Abstract
Combined effects of controlled atmosphere and different postharvest treatment (salicylic acid, oxalic acid and putrescine) on bioactive compounds and quality of pomegranate cv. Hicaznar were investigated. Pomegranates were harvested at commercial harvest stage and transported immediately to postharvest physiology laboratory. Fruit were divided into four groups. 1 Control: Dipped into distilled water + 0.01% Tween-20 solution for 10 min. 2 Oxalic acid (OA): Dipped into 6 mM OA + Tween-20 solution for 10 min. 3 Salicylic acid (SA): Dipped into 2 mM SA + Tween-20 solution for 10 min. 4 Putrescine (PUT): Dipped into 2 mM PUT + Tween-20 solution for 10 min. After treatments, pomegranates were stored at 6 °C and 90 ± 5% relative humidity for 6 months in controlled atmosphere (5% O2 + 15% CO2). Weight loss, color, total soluble solids content, titretable acidity (TA), total phenolic content, vitamin C, antioxidant activity and sugar content (glucose and fructose) were determined at 0th, 2th, 4th and 6th month of cold storage. Generally, weight losses were minimized by treatments, especially PUT, compared to control. The level of ascorbic acid significantly tended to decrease throughout the storage in all treatments. Treated pomegranate exhibited higher titratable acidity, total phenolic contents and antioxidant activity compared to control samples. However, PUT was the best among all treatments. The results suggest that SA, OA and PUT have the potential to extend the storage life of pomegranate by delaying quality loss and maintaining some bioactive compound and antioxidant activity.
Keywords: Punica granatum, Controlled atmosphere, Phenolic content, Antioxidant activity, Sugar, Ascorbic acid
Introduction
The pomegranate, considered as a tropical and subtropical climate fruit, is one of the oldest known edible fruit originated from Turkey (Selçuk and Erkan 2015). Pomegranate is also gaining popularity in recent years due to its high economic, nutritive and medicinal attributes (Barman et al. 2011). Due to increasing awareness of healthy nutrition in the world, studies on functional foods and components are increasing. As a result of these studies, it is stated that pomegranate (Punica granatum L.) is a fruit in the functional foods class owing to containing high level of vitamin C, antioxidants compound and polyphenol content (Varasteh et al. 2012).
Pomegranate is a non-climacteric and a highly perishable fruit during storage. Postharvest losses are still about 30% because of weight loss, discoloration of skin color and decay of fruit (Barman et al. 2011; Kumar and Kalita 2017; Porat et al. 2018). These symptoms reach the arils, which results in a reduction in both internal and external fruit quality (Mirdehghan et al. 2007). Recent studies on pomegranate have been focused on prolonging the storage life and preserve the storage quality of fruit (Selçuk and Erkan 2014). To extend storability and marketing of several fruit species, good results were obtained with polyamines (Mirdehghan et al. 2007; Sharma et al. 2017), SA (Sayyari et al. 2009), edible coating (Ghasemnezhad et al. 2013) and heat treatments (Mirdehghan et al. 2006). And also, these treatments have been made to alleviate the severity of these symptoms (weight loss, discoloration of skin color and decay of fruit) with satisfactory results.
SA, OA and PUT are natural organic compounds that are found ubiquitously in plant species, including some important crops, and play different roles (such as fruit tissue browning, fruit ripening, controlling fruit decay, suppressing ethylene production, maintaining firmness, preserving fruit color) in the living organisms (Smith 1985; Sayyari et al. 2009, 2010; Barman et al. 2011). Exogenous application of PUT has been reported to improve storage life and storage quality attributes in many fruit like apricot (Martinez-Romero et al. 2002; Zokaee Khosroshahi and Esna-Ashari 2007), plum (Perez-Vicente et al. 2002; Khan et al. 2008) and pomegranate (Barman et al. 2014). Pre- and postharvest SA also has several physiological and biochemical effects (Raskin 1992), including ethylene synthesis and chilling injury (Leslie and Romani 1986). Similarly, OA and SA have been reported as anti-senescence agents, the main effects in fruit being delayed postharvest ripening process (Gimenez et al. 2017), reduced chilling injury (Luo et al. 2011), extended the storability (Valero et al. 2011) and increased disease resistance (Zheng et al. 2007).
To the best of our knowledge, there are a few literatures available about the beneficial effects of only one of these materials (SA, PUT, OA) on pomegranates in each research. But as far as we know, no study has been conducted yet on the combined effects of controlled atmosphere storage and all these materials on Hicaznar pomegranate. Therefore, the main purpose of this study was to investigate the combined effects of controlled atmosphere and different postharvest treatments (SA, OA and PUT) on bioactive compounds and quality of pomegranate cv. Hicaznar.
Material and methods
Material
Pomegranates (Punica granatum cv. Hicaznar) were harvested manually at commercial maturity from a commercial orchard and transported to the postharvest physiology laboratory immediately. Fruit with signs of sunburn, bruises, mechanical damage and disease were eliminated, and samples were selected for uniformity in size, shape and color.
