Abstract
Sprouted and fermented foods have shown hypoglycemic effects on humans and animals, by reducing concentrations of soluble carbohydrates, and increasing dietary fiber and resistant starch content. In this study, diets with high levels of simple carbohydrates supplemented with toasted quinoa flour, sprouted and toasted quinoa flour, fermented and toasted quinoa flour or sprouted/fermented and toasted quinoa flour were given to Wistar rats. During the experiment, the glycemic index (GI) of the diets were measure and, at the end of 47 days of feeding, the effects of the diets on physical and biochemical parameters of the animals were evaluated. Results indicated that the processes of sprouting and/or fermentation potentiate the ability of quinoa to reduce GI of diets with high levels of simple carbohydrates. Moreover, food intake, blood glucose and lipid levels, and accumulation of epididymal adipose tissue were reduced in rats fed diets supplemented with quinoa. These effects may be due to the nutritional composition of the supplemented diets, besides the chemical changes promoted by processing quinoa. These results are particularly relevant once sprouted and fermented quinoa could be an alimentary source of interest, especially for disease risk prevention such as diabetes, obesity and dyslipidemias.
Keywords: Adipose tissue, Blood glucose, Cholesterol, Glycated hemoglobin, Glycemic index
Introduction
Quinoa (Chenopodium quinoa) is a pseudocereal of Andean origin that is being widely disseminated across continents because it has key nutritional qualities (Navarro-Perez et al. 2017). It is classified as a low glycemic index (GI) food because it provides slow glucose release into the bloodstream following its intake (Onwulata et al. 2008).
Low-GI foods have beneficial physiological effects related to the reduction of glucose and insulin responses; the production of hypocholesterolemic responses; protection against weight gain, obesity and colon cancer; and reducing the risk of developing type II diabetes (Augustin et al. 2015; Sacks et al. 2014).
The GI of a food depends on the chemical and structural properties of its granules, which may undergo changes resulting from processing, including milling, baking, sprouting and fermenting (Abdel-Aal and Rabalski 2008). More specifically, sprouted and fermented foods have shown hypoglycemic effects on humans and animals, and the likely mechanisms include reduced concentrations of soluble carbohydrates, increased dietary fiber levels and increased levels of resistant starch (Östman et al. 2001; Hsu et al. 2008; Scazzina et al. 2009). In addition, the fermentation and/or germination are viewed as desirable methods for seeds processing because of their low cost and low energy requirements, besides contributing to acceptable and diversified flavours for human consumption (Singh et al. 2015).
Sprouted seeds are well known sources of enzymes and nutritive compounds that have potential to stimulate the growth and metabolism of microorganisms (Diowksz et al. 2014). Sprouting seeds requires the expenditure of energy derived from catabolism of their own reserves, which are mainly carbohydrates, lipids and proteins. The reduced GI of sprouted quinoa seeds presumably results from the metabolism of soluble sugars and soluble starch for the provision of energy to plant cells and for the synthesis of fibers, which are important for new tissues (Silva et al. 2008). Conversely, the fermentation process increases levels of resistant starch and delays gastric emptying caused by organic acids derived from fermentation, which tend to reduce the GI (Östaman et al. 2002; Scazzina et al. 2009).
Given the above, this study aimed to assess the GI of diets containing sprouted and fermented quinoa and to evaluate changes in physiological parameters of Wistar rats fed the aforementioned diets.
Materials and methods
Flour production
Quinoa seeds (14 kg) were washed in potable water, using fine frame sieves. This process aimed at removing saponins and were conducted until there were no more foaming. Then, seeds were divided in two fractions (7 kg each one). One fraction was dehydrated in forced air circulation oven at 35 °C for 72 h, and milling in knife and sieving mill, to obtain quinoa flour. The other fraction of seeds was reserved for sprouting.
The sprouting of quinoa seeds was performed in sprouters using paper substrate according to the method described by Schabes and Sigstad (2004) at 25 ± 2 °C. The sprouted seeds were dehydrated in a forced-air oven at 35 °C for 72 h and were subsequently ground and sieved, thus producing the sprouted quinoa flour.
A portion of both flour fractions was naturally fermented by adding 0.5% of glucose in relation to the total mass of flour, in glass recipients, containing distilled water (Avancini 2007) at 26 °C ± 2 °C. Measures of pH, soluble solids and titratable acidity of were taken daily. The process finished when titratable acidity stabilized (AOAC 2007).
The fermented quinoa flour and the sprouted/fermented quinoa flour underwent a drying process in a forced-air oven at 35 °C for 72 h after reaching the maximum degree of fermentation. A dough was prepared from each flour, that were subsequently toasted. The dough was produced by adding distilled water to the raw flour in sufficient quantity to mold it into the shape of a biscuit and then placed in the oven for 40 min at the temperature of 200 °C (Cereda 1983). After cooling, the cookies were ground in a knife mill, thus producing toasted quinoa flour (TQF), sprouted and toasted quinoa flour (STQF), fermented and toasted quinoa flour (FTQF), and sprouted/fermented and toasted quinoa flour (SFTQF). The finished flour was stored in rigid polyethylene containers at 5 °C, protected from moisture and light until preparing of the experimental diets.
