Abstract
Beef jerky is a ready-to-eat product that does not require refrigeration at the point of sale. Here, we evaluated the occurrence of Listeria monocytogenes in the production process of beef jerky, the presence of virulence genes and the genomic relatedness of the isolates, to assess the safety of the final product. The raw material, surfaces with and without contact with the product and the final product were evaluated along the beef jerky processing line. The samples were evaluated by VIDAS immunoassay system, and the L. monocytogenes isolates were confirmed and evaluated for the presence of several virulence genes by PCR. Listeria monocytogenes was identified in six of the 84 samples (7.14%), and no genetic relationship was observed among isolates. Samples of raw material (2/7), food contact surface (1/56), and work surfaces without contact with food (3/14) presented contamination by L. monocytogenes. The final product was not contaminated, demonstrating that barriers to multiplication of pathogens used during the production process were effective for its control.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3505-3) contains supplementary material, which is available to authorized users.
Keywords: Meat product, Ready-to-eat, Foodborne pathogen, Virulence genes, PFGE
Introduction
Beef jerky is a very popular RTE product in the United States of America (USA) and Canada, where it is available in nearly every convenience store, gas station, supermarket, and variety shop. In Brazil, beef jerky consumption is low and production is mainly destined for exportation, generally to the USA, United Kingdom (UK) and Japan (Fernandes et al. 2017).
Beef jerky is a snack food in high demand owing to its rich flavor, nutritional value, and storage stability without refrigeration (Kim et al. 2014). Meat products with intermediate moisture, such as beef jerky, are the result of application of what is known as hurdle technology, which involves factors such as temperature, water activity, and preservatives in the preparation (Leistner 1987). Traditionally, the production of jerky consists of slicing a whole muscle or ground beef, followed by marinating it with nitrite and spices, drying and packing of the final product (Choi et al. 2008). According to the US Department of Agriculture (USDA 2014), the standard of identity for beef jerky requires a moisture-to-protein ratio value of ≤ 0.75:1, whereas to be shelf-stable, jerky must have a water activity (aw) value of ≤ 0.85. Also, the product has a shelf life of approximately 2 years when vacuum packaged and stored at room temperature (USDA 2014).
Although the manufacturing process provides barriers to microbial growth, foodborne outbreaks have been epidemiologically related with beef jerky consumption. These events were linked to Escherichia coli O157:H7, Listeria monocytogenes, Staphylococcus aureus, and several Salmonella serotypes (Dierschke et al. 2010; Kim et al. 2014). Following a 2003 salmonellosis outbreak from Salmonella Kiambu in beef jerky produced in New Mexico, the US Food Safety and Inspection Service (FSIS) published the first version of the “Compliance Guideline for Meat and Poultry Jerky Produced by Small and Very Small Establishments”. The compliance guideline is designed to help establishments that manufacture jerky to identify the critical steps needed to ensure safety and eliminate foodborne pathogens (USDA 2014).
One of the biological hazards of major concern to food-producing industries is L. monocytogenes, which is a Gram-positive, facultative anaerobic, non-spore forming rod and the etiologic agent of listeriosis. Although human listeriosis is a relatively rare disease, it is of particular concern for pregnant women, the elderly and immunocompromised individuals, with fatality rates of 20–30% being common among hospitalized patients (Vázquez-Boland et al. 2001). In the USA, L. monocytogenes is estimated to cause about 1600 cases of listeriosis with more than 1500 related hospitalizations and 260 related deaths (Scallan et al. 2011). Listeria monocytogenes is a concern for many RTE meats in view of the ability of this microorganism to survive in food processing environments and due to post-processing contamination (Gómez et al. 2015; Lansini et al. 2017; Selvaganapathi et al. 2018). Therefore, it is necessary to use fast methods of identification for this pathogen, since traditional methods require a long time for confirmation (around 8 days). For example, the immunoassay method VIDAS® (BioMérieux) is one of the fastest methods, with identification time for L. monocytogenes of only 2 days. Considering the importance for public health of contamination by L. monocytogenes in RTE products, this study aimed to evaluate the occurrence of this pathogen during the production process of beef jerky, as well as evaluate virulence genes and the genotypic profiles of the isolates to determine the relationship and spread of the pathogen in the processing line.