Hicaznar, with its sour–sweet taste, is the most popular export pomegranate cultivar in Turkey and is cultivated widely in the west Mediterranean coast of the country. The fruit is large size with dark red skin color and the arils are tender, with deep violet–red color, delicious sour–sweet (Selçuk and Erkan 2015).
Chemicals
SA (≥ 99.0%), OA (97%) and PUT (> 98%) were procured from Sigma-Aldrich. SA, which belongs to a group of phenolic, is widely distributed in plants and it is now considered as a hormonal substance (Luo et al. 2011). OA, a natural organic acid, is a final metabolite product in plants (Zheng et al. 2007). Polyamines (PAs) are a class of positively charged small aliphatic amines that are ubiquitous in living organisms. PUT, spermidine and spermine are the major forms of PAs found in plants (Khan et al. 2008).
Treatments and storage conditions
Selected pomegranates were randomly divided into four groups (each group contained 135 fruit). 1 Control (C): Dipped into distilled water + 0.01% Tween-20 solution for 10 min. 2 Oxalic acid (OA): Dipped into 6 mM OA + Tween-20 solution for 10 min. 3 Salicylic acid (SA): Dipped into 2 mM SA + Tween-20 solution for 10 min. 4 Putrescine (PUT): Dipped into 2 mM PUT + Tween-20 solution for 10 min. After dipping treatments, fruit placed on kraft paper were allowed to dry at 20 °C overnight. Dried pomegranates were placed in plastic boxes and stored at 6 °C and 90 ± 5% relative humidity for 6 months in controlled atmosphere condition (5% O2 + 15% CO2). During cold storage, 15 fruit of each replicate were analyzed at 60 days intervals. Other group of 30 fruit was used for initial analyses.
Quality and biochemical analysis
Weight loss of pomegranates was expressed as the percentage of loss of weight with respect to the initial weight. Weight loss was determined by the formula;
Color was measured at two points on the fruit surface with a colorimeter (Minolta CR-400, Japan) over 15 pomegranates in each replicate. The colorimeter was calibrated using the manufacturer’s standard white plate (Y = 92.3, x = 0.3136 and y = 0.3194). The values were expressed by the CIE L* (indicates the lightness, 0–100 representing dark to light), a* (determines the degree of red-green color, with a higher positive a* value indicating more red) and b* (indicates the degree of yellow–blue color, with a higher positive b* value indicating more yellow) (Parmar et al. 2017) and the values were evaluated as L*, chroma (C*) and hue angle (h°).
Total soluble solids (TSS) content was measured using a digital refractometer (Atago Pocket PAL-1) and expressed as g kg−1.
Titratable acidity (TA) was determined by a digital pH meter (Hanna Instruments) and titrimeter (Digitrat, Isolab), and expressed as g citric acid kg−1.
Fruit decay was determined by hedonic test with 9 panelists (panelists were mixed age (25–50), genders and cultural background) using decay index. Decay index was assessed on a 1–5 scale, describing the severity of postharvest fungal decay (1 = no decay; 2 = 25%; 3 = 50%; 4 = 75% of the fruit surface affected, and 5 = entire fruit decayed) (Selçuk and Erkan 2014).
Extraction of pomegranate samples for total phenolic content and antioxidant activity The pomegranate sample mixed with 25 mL methanol solution was crushed by the aid of homogenizer. It was left in dark conditions for 14–16 h at 4 °C and then filtered with filter paper (Thaipong et al. 2006).
Determination of total phenolic content The total phenolic substance content was determined by spectrophotometer by modifying the Folin-Ciocalteu colorimetric method (Swain and Hillis 1959). Extract (0.15 mL) was vortexed for 30–40 s by adding 2.4 mL of purified water and 0.15 mL of Folin-Ciocalteu (1:10) solution. After 3–4 min 0.3 mL sodium carbonate (Na2CO3, 1 N) was added and allowed to stand at 20 °C for 2 h in dark condition. The absorbance was read at 725 nm wavelength by spectrophotometer. The total amount of phenolic substance (per fresh weight) was expressed as gallic acid equivalent (GAE) g kg−1.
Determination of antioxidant activity by FRAP method Ferric Reducing Antioxidant Power (FRAP) method was used to determine antioxidant activity. To 0.15 mL extract 2850 FRAP working solution was added and incubated for 30 min at 20 °C in dark condition. The absorbance was read at 593 nm wavelength by spectrophotometer. The antioxidant activity values (per fresh weight) were expressed as trolox equivalent (TE) mmol kg−1 (Benzie and Strain 1996).
Extraction and HPLC analysis of ascorbic acid and sugars Ascorbic acid (vitamin C) was determined following a modified method of Watada (1982). Pomegranate samples were crushed by a mixer. The crushed samples were filtered with filter paper to remove water. Sample (5 mL) was mixed with 6% (w v−1) aqueous solution of metaphosphoric acid, then centrifuged in a refrigerated centrifuge. The extract was filtered through 450 nm pore filters and a 0.01 mL sample used for HPLC analysis of AA. Extracts were analyzed using a liquid chromatograph equipped with a diode array detector monitoring at 210 nm. The HPLC conditions were as follows: column, ODS-3 C-18 (5.000 nm, 250 × 4.6 i.d.); solvent 2% (w v−1) KH2PO4/H2O as mobile phase, at a flow rate of 0.5 mL min−1, at 25 °C.
