Abstract
Apricot (Prunus sp.) is an important fruit crop worldwide. Despite recent advances in apricot research, much is still to be done to improve its productivity and environmental adaptability. The availability of wild apricot germplasms with economically interesting traits is a strong incentive to increase research panels toward improving its economic, environmental and nutritional characteristics. New technologies and genomic studies have generated a large amount of raw data that the mining and exploitation can help decrypt the biology of apricot and enhance its agronomic values. Here, we outline recent findings in relation to apricot production, pomological and nutraceutical properties. In particular, we retrace its origin from central Asia and the path it took to attain Europe and other production areas around the Mediterranean basin while locating it in the rosaceae family and referring to its genetic diversities and new attempts of classification. The production, nutritional, and nutraceutical importance of apricot are recapped in an easy readable and comparable way. We also highlight and discuss the effects of late frost damages on apricot production over different growth stages, from swollen buds to green fruits formation. Issues related to the length of production season and biotic and abiotic environmental challenges are also discussed with future perspective on how to lengthen the production season without compromising the fruit quality and productivity.
Keywords: Apricot kernel oil, Plum pox virus, Prunus armeniaca, Spring frost, Stone fruit, Sharka
Apricot: a stone fruit in a large family
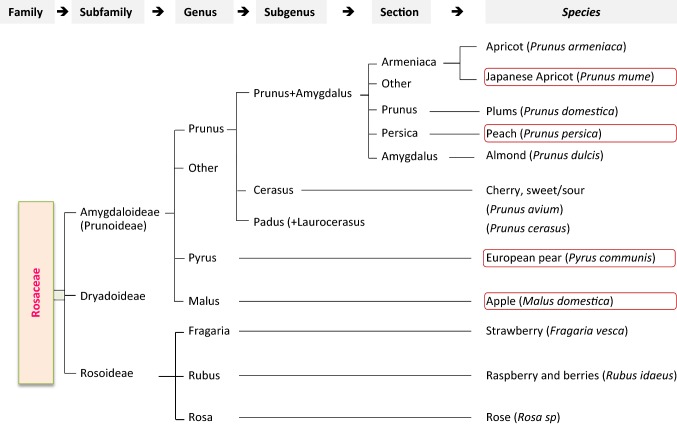
Apricot (Prunus sp.) is an important fruit crop worldwide, belonging to a large family, the Rosaceae, that contains about 100 genera and 2830–3100 species (Hummer and Janick 2009). Different physiological and morphological characteristics are the origin of a complex taxonomy pattern of this family that includes many taxon levels (i.e., subfamilies, tribes, subgenera, and sections) (Fig. 1). Although molecular genetics findings have introduced new taxonomical rearrangements, the classification is still controversial at least for some taxon groups in the Rosaceae (Potter et al. 2007; Shi et al. 2013). The number of subfamilies for example has been reduced into three: Rosoideae, Amygdaloideae, and Dryadoideae (Potter et al. 2007). The first subfamily, Rosoideae, harbors several notable plants such as roses and berries. The subfamily Amygdaloideae (also known as Prunoideae) contains the most common stone fruits genera: Pyrus (e.g., pear), Malus (e.g., apple), and Prunus (e.g., apricot). The genus Prunus has been recently divided into three subgenera after it was containing five previously (Shi et al. 2013). Almonds and peaches, formerly placed under the subgenus Amygdalus, are now placed under the subgenus Prunus, which is also divided into different sections. Both common apricot (P. armeniaca) and Japanese apricot (Prunus mume) are parts of the section Armeniaca.
Fig. 1.
Classification of the Rosaceae family. Most common genera and species of Rosaceae are shown according to Potter et al. (2007) and Shi et al. (2013). Subtribes were omitted for simplification purposes. The genomes of boxed species have been already sequenced
Apricot deep roots in Asia
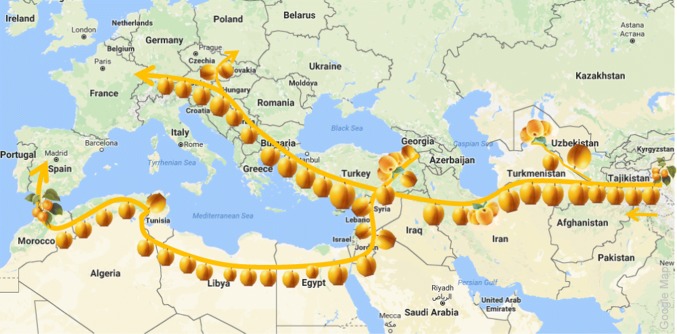
The origin of apricot (Fig. 2) seems to be deeply rooted in three Asian interconnected regions (Vavilov 1951; Faust et al. 1998): the first is China where some wild type varieties are still growing there naturally (Li et al. 2013). The second is central Asia, and the third is the Near East region, including the Irano-Caucasian territory, which is considered as a second center of origin of apricots. The domestication of apricot would have taken at the first place in China (Janick and Moore 1996) where Japanese varieties would originate too (Yoshida 1998). The European apricots in turn seem to be originated from the Irano-Caucasian region by two ways: through a North Africa road to South Europe and from Central Europe to Western Europe (Bourguiba et al. 2012). European varieties have then been introduced into the Americas. However, some classifications show complex taxonomy patterns between European and American varieties (Hagen et al. 2002). Some Southern Europe varieties also seem to be reintroduced back into North Africa (Bourguiba et al. 2013). The name of apricot in Latin languages derives from the Arabic “al-barquq”.
Fig. 2.
Origin and dissemination of Apricot. Apricots originated on the region stretching from China-to-central Asia from which it has spread to Armenia and then into Europe alongside the “Silk Road”. It is supposed that apricot was introduced into Europe by two ways: by the Romans through the central Asia and by the Arabs through southern Europe (Faust et al. 1998)
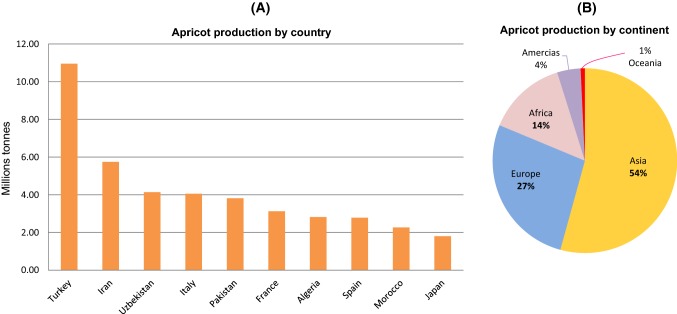
Nowadays, apricot is mainly grown in the Mediterranean Basin and moderate regions including ex-Soviet Union countries, Iran, China, Japan, South Africa and the United States (Asma 2007). The apricot worldwide production varies from region to another according to the Food and Agriculture Organization (FAO)* of the United Nations, the top producers over 20 years (from 1994 to 2014) (Fig. 3a) are Turkey with up to 10 million tonnes, followed by Iran (about 6 million tonnes) and Uzbekistan (about ~ 4 million tonnes), making Asia as the first producing region of apricots with more than 50% of the worldwide production, followed by Europe (27%) and Africa − 14%) (Fig. 3b).