Experimental animals and diets
The study was conducted according to the Ethical Principles for Animal Experimentation and it was previously approved by the Ethics Committee on Animal Use of the Federal University of Lavras, protocol n. 2010038.
Thirty-six adult male Wistar rats aged 56 days, normoglycemic and with an average weight of 233.27 ± 27.2 g were used for the in vivo assay. The animals were weighed and randomized into six groups with six rats in each (n = 6), named Group 1, Group 2, Group 3, Group 4, Group 5 and Group 6, which were fed the diets A, B, C, D, E and F, respectively. The composition of the diets followed the AIN-93M standards (Reeves et al. 1993) for Group 1, and AIN-93M standards with adaptations regarding starch and glucose content (for the other groups). These compositions are outlined in Table 1.
Table 1.
Composition of the standard control diet (AIN-93M), control diet with high glycemic index and diets with high glycemic index and supplementation with processed quinoa flour (AIN-93M adapted to experimental conditions) (g/kg)
| Ingredients | Diets (g/kg) | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| Corn starch | 465.62 | 150 | – | – | – | – |
| Casein | 140 | 140 | 140 | 140 | 140 | 140 |
| Dextrin | 155 | 155 | 155 | 155 | 155 | 155 |
| Sucrose | 100 | 100 | 100 | 100 | 100 | 100 |
| Soybean oil | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 |
| Cellulose | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 |
| Mineral pre-mix | 35.00 | 35.00 | 35.00 | 35.00 | 35.00 | 35.00 |
| Vitamin pre-mix | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| l-Cysteine | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 |
| Choline bitartrate | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Tert-butylhydroquinone | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 |
| Glucose | – | 315.62 | 315.62 | 315.62 | 315.62 | 315.62 |
| TQF | – | – | 150 | – | – | – |
| STQF | – | – | – | 150 | – | – |
| FTQF | – | – | – | – | 150 | – |
| SFTQF | – | – | – | – | – | 150 |
| Total (g) | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
TQF toasted quinoa flour, STQF sprouted and toasted quinoa flour, FTQF fermented and toasted quinoa flour, SFTQF sprouted/fermented and toasted quinoa flour
The animals were kept in individual metabolic cages under controlled temperature (21 ± 3 °C) and 12-h light/dark cycle. The experiment was conducted for 47 days and was divided into three phases. The first phase occurred from the 1st to the 3rd day and corresponded to an adaptation phase and establishment of standard nutrients in the body of the animals. In this period, the animals fed the standard diet (diet A ad libitum). The next phase started on the 4th day and corresponded to the glycemic index assay stage, using the experimental diets, which lasted until the 7th day of experiment. Further details are presented below. The third phase started on the 8th day and extended until the 47th day, when biochemical analysis and tissue evaluation were conducted.
Food intake and weight gain
Animal intake and weight gain were evaluated for 41 days every 3 days from the start of the third experimental phase to enable evaluating animal development, average daily intake (ADI), average daily weight gain (ADWG) and the feed efficiency coefficient (FEC). These indexes were calculated according to method proposed by Pellet and Young (1980). All of these indices were calculated individually, enabling the mean and coefficient of variation to be found for each group. The animals were provided water ad libitum throughout the experiment.
Fasting blood glucose, postprandial blood glucose and glycemic index
This phase started at day 4 and lasted 4 days, being the first 3 days a training period, before to obtain the glycemic measures on day 7. In this period, a total of 3 g of experimental diets A, B, C, D, E and F was provided to groups 1, 2, 3, 4, 5, and 6, respectively within a pre-set time (15 min).
During the 4 days, the animals were deprived of nocturnal feeding during this phase, i.e. from 8:00 pm till 8:00 am (12 h). Thus, they would ingest the 3 g of experimental diet provided within a 15-min time period at 8:00 am. The feeder was removed after 15 min, even if the animal had not fully consumed the portion provided. The animals were then fed ad libitum for a 6-h period, from 2:00 pm to 8:00 pm (Lerer-Metzger et al. 1996; Kabir et al. 2000). On the 7th experimental day, after 3 days of training and 12 h of fasting, measures of blood glucose were taken to assess the glycemic response and to calculate the glycemic index of the diets.
For this, measurements of capillary glucose levels from the tail vein of each animal were taken, following the method proposed by de Angelis-Pereira et al. (2016). Blood glucose levels were monitored using glucometers and Accu-Chek® Compact Plus® (Roche Diagnostics, Japan) glucose test strips. Then, the animals were subsequently fed ad libitum their respective diet at the pre-established amount of 3 g for a time period of 15 min, thus enabling postprandial blood glucose to be evaluated. The first puncture performed at the end of the pre-set 15 min was defined as time 0 (zero). The analyses continued thenceforth every 15 min until 3 h (180 min) had elapsed, totaling 12 evaluations per animal with values expressed as mg/dl blood.
To calculate postprandial blood glucose, the blood glucose results (mg/dl) of each animal were added and divided by 12, which corresponded to the number of evaluations. The glycemic indices (GI) of the respective experimental diets were calculated based on the increase in the area under the glycemic curves recorded from the postprandial blood glucose concentration evaluation of the animals (de Angelis-Pereira et al. 2016).