Sugars were determined following a modified method of Melgarejo et al. (2000). Pomegranate samples were crushed by a mixer. The crushed samples were filtered with filter paper to remove water. Sample extract (1 mL) and nanopure water (9 mL) was added to a 15 mL volumetric flask, and then centrifuged in a refrigerated centrifuge at 3000 g for 10 min at 200 °C. Acetonitrile (up to a final volume of 5 mL) was added to 1 mL of supernatant. The extract was filtered through 450 nm pore filters and a 0.01 mL sample used for HPLC analysis of sugars (glucose, fructose). The analysis was done in triplicate. Extracts were analyzed using a liquid chromatograph equipped with a refractive index detector. The HPLC conditions were as follows: column, Inertsil NH2 (5.000 nm, 250 × 4.6 i.d.); solvent 70% (v v−1) ACN/H2O as mobile phase, at a flow rate of 0.9 mL min−1, at 25 °C.
For AA and sugars quantification, external standard calibration curves were used for the identified components. Five injections were made for each calibration level. For the linear regression of the curves of external calibration standards, R2 values were between 0.995 and 0.999.
Statistical analysis
The experiment was set up according to the factorial randomized design with 3 replications (15 fruit per replication). Data were expressed as the mean ± SE for all parameters. Sources of variation for cultivar were treatments and storage period. Main effects and interactions were analyzed and means were compared by Tukey’s test at a significance level of 0.05 (Table 2). All analyses were performed with JMP software package by General Linear Model (GLM) univariate test.
Table 2.
ANOVA for dependent variables for treatments, storage period and their interactions for pomegranates
| Parameters | Treatments (T) | Storage period (SP) | T × SP |
|---|---|---|---|
| Weight loss | ** | ** | NS |
| Total soluble solid | ** | ** | ** |
| Titratable acidity | ** | ** | ** |
| Colour L* | ** | ** | NS |
| Chroma | ** | ** | ** |
| Hue angle | ** | NS | * |
| Fructose | NS | ** | NS |
| Glucose | NS | ** | NS |
| Ascorbic acid content | NS | ** | NS |
| Total phenolic content | NS | ** | NS |
| Antioxidant activity | NS | ** | NS |
| Decay index | ** | ** | ** |
NSRepresents non-significance at p < 0.05; ** Represents significance at the 0.01 level; * Represents significance at the 0.05 level
Results and discussion
Weight loss
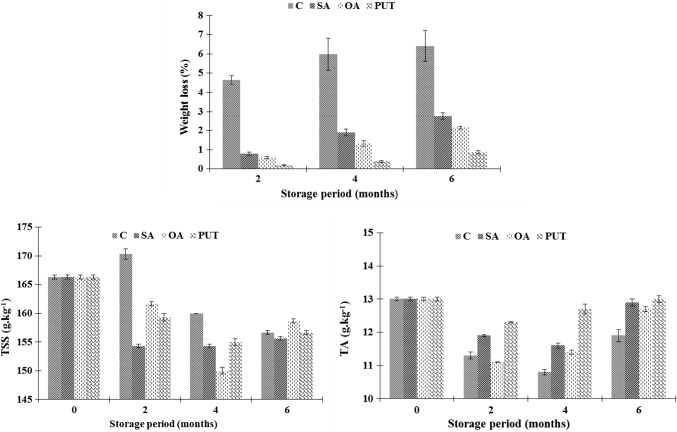
Visual quality of pomegranates, which is main quality parameter for marketing, is affected by weight loss of fruit during storage. Pomegranates are highly susceptible to weight loss because of high porosity of the skin (Selçuk and Erkan 2015). In this research, treatments and storage periods significantly (p < 0.05) affected the weight loss of pomegranates (Table 2). The weight loss of pomegranates progressively increased with increased storage period and reached 6.41% in control group at the end of storage. PUT was the most effective treatment in controlling weight loss of pomegranates, followed by OA and SA. PUT treated pomegranate lost 0.86% of their initial weight, whereas these values were 2.15% and 2.76% for OA and SA, respectively at the end of experiment (Fig. 1). The lower weight loss in treated pomegranates might be attributed to stabilization or maintenance of cell integrity and the permeability of the tissues with these applications (Mirdehghan et al. 2007). In addition, the suppressing effect of these treatments on metabolic activity of fruit after harvest can be responsible for lower weight losses. Comparing the effects of treatments, the combination of PUT and CA storage was more effective for weight loss than the others. This can be explained by strong delaying effect of PUT on cell integrity and senescence process of fruit. It is clear from this study that the integration of postharvest PUT treatments with CA storage can be promising application for restricting weight loss of pomegranate cv. Hicaznar.
Fig. 1.