Fig. 3.
Apricot production worldwide from 1994 to 2014. a Top 10 countries producers, b production share of Apricots by continent.
Source: http://www.fao.org/faostat/en/#data/QC/visualize Accessed 6 October 2018
Each of these regions has its specific varieties that have been progressively adapted to the prevailing climate conditions. In India, apricot trees growing at 6000 m of altitude can survive at severe cold temperatures as low as − 35 °C (Kumar et al. 2009). Southern European varieties, in turn, could survive at warm temperatures. According to their geographic origins, apricots varieties are usually divided into different groups: Central Asian group, Irano-Caucasian group, European group, and Dzhungar-Zailij group. A fifth group composed of North and East Chinese varieties and a non-geographically based group, called “adaptable group” were also introduced to include apricot varieties that could adapt to a large spectrum of geographic areas (Hagen et al. 2002). However, most classifications harbor a certain number of outliers. Other apricot classifications are based on reproduction methods, such as sexual or asexual methods (i.e., grafting versus seeds) (Bourguiba et al. 2010) or fruit processing types or purposes (Yılmaz et al. 2009).
Apricot diversity and domestication
The first step in apricot domestication is the selection, among a large wild type population, of some varieties with desirable characteristics. Then, the selected variety is maintained and optimized over generations. One of the first places of domestication was Anatolia, in Turkey, more than 2000 years ago where apricot was introduced either by Alexander the Great during his conquer campaigns of Asia (334 BC) or by merchants who trade with apricot seeds from China and Central Asia alongside the Silk Road (Fig. 2) or by Romans during their campaigns to the Near East region (Syria, Iran, and Caucasus) (Yilmaz and Gurcan 2012).
Whatever the road of apricot domestication, the introduction of apricot into new ecological destinations has contributed to the enrichment of apricot varieties with enhanced genetic diversities (i.e., allelic polymorphism, heterozygosity, etc.). Nonetheless, China, Central Asia, and Irano-Caucasian regions are still harboring important diverse populations of wild types compared with central Europe (Akpınar et al. 2010; Yilmaz and Gurcan 2012). However, the genetic diversity of apricot tends to decrease from the Irano-Caucasian region up to Europe (Bourguiba et al. 2012) (Table 1). Among the potential reasons behind genetic impoverishment in central Europe is that most of the apricot species grown in this region would have been originated from three cultivars only and/or from self-compatible cultivars (Pedryc et al. 2009). A poor genetic variation is also observed in North Africa that has witnessed several waves of apricot domestication (Bourguiba et al. 2013). The genome of apricot is still not fully sequenced. However, as Peach and Japanese apricot seem to share high sequence similarity (Cross 2015), important information on apricot diversity could be obtained from relative pedigree species that the genomic sequences are already available.
Table 1.
Apricot populations with some genetic characteristics (n° of genetic markers, alleles, and heterozygosity)
| Population | Marker | N° alleles | Range He | Range Ho | Ho/He | References |
|---|---|---|---|---|---|---|
| China (83)/other (11) | SSR (21/21) | 7–24 (15.14) | 0.353–0.927 (0.792) | 0.108–0.753 (0.523) | 0.9–1.1 (2) 0.7–0.9 (9) 0.5–0.7 (6) < 0.5 (4) |
Zhang et al. (2014) |
| China (23) | SSR (6/8) | 3–11 (8) | 0.325–0.892 (0.763) | NA | NA | Pedryc et al. (2009) |
| Central Europe (24) | 2–5(3.3) | 0.264–0.551 (0.440) | NA | NA | Pedryc et al. (2009) | |
| West Europe/Mediterranean (8) | 2–8 (5.8) | 0.240–0.770 (0.616) | NA | NA | Pedryc et al. (2009) | |
| North America (12) | 3–9 (6.2) | 0.236–0.859 (0.717) | NA | NA | Pedryc et al. (2009) | |
| Turkish (29) | SSR (8/8) | 4–10 (6.37) | 0.392–0.839 (0.657) | 0.379–0.896 (0.648) | > 1.1 (3) 0.9–1.1 (3) 0.7–0.9 (1) 0.5–0.7 (1) |
Akpınar et al. (2010) |
| Iran/Turkey (46) | SSR (25/25) | 7.43* | 0.596 | 0.645 | 0.9–1.1 (6) 0.8–0.9 (17) 0.7–0.9 (2) |
Bourguiba et al. (2010) |
| Europe (51) | 5.77* | 0.624 | 0.623 | |||
| Spain/North Africa (110) | 5.43* | 0.488 | 0.569 | |||
| North Africa (183) | SSR (24/24) | 2–16 (7.95) | 0.144–0.87 (0.593) | 0.12–0.71 (0.504) | 0.9–1.1 (7) 0.8–0.9 (12) 0.7–0.8 (5) |
Bourguiba et al. (2013) |
| North Africa (oasis1/seed) | SSR (24/24) | 3.46** | 0.549 | 0.480 | 0.9–1.1 (13) 0.8–0.9 (9) 0.7–0.8 (2) |
Bourguiba et al. (2010) |
| North Africa (oasis2/seed) | 3.39** | 0.498 | 0.485 | |||
| North Africa (Graft1/N) | 3.44** | 0.551 | 0.572 | |||
| North Africa (Graft2) | 3.34** | 0.531 | 0.488 | |||
| North Africa (Graft3/S) | 3.08** | 0.508 | 0.576 |
The number of individuals of each population is given in parentheses (first column) and SSR (simple sequence repeats) markers provided in the second column. Allele numbers are provided as absolute values or allelic richness (*, **) (i.e. the average number of alleles per locus for a given population [19* or 16**]). Ranges of the heterozygosity observed (Ho) over the heterozygosity expected (He) are also provided with the number of markers within that span
Apricot fruit development
Depending on the regions and varieties, the apricots start to bloom usually around mid-March. The number of blooming flowers depends on environmental and genetic factors and on the nutrient reserves that have been accumulated during the previous season. Consequently, the overall yield of apricot depends on the total number of flowers, the variety, the rate of successful pollination, the environmental biotic and abiotic stresses, and the percentage of fruits that reach full development stages (Alburquerque et al. 2004; Ruiz and Egea 2008).
The fruits of the Prunus genera develop in general in three distinct stages. The first is a rapid growth phase (of about 3–4 weeks) controlled by cell division and cell expansion mechanisms. The second is a slow growth stage during which the fruit stone starts to harden gradually by lignification of cell walls. This stage could last several weeks in early-maturing varieties and longer in late-maturing varieties. The third stage is a maturation stage corresponding to rapid fruit growth that usually begins 4–6 weeks before harvest, during which sugars, ethylene and aroma compounds accumulate rapidly (Durmaz et al. 2010; Baldicchi et al. 2015). The three stages vary in length and intensity, depending on species and environmental conditions (temperatures, light, etc.). While the first and third stages in Prunus mume are relatively long, the second phase is short and almost invisible (Yamaguchi et al. 2004). However, high rates of cell division in the first stage are observed in different varieties of peach, apricot, and Prunus mume with high yields (Scorzal et al. 1991; Yamaguchi et al. 2004; Olmstead et al. 2007; Baldicchi et al. 2015). The numbers of cells but not their sizes also seem to contribute to the final apricot yield (Scorzal et al. 1991; Yamaguchi et al. 2004; Olmstead et al. 2007). The colors of developed or mature fruits vary from yellow to golden and from orange to red, and the ratio of fruit flesh to seed is an important determinant factor of fruit quality and maturation.