Materials and methods
Sampling
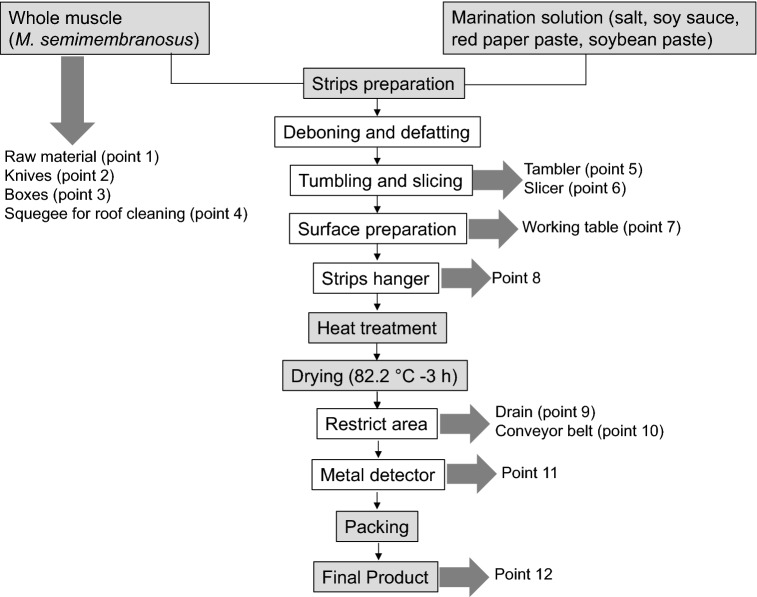
Seven sampling events were carried out in a beef jerky processing plant located in the town of Bagé, Rio Grande do Sul, Brazil. During each sampling event, samples were collected in 12 points, as described in Fig. 1. Eighty-four samples, totalizing 56 from food contact surfaces (knives, boxes, tambler, slicer, working table, strips hanger, conveyor belt, and metal detector), 14 from non-food contact surfaces (squeegee and drain), 7 from raw material, and 7 from final product were evaluated. Surfaces were sampled with swab (3 M, Minnesota, USA) using a sterilized stainless steel frame measuring 100 cm2 to delineate the sampling area. Raw material from the boning section was processed using samples of 25 g, which were placed in sterile bags (3 M, Minnesota, USA) and packaged in ice-containing boxes. Samples of the final product (beef jerky) in the packaging ready for commercialization (packaging with modified atmosphere) were also collected.
Fig. 1.
Diagram illustrating the experimental sampling procedure of the present study
Microbiological analysis
Samples from each sampling point were evaluated by VIDAS® immunoassay method (BioMérieux, Marcy-l’Étoile, France) in the laboratory of the industrial plant. The pre-enrichment and enrichment steps were performed according to the manufacturer’s instructions. All samples with positive results in VIDAS® were stored in Fraser enrichment medium (BioMérieux, Marcy-l’Étoile, France) and kept refrigerated until the biochemical confirmation at the Laboratory of Food Microbiology (DCTA/FAEM/UFPel).
Biochemical confirmation of Listeria monocytogenes
The positive samples were inoculated in Tryptone Soy broth (TSB, Neogen, Indaiatuba, Brazil) supplemented with 0.6% (w/v) yeast extract (YE, Himedia, Mumbai, India) and incubated at 37 °C for 24 h. A loopful of the culture in TSB-YE was streaked onto Oxford agar plates (OXA, Oxoid, Hampshire, UK) and Chromogenic agar plates (CHRO, Oxoid, Hampshire, UK), incubated at 37 °C for 48 h. The presumptive colonies were randomly selected and streaked onto Tryptone Soy agar (TSA, Oxoid, Hampshire, UK) supplemented with 0.6% (w/v) of YE (TSA-YE) and incubated at 37 °C for 24 h. Listeria identification was performed by biochemical tests based on the production of catalase and β-hemolysis, and fermentation of dextrose, xylose, rhamnose, and mannitol (Labsynth, Diadema, Brazil). Moreover, all the six L. monocytogenes isolates were characterized by serotyping at the Fundação Oswaldo Cruz (FIOCRUZ), Rio de Janeiro, Brazil.