Weight loss, total soluble solid (TSS) and titratable acidity (TA) content of control and treated pomegranates during storage. C: (Dipped into distilled water + 0.01% Tween-20 for 10 min), SA: (Dipped into 2 mM SA+ Tween-20 solution for 10 min), OA: (Dipped into 6 mM OA+ Tween-20 solution for 10 min), PUT: (Dipped into 2 mM PUT + Tween-20 solution for 10 min). Vertical bars represent the standard error of the mean (n = 3)
Total soluble solid, titratable acidity, color and sugar
The effects of treatments and storage time on the total soluble solid contents of pomegranates were statistically (p < 0.05) significant (Table 2). TSS of fruit fluctuated during storage and decreased compared to initial values, regardless of treatments. At the end of storage, TSS contents of pomegranates varied between 155.7 g kg−1 (SA) and 158.7 g kg−1 (OA), while the initial value was 166.3 g kg−1 (Fig. 1). This reduction can be due to utilization of sugars in respiration (Ramesh et al. 2016; Shaarawi and Nagy 2017). Our results are in agreement with Artes et al. (1996, 1998). However, some previous studies reported increases in TSS contents of pomegranate throughout storage, depending on higher moisture loss (Koksal 1989; Ghafir et al. 2010).
In present study, the initial titratable acidity (as citric acid) of pomegranates was 13.0 g kg−1. The TA contents of fruit significantly decrease over time regardless of treatments, especially in the first two months. Generally, the decreased in acidity with ripening could be attributed to factors, such as transformation of acids to other compounds and reduced ability of fruit to synthesize acids with maturity (Shaarawi and Nagy 2017). PUT was the best treatment in maintaining TA, while control samples had the lowest values at the end of storage. SA and OA also delayed acidity losses compared with non-treated fruit (Fig. 1). These positive effects of treatments and controlled atmosphere storage on TA might be due to the slowing down of fruit metabolism by them. It is well known that CA and PUT treatments alone have delaying effect on TA contents of pomegranate during cold storage (Artes et al. 1996; Barman et al. 2011). But as far as we know, there is no research conducted on combined effect of these treatments on pomegranate quality loss after harvest. This research revealed that PUT and CA combination has potential for maintaining TA of pomegranate compared to other treatments. This can be ascribed to the higher suppressing effects of PUT on metabolism and senescence process of fruit. It is known that organic acids are the major respiratory substrates in fruit (Echeverria and Valich 1989; Sayyari et al. 2011). Selçuk and Erkan (2015) reported that the decrease of TA in pomegranate could be due to the use of citric acid in respiratory process of fruit during storage. It is concluded that treatments, especially PUT, suppressed the metabolic activity and respiration rate of pomegranates, which are accordance with previous studies (Sayyari et al. 2009; 2010; Barman et al. 2011).
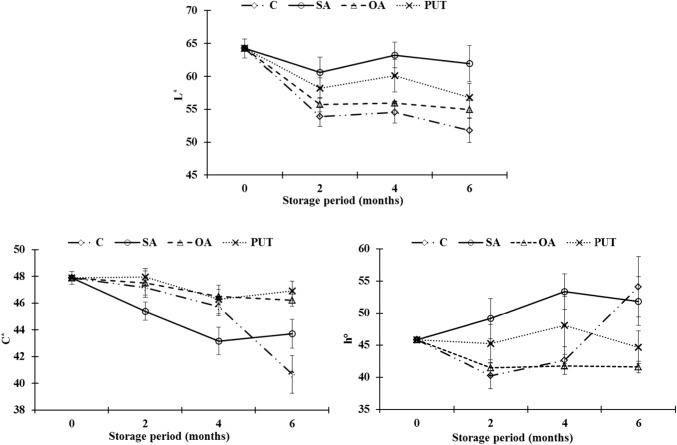
Colour changes of pomegranates peel are presented in Fig. 2. Skin colour, which influences consumer demand, is very important quality parameter for pomegranates. When compared to values at harvest, L* and C* values significantly (p < 0.05) decreased in all treatments at the end of cold storage. However, it was not observed same decrease for ho values in all applications. The effects of treatments on L*, C* and ho values were statistically (p < 0.05) significant (Table 2). All treatments, especially SA, maintained skin brightness compared to control samples with lower L* value changes during storage (Fig. 2). Treated fruit had higher L* values (less dark peel) compared to control group. It is explained that the higher loss of peel luminosity in control groups was possibly related to the higher water loss of control fruit. The average ho values of skin were higher in SA and PUT treated fruit than those of OA and control. Similarly Selçuk and Erkan (2015) reported that control fruit had lower ho values (dark-reddish colour) than treated pomegranate related to water loss. PUT and OA treated pomegranates maintained C* values compared to SA and control group. Higher C* values represent better colour (vivid) for pomegranate peel. Control groups had less h° values as they were dark-reddish, while treated fruits were generally shiny red. Therefore, it can be concluded from this research that PUT and OA are better treatments to maintain the skin colour of pomegranates compared with control and SA treated samples. As far as we know, there are not detailed results about the effects of OA on colour changes of pomegranates during storage. But it is known that PUT treatments preserved skin colour (through inhibiting chlorophyll and other color material degradation) of pomegranates better than those of control samples (Barman et al. 2011, 2014). Our results related to the effects of PUT on skin colour of fruit are in agreement with previous findings (Mirdehghan et al. 2007).
Fig. 2.