Nutritional and pharmaceutical values of apricot
Apricot is considered as one of the most delicious fruits of moderate regions (Faust et al. 1998). The fruits can be consumed fresh, dried or conserved in jam or marmalades or fruit bars. Fruit drying could be performed either manually or traditionally on large outspread sheets under natural insolation conditions, without any treatment, or after treatment with sulfur vapor that help preserve fruit colors and extends their storage period. Naturally dried fruits turn into brown color but the drying method has many important advantages over industrial or sulfur dioxide (SO2) processing including, for example, simplicity, cost effectiveness and safety. Sulfur-treatment approach, on the other hand, keeps fruit brighter with more consistent textures, but with sulfur traces that raise some health concerns. The level of sulfur dioxide allowed in dried fruits is regulated and varies from country to country (usually between 2500 and 3000 ppm) (Somogyi et al. 1996), therefore the amount of SO2 used must be carefully controlled. Fruit drying by combining osmo-air dehydration (dipping fruits in sucrose syrup followed by air drying) is also used (Raj et al. 2015; İspir and Toğrul 2009; Riva et al. 2005).
Apricot fruits contain good amounts of sugars, fibers, proteins, minerals and vitamins (Table 2). These variable nutrients make the apricot as a “golden” food crop from nutritional and medicinal viewpoints. The pomological and nutraceutical properties of apricot vary depending on apricot varieties, cultivation systems, storage conditions fruit and developmental stages (Ruiz et al. 2005a, b; Drogoudi et al. 2008; Goliáš et al. 2013; Bae et al. 2014). During the maturation phase, for example, the rates of organic acids (i.e., citric and malic acids) decrease while those of sugars, particularly sucrose and glucose rise noticeably
Table 2.
Nutritional values of apricot (fresh and dried fruits) in regard with sugars, proteins, lipids, vitamins and minerals
| Nutritional values per 100 g | ||
|---|---|---|
| Raw apricot | Dried apricot | |
| Nutrient | ||
| Water | 86.35 g | 30.89 g |
| Sugars | 9.24 g | 53.44 g |
| Fibers | 2 g | 7.3 g |
| Proteins | 1.4 g | 3.39 g |
| Lipids | 0.39 g | 0.51 g |
| Minerals | ||
| Calcium | 13 mg | 55 mg |
| Iron | 0.39 mg | 2.66 mg |
| Magnesium | 10 mg | 32 mg |
| Phosphorus | 23 mg | 71 mg |
| Potassium | 259 mg | 1162 mg |
| Sodium | 1 mg | 10 mg |
| Zinc | 0.2 mg | 0.39 mg |
| Vitamins | ||
| Vitamin C | 10 mg | 1 mg |
| Thiamin | 0.03 mg | 0.015 mg |
| Riboflavin | 0.04 mg | 0.074 mg |
| Niacin | 0.6 mg | 2.589 mg |
| Vitamin B6 | 0.054 mg | 0.143 mg |
| Vitamin E | 0.89 mg | 4.33 mg |
| Folate | 9 µg | 10 µg |
| Vitamin A | 96 µg | 180 µg |
| Vitamin K | 3.3 µg | 3.1 µg |
| Vitamin A, International Unit (IU) | 1926 IU | 3604 IU |
Compiled from: https://ndb.nal.usda.gov/ndb/search/list?qlookup=09021&format=Full, https://ndb.nal.usda.gov/ndb/search/list?qlookup=09032&format=Ful. Accessed 6 October 2018
Antioxidants in general are known for their positive effects against many diseases such as cancers and diabetes. Such important proprieties make many apricot products as functional or medicinal food (Ruiz et al. 2005a, b). The fruit contains more than 200 volatile compounds including, for example, esters, alcohols, and aldehydes whose concentrations vary between cultivars (Guillot et al. 2006). The total carotenoid content could range between 1512 and 16,500 μg 100 g−1 of edible portion, with β-carotene as a main pigment followed by β-cryptoxanthin and γ-carotene (Ruiz et al. 2005a, b). Most apricot varieties are good sources of phenolic compounds (4233.70–8180.49 mg of gallic acid equiv/100 g of dry weight), carotenoids (14.83–91.89 mg of β-carotene equiv/100 g of dry weight), and β-carotene (5.74–48.69 mg/100 g of dry weight) (Akin et al. 2008). The total sugar content could reach up to 90 mg/100 g in some varieties and vitamin C up to 100 mg/100 g of dry weights (Akin et al. 2008). In a recent study (Karabulut et al. 2014), it was reported that apricot could attenuate apoptosis and oxidative stress in liver carcinogenesis when combined with radiotherapy (Karabulut et al. 2014). Another study reports that 5% of sun-dried organic apricot food supplementation over 3 weeks has beneficial effects on liver regeneration (Yilmaz et al. 2013). Previously, it was reported that apricot-rich diet could have a preventive role on histopathological changes caused by alcohol in rat testes (Kurus et al. 2009). Except for vitamin C, dried fruits are richer in nutritional values than the same weight of raw fruits (Table 1). About 30 g of dried fruits could supply the recommended daily value of vitamin A (Drogoudi et al. 2008).
Although some rare cases of cyanide toxicity in kernels were reported (Kurus et al. 2009; Akil et al. 2013), though some of them are contested (Senthilkumaran et al. 2015), the kernel oil could be used in cosmetics and medicinal admixtures as a source of lipophilic compounds, such as tocopherols, phytosterols, and fatty acids. The use of apricot kernel oil as a nutritional supplement can improve the chemotherapy-associated immunosuppression in rats (Tian et al. 2016) and inhibit the growth of carcinoma tumors (Yamshanov et al. 2016). Apricot kernel extracts (with or without oil) also seem to have anti-inflammatory effects and could improve bowel disorders such as ulcerative colitis (Minaiyan et al. 2014). Finally, the kernel press cake (the solid remains after pressing the kernel to extract oil) contains up to 70% proteins and can be used in preparations of protein isolates to improve the nutritional status of some food supplements (Sharma et al. 2010). However, the nutritional and nutraceutical mentioned above vary largely as a function of the approaches being used to process and storage the apricot fruits. For example, canning and freezing can retain apricot bioactive compounds while preserving the fruit pulp up to 12 months (Wani et al. 2018). By contrast, thermal and mechanical processing approaches reduce the bioactivity of many fruit compounds compared with non-thermal approaches, such as gamma and ultraviolet sterilizations (Al Juhaimi et al. 2018a) or cold (Al Juhaimi et al. 2018b).