Molecular characterization
Genomic DNA of L. monocytogenes isolates was extracted according to the protocol described by Green and Sambrook (2012) with minor adaptations. The confirmation of Listeria genus was performed by the presence of the prs gene. Furthermore, the identification of virulence genes hlyA, prfA, plcA, plcB, actA, mpl, iap, inlA, inlB, inlC, and inlJ was also performed. Oligonucleotide sequences used are listed in Table S1. The reactions were performed using a final volume of 25 µL with 12.5 µL GoTaq Green Master Mix (Promega, Wisconsing, USA), 20 pmol of each oligonucleotide and 10 ng/µL DNA template.
The PCR products were submitted to electrophoresis on 1.5% (w/v) agarose gel (Invitrogen, Waltham, USA) stained with GelRed™ (Biotium, Fremont, USA), with a 1 kb molecular weight marker (Invitrogen, Waltham, USA). The amplification products were visualized under UV light in a transilluminator (Loccus, Cotia, Brazil).
Sequencing
Two isolates that were not identified by conventional standard serological test were submitted to sequencing of the inlC gene at the genomic facility of the Unidade de Análises Moleculares e de Proteínas, Centro de Pesquisa Experimental, Hospital de Clínicas de Porto Alegre, Porto Alegre, RS, Brazil, using an ABI 3500 Genetic Analyzer (Applied Biosystems, Foster City, USA). The obtained sequences were confirmed for L. monocytogenes species using Basic Local Alignment Search Tool (Nucleotide BLAST®, https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Pulsed-field gel electrophoresis (PFGE)
The genomic relatedness of L. monocytogenes isolates was investigated by macrorestriction analysis with ApaI and AscI endonucleases (Promega, Wisconsin, USA). For this, the standardized laboratory protocol for L. monocytogenes of PulseNet was followed (Graves and Swaminathan 2001). The DNA fragments were separated by PFGE using the CHEF DR II system (Bio-Rad, Hercules, USA) at 6.0 V/cm with 0.5X TBE as the running buffer. Whole cell DNA of Salmonella Branderup H9812 digested with XbaI endonuclease served as size marker. Macrorestriction patterns were analyzed using the BioNumerics software package (Applied Maths, Sint-Martens Latem, Belgium), and the similarities between the patterns were compared based on the Dice correlation coefficient, with a maximal position tolerance of 1.5%. Patterns were clustered according to the unweighted pair group method with arithmetic averages (UPGMA). Fragments smaller than 20 kb were not considered for cluster analysis.
Results and discussion
Considering the ever-increasing attention to food safety issues, there is a growing need for fast and reliable methods for detecting pathogens such as L. monocytogenes in food samples. In this study, the automated food pathogen detection system VIDAS® was used to isolate L. monocytogenes from samples obtained from a beef jerky industry. Among the seven sampling events carried out, L. monocytogenes was isolated just in the first and second sampling (Table 1). VIDAS® identified six (7.14%) of the 84 samples from beef jerky production line positive for L. monocytogenes (Table 2). These positive samples were immediately sent to the Laboratory of Food Microbiology (UFPel) for biochemical characterization and PCR confirmation. Both biochemical characterization and PCR assays confirmed the presence of L. monocytogenes in the six samples tested positive in VIDAS®. Meyer et al. (2011) detected L. monocytogenes in 4% of beef and pork samples by using VIDAS®, while just 3% were confirmed in selective agars.
Table 1.
Occurrence of Listeria monocytogenes in a beef jerky processing line during seven sampling events
| Origin | Sampling event | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | ||
| Raw material | 1/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 2/7 (28.6%) |
| Food contact surface | 1/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 1/56 (1.8%) |
| Non-food contact surface | 2/2 | 1/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 3/14 (21.4%) |
| Final product | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/7 |
| Total | 4/12 (33.3%) | 2/12 (16.7%) | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 6/84 (7.1%) |
Table 2.