L*, chroma (C*) and hue angle (h°) value of control and treated pomegranates during storage. C: (Dipped into distilled water + 0.01% Tween-20 for 10 min), SA: (Dipped into 2 mM SA+ Tween-20 solution for 10 min), OA: (Dipped into 6 mM OA+ Tween-20 solution for 10 min), PUT: (Dipped into 2 mM PUT + Tween-20 solution for 10 min). Vertical bars represent the standard error of the mean (n = 3)
At harvest fructose and glucose contents of pomegranates were 79.05 and 74.24 mg kg−1, respectively. The level of these predominant sugars significantly decreased with increased storage period in all treatments. The combinational impact of PUT application and CA storage delayed both fructose and glucose losses during storage compared to other treatments, though there were no significant differences (p < 0.05) among treatments. Control group fruit had the lowest sugar contents at the end of storage (Fig. 3, Table 2). This can be due to suppressing effects of treatments on metabolic activity of fruit in comparison to non-treated pomegranates. Similarly, Barman et al. (2011) reported that lower level sugar might be attributed to higher respiration rate of non-treated pomegranates compared to PUT treated. It is known that, fruit sugar is used in respiration process during storage. The suppressing effect of PUT on respiration rate of pomegranates was also reported by Mirdehghan et al. (2007).
Fig. 3.
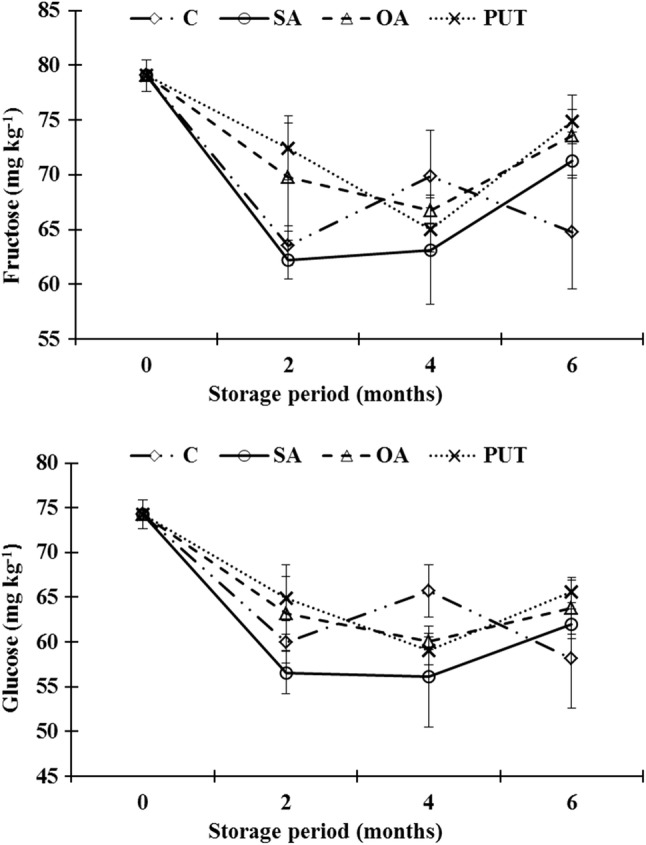
Fructose and glucose value of control and treated pomegranates during storage. C: (Dipped into distilled water + 0.01% Tween-20 for 10 min), SA: (Dipped into 2 mM SA+ Tween-20 solution for 10 min), OA: (Dipped into 6 mM OA+ Tween-20 solution for 10 min), PUT: (Dipped into 2 mM PUT + Tween-20 solution for 10 min). Vertical bars represent the standard error of the mean (n = 3)
Ascorbic acid, total phenolic and antioxidant activity
Vitamin C, including AA and dehydroascorbic acid, is one of the most important nutritional quality factors in many horticultural crops (Lee and Kader 2000). The effects of treatments and storage period on AA were given in Tables 1 and 2. There was a significant decrease in AA contents of pomegranates regardless of treatments during cold storage. All these treatments had no significant effects to maintain AA in comparison to control. The AA contents of fruit changed from 35.4 mg kg−1 (C) to 31.0 mg kg−1 (OA) at the end of experiment, while initial value was 59.6 mg kg−1. The results of this research are in agreement with previous studies (Artes et al. 1996; Nanda et al. 2001; Aarabi et al. 2008; Barman et al. 2014) in which significant decrease in AA content of pomegranates was reported during storage. Whereas Miguel et al. (2006) found that AA content of pomegranate increased after 4 months storage. On the other hand, Sayyari et al. (2010) noted that control pomegranates showed a remarkable reduction in the content of AA but OA led to a significant increase in AA after almost 3 months cold storage. These various results can be attributed to different combinations of cultivar, maturity, treatment and application doses as reported by Lee and Kader (2000) for horticultural crops. Sayyari et al. (2009) found that the AA contents of pomegranates decreased in control and lower dose SA-treated (0.7 and 1.4 mM) fruit, but remained unchanged in fruit treated with the highest dose (2 mM).
Table 1.