Mechanisms to improve apricot yield under environmental constraints
The yield of apricot could be affected by many genetic and environmental factors (biotic and abiotic conditions). The Sharka, for example, is a major viral disease that severely affects apricot and other stone fruits (Levy et al. 2000; Cambra et al. 2006). The first description of this illness was reported in 1915 on plum trees in Bulgaria, where it was named as “Sarka po slivite” (“pox of plum”). Then, it has appeared in Turkey in mid-1960s and in the Americas in 1990s. The pathogenic agent of the Sharka is a Potyvirus, known as Plum Pox Virus (PPV). Many strains of the virus have been identified so far, such as the PPV-rec and the PPV-T that could be transmitted and infect new tree populations through aphids (sap-sucking insects). Four to six aphid species are considered as major transmitters that could acquire the virus as quickly as 30 s of feeding on infected plant sap (Levy et al. 2000). The infection symptoms are variable, depending on the host, the viral strain, the environment and time of infection, and could manifest by the appearance of visible yellow or brown blotches or rings on the infected tissues and the deformation of leaves, seeds and fruits (bumpy fruits). A long latency between infection and symptoms may reach up 2 years, making the diagnosis of the disease somewhat tricky (Rubio et al. 2014). In other words, the disease can be chronic or latent so that only molecular approaches could detect the presence of the virus.
No efficient treatments exist so far to eradicate the disease completely, though insecticides are commonly used against the infection and transmission agents (aphid populations) but with mixed efficiency due to the non-persistent transmission manner of the virus. However, the risk of infection could be reduced by applying preventive procedures such as the removal of infected plants and tissues and the use of virus-free certified materials by the application of quarantine precaution. Other approaches consist to place the plants in cold conditions (vernalization chamber) to impose dormancy and fulfill chilling requirements and then record any potential symptoms that may appear as a sign of latent infections (Rubio et al. 2014).
Some peach and apricot cultivars are partially resistant to the virus where no symptoms or limited symptoms only appear while plants harbor the virus at a level that it is usually harmful for sensitive cultivars (Escalettes et al. 2006). Some contradictory results were observed in some cultivars between field and greenhouse conditions (Rubio et al. 2014). The cultivar Goldrich, for example, is classified by some as partially resistant to the Sharka virus (Escalettes et al. 2006) but others consider it as a fully resistant cultivar (Rubio et al. 2014). Biotechnology approaches (Ilardi and Tavazza 2015) and programs to breed Sharka-resistant apricots are under investigation worldwide (Fideghelli and Della Strada 2010; Yilmaz and Gurcan 2012; Çağlayan et al. 2013). Transformation methods have been reported for several Prunus species including apricot, but the efficiency is still non-conclusive (Yamane 2014). Some strategies to increase resistance to the Sharka virus consist of breaking down the viral genes (gene silencing), or targeting plant proteins that potentially interact with viral components. The first approach was successfully used to create a resistant plum variety called HoneySweet, which has been approved for cultivation in the United States of America. Other plum transformants show resistance to the virus in greenhouse conditions (Ravelonandro et al. 2014; García-Almodóvar et al. 2015). Although the transformation and regeneration of commercial varieties of woody fruit crops including apricot (Prunus armeniaca L.) are not easy, some studies have reported the transformation of apricot leave explants using Agrobacterium tumefaciens (Petri et al. 2008, 2004). The transformation approach could consist of infecting apricot leaves for 10 min in a bacterial suspension (cultured for 24 h in a medium containing 500 1/4M AS and diluted to about 107 cfu ml−1), with a co-culture time of 4 days in the presence of l00 ¼M acetosyringone (Petri et al. 2004). Recently, an RNAi-mediated gene silencing approach was also used to produce plum rootstocks that seem to be resistant to the crown gall disease but failed to produce the same trait in apricot (Alburquerque et al. 2017). The advantage of using genetically engineered rootstocks in rosaceae species is that, rootstocks could be grafted with standard non-transgenic commercial scion cultivars to produce non-genetically modified varieties to address the public’s concerns regarding genetically modified fruits (Alburquerque et al. 2017).
In sum, as there is no efficient treatment for viral diseases in general, the prevention and control measures of the Sharka disease in apricot could comprise the use of certified virus-free nursery stocks, the use of resistant species or varieties (when available), the control of insect vectors (aphid populations), the suppression of contaminated trees, and the enhancement of national and international quarantine regulations.
Effects of spring frost on apricot yield
Cold has positive and negative effects on apricot. The hibernal cold dormancy plays a vital role in crop survival during the cold season. Dormancy is defined as a period of stopped or reduced metabolic and physiological activities to cope with internal and external factors associated with chilling requirements. Different types of dormancy have been described, on the basis of organ or growth-inhibiting factors (Zhong et al. 2013), including for example organ dormancy (seed or bud dormancy) and environmental dormancy (winter or summer dormancy). Buds enter an endo-dormancy by internal growth inhibition mechanisms, but they keep their ability to grow when the surrounding environmental conditions are favorable. In Prunus mume, some flowers and vegetative buds start to develop as early as June (Yamane 2014) but they remain dormant until the next growth season. In the Northern Hemisphere, however, the ecodormancy in Prunus mume, peaches and apricots start typically in winter, from the end of December to the end of January (Yamane 2014; Zhong et al. 2013; Szymajda et al. 2013).
Apricot has mild-to-high chilling requirements that vary among cultivars (Ruiz et al. 2007) and play important roles to ensure simultaneous flowering and synchronized fruit harvesting. By contrast, warm winters that disturb the chilling requirements cycles reduce the annual yield and degrade fruit quality (Ghrab et al. 2014). Campoy and colleagues (Campoy et al. 2013) report that artificial exposure of apricot shoots to warm temperature degrees results in negative effects on the fruit production (Campoy et al. 2013).
Another positive effect of cold is that, plants exposed to early moderate non-freezing cold temperatures tend to develop resistance to future freezing temperatures (cold acclimation). Extended moderate temperatures in early autumn could enhance plant survival during harsh winters later. The positive effects of cold acclimation, however, depend on species and intensity of acclimation conditions. Similar conditions can improve the acclimation capacity in one species, but reduce it in another species. Differential cold-sensing mechanisms could affect the start point of the dormancy. In this context, different models to calculate chilling requirement units have been suggested, taking into account the negative effects of the temperatures above and below the optimal range, in addition to the potential reversibility of cold accumulation (Ghrab et al. 2014; Yamane 2014). It is worth noting, however, that plant response to its environment is not triggered only at the beginning of the acclimation process but also during the acclimation process itself. Apricot trees could adjust their endodormancy as a function of ambient temperatures (Campoy et al. 2013).
