scholar]
- Vazquez LC, Alanon ME, Robledo VR, Coello MSP, Gutierrez IH, Maroto MCD, Jordan J, Galindo MF, Jiménez MMA. Bioactive flavonoids, antioxidant behaviour, and cytoprotective effects of dried grapefruit peels (Citrus paradisi Macf) Oxid Med Cell Longev. 2016;2016:1–12. doi: 10.1155/2016/8915729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Chuang YC, Ku YH. Quantitation of bioactive compounds in citrus fruits cultivated in Taiwan. Food Chem. 2007;102:1163–1171. doi: 10.1016/j.foodchem.2006.06.057. [DOI] [Google Scholar]
- Wattanasiritham L, Taweesuk K, Ratanachinakorn B (2005) Limonin and Naringin in Pummelos (Citrus grandis (L.) Osbeck) In: 31st Congress on science and technology of Thailand, Suranaree University of Technology, Thailand
- Wilson KW, Crutichfield CA. Spectrophotometric determination of limonin in orange juice. J Agric Food Chem. 1968;16(1):119. doi: 10.1021/jf60155a013. [DOI] [Google Scholar]
- Xu G, Liu D, Chen J, Ye X, Ma Y, Shi J. Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem. 2008;106:545–551. doi: 10.1016/j.foodchem.2007.06.046. [DOI] [Google Scholar]
- Zarina Z, Tan SY. Determination of flavonoids in Citrus grandis (pomelo) peels and their inhibition activity on lipid peroxidation in fish tissue. Int Food Res J. 2013;20(1):313–317. [Google Scholar]