Total phenolic content, antioxidant activity and ascorbic acid content of control and treated pomegranates during storage
| Treatments | 0 days | 60 days | 120 days | 180 days | Means |
|---|---|---|---|---|---|
| Ascorbic acid (mg kg−1) | |||||
| C | 59.63 ± 4.19 | 54.67 ± 0.32 | 30.15 ± 0.11 | 35.36 ± 0.38 | 44.95NS |
| SA | 59.63 ± 4.19 | 40.78 ± 1.29 | 29.16 ± 0.32 | 31.22 ± 0.52 | 40.20 |
| OA | 59.63 ± 4.19 | 42.90 ± 2.06 | 32.68 ± 8.36 | 31.05 ± 0.66 | 41.57 |
| PUT | 59.63 ± 4.19 | 44.78 ± 0.53 | 29.98 ± 0.22 | 33.64 ± 0.50 | 42.01 |
| Means | 59.63a | 45.78b | 30.49c | 32.82c | |
| Total phenolic content (g kg−1) | |||||
| C | 3.21 ± 0.37 | 4.46 ± 0.04 | 3.11 ± 0.22 | 4.40 ± 0.10 | 3.80NS |
| SA | 3.21 ± 0.37 | 4.57 ± 0.11 | 3.39 ± 0.31 | 4.71 ± 0.13 | 3.97 |
| OA | 3.21 ± 0.37 | 4.63 ± 0.06 | 3.47 ± 0.37 | 4.29 ± 0.56 | 3.90 |
| PUT | 3.21 ± 0.37 | 4.97 ± 0.03 | 3.52 ± 0.50 | 4.71 ± 0.06 | 4.10 |
| Means | 3.21b | 4.66a | 3.37b | 4.53a | |
| Antioxidant activity (mmol kg−1) | |||||
| C | 27.20 ± 3.71 | 39.22 ± 0.84 | 26.15 ± 2.17 | 39.04 ± 1.01 | 32.90NS |
| SA | 27.20 ± 3.71 | 40.75 ± 1.06 | 28.98 ± 3.12 | 42.17 ± 1.28 | 34.77 |
| OA | 27.20 ± 3.71 | 41.38 ± 0.58 | 29.71 ± 3.75 | 37.91 ± 5.59 | 34.05 |
| PUT | 27.20 ± 3.71 | 44.74 ± 0.33 | 30.28 ± 4.96 | 42.19 ± 0.57 | 36.10 |
| Means | 27.20b | 41.52a | 28.78b | 40.33a | |
C: (Dipped into distilled water + 0.01% Tween-20 for 10 min), SA: (Dipped into 2 mM SA + Tween-20 solution for 10 min), OA: (Dipped into 6 mM OA + Tween-20 solution for 10 min), PUT: (Dipped into 2 mM PUT + Tween-20 solution for 10 min). Means followed by different letters within the same row are significantly different at p < 0.05, n = 3, Tukey’s; NS: non significant
Values are mean ± standard error of triplicate determinations
At the end of cold storage, no statistical effects due to treatments on changes in total phenolic and antioxidant activity of pomegranate were found. However, storage period significantly affected total phenolic contents and antioxidant activity of samples (Tables 1, 2). As can be seen in Table 1 total phenolic contents fluctuated throughout storage but an increase was observed at the end of 6 months according to initial value (3.21 g kg−1). In agreement with our results, Selçuk and Erkan (2015) reported that the total phenolic content of Hicaznar pomegranates fluctuated during cold storage and exhibited an increase at the end of 180 days. In this study, the application of PUT, SA and OA led to a significant increase of the total phenolic contents with final average values of 4.10, 3.97 and 3.90 g kg−1, respectively, compared to control (3.80 g kg−1). PUT and CA combination was the best treatment to maintain or increase of total phenol content of pomegranate followed by SA and OA (Table 1). It is known that because of anti-senescence and anti-respiration properties of PUT and SA, they retard many post-harvest physiological activities responsible for quality losses of fruit (Martinez-Romero et al. 2002; Asghari and Aghdam 2010; Onursal et al. 2015). It might also be argued that inhibition of respiration rate and ethylene production with PUT may be due to the inhibition of activities of enzymes related to these processes (Kakkar and Rai 1993; Khan et al. 2008). On the other hand, Sayyari et al. (2010) indicated that OA treatment induced lower losses of total phenolic compound of pomegranates. The increasing mechanism of OA on the bioactive compounds and antioxidant properties of fruit is not well known. But it has been reported as a natural antioxidant by suppressing lipid peroxidation and reducing the AA oxidation (Kayashima and Katayama 2002).
The same trend was determined for antioxidant activity, for which PUT, SA and OA treated pomegranates showed higher antioxidant activity with average values of 36.10, 34.77 and 34.05 mmol kg−1 respectively, in comparison to control samples (32.90 mmol kg−1), although there were no significant differences between treatments and control samples (Tables 1, 2). CA storage with lower O2 has a role for lowering oxidation and its combined application with treatments, especially PUT, might contribute higher antioxidant activity retention. Pomegranates, depending on varieties, are rich in antioxidant compounds including phenolic compounds such as AA and anthocyanins. It was thought that the increasing of total phenolic, especially in PUT and CA combination, resulted in an increase of antioxidant activity in pomegranates examined in this study. Correspondingly, Mirdehghan et al. (2006) reported that the total antioxidant activity of Mollar de Elche pomegranate was highly correlated with total phenolic of fruits, while total anthocyanins and AA had less contribution on antioxidant activity. Sayyari et al. (2010) found a significant increase in antioxidant activity of pomegranates treated with OA, especially for the 6 mM dose, during cold storage although it was not known exact mechanism of OA. Similarly Selçuk and Erkan (2014) reported an increase in antioxidant activity of pomegranates cv. Beynar after 120 days cold storage. These findings related to higher total antioxidant activity of pomegranates at the end of storage might be explained by lowering losses of bioactive compound such as phenolics, anthocyanins and AA (Barman et al. 2014).