Fasting glucose was measured after 12 h of fasting on the 1st and 47th days of experiment, by taking a sample of blood from the tail vein of each animal, to measure capillary glucose levels, following the method proposed by de Angelis-Pereira et al. (2016), using glucometers and Accu-Chek® Compact Plus® (Roche Diagnostics, Japan) glucose test strips.
Glycated hemoglobin, triglycerides, total cholesterol and cholesterol fractions
The animals were sedated and anesthetized with sodium thiopental (40 mg/kg) after the blood glucose test on the 47th day. The abdominal aorta was sectioned to collect approximately 6 ml of blood sample. Of these, 3 ml were collected in a heparinized tube to assess the glycated hemoglobin concentration (HbA1) using enzymatic kit (HBA1C TURBIQUEST, LABTEST, Lagoa Santa, MG, Brazil) for whole blood by immunoturbidimetry, with limits of assay range between 3 and 13%. Analysis was conducted according to instructions provided by manufacturer.
Another fraction of 3 ml of blood sample were collected in a nonheparinized tube to assess total cholesterol, cholesterol fraction and triglycerides; the nonheparinized tube was centrifuged to separate the serum used in the analysis. The concentrations of the very low- (VLDL-c) and high-density (HDL-c) lipoprotein–cholesterol fractions were determined using enzymatic kit (LABTEST, Lagoa Santa, MG, Brazil) and calculated using the equation of Friedewald, wherein:
Euthanasia and organ dissection
The animals, after being sedated and anesthetized, undergone cardiac arrest followed by death due to hypovolemia after blood collection. After death, liver and epididymal adipose tissue were dissected, washed with saline, and immediately weighted using an analytical scale. Mean weight of the liver and adipose tissue where then calculated for each group.
Experimental design and statistical analyses
The experimental design was a completely randomized (CRD), including six treatments that were analyzed in six replicates. A 6 × 2 factorial scheme was applied to the fasting blood glucose analysis, including 6 types of diets and 2 time periods (start and end of the experiment). The results were expressed as means and percentage of the coefficient of variation (CV%). Analysis of variance was used, followed by Tukey’s test at 5% significance in the Sisvar software (Ferreira 2008). The assumptions of ANOVA were checked through Shapiro–Wilk’s and Bartlett’s tests.
Results
Among the six different diets prepared in this study, the animals consuming diets D and F, which contained sprouted quinoa flour, showed significantly lower ADI (16.11 and 14.86 g, respectively) (p ≤ 0.05) than the animals consuming diets A and B (22.90 and 18.79 g). These results are in accordance with the ADWG and FEC, which were higher in groups D and F (Table 2). FEC is inversely proportional to ADI and directly proportional to ADWG, that is, the smaller the ADI and the greater the ADWG are, the greater the FEC will be.
Table 2.
Average daily intake (ADI), average daily weight gain (ADWG) and feed efficiency coefficient (FEC) of experimental animals during the second stage of treatment and average weight of epididymal adipose tissue (g) and the liver (g) at the end of the study followed by the coefficient of variation expressed as a percentage (CV%)
| Group | Dietary intake control | Liver (g) | Epididymal adipose tissue (g) | ||
|---|---|---|---|---|---|
| ADI (g) | ADWG (g) | FEC | |||
| 1 (diet A) | 22.90a | 1.87b | 0.094b,c | 8.15 | 5.11b |
| 2 (diet B) | 18.79a,b,c | 2.22a | 0.130a | 8.36 | 5.65b |
| 3 (diet C) | 17.99a,b,c | 1.94a,b | 0.102b | 8.29 | 1.69a |
| 4 (diet D) | 16.11b,c | 1.70b,c | 0.098b,c | 6.56 | 1.88a |
| 5 (diet E) | 20.27a,b | 1.30d | 0.073d | 6.71 | 1.48a |
| 6 (diet F) | 14.86c | 1.45c,d | 0.084c,d | 7.54 | 1.75a |
| CV% | 16.86 | 29.83 | 10 | 15.8 | 26.2 |
Group 1 (standard diet), group 2 (diet with high glucose levels, 31.5%), group 3 (diet with high glucose levels and 15% toasted quinoa flour), group 4 (diet with high glucose levels and 15% sprouted and toasted quinoa flour), group 5 (diet with high glucose levels and 15% fermented and toasted quinoa flour) and group 6 (diet with high glucose levels and 15% sprouted/fermented and toasted quinoa flour). Means followed by the same letter in columns are not significantly different from each other according to Tukey’s test at 5% significance
The average weight of epididymal adipose tissue in animals from groups 3, 4, 5 and 6 (1.69, 1.88, 1.48 and 1.75) fed diets C, D, E and F, respectively, with high levels of simple carbohydrates and supplementation with processed quinoa flour, was significantly (p ≤ 0.05) lower than that of groups 1 and 2 (5.11 and 5.65), which were fed the control diets A and B (Table 2).
The average liver weight of the animals did not differ between the groups, suggesting that none of the six diets promoted some type of alteration because they caused no liver hypertrophy, which could be indicative of toxicity. Details are shown in Table 2.