The mechanisms of acclimation (freezing tolerance) and deacclimation (loss of freezing tolerance) are usually associated with each other. Cold acclimation is a gradual increase of tolerance to freezing during exposure to low non-freezing temperatures. There is ample evidence that during this process, numerous endogenous metabolic changes accompany the transition to the cold hardiness state such as the synthesis and accumulation of compatible solutes compounds and cryoprotectants (e.g., amino acids, carbohydrates, sugars and proteins) that play protective roles of the photosynthetic machinery and cell membranes stability (Stushnoff et al. 1993). Conversely, during the deacclimation process, there is a substantial reduction in compatible solute compounds that explain, at least in part, the susceptibility of plant tissues to low temperatures. The comparison of four different peach cultivars showed that peach varieties of different cold-tolerance capabilities displayed variable responses to cold/warm cycles during ecodormancy (Shin et al. 2015). While cold tolerant peaches withstood 4 cycles before bud burst, cold sensitive peaches enter a bud burst phase after two cold/warm cycles with buds that are still moderately resistant (Shin et al. 2015).
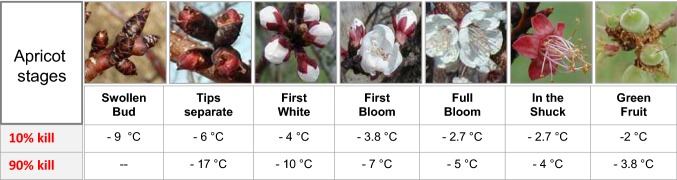
As apricots start to bloom by the end of March, they become completely deacclimated and sensitive to low temperatures. Frosts in this period (spring frost) could be extremely harmful with up to 90% yield loss. Spring frost occurs sometimes later, by the end of April, causing severe damages to both flowers and freshly produced fruits. Figure 4 illustrates the range of temperatures that kills up to 10 or 90% of flowers and fruits during the different developmental stages. For example, an exposure to 3.8 °C for 30 min kills up to 90% of freshly formed fruits (Fig. 4).
Fig. 4.
Critical temperatures of frost damages on apricot trees in °C (celsius). 10% and 90% of flowers/buds/fruits will be killed after 30 min exposure during the indicated fruit developmental stages (from swollen buds to green fruit onset).
Adapted from (Washington University data): http://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=1643&context=extension_curall. Accessed 6 October 2018
On another hand, drought stresses and water deficits also affect the rates and composition of membrane lipids, fatty acids, ethylene release and lipoxygenase activity in stressed apricot plants (Guo and Li 2002). Water-stressed trees also suffer from a decreased water potential (ψw) and water conductance (Ruiz-Sánchez et al. 2007) that could affect both plant growth and fruit yield (Pérez-Pastor et al. 2009). To improve drought resistance in apricot, water stress preconditioning could be considered in young plants (Ruiz-Sanchez et al. 2000) by for example reducing the amount of irrigation water while increasing its frequency over a month or so, which could promote plant hardening in later stages (Ruiz-Sanchez et al. 2000). However, some conditions of deficit irrigations seem to be commercially advantageous to produce fruits with higher values of total soluble solids (TSS), titratable acidity and hue angle while saving important amount of irrigation water (Pérez-Pastor et al. 2007).
Harvest later, benefit better?
The maturation of apricot fruits in low altitude regions is in general earlier than in high altitudes. However, and as mentioned above, in moderate regions spring frost is one of the main factors that limit the annual yield of apricot and other stone fruit trees that sometimes bloom early under favorable spring warm days. To reduce the damages of such freezes and maximize the fruit profit, old and new approaches ranging from simple and cheap to complicated and costly are being used with plus or minus efficiency. These include for example but not limited to the selection of appropriate cultivation site, the use of orchard heaters or wind machines, or the use of water sprinklers prior and at the expected date of frost. However, the selection of late-blooming cultivars or delaying blooming can be cheaper and more effective than other methods. Although trees or buds are rarely injured by cold during dormancy, they become more sensitive when they are near blooming and afterwards. Delaying flowering and extending the maturing seasons is thus of a particular interest to protect apricots from late frosts and to meet both farmers’ and consumers’ requirements. One of the potential methods toward such objective is to combine as many quality and quantity characteristics in the bred varieties. This could be achieved for example by taking advantage of wild apricot germplasms to introduce new characteristics to the cultivated varieties. A cross between wild late and early blooming varieties for early selection of the latest flowering variety in a nursery, which could then be planted in experimental orchards for further selection, could produce new intermediate varieties with late and good quality fruits.
Although some apricot varieties mature as late as September, the fruit quality and nutritional values, such as fruit weight and size—which are important quality traits in fresh fruits- and the total soluble solids “TSS”—which influence the fruit taste- are sometimes poor. Under favorable weather conditions, some apricot varieties also produce a large number of fruits that will result in many but small fruits at harvest. To prevent this and improve fruit quality in late-maturing varieties, an early removal of some immature and small-sized fruits could be applied. This approach, called fruit thinning, could maximize fruit value by optimizing fruit size, color, shape and quality while maintaining a good balance between tree growth, leave photosynthesis surface and fruit yields (Costa and Vizzotto 2010). Fruit thinning could be achieved by different strategies, either manually, mechanically or chemically, for example, to inhibit flowering or prevent fruit set or to stimulate the abscission of fruitlets (immature or small fruits) (Webster and Spencer 2000).
Thus, to benefit better from late harvests physiological parameters that control fruit quality and quantity need to be taken into consideration and combined in any breeding programs. However, this will largely depend on the type of physiological control of fruit numbers, the ability to transport nutrients to developing fruits and the rate of development of young fruits. As leaves are fully expanded about a month after flowering onset, an extension of the maturation timeline would cause some changes in the rate of accumulating nutritional reserves, which might explain the poor organoleptic properties of late maturing fruits in which little sugars are accumulated. Consequently, switching from late and small fruits to late and large fruits require full comprehension of many physiological mechanisms that are still not fully dissected. Extended flowering season (up to late May) and fruit maturity (to September) was recently observed in genotypes growing in the trans-Himalayan Ladakh region with total soluble solids (TSS) up to 37.9° Brix (Angmo et al. 2017) the native genotypes for fruit quality improvement programs worldwide.
As apricot wild types naturally harbor extensive variations in fruit, developmental, and stress tolerance traits, apricot breeding programs should take advantage of such variations to extend apricot flowering and fruiting seasons before they disappear by industrial or human activities (Li et al. 2013). However, the potential loss of genetic diversity in wild populations is difficult to tackle as most of them are not fully characterized yet. Another important hurdle is that the number of varieties registered in local repositories is decreasing continuously at the expense of a few commercial varieties adopted instead (Bourguiba et al. 2013; Zhang et al. 2014). The damage here is double: a scientific harm because an important wild germplasm with potential useful traits is getting lost, and an economic harm as potential sources of cold or Sharka resistance characteristics are bypassed. Priority efforts and coordination between various research centers might be necessary to collect and share experience and information to characterize apricot wild type varieties as an attempt toward new domestications and taking advantages of valuable apricot germplasm that could help breeding new late-ripening varieties with good fruit quality for the best agricultural and economic benefits possible.