Decay index and chilling injury
During 6 months storage in CA, no chilling injury incidence was recorded. This can be explained with the resistance of Hicaznar variety to chilling injury and appropriate storage temperature (6 °C). The effects of treatments and storage period on decay index values were significant (p < 0.05) (Table 2). In the first 60 days, no fruit decay was observed in all treatments. The first decay incidence was determined at the 120 days of storage varying between 1.22 (index value) and 1.78. At the end of storage, the highest decay rate (as index) was obtained from control samples (3.11), while pomegranates treated with SA gave the lowest (1.89) value followed by OA (Fig. 4). The positive effect of CA combination with SA and OA on decay rate, as expected, can be attributed to its antifungal effects. Similarly, Zheng et al. (2005) reported that OA treatment in combination with CA storage decreased the decay incidence and extended the storage life of fruit.
Fig. 4.
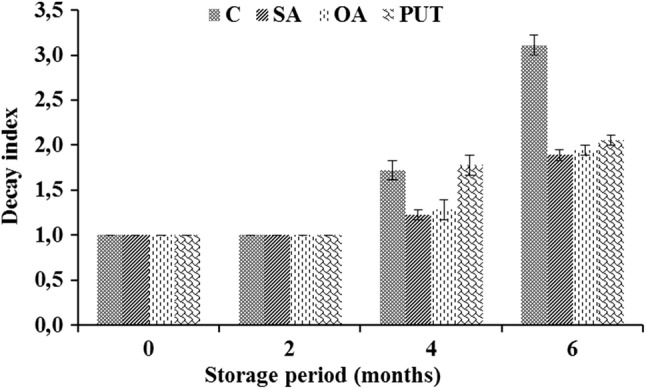
Decay index of control and treated pomegranates during storage. C: (Dipped into distilled water + 0.01% Tween-20 for 10 min), SA: (Dipped into 2 mM SA+ Tween-20 solution for 10 min), OA: (Dipped into 6 mM OA+ Tween-20 solution for 10 min), PUT: (Dipped into 2 mM PUT + Tween-20 solution for 10 min). Decay index scale: 1 = no decay; 2 = 25%; 3 = 50%; 4 = 75% of the fruit surface affected, and 5 = entire fruit decayed. Vertical bars represent the standard error of the mean (n = 3)
Conclusion
In conclusion, weight loss of pomegranates was decreased by PUT, SA and OA during storage, while these treatments had not an obvious effect on TSS and AA contents. PUT and OA maintained skin color better than SA and control group samples. All treatments delayed sugar loss and increased total phenolic and antioxidant activity of pomegranates compared to control. However, PUT was the best among all treatments in terms of weight loss, TA, sugar, total phenolic and antioxidant activity. These treatments, especially PUT, could be a promising postharvest tool for delaying quality loss and maintaining or enhancing some bioactive compound and antioxidant activity of pomegranates during cold storage. In summary, pomegranate cv. Hicaznar could be stored up to 5 months under combination of PUT and CA (5% O2 + 15%CO2) with minimum quality losses. However, more in-depth research is needed to better understand the mechanism of these treatments on postharvest physiology of pomegranates.
Contributor Information
Mehmet Ali Koyuncu, Phone: +90 246 211 85 29, Email: koyuncu.ma@gmail.com.
Derya Erbas, Email: deryabyndr@gmail.com.
Cemile Ebru Onursal, Email: ebru.onursal@gmail.com.
Tuba Secmen, Email: secmentuba@gmail.com.
Atakan Guneyli, Email: atakangnyl@gmail.com.
Seda Sevinc Uzumcu, Email: sedasevincuzumcu@gmail.com.