Initial and final fasting blood glucose, postprandial blood glucose and GI
Processed quinoa flour added to the experimental diets had a protective effect against increased blood glucose in the animals. The maintenance or reduction of the mean initial fasting blood glucose in groups 3, 4, 5 and 6 (81.75, 77.77, 81.50 and 80.40 mg/dl, respectively) compared to the final fasting blood glucose (69.60, 69.58, 73.83 and 75 mg/dl, respectively) may be due to incorporation of quinoa flour in the diet, once control groups 1 and 2 showed an increase in blood glucose over time (from 77.4 and 72 to 78 and 79.4 mg/dl, respectively) (Table 3).
Table 3.
Initial and final mean fasting blood glucose (mg/dl) and postprandial blood glucose of animals subjected to treatment with the experimental diets and coefficient of variation in percentage (CV%)
| Groups | Inicial blood glucosea (mg/dl) | Final blood glucosea (mg/dl) | Postprandial blood glucose (mg/dl) |
|---|---|---|---|
| 1 (diet A) | 77.40a | 78.00a | 142.86a |
| 2 (diet B) | 72.00b | 79.40ª | 140.88a,b |
| 3 (diet C) | 81.75ª | 69.60ª | 135.47a,b,c |
| 4 (diet D) | 77.66ª | 69.58b | – |
| 5 (diet E) | 81.50ª | 73.83ª | 135.80a,b,c |
| 6 (diet F) | 80.30ª | 75.00a | 132.12b,c |
| CV% | – | – | 10.73 |
aComparison between the fasting blood glucose of animals on the first and the last days of treatment. Group 1 (diet A, standard), group 2 (diet B, with high glucose levels, 31.5%), group 3 (diet C, with 31.5% glucose and 15% toasted quinoa flour), group 4 (diet D, with 31.5% glucose and 15% sprouted and toasted quinoa flour), group 5 (diet E, with 31.5% glucose and 15% fermented and toasted quinoa flour) and group 6 (diet F, with 31.5% glucose and 15% sprouted/fermented and toasted quinoa flour). Means of postprandial blood glucose followed by the same letter in columns and means of initial and final fasting blood glucose followed by the same letter in rows are not significantly different from each other according to Tukey’s test at 5% significance
Animals from groups 3, 4, 5 and 6 fed diets C, D, E and F, respectively, showed lower postprandial blood glucose in comparison to animals from groups 1 and 2 fed the control diets A and B, respectively. Group 1 showed the greatest increase (142.86 mg/dl), and this result was significantly (p ≤ 0.05) greater than that of group 6 (132.12 mg/dl), supplemented with SFTQF.
Glycemic index (GI)
Supplementation with processed quinoa flour tended to decrease the GI of the experimental diets. These values were even lower for diet F (p ≤ 0.05), with 15% SFTQF, which showed the lowest GI (93.71 GI). The GI of that diet was 7.57 points lower than that of diet A (control) and 5.81 points lower than that of diet B (supplemented with 31.5% simple carbohydrates replacing complex carbohydrates).
The lower GI of diet F in comparison to diets C and E may be explained by the different processes applied to the quinoa. GI of diet D, in turn, could not be evaluated because none of the six animals from group 4 consumed the 3 g of the diet provided within the 15-min period, which precluded the test. This could be an indicative that, during the second phase, this diet was not very well acceptable by the animals, regarding the sensory aspect. In the following days of experiment, however, feed intake was noted probably because it was the only source of energy available, although this fact may be better elucidated in further studies aiming to evaluate sensory aspects of the product. A graphical representation of the mean GI values of the experimental diets is shown in Fig. 1.
Fig. 1.
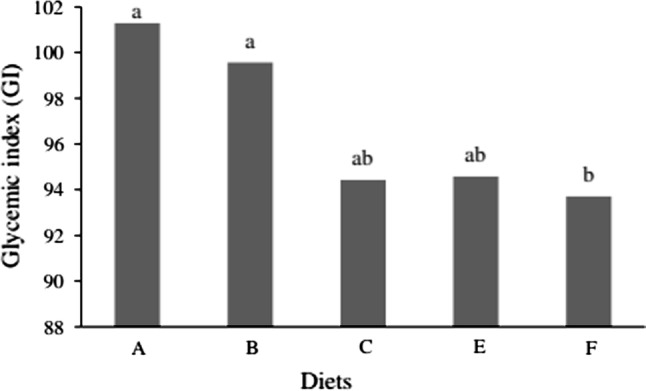
Glycemic index (GI) of diets A (control), B (control diet with high glucose levels), C (high glucose levels and 15% toasted quinoa flour), E (high glucose levels and 15% fermented and toasted quinoa flour) and F (high glucose levels and 15% sprouted/fermented and toasted quinoa flour). Means followed by the same letter in columns are not significantly different from each other according to Tukey’s test at 5% probability
Biochemical parameters
The experimental diets supplemented with TQF, STQF, FTQF and SFTQF triggered significant (p ≤ 0.05) decreases in total cholesterol, LDL-c and triacylglycerols, while HDL-cholesterol was maintained or increased (p ≤ 0.05) in groups 3, 4, 5 and 6 compared to the control diets provided to groups 1 and 2. Group 6, which was fed diet F containing SFTQF, showed greater improvements in these parameters.
The replacement of complex carbohydrates in diet A with simple carbohydrates in diet B promoted increasing in mean LDL-c (from 77.07 to 80.47 mg/dl) and VLDL-c (from 27.44 to 37.48 mg/dl), and decreased mean HDL-c (from 44.89 to 40.62 mg/dl). These changes resulted in an increase of total cholesterol from 146.48 to 151.20 mg/dl.