Conclusion and future perspective
Apricot is an important fruit crop in moderate and cold regions. Its nutritional and pomological value is gaining attention. However, its cultivation and yields are threatened by many biotic and abiotic environmental conditions, such as late spring frosts and viral diseases. A wide range of flowering, fruiting and maturing timescales of apricot makes it possible to combine important agronomical and economic traits to fulfill both farmer and consumer needs. To achieve this goal, the wild varieties of apricot are an important germplasm source to focus on and to improve cultivated cultivars in terms of quality, productivity and adaptation to diverse environmental conditions and nutrients content. Classical and modern breeding approaches based, for example, on crossing, back-crossing, reverse genetics and genetic dissection to better understand early and late flowering and maturing mechanisms are also good research clues in breeding programs to consolidate and select as many desirable traits as possible. The completion of genome sequencing of apricot may also help achieve such goals and better understand the apricot genetic diversity and its relationship with other stone fruit species. Deeping and extrapolating knowledge from other related species on apricot is another venue of interest. We also think that endeavors toward breeding small size apricot-trees with late-ripping and high yield traits would be a good objective to focus on. Obviously, this will need to combine traditional and modern breeding approaches to produce the best outcome possible.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Akil M, Kaya A, Ustyol L, Aktar F, Akbayram S. Acute cyanide intoxication due to apricot seed ingestion. J Emerg Med. 2013;44(2):e285–e286. doi: 10.1016/j.jemermed.2012.05.041. [DOI] [PubMed] [Google Scholar]
- Akin EB, Karabulut I, Topcu A. Some compositional properties of main Malatya apricot (Prunus armeniaca L.) varieties. Food Chem. 2008;107(2):939–948. [Google Scholar]
- Akpınar A, Koçal H, Ergül A, Kazan K, Şelli M, Bakır M, Aslantaş Ş, Kaymak S, Sarıbaş R. SSR-based molecular analysis of economically important Turkish apricot cultivars. Genet Mol Res. 2010;9(1):324–332. doi: 10.4238/vol9-1gmr727. [DOI] [PubMed] [Google Scholar]
- Al Juhaimi F, Ghafoor K, Özcan MM, Jahurul MHA, Babiker EE, Jinap S, Sahena F, Sharifudin MS, Zaidul ISMJ. Effect of various food processing and handling methods on preservation of natural antioxidants in fruits and vegetables. J Food Sci Technol. 2018;8:9–10. doi: 10.1007/s13197-018-3370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Juhaimi F, Ozcan MM, Ghafoor K, Babiker EE, Hussain S. Comparison of cold-pressing and soxhlet extraction systems for bioactive compounds, antioxidant properties, polyphenols, fatty acids and tocopherols in eight nut oils. J Food Sci Technol. 2018;55(8):3163–3173. doi: 10.1007/s13197-018-3244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alburquerque N, Burgos L, Egea J. Influence of flower bud density, flower bud drop and fruit set on apricot productivity. Sci Hortic. 2004;102(4):397–406. [Google Scholar]
- Alburquerque N, Faize L, Burgos L. Silencing of Agrobacterium tumefaciens oncogenes ipt and iaaM induces resistance to crown gall disease in plum but not in apricot. Pest Manag Sci. 2017;73(10):2163–2173. doi: 10.1002/ps.4600. [DOI] [PubMed] [Google Scholar]
- Angmo P, Angmo S, Upadhyay SS, Targais K, Kumar B, Stobdan T. Apricots (Prunus armeniaca L.) of trans-Himalayan Ladakh: potential candidate for fruit quality breeding programs. Sci Hortic. 2017;218:187–192. [Google Scholar]
- Asma BM. Malatya: world’s capital of apricot culture. Chron Hortic. 2007;47:20–24. [Google Scholar]
- Bae H, Yun SK, Yoon IK, Nam EY, Kwon JH, Jun JH. Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. J Appl Bot Food Qual. 2014;87:24–29. [Google Scholar]
- Baldicchi A, Farinelli D, Micheli M, Di Vaio C, Moscatello S, Battistelli A, Walker RP, Famiani F. Analysis of seed growth, fruit growth and composition and phospoenolpyruvate carboxykinase (PEPCK) occurrence in apricot (Prunus armeniaca L.) Sci Hortic. 2015;186:38–46. [Google Scholar]
- Bourguiba H, Khadari B, Krichen L, Trifi-Farah N, Santoni S, Audergon J-M. Grafting versus seed propagated apricot populations: two main gene pools in Tunisia evidenced by SSR markers and model-based Bayesian clustering. Genetica. 2010;138(9–10):1023–1032. doi: 10.1007/s10709-010-9488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguiba H, Audergon J-M, Krichen L, Trifi-Farah N, Mamouni A, Trabelsi S, D’Onofrio C, Asma BM, Santoni S, Khadari B. Loss of genetic diversity as a signature of apricot domestication and diffusion into the Mediterranean Basin. BMC Plant Biol. 2012;12(1):1. doi: 10.1186/1471-2229-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguiba H, Khadari B, Krichen L, Trifi-Farah N, Mamouni A, Trabelsi S, Audergon J-M. Genetic relationships between local North African apricot (Prunus armeniaca L.) germplasm and recently introduced varieties. Sci Hortic. 2013;152:61–69. [Google Scholar]
- Çağlayan K, Asma B, Badenes M, Ulubaş Serçe C, Gazel M (2013) Screening for resistance to plum pox virus in some local turkish apricot cultivars and their crosses by molecular markers. In: II International symposium on plum pox virus 1063
- Cambra M, Capote N, Myrta A, Llácer G. Plum pox virus and the estimated costs associated with sharka disease. EPPO Bull. 2006;36(2):202–204. [Google Scholar]
- Campoy J, Ruiz D, Nortes M, Egea J. Temperature efficiency for dormancy release in apricot varies when applied at different amounts of chill accumulation. Plant Biol. 2013;15(s1):28–35. doi: 10.1111/j.1438-8677.2012.00636.x. [DOI] [PubMed] [Google Scholar]
- Costa G, Vizzotto G. Flower and fruit thinning of peach and other Prunus. Hortic Rev. 2010;28:351. [Google Scholar]
- Cross JM. Gene comparison between arabidopsis thaliana, prunus mume and prunus persica. Res J Biol Sci. 2015;10(4–5):44–55. [Google Scholar]
- Drogoudi PD, Vemmos S, Pantelidis G, Petri E, Tzoutzoukou C, Karayiannis I. Physical characters and antioxidant, sugar, and mineral nutrient contents in fruit from 29 apricot (Prunus armeniaca L.) cultivars and hybrids. J Agric Food Chem. 2008;56(22):10754–10760. doi: 10.1021/jf801995x. [DOI] [PubMed] [Google Scholar]