Occurrence of Listeria monocytogenes in a beef jerky processing line
| Samples | Number of analyzed samples | Number of positive samples (%) | Sampling event |
|---|---|---|---|
| Raw material | 7 | 2 (28.6%) | 1st and 2nd |
| Food contact surface | |||
| Meat knives | 7 | 0 | – |
| Boxes | 7 | 0 | – |
| Tambler | 7 | 0 | – |
| Slicer | 7 | 0 | – |
| Working table | 7 | 1 (14.3%) | 1st |
| Strips hanger | 7 | 0 | – |
| Conveyor belt | 7 | 0 | – |
| Metal detector | 7 | 0 | – |
| Non-food contact surface | |||
| Squeegee | 7 | 2 (28.6%) | 1st and 2nd |
| Drain | 7 | 1 (14.3%) | 1st |
| Final product | 7 | 0 | – |
| Total | 84 | 6 (7.14%) | 1st and 2nd |
The results of serotyping showed that one isolate belonged to serotype 4b, three to 1/2a and two isolates were not identified by the serotyping test. It should be noted that although any L. monocytogenes can be potentially virulent, the serotypes 1/2a, 1/2b and 4b are the main serotypes associated with human listeriosis, suggesting risks of L. monocytogenes infection to exposed consumers (Haubert et al. 2016; Orsi et al. 2011). The two isolates that were not identified by serotyping (Lm3 and Lm4) were subjected to sequencing of the inlC, a conserved and specific gene of L. monocytogenes species (Liu et al. 2007). According to Nucleotide BLAST®, the isolates showed 99% of similarity with other L. monocytogenes deposited in the database. Thereafter, the identified sequences of these two isolates were added to the GenBank of the National Center for Biotechnology Information (NCBI©, htttp://www.ncbi.nlm.nih.gov/genbank) under accession numbers MH190784 and MH190785.
The prs gene was used to identify the Listeria genus, and all six isolates representative of each sample amplified a 370 bp amplicon specific for this gene. The inlA, inlB, inlC and inlJ genes, encoding inlA, inlB, inlC and inlJ proteins, which are involved in cell invasion (Doumith et al. 2004; Liu et al. 2007) were also identified in all isolates. Four L. monocytogenes isolates (66.7%) carried genes for LIPI-1 (hlyA, prfA, plcA, plcB, actA, and mpl) as well as iap gene; however, one isolate was negative for the prfA gene. Two isolates (33.3%) were negative for LIPI-1 and the iap gene; however, one of them was positive for the plcA gene.
Probably the non-amplification of some virulence genes in the isolates can correspond to mutations in the specific gene regions, leading to non-amplification of the respective gene by PCR assay (Maury et al. 2017). Further studies are necessary to unravel these points. On the other hand, Iglesias et al. (2017) evaluated the same 11 virulence genes assessed in this study and verified that all 12 L. monocytogenes isolates from bovine carcasses carried these genes.
The occurrence of L. monocytogenes in beef and meat products is described in several countries, emphasizing the possibility of contamination during processing, even in the final and RTE products (Gómez et al. 2015; Meloni et al. 2014). On the other hand, there are only limited data in the literature describing L. monocytogenes contamination in dried foods such as beef jerky. The USDA conducted microbiological analysis in RTE meat and poultry products at production facilities and reported four of 770 beef jerky samples contaminated with L. monocytogenes over a 10-year period (Levine et al. 2001). It is interesting to note that most countries apply a “zero-tolerance” policy for L. monocytogenes in RTE meat products, including beef jerky.
In our study, L. monocytogenes was found in raw material (2/7), food contact surface (1/56), and work surfaces without contact with food (3/14). The final product was not contaminated (0/7) (Table 2), highlighting that the barriers were adequate to ensure product safety. Moreover, the steps in the production of beef jerky, including heat treatment and marinade solution, which promote high salt concentration, sodium nitrite content and water activity reduction, were able to inhibit L. monocytogenes.
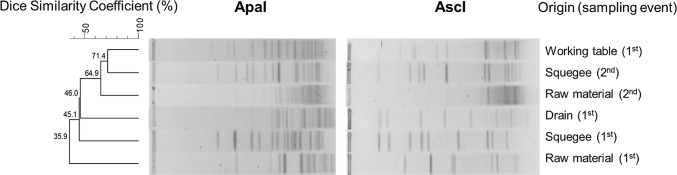
Among the raw material (whole muscle), two of the seven samples showed L. monocytogenes contamination. It is a worrying result, considering the possibility of cross-contamination on work surfaces, as well as to manipulators and in the final product. Our results reinforce the importance of proper supplier selection (Daelman et al. 2013) and of microbiological control of the raw material used for the manufacture of meat products, especially in those that will be commercialized as RTE, such as beef jerky (Todd and Notermans 2011). The two isolates from raw material belonged to different PFGE profiles, indicating different contamination sources (Fig. 2).
Fig. 2.