Diets containing processed quinoa flour also promoted a significant reduction in glycated hemoglobin (HbA1) in the animals. Diet F was the most efficient, reducing the HbA1 of group 6 by approximately 2 times compared to group 2, which was fed the control diet containing high levels of simple carbohydrates.
The results of the biochemical parameters are shown in Table 4.
Table 4.
Biochemical parameters of animals subjected to treatment with the experimental diets and coefficient of variation in percentage (CV%)
| Groupa | Total cholesterol | LDL-c | HDL-c | VLDL-c | Triacylglycerol | Glycated hemoglobin |
|---|---|---|---|---|---|---|
| 1 (diet A) | 146.48a.b | 77.07a.b | 44.89c | 27.44b | 137.24b | 4.27a |
| 2 (diet B) | 151.20ª | 80.47a | 40.62c | 37.48ª | 184.1a | 4.95a,b |
| 3 (diet C) | 136.47b.c | 58.56b | 58.68a | 19.29c | 96.49c | 3.35b,c |
| 4 (diet D) | 140.96a.b.c | 62.21a.b | 55.13a.b | 23.85b.c | 119.23b.c | 3.34b,c |
| 5 (diet E) | 138.75b.c | 60.57b | 57.98a | 19.97c | 99.88c | 3.5b,c |
| 6 (diet F) | 133.0c | 66.36a.b | 48.27b.c | 19.52c | 97.63c | 2.58c |
| CV% | 3.84 | 10.6 | 6.5 | 10.9 | 10.3 | 12.65 |
aGroup 1 (diet A, standard), group 2 (diet B, with high glucose levels, 31.5%), group 3 (diet C, with 31.5% glucose and 15% toasted quinoa flour), group 4 (diet D, with 31.5% glucose and 15% sprouted and toasted quinoa flour), group 5 (diet E, with 31.5% glucose and 15% fermented and toasted quinoa flour) and group 6 (diet F, with 31.5% glucose and 15% sprouted/fermented and toasted quinoa flour). Means of postprandial blood glucose followed by the same letter in columns and means of initial and final fasting blood glucose followed by the same letter in rows are not significantly different from each other according to Tukey’s test at 5% significance
Discussion
The present study showed that the processes of sprouting and/or fermentation potentiate the ability of quinoa to reduce the GI of diets with high levels of simple carbohydrates and to decrease blood glucose and lipid levels, food intake and the accumulation of epididymal adipose tissue of Wistar rats fed these diets during 47 days. These effects were mainly related to the changes in nutritional composition promoted by incorporation of processed quinoa in the diets, mainly regarding its fiber content, and to the chemical changes that occurred in the quinoa due its processing.
Quinoa is a pseudocereal containing low glycemic index carbohydrates, dietary fiber, high biological value protein, phytosterols, and n-3 and n-6 fatty acids (Abellán Ruiz et al. 2017). A greater feeling of fullness and satiety provided by meals containing quinoa was observed by Berti et al. (2004) and Berti et al. (2005), proving that the satiating efficiency index (SEI) of this seed in humans is higher than that of foods prepared using traditional cereals, such white bread. The SEI is used to relate the energetic load of a particular “satiating” food with the total consumption of ad libitum energy at the next meal. According to these authors, “satiating” food should be consumed in the first meal (pre load in pre-set amount) once it is expected that it induces less subsequent total energy consumption. The authors related this effect to the lower glycemic index (GI) of dough containing quinoa flour. They proved that foods with low GI are relatively more satiating than foods with high GI. This could explain the GI results obtained in the present study and may explain why diets with sprouted quinoa flour showed significantly lower ADI than the animals consuming control diets.
In fact, studies have shown that sprouting and fermentation processes contribute to reducing the GI of foods. Hsu et al. (2008) suggest that diet containing pre-sprouted whole-grain rice seeds delayed digestion and reduced carbohydrate absorption when compared to diets containing white rice. These effects are mainly due to ability of dietary fiber in preclude absorption and digestion of starch granules. Conversely, Seki et al. (2005) attributed to soluble and insoluble fiber the blood glucose-lowering ability on animals fed sprouted rice seeds. These evidences corroborate our results.
Regarding the effect of food fermentation on reducing the glycemic response, certain organic acids, including acetic acid and lactic acid, are known to have the ability to decrease the GI of foods (Östman et al. 2005; Brighenti et al. 2006; Scazzina et al. 2009; Stamataki et al. 2017). Östaman et al. (2002) conducted studies with humans and evidenced that the reduction of blood glucose caused by the intake of fermented bread supplemented with acetic acid was associated with a reduction in the rate of gastric emptying of those individuals caused by acetic acid. Although the studies proved the effects of fermented foods and organic acids on the reduction of the area under the glycemic curve, these mechanisms of action have not been completely understood.
Brighenti et al. (2006) noticed that fermentation promoted decreases in GI of high GI foods. The glucose-lowering effect shown by these fermented foods was also associated with delayed gastric emptying. Scazzina et al. (2009), in turn, related the low digestibility of starch from fermented foods with the presence of lactic acid during cooking process. The authors proposed that lactic acid increases starch retrogradation, and therefore, increases resistant starch formation.