- Durmaz G, Cam M, Kutlu T, HIşIL Y. Some physical and chemical changes during fruit development of five common apricot (Prunus armeniaca L.) cultivars. Food Sci Technol Res. 2010;16(1):71–78. [Google Scholar]
- Escalettes VS-L, Hullot C, Wawrzy’nczak D, Mathieu E, Eyquard J-P, Le Gall O, Decroocq V. Plum pox virus induces differential gene expression in the partially resistant stone fruit tree Prunus armeniaca cv. Goldrich. Gene. 2006;374:96–103. doi: 10.1016/j.gene.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Faust M, Suranyi D, Nyujto F. Origin and dissemination of apricot. Hortic Rev Westport N Y. 1998;22:225–260. [Google Scholar]
- Fideghelli C, Della Strada G. The breeding activity on apricot in the world from 1980 through today. Acta Hort. 2010;862:93–98. [Google Scholar]
- García-Almodóvar R, Clemente-Moreno M, Díaz-Vivancos P, Petri C, Rubio M, Padilla I, Ilardi V, Burgos L. Greenhouse evaluation confirms in vitro sharka resistance of genetically engineered h-UTR/P1 plum plants. Plant Cell Tissue Org Cult. 2015;120(2):791–796. [Google Scholar]
- Ghrab M, Mimoun MB, Masmoudi MM, Mechlia NB. Chilling trends in a warm production area and their impact on flowering and fruiting of peach trees. Sci Hortic. 2014;178:87–94. [Google Scholar]
- Goliáš J, Létal J, Dokoupil L, Krška B. Physico-chemical changes and volatile constituents observed in 10 apricot cultivars (Prunus armeniaca L.) during post-harvest ripening. Hortic Sci. 2013;40(3):102–110. [Google Scholar]
- Guillot S, Peytavi L, Bureau S, Boulanger R, Lepoutre J-P, Crouzet J, Schorr-Galindo S. Aroma characterization of various apricot varieties using headspace–solid phase microextraction combined with gas chromatography–mass spectrometry and gas chromatography–olfactometry. Food Chem. 2006;96(1):147–155. [Google Scholar]
- Guo Y, Li J. Changes of fatty acid composition of membrane lipid, ethylene release and lipoxygenase activity in leaves of apricot under drought stress. J Zhejiang Univ (Agriculture and Life Sciences) 2002;28(5):513–517. [Google Scholar]
- Hagen L, Khadari B, Lambert P, Audergon J-M. Genetic diversity in apricot revealed by AFLP markers: species and cultivar comparisons. Theor Appl Genet. 2002;105(2–3):298–305. doi: 10.1007/s00122-002-0910-8. [DOI] [PubMed] [Google Scholar]
- Hummer KE, Janick J (2009) Rosaceae: taxonomy, economic importance, genomics. In: Folta KM, Gardiner SE (eds) Genetics and genomics of rosaceae. Plant genetics and genomics: crops and models, vol 6. Springer, New York, NY
- Ilardi V, Tavazza M. Biotechnological strategies and tools for Plum pox virus resistance: trans-, intra-, cis-genesis, and beyond. Front Plant Sci. 2015;6:379. doi: 10.3389/fpls.2015.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İspir A, Toğrul İT. Osmotic dehydration of apricot: kinetics and the effect of process parameters. Chem Eng Res Des. 2009;87(2):166–180. [Google Scholar]
- Janick J, Moore JN. Fruit breeding, tree and tropical fruits. New York: Wiley; 1996. [Google Scholar]
- Karabulut AB, Karadag N, Gurocak S, Kiran T, Tuzcu M, Sahin K. Apricot attenuates oxidative stress and modulates of Bax, Bcl-2, caspases, NFκ-B, AP-1, CREB expression of rats bearing DMBA-induced liver damage and treated with a combination of radiotherapy. Food Chem Toxicol. 2014;70:128–133. doi: 10.1016/j.fct.2014.04.036. [DOI] [PubMed] [Google Scholar]
- Kumar M, Mishra GP, Singh R, Kumar J, Naik PK, Singh SB. Correspondence of ISSR and RAPD markers for comparative analysis of genetic diversity among different apricot genotypes from cold arid deserts of trans-Himalayas. Physiol Mol Biol Plants. 2009;15(3):225–236. doi: 10.1007/s12298-009-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurus M, Ugras M, Ates B, Otlu A. Apricot ameliorates alcohol induced testicular damage in rat model. Food Chem Toxicol. 2009;47(10):2666–2672. doi: 10.1016/j.fct.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Levy L, Damsteegt V, Scorza R, Kolber M (2000) Plum pox potyvirus disease of stone fruits. American Phytopathological Society. http://www.apsnet.org/online/feature/PlumPox
- Li M, Zhao Z, Miao XJ. Genetic variability of wild apricot (Prunus armeniaca L.) populations in the Ili Valley as revealed by ISSR markers. Genet Resour Crop Evol. 2013;60(8):2293–2302. [Google Scholar]
- Minaiyan M, Ghannadi A, Asadi M, Etemad M, Mahzouni P. Anti-inflammatory effect of Prunus armeniaca L. (Apricot) extracts ameliorates TNBS-induced ulcerative colitis in rats. Res Pharm Sci. 2014;9(4):225–231. [PMC free article] [PubMed] [Google Scholar]
- Olmstead JW, Iezzoni AF, Whiting MD. Genotypic differences in sweet cherry fruit size are primarily a function of cell number. J Am Soc Hortic Sci. 2007;132(5):697–703. [Google Scholar]
- Pedryc A, Ruthner S, Hermán R, Krska B, Hegedűs A, Halász J. Genetic diversity of apricot revealed by a set of SSR markers from linkage group G1. Sci Hortic. 2009;121(1):19–26. [Google Scholar]
- Pérez-Pastor A, Ruiz-Sánchez MC, Martínez JA, Nortes PA, Artés F, Domingo R. Effect of deficit irrigation on apricot fruit quality at harvest and during storage. J Sci Food Agric. 2007;87(13):2409–2415. [Google Scholar]
- Pérez-Pastor A, Domingo R, Torrecillas A, Ruiz-Sánchez MC. Response of apricot trees to deficit irrigation strategies. Irrig Sci. 2009;27(3):231–242. [Google Scholar]
- Petri C, Alburquerque A, Garcéa-Castillo S, Egea J, Burgos L. Factors affecting gene transfer efficiency to apricot leaves during early agrobacterium-mediated transformation steps. J Hortic Sci Biotechnol. 2004;79(5):704–712. [Google Scholar]
- Petri C, Wang H, Alburquerque N, Faize M, Burgos L. Agrobacterium-mediated transformation of apricot (Prunus armeniaca L.) leaf explants. Plant Cell Rep. 2008;27(8):1317–1324. doi: 10.1007/s00299-008-0550-9. [DOI] [PubMed] [Google Scholar]
- Potter D, Eriksson T, Evans RC, Oh S, Smedmark J, Morgan DR, Kerr M, Robertson KR, Arsenault M, Dickinson TA. Phylogeny and classification of Rosaceae. Plant Syst Evol. 2007;266(1–2):5–43. [Google Scholar]