Dendogram of Listeria monocytogenes isolates based on ApaI and AscI-macrorestriction (PFGE) patterns. The similarity analysis was performed using the Dice coefficient and UPGMA method (tolerance 1%). The origin of the isolates and sampling events are shown
Only one (1.78%) of the 56 samples from food contact surfaces collected showed L. monocytogenes. The microorganism was isolated from the table (point 7) in the preparation area for marinated strips at the first sampling event. The PFGE pattern observed in this isolate differed (35.9% of similarity) from that found in the raw material at the same sampling event, suggesting that there was no cross-contamination between the raw material and the table. Similarly, L. monocytogenes was detected in four of 226 environmental food contact surface samples (1.8%) in the production process of processed foods of extended durability (Daelman et al. 2013). It is noteworthy that the contamination rates of food contact surfaces by L. monocytogenes is quite controversial among different studies, because they depend on variables such as plant structure, implementation and execution of quality programs in the industry. For example, Nalério et al. (2009) found 29.2% of the food contact surfaces contaminated by L. monocytogenes in a broiler slaughterhouse, while Meloni et al. (2014) observed 9.7% in a fermented sausage processing plant.
Regarding the surfaces without contact with food, L. monocytogenes was present in three of 14 samples, two from the squeegee and one from the drain in the restricted area (Fig. 1). The two positive squeegee samples were obtained at different sampling events (1st and 2nd), demonstrating the importance of this point for the contamination in this processing line. In addition, the presence of L. monocytogenes at the same point during two sampling events is an important result, since there are strains that become persistent in food processing plants, remaining for years in the industrial environment and causing recurrent contamination of the final product (Warriner and Namvar 2009). However, the PFGE analysis demonstrated that these two positive squeegee samples were not genetically related (46% of similarity), and probably are from different contamination sources. The isolation of L. monocytogenes from the drain in the restricted area confirms that drains, floors, curved pipes and air systems can be reservoirs and sources of contamination by this microorganism to the final product (Meloni et al. 2014).
Although L. monocytogenes has been isolated from non-food contact surfaces, these can be sources of contamination for the final product and contribute to the persistence of this microorganism in the environment of the food processing plant. According to Carpentier and Cerf (2011), the persistence of L. monocytogenes in food processing plants is related to the difficulty of cleaning in places where these microorganisms are housed, as well as their ability to multiply at low temperatures. In 2017, an outbreak of listeriosis occurred due to the consumption of raw milk soft cheeses (CDC 2017), in which the main source of contamination was the processing environment. Control and prevention measures to avoid contamination by L. monocytogenes between surfaces and products should be standardized in industries producing RTE foods (Luber et al. 2011). It should be noted that although the beef jerky processing plant evaluated in this study follows the USDA/FSIS (2014) recommendations regarding the implementation of monitoring, control and prevention programs, L. monocytogenes was detected in the industry environment samples.
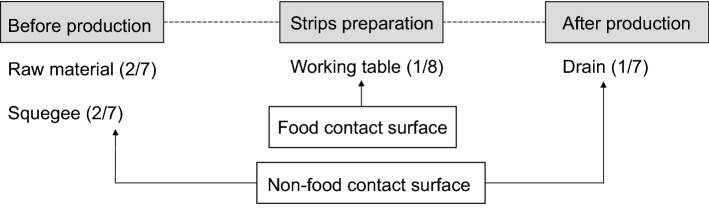
On the other hand, no contamination by L. monocytogenes was found in the final product. The barriers such as temperature (82.2 °C for approximately 3 h), marination and reduction of water activity (less than 0.80), ensured a final product with satisfactory sanitary quality and safety for the consumer. In addition, the use of organic acids, nitrite and spices, helps in the process of microbial reduction, considering that all these components form barriers preventing the growth of microorganisms (Freitas de Macedo et al. 2013). This was observed in this study, considering that although L. monocytogenes was isolated from the raw material (28.57%) and on surfaces within the industry (5.71%), no contamination of the final product was found in any sample of the final product. It is noteworthy that L. monocytogenes was isolated from a drain in the restricted area; therefore, this occurred after heat treatment and drying of the beef jerky (Fig. 3), which highlights concerns about the possibility of cross-contamination to final product.
Fig. 3.