Maintaining the low glycemic index of the diet has been a healthier and more effective strategy in the prevention and control of obesity and other noncommunicable diseases. Evidences also highlight that diets with low GI are effective in the prevention and control of diabetes, cardiovascular diseases and obesity, and therefore should be considered by the public health strategies in the context of diets that promote the population’s health, besides being important for individuals with insulin resistance (Augustin et al. 2015).
In contrast, the intake of high GI meals relates to increased hunger in the middle of the postprandial period due to insulin-induced hypoglycemia, and it apparently causes prolonged and persistent hyperphagia, even after normal blood glucose levels are restored. The rate of carbohydrate absorption after a meal, quantified by the GI, has significant effects on postprandial hormonal and metabolic responses and may increase the risk of developing obesity, type-2 diabetes and heart disease (Ludwig 2002; Pasko et al. 2010).
Increased fat deposition in animals consuming a high GI diet was proven in a study by Lerer-Metzger et al. (1996). They fed rats with diets rich in amylopectin (high GI starch) or amylose (low GI starch) and observed the first physiological changes favoring fat deposition, including increased fat incorporation, greater adipocyte diameter, increased glucose incorporation in lipids and greater fatty acid synthesis after 3–5 weeks. The researchers noted increased epididymal fat mass at the 7th week, and the animals developed obesity at weeks 32–51.
Pasko et al. (2010) observed a significant glucose reduction in groups of animals fed diets containing quinoa seeds compared to standard and high GI (62% fructose) control groups. Researchers have associated the glucose-lowering effect of quinoa with the presence of chemical compounds in the seeds, namely tocopherols and polyphenols, and the lower digestibility of starch from these seeds (Berti et al. 2004; Pasko et al. 2010).
On the other hand, the increased production of triacylglycerols in hepatocytes resulting from glucose overload (as are the cases of the diets with high levels of simple carbohydrates used in this study) causes increased lipogenesis, overproduction of VLDL-cholesterol and higher average of HbA1 in individuals (Ludwig 2002).
However, the hypocholesterolemic effect of the diets containing quinoa evidenced in the present study may be related to its dietary fiber content. Reduction in the absorption of triacylglycerols in the large intestine, increased synthesis and excretion of bile acids, inhibition of the synthesis of cholesterol by short-chain fatty acids generated during fermentation, and modifications in the metabolism of lipoproteins through an increase in the amount of hepatic receptors of LDL-c have been suggested as the main mechanisms of dietary fiber in promoting hypocholesterolemic effects (Lattimer and Haub 2010; Pasko et al. 2010). This could explain the reductions in total cholesterol, LDL-c and triacylglycerols observed in the groups fed diets supplemented with quinoa.
The ability of quinoa flour to reduce serum triacylglycerols may also be related to proteins. Takao et al. (2005) observed improvement in triacylglycerols levels in rats fed diets with protein isolated from quinoa seeds. In addition, considering that extra glucose is converted to triglycerides via lipogenesis and is stored in the lipid droplets of adipocytes (Dashty 2013), these results may be directly related to that found for GI, i.e., lower GI diets induces decreased or slow glucose absorption, not favoring the synthesis of triacylglycerols.
Conversely, Berti et al. (2005) observed such effects in healthy humans after the intake of pasta prepared with quinoa, and they attributed that phenomenon to the dietary fiber present in quinoa and the slow release of starch in the small intestine, a process proven to suppress the blood levels of free fatty acids (FFAs) and induce lower blood triacylglycerol levels. The authors stated that the suppression of FFA through low-glycemic meals may lead to increased insulin sensitivity and lower blood concentrations of glucose and triacylglycerols. Therefore, the intake of a low GI diet contributes to the control blood lipid levels, which also corroborates the results present in this study.
Diets containing processed quinoa flour also promoted a significant reduction in glycated hemoglobin (HbA1). The assessment of HbA1 levels is the best option for evaluating medium- and long-term glycemic control (Bem and Kunde 2006). According to Yamakawa et al. (2018), dietary carbohydrate to energy ratio has a positive correlation with HbA1c, suggesting that avoiding excessive carbohydrate intake (> 60%) may help foster glycemic control in humans. These results also corroborate those from Abellán Ruiz et al. (2017), who shown that processed quinoa intake during 28 days decreased HbA1c levels and the satiation and fullness (complete) degree in prediabetic patients.
It is important to highlight, however, the methods described here to evaluate blood glucose and HbA1c are calibrated for human blood samples, and the assumption that the absolute values of the obtained results can have systematic error (systematic shift) must be taken into consideration when evaluating these results. Thus, further tests with humans should be performed to better understand the reproducibility and consistency of the results presented here.
Conclusion
The processes of quinoa sprouting and fermentation potentiate its ability to reduce GI of diets with high levels of simple carbohydrates and to reduce blood glucose levels and lipid levels, food intake and the accumulation of epididymal adipose tissue in Wistar rats. That effect is associated with satiety and the chemical changes that occurred in the quinoa resulting from processing. These results are particularly relevant considering sprouted and fermented quinoa could be an alimentary source of interest, mainly when the focus in on disease risk prevention such as diabetes, obesity and dyslipidemias.