- Raj D, Sharma PC, Sharera SK. Studies on Osmo-air dehydration of different Indian apricot (Prunus armeniaca L.) cultivars. J Food Sci Technol. 2015;52(6):3794–3802. doi: 10.1007/s13197-014-1443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravelonandro M, Scorza R, Michel HJ, Briard P. The efficiency of RNA interference for conferring stable resistance to Plum pox virus. Plant Cell Tissue Org Cult. 2014;118(2):347–356. [Google Scholar]
- Riva M, Campolongo S, Leva AA, Maestrelli A, Torreggiani D. Structure–property relationships in osmo-air-dehydrated apricot cubes. Food Res Int. 2005;38(5):533–542. [Google Scholar]
- Rubio M, Ruiz D, Egea J, Martínez-Gómez P, Dicenta F. Opportunities of marker-assisted selection for Plum pox virus resistance in apricot breeding programs. Tree Genet Genom. 2014;10(3):513–525. [Google Scholar]
- Ruiz D, Egea J. Analysis of the variability and correlations of floral biology factors affecting fruit set in apricot in a Mediterranean climate. Sci Hortic. 2008;115(2):154–163. [Google Scholar]
- Ruiz D, Egea J, Gil MI, Tomás-Barberán FA. Characterization and quantitation of phenolic compounds in new apricot (Prunus armeniaca L.) varieties. J Agric Food Chem. 2005;53(24):9544–9552. doi: 10.1021/jf051539p. [DOI] [PubMed] [Google Scholar]
- Ruiz D, Egea J, Tomás-Barberán FA, Gil MI. Carotenoids from new apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J Agric Food Chem. 2005;53(16):6368–6374. doi: 10.1021/jf0480703. [DOI] [PubMed] [Google Scholar]
- Ruiz D, Campoy JA, Egea J. Chilling and heat requirements of apricot cultivars for flowering. Environ Exp Bot. 2007;61(3):254–263. [Google Scholar]
- Ruiz-Sanchez MC, Domingo R, Torrecillas A, Perez-Pastor A. Water stress preconditioning to improve drought resistance in young apricot plants. Plant Sci. 2000;156(2):245–251. doi: 10.1016/s0168-9452(00)00262-4. [DOI] [PubMed] [Google Scholar]
- Ruiz-Sánchez MC, Domingo R, Pérez-Pastor A. Daily variations in water relations of apricot trees under different irrigation regimes. Biol Plant. 2007;51(4):735–740. [Google Scholar]
- Scorzal R, May LG, Purnell B, Upchurch B. Differences in number and area of mesocarp cells between small-and large-fruited peach cultivars. J Am Soc Hortic Sci. 1991;116(5):861–864. [Google Scholar]
- Senthilkumaran S, Menezes RG, Jayaraman S, Thirumalaikolundusubramanian P. Acute cyanide intoxication due to apricot seeds: Is “evidence” countable? J Emerg Med. 2015;48(1):82–83. doi: 10.1016/j.jemermed.2013.08.150. [DOI] [PubMed] [Google Scholar]
- Sharma PC, Tilakratne BM, Gupta A. Utilization of wild apricot kernel press cake for extraction of protein isolate. J Food Sci Technol. 2010;47(6):682–685. doi: 10.1007/s13197-010-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Li J, Sun J, Yu J, Zhou S. Phylogeny and classification of Prunus sensu lato (Rosaceae) J Integr Plant Biol. 2013;55(11):1069–1079. doi: 10.1111/jipb.12095. [DOI] [PubMed] [Google Scholar]
- Shin H, Oh Y, Kim D. Differences in cold hardiness, carbohydrates, dehydrins and related gene expressions under an experimental deacclimation and reacclimation in Prunus persica. Physiol Plant. 2015;154(4):485–499. doi: 10.1111/ppl.12293. [DOI] [PubMed] [Google Scholar]
- Somogyi L, Barrett DM, Hui YH. Processing fruits. Boca Raton: CRC Press; 1996. [Google Scholar]
- Stushnoff C, Remmele RL, Essensee V, McNeil M. Low temperature induced biochemical mechanisms: implications for cold acclimation and de-acclimation. In: Jackson MB, Black CR, editors. Interacting stresses on plants in a changing climate. Berlin: Springer; 1993. pp. 647–657. [Google Scholar]
- Szymajda M, Pruski K, Żurawicz E, Sitarek M. Freezing injuries to flower buds and their influence on yield of apricot (Prunus armeniaca L.) and peach (Prunus persica L.) Can J Plant Sci. 2013;93(2):191–198. [Google Scholar]
- Tian H, Yan H, Tan S, Zhan P, Mao X, Wang P, Wang Z. Apricot kernel oil ameliorates cyclophosphamide-associated immunosuppression in rats. Lipids. 2016;51(8):931–939. doi: 10.1007/s11745-016-4166-5. [DOI] [PubMed] [Google Scholar]
- Vavilov NI. The origin, variation, immunity and breeding of cultivated plants. Soil Sci. 1951;72(6):482. [Google Scholar]
- Wani SM, Masoodi FA, Ahmad M, Mir SA. Processing and storage of apricots: effect on physicochemical and antioxidant properties. Journal of food science and technology. 2018;55:4505. doi: 10.1007/s13197-018-3381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster AD, Spencer JE. Fruit thinning plums and apricots. Plant Growth Regul. 2000;31(1):101–112. [Google Scholar]
- Yamaguchi M, Haji T, Yaegaki H. Differences in mesocarp cell number, cell length and occurrence of gumming in fruit of Japanese apricot (Prunus mume Sieb. et Zucc.) cultivars during their development. J Jpn Soc Hortic Sci. 2004;73(3):200–207. [Google Scholar]
- Yamane H. Regulation of bud dormancy and bud break in Japanese apricot (Prunus mume Siebold & Zucc.) and peach [Prunus persica (L.) Batsch]: a summary of recent studies. J Jpn Soc Hortic Sci. 2014;83:187–202. [Google Scholar]
- Yamshanov VA, Kovan’ko EG, Pustovalov YI. Effects of amygdaline from apricot kernel on transplanted tumors in mice. Bull Exp Biol Med. 2016;160(5):712–714. doi: 10.1007/s10517-016-3257-x. [DOI] [PubMed] [Google Scholar]
- Yilmaz KU, Gurcan K (2012) Genetic diversity in apricot. In: Genetic diversity in plants. InTech, pp 249–270
- Yilmaz I, Karaman A, Vardi N, Cetin A, Erdemli E. Effects of organic apricot on liver regeneration after partial hepatectomy in rats. Transplant Proc. 2013;45(6):2455–2460. doi: 10.1016/j.transproceed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Yılmaz KU, Ercişli S, Asma BM, Doğan Y, Kafkas S. Genetic relatedness in Prunus genus revealed by inter-simple sequence repeat markers. HortScience. 2009;44(2):293–297. [Google Scholar]
- Yoshida M. Classification of apricot varieties by RAPD analysis. J Jpn Soc Hort Sci. 1998;67:2127. [Google Scholar]
- Zhang Q-P, Liu D-C, Liu S, Liu N, Wei X, Zhang A-M, Liu W-S. Genetic diversity and relationships of common apricot (Prunus armeniaca L.) in China based on simple sequence repeat (SSR) markers. Genet Resour Crop Evol. 2014;61(2):357–368. [Google Scholar]
- Zhong W, Gao Z, Zhuang W, Shi T, Zhang Z, Ni Z. Genome-wide expression profiles of seasonal bud dormancy at four critical stages in Japanese apricot. Plant Mol Biol. 2013;83(3):247–264. doi: 10.1007/s11103-013-0086-4. [DOI] [PubMed] [Google Scholar]