Stages and number of positive samples of L. monocytogenes in the beef jerky production line
Conclusion
Listeria monocytogenes was isolated in raw material and on surfaces with or without food contact in the industrial plant during the production process of beef jerky. The raw material is an important source of contamination and introduction of L. monocytogenes in this industry. In addition, the presence of this microorganism on surfaces with and without contact with food reinforces the importance of hygiene programs as a way of avoiding cross-contamination of the final product. Most isolates harbored all the virulence genes evaluated; however, the isolates showed a low genetic relationship, indicating different contamination sources.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The authors extend their thanks to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (309101/2016-6) and Programa Nacional de Pós-Doutorado (PNPD).
References
- Carpentier B, Cerf O. Review—persistence of Listeria monocytogenes in food industry equipment and premises. Int J Food Microbiol. 2011;145:1–8. doi: 10.1016/j.ijfoodmicro.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Center for Diseases Control and Prevention (CDC) (2017) Available at: https://www.cdc.gov/listeria/outbreaks/soft-cheese-03-17/index.html
- Choi JH, Jeong JY, Han DJ, Choi YS, Kim HY, Lee MA, Lee ES, Paik HD, Kim CJ. Effects of pork/beef levels and various casing on quality properties of semi-dried jerky. Meat Sci. 2008;80:278–286. doi: 10.1016/j.meatsci.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Daelman J, Jacxsens L, Lahou E, Devleighere F, Uyttendaele M. Assessment of the microbial safety and quality of cooked chilled foods and their production process. Int J Food Microbiol. 2013;60:193–200. doi: 10.1016/j.ijfoodmicro.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Dierschke S, Ingham SC, Ingham BH. Destruction of Escherichia coli O157:H7, Salmonella, Listeria monocytogenes, and Staphylococcus aureus achieved during manufacture of whole-muscle beef jerky in home-style dehydrators. J Food Prot. 2010;73:2034–2042. doi: 10.4315/0362-028X-73.11.2034. [DOI] [PubMed] [Google Scholar]
- Doumith M, Buchrieser C, Glaser P, Jacquet C, Martin P. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J Clin Microbiol. 2004;42:3819–3822. doi: 10.1128/JCM.42.8.3819-3822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes FP, Voloski FLS, Ramires T, Haubert L, Reta GG, Mondadori RG, Silva WP, Conceição RCS, Duval EH. Virulence and antimicrobial resistance of Salmonella spp. and Escherichia coli in the beef jerky production line. FEMS Microbiol Lett. 2017;364:1–8. doi: 10.1093/femsle/fnx083. [DOI] [PubMed] [Google Scholar]
- Freitas de Macedo RE, Miyague L, Costa LB, Luciano FB. Control of Listeria monocytogenes growth by bacteriocin-producing starter cultures in the manufacturing of dry fermented sausage. Afr J Microbiol. 2013;7:710–718. [Google Scholar]
- Gómez D, Iguácel LP, Rota MC, Carraminãna JJ, Ariño A, Yangüela J. Occurrence of Listeria monocytogenes in ready-to-eat meat products and meat processing plants in Spain. Foods. 2015;4:271–282. doi: 10.3390/foods4030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves LM, Swaminathan B. PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int J Food Microbiol. 2001;65:55–62. doi: 10.1016/S0168-1605(00)00501-8. [DOI] [PubMed] [Google Scholar]
- Green MR, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2012. [Google Scholar]
- Haubert L, Mendonça M, Lopes GV, Cardoso MRI, Silva WP. Listeria monocytogenes isolates from food and food environment harbouring tetM and ermB resistance genes. Lett Appl Microbiol. 2016;62:23–29. doi: 10.1111/lam.12516. [DOI] [PubMed] [Google Scholar]
- Iglesias MA, Kroning IS, Decol LT, Franco BDGM, Silva WP. Occurrence and phenotypic and molecular characterization of Listeria monocytogenes and Salmonella spp. in slaughterhouses in southern Brazil. Food Res Int. 2017;100:96–101. doi: 10.1016/j.foodres.2017.06.023. [DOI] [PubMed] [Google Scholar]