Further studies about the ingredients used and their incorporation in foods need to be conducted for better comprehension of the results found in the present work. This is particularly important for sprouted and toasted quinoa flour, because intake of the diet with incorporation of this treatment was probably not well accepted by the animals and no results for the glycemic assay could be acquired.
Acknowledgements
The authors thank the Federal University of Lavras (Universidade Federal de Lavras, UFLA), Lavras/Minas Gerais (MG) and the Minas Gerais Research Foundation (Fundação de Amparo a Pesquisa de Minas Gerais, FAPEMIG) for the support for the development of this study.
References
- Abdel-Aal ESM, Rabalski I. Effect of baking on nutritional properties of starch in organic spelt whole grain products. Food Chem. 2008;111(4):150–156. doi: 10.1016/j.foodchem.2008.03.050. [DOI] [Google Scholar]
- Abellán Ruiz MS, Barnuevo Espinosa MD, García Santamaría C, Contreras Fernández CJ, Aldeguer García M, Soto Méndez F, Guillén Guillén I, Luque Rubia AJ, Quinde Ràzuri FJ, Martínez Garrido A, López Román FJ. Effect of quinua (Chenopodium quinoa) consumption as a coadjuvant in nutritional intervention in prediabetic subjects. Nutr Hosp. 2017;34(5):1163–1169. doi: 10.20960/nh.843. [DOI] [PubMed] [Google Scholar]
- AOAC International . Official methods of analysis. 18. Gaithersburg: AOAC International; 2007. [Google Scholar]
- Augustin LS, Kendall CW, Jenkins DJ, Willett WC, Astrup A, Barclay AW, et al. Glycemic index, glycemic load and glycemic response: an International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC) Nutr Metab Cardiovasc Dis. 2015;25(9):795–815. doi: 10.1016/j.numecd.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Avancini SRP. Caracterização química, microbiológica e toxicológica da água da fermentação do amido de mandioca. Florianópolis: Universidade Federal de Santa Catarina; 2007. p. 104. [Google Scholar]
- Bem AF, Kunde JA. Importância da hemoglobina glicada no monitoramento das complicações crônicas do diabetes mellitus. J Bras Patol Med Lab. 2006;42(3):185–191. doi: 10.1590/S1676-24442006000300007. [DOI] [Google Scholar]
- Berti C, Riso P, Monti LD, Porrini M. In vitro starch digestibility and in vivo glucose response of gluten-free foods and their gluten counterparts. Eur J Nutr. 2004;43(4):198–204. doi: 10.1007/s00394-004-0459-1. [DOI] [PubMed] [Google Scholar]
- Berti C, Riso P, Brusamolino A, Porrini M. Effect on appetite control of minor cereal and pseudocereal products. Br J Nutr. 2005;94(5):850–858. doi: 10.1079/BJN20051563. [DOI] [PubMed] [Google Scholar]
- Brighenti F, Benini L, Del Rio D, Casiraghi C, Pellegrini N, Scazzina F, Jenkins DJ, Vantini I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am J Clin Nutr. 2006;83(4):817–822. doi: 10.1093/ajcn/83.4.817. [DOI] [PubMed] [Google Scholar]
- Cereda MP. Padronização para ensaios de qualidade de fécula fermentada de mandioca (polvilho azedo): I. Formulação e preparo de biscoitos. B Soc Bras Ciênc Tecnol Alimen. 1983;17(3):287–295. [Google Scholar]
- Dashty M. A quick look at biochemistry: carbohydrate metabolism. Clin Biochem. 2013;46(15):1339–1352. doi: 10.1016/j.clinbiochem.2013.04.027. [DOI] [PubMed] [Google Scholar]
- De Angelis-Pereira MC, Barcelos MFP, Pereira JAR, Pereira RC, de Sousa RV. Chemical composition of unripe banana peels and pulps flours and its effects on blood glucose of rats. Nutr Food Sci. 2016;46(4):504–516. doi: 10.1108/NFS-11-2015-0150. [DOI] [Google Scholar]
- Diowksz A, Kordialik-Bogacka E, Ambroziak W. Se-enriched sprouted seeds as functional additives in sourdough fermentation. LWT Food Sci Technol. 2014;56:524–528. doi: 10.1016/j.lwt.2013.11.023. [DOI] [Google Scholar]
- Ferreira DF. Sisvar: um programa para análise e ensino de estatística. Rev Symp. 2008;6(4):36–41. [Google Scholar]
- Hsu TF, Kise M, Wang MF, Ito Y, Yang MD, Aoto H, Yoshihara R, Yokoyama J, Kunii D, Yamamoto S. Effects of pre-sprouted brown rice on blood glucose and lipid levels in free-living patients with impaired fasting glucose or type 2 diabetes. J Nutr Sci Vitaminol. 2008;54(2):163–168. doi: 10.3177/jnsv.54.163. [DOI] [PubMed] [Google Scholar]
- Kabir M, Guerre-Millo M, Laromiguiere M, Slama G, Rizkalla SW. Negative regulation of leptin by chronic high-glycemic index starch diet. Metabolism. 2000;49(6):764–769. doi: 10.1053/meta.2000.6258. [DOI] [PubMed] [Google Scholar]
- Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2(12):1266–1289. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer-Metzger M, Rizkalla SW, Luo J, Champ M, Kabir M, Bruzzo F, Bornet F, Slama G. Effects of long-term low-glycaemic index starchy food on plasma glucose and lipid concentrations and adipose tissue cellularity in normal and diabetic rats. Br J Nutr. 1996;75(5):723–732. doi: 10.1079/BJN19960176. [DOI] [PubMed] [Google Scholar]
- Ludwig DS. The glycemic index physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- Navarro-Perez D, Radcliffe J, Tierney A, Jois M. Quinoa seed lowers serum triglycerides in overweight and obese subjects: a dose–response randomized controlled clinical trial. Curr Dev Nutr. 2017;1(9):e001321. doi: 10.3945/cdn.117.001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onwulata C, Thomas-Gahring A, Cooke P, Phillips J, Carvalho CW, Ascheri JL, Tomasula P. Production of extruded barley, cassava, corn and quinoa enriched with whey proteins and cashew pulp. Int J Food Prop. 2008;37(8):362–371. [Google Scholar]
- Östaman EM, Nilsso M, Liljeberg Elmstahl HGM, Molin G, Bjorck IME. On the effect of lactic acid on blood glucose and insulin responses to cereal products: mechanistic studies in healthy subjects and in vitro. J Cereal Sci. 2002;36(2):339–346. doi: 10.1006/jcrs.2002.0469. [DOI] [Google Scholar]
- Östman EM, Liljeberg Elmståhl HG, Björck IM. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am J Clin Nutr. 2001;74(1):96–100. doi: 10.1093/ajcn/74.1.96. [DOI] [PubMed] [Google Scholar]
- Östman EM, Granfeldt Y, Persson L, Björck IM. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr. 2005;59(9):983–988. doi: 10.1038/sj.ejcn.1602197. [DOI] [PubMed] [Google Scholar]
- Paśko P, Zagrodzki P, Bartoń H, Chłopicka J, Gorinstein S. Effect of quinoa seeds (Chenopodium quinoa) in diet on some biochemical parameters and essential elements in blood of high fructose-fed rats. Plant Foods Hum Nutr. 2010;65(4):333–338. doi: 10.1007/s11130-010-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellet PL, Young VR. Nutritional evaluation of protein foods. Tokyo: The United Nations University; 1980. Evaluation of protein quality in experimental animals. [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Carey VJ, Anderson CA, Miller ER, Copeland T, Charleston J, Harshfield BJ, Laranjo N, McCarron P, Swain J, White K, Yee K, Appel LJ. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: the OmniCarb randomized clinical trial. JAMA. 2014;312(23):2531–2541. doi: 10.1001/jama.2014.16658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scazzina F, Del Rio D, Pellegrini N, Brighenti F. Sourdough bread: starch digestibility and postprandial glycemic response. J Cereal Sci. 2009;49(11):419–421. doi: 10.1016/j.jcs.2008.12.008. [DOI] [Google Scholar]
- Schabes FI, Sigstad EE. Calorimetric studies of quinoa (Chenopodium quinoa Willd.) seed germination under saline stress conditions. Thermochim Acta. 2004;428(8):71–75. [Google Scholar]
- Seki T, Nagase R, Torimitsu M, Yanagi M, Ito Y, Kise M, Mizukuchi A, Fujimura N, Hayamizu K, Ariga T. Insoluble fiber is a major constituent responsible for lowering the post-prandial blood glucose concentration in pre-sprouted brown rice. Biol Pharm Bull. 2005;28(8):1539–1541. doi: 10.1248/bpb.28.1539. [DOI] [PubMed] [Google Scholar]
- Silva RN, Duarte GL, Lopes NF, de Moraes DM, Pereira AAA. Composição química de sementes de trigo (Triticum aestivum L.) submetidas a estresse salino na germinação. Rev Bras Sementes. 2008;30(1):148–155. [Google Scholar]
- Singh AK, Rehal J, Kaur A, Jyot G. Enhancement of attributes of cereals by germination and fermentation: a review. Crit Rev Food Sci Nutr. 2015;55:1575–1589. doi: 10.1080/10408398.2012.706661. [DOI] [PubMed] [Google Scholar]
- Stamataki NS, Yanni AE, Karathanos VT. Bread making technology influences postprandial glucose response: a review of the clinical evidence. Br J Nutr. 2017;117(7):1001–1012. doi: 10.1017/S0007114517000770. [DOI] [PubMed] [Google Scholar]
- Takao T, Watanabe N, Yuhara K, Konishi Y. Hypocholesterolemic efect of protein isolated from quinoa (Chenopodium quinoa Willd.) seeds. Food Sci Technol Res. 2005;11(2):161–167. doi: 10.3136/fstr.11.161. [DOI] [Google Scholar]
- Yamakawa T, Sakamoto R, Takahashi K, Suzuki J, Matuura-Shinoda M, Takahashi M, Shigematsu E, Tanaka S, Kaneshiro M, Asakura T, Kawata T, Yamada Y, Osada UN, Isozaki T, Takahashi A, Kadonosono K, Terauchi Y. Dietary survey in Japanese patients with type 2 diabetes and influence of dietary carbohydrate on hemoglobin A1c: the Soreka study. J Diabetes Investig. 2018 doi: 10.1111/jdi.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]