- Kim JS, Lee EJ, Choi EH, Kim YJ. Inactivation of Staphylococcus aureus on the beef jerky by radio-frequency atmospheric pressure plasma discharge treatment. Innov Food Sci Emerg Technol. 2014;22:124–130. doi: 10.1016/j.ifset.2013.12.012. [DOI] [Google Scholar]
- Lansini V, Maia DSV, Prates DF, Lima AS, Silva WP. Antibacterial activity of Timsen® (n-alkyl dimethyl benzyl ammonium chloride-40%) in scalding and precooling water in poultry slaughterhouses. J Food Sci Technol. 2017;54:2607–2612. doi: 10.1007/s13197-017-2660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistner L. Shelf stable product and intermediate moisture foods based on meat. In: Rockland L, Beuchat LB, editors. Water activity theory and application to foods. New York: Marcel Dekker Inc.; 1987. pp. 295–328. [Google Scholar]
- Levine P, Rose B, Green S, Ranson G, Hill W. Pathogen testing of ready-to-eat meat and poultry products collected at federally inspected establishments in the United States, 1990 to 1999. J Food Prot. 2001;64:1188–1193. doi: 10.4315/0362-028X-64.8.1188. [DOI] [PubMed] [Google Scholar]
- Liu D, Lawrence ML, Austin FW, Ainsworth AJ. A multiplex PCR for species and virulence-specific determination of Listeria monocytogenes. J Microbiol Methods. 2007;71:133–140. doi: 10.1016/j.mimet.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Luber P, Crerar S, Dufour C, Farber J, Datta A, Todd ECD. Controlling Listeria monocytogenes in ready-to-eat foods: working towards global scientific consensus and harmonization—recommendations for improved prevention and control. Food Control. 2011;22:1535–1549. doi: 10.1016/j.foodcont.2011.01.008. [DOI] [Google Scholar]
- Maury MM, Chenal-Francisque V, Bracq-Dieye H, Han L, Leclercq A, Vales G, Moura A, Gouin E, Scortti M, Disson O, Vázquez-Boland JA, Lecuit M. Spontaneous loss of virulence in natural populations of Listeria monocytogenes. Infect Immun. 2017;85:e00541-17. doi: 10.1128/IAI.00541-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni D, Cosolati SG, Muredu A, Mazza R, Fois FP, Mazzette R. Presence and molecular characterization of the major serovars of Listeria monocytogenes in ten Sardinian fermented sausage processing plants. Meat Sci. 2014;97:443–450. doi: 10.1016/j.meatsci.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Meyer C, Fredriksson-Ahomaa M, Sperner B, Märtlbauer E. Detection of Listeria monocytogenes in pork and beef using VIDAS® LM02 automated enzyme linked immunoassay method. Meat Sci. 2011;88:594–596. doi: 10.1016/j.meatsci.2011.01.035. [DOI] [PubMed] [Google Scholar]
- Nalério ES, Araújo MR, Mendonça KS, Bassani MT, Silva WP. Listeria monocytogenes: monitoramento desse perigo biológico na cadeia produtiva de frangos do sul do Rio Grande do Sul. Food Sci Technol. 2009;29:626–630. doi: 10.1590/S0101-20612009000300026. [DOI] [Google Scholar]
- Orsi RH, Den Bakker HC, Wiedmann M. Listeria monocytogenes lineages: genomics, evolution, ecology, and phenotypic characteristics. Int J Med Microbiol. 2011;301:79–96. doi: 10.1016/j.ijmm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaganapathi R, Jeyasekaran G, Shakila RJ, Sukumar D, Kumar MP, Sivaraman B. Occurrence of Listeria monocytogenes on the seafood contact surfaces of Tuticorin Coast of India. J Food Sci Technol. 2018;55:1–5. doi: 10.1007/s13197-018-3230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd ECD, Notermans S. Surveillance of listeriosis and its causative pathogen, Listeria monocytogenes. Food Control. 2011;22:1484–1490. doi: 10.1016/j.foodcont.2010.07.021. [DOI] [Google Scholar]
- USDA—United States Department of Agriculture. Food Safety and Inspection Service (USDA–FSIS) (2014) Compliance guideline for meat and poultry jerky produced by small and very small establishments compliance guideline. Available at: https://www.fsis.usda.gov/wps/wcm/connect/5fd4a01d-a381-4134-8b91-99617e56a90a/Compliance-GuidelineJerky2014.pdf?MOD=AJPERES
- Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, González-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warriner K, Namvar A. What is the hysteria with Listeria? Food Sci Technol. 2009;20:245–254. doi: 10.1016/j.tifs.2009.03.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.