References
- Aarabi A, Barzegar M, Azizi MH. Effect of cultivar and cold storage of pomegranate (Punica granatum L.) juices on organic acid composition. ASEAN Food J. 2008;15:45–55. [Google Scholar]
- Artes F, Marin JG, Martinez JA. Controlled atmosphere storage of pomegranate. Eur Food Res Technol. 1996;203:33–37. [Google Scholar]
- Artes F, Tudela JA, Gil MI. Improving the keeping quality of pomegranate fruit by intermittent warming. Eur Food Res Technol. 1998;207:316–321. [Google Scholar]
- Asghari M, Aghdam MS. Impact of salicylic acid on postharvest physiology of horticultural crops. Trend Food Sci Technol. 2010;2:502–509. doi: 10.1016/j.tifs.2010.07.009. [DOI] [Google Scholar]
- Barman K, Asrey R, Pal RK. Putrescine and carnauba wax pretreatments alleviate chilling injury, enhance shelf life and preserve pomegranate fruit quality during cold storage. Sci Hort. 2011;130:795–800. doi: 10.1016/j.scienta.2011.09.005. [DOI] [Google Scholar]
- Barman K, Asrey R, Pal RK, Kaur C, Jha SK. Influence of putrescine and carnauba wax on functional and sensory quality of pomegranate (Punica granatum L.) fruits during storage. J Food Sci Technol. 2014;51:111–117. doi: 10.1007/s13197-011-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Echeverria E, Valich J. Enzymes of sugar and acid metabolism in stored Valencia organs. J Am Soc Hortic Sci. 1989;114:445–449. [Google Scholar]
- Ghafir SAM, Ibrahim IZ, Abusrewel GS, Suaad ZA. Response of local variety ‘Shlefy’ pomegranate fruits to packaging and cold storage. Acta Hortic. 2010;877:427–432. doi: 10.17660/ActaHortic.2010.877.55. [DOI] [Google Scholar]
- Ghasemnezhad M, Zareh S, Rassa M, Sajedi RH. Effect of chitosan coating on maintenance of aril quality, microbial population and PPO activity of pomegranate (Punica granatum L. cv. Tarom) at cold storage temperature. J Sci Food Agric. 2013;93:368–374. doi: 10.1002/jsfa.5770. [DOI] [PubMed] [Google Scholar]
- Gimenez MJ, Serrano M, Valverde JM, Martinez-Romero D, Castillo S, Valero D, Guillen F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J Sci Food Agric. 2017;97:1220–1228. doi: 10.1002/jsfa.7853. [DOI] [PubMed] [Google Scholar]
- Kakkar RK, Rai VK. Plant polyamines in flowering and fruit ripening. Phytochemistry. 1993;33:1281–1288. doi: 10.1016/0031-9422(93)85076-4. [DOI] [Google Scholar]
- Kayashima T, Katayama T. Oxalic acid is available as a natural antioxidant in some systems. BBA-General Subjects. 2002;1573:1–3. doi: 10.1016/S0304-4165(02)00338-0. [DOI] [PubMed] [Google Scholar]
- Khan AS, Singh Z, Abbasi NA, Swinny EE. Pre-or post-harvest applications of putrescine and low temperature storage affect fruit ripening and quality of ‘Angelino’ plum. J Sci Food Agric. 2008;88:1686–1695. doi: 10.1002/jsfa.3265. [DOI] [Google Scholar]
- Koksal AI. Research on the storage of pomegranate (cv. Gök Bahçe) under different conditions. Acta Hortic. 1989;258:299–302. [Google Scholar]
- Kumar D, Kalita P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods. 2017;6(1):8. doi: 10.3390/foods6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Kader AA. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol. 2000;20:207–220. doi: 10.1016/S0925-5214(00)00133-2. [DOI] [Google Scholar]
- Leslie CA, Romani RJ. Salicylic acid: a new inhibitor of ethylene biosynthesis. Plant Cell Rep. 1986;5:144–146. doi: 10.1007/BF00269255. [DOI] [PubMed] [Google Scholar]
- Luo Z, Chen C, Xie J. Effect of salicylic acid treatment on alleviating postharvest chilling injury of ‘Qingnai’ plum fruit. Postharvest Biol Technol. 2011;62:115–120. doi: 10.1016/j.postharvbio.2011.05.012. [DOI] [Google Scholar]
- Martinez-Romero D, Serrano M, Carbonell A, Burgos L, Riquelme F, Valero D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J Food Sci. 2002;67:1706–1712. doi: 10.1111/j.1365-2621.2002.tb08710.x. [DOI] [Google Scholar]
- Melgarejo P, Salazar DM, Artes F. Organic acids and sugars composition of harvested pomegranate fruits. Eur Food Res Technol. 2000;211:185–190. doi: 10.1007/s002170050021. [DOI] [Google Scholar]
- Miguel MG, Fontes C, Martins D, Neves A, Antunes D. Effects of postharvest treatment and storage time on the organic acid content in ‘Assaria’ and ‘Mollar’ pomegranate (Punica granatum L.) fruit. Ital J Food Sci. 2006;18:317–322. [Google Scholar]
- Mirdehghan SH, Rahemi M, Serrano M, Guillen F, Martinez-Romero D, Valero D. Prestorage heat treatment to maintain nutritive and functional properties during postharvest cold storage of pomegranate. J Agric Food Chem. 2006;54:8495–8500. doi: 10.1021/jf0615146. [DOI] [PubMed] [Google Scholar]
- Mirdehghan SH, Rahemi M, Martínez-Romero D, Guillen F, Valverde JM, Zapata PJ, Serrano M, Valero D. Reduction of pomegranate chilling injury during storage after heat treatment: role of polyamines. Postharvest Biol Technol. 2007;44:19–25. doi: 10.1016/j.postharvbio.2006.11.001. [DOI] [Google Scholar]
- Nanda S, Rao DVS, Krishnamurthy S. Effects of shrink film wrapping and storage temperature on the shelf life and quality of pomegranate fruits cv. Ganesh. Postharvest Biol Technol. 2001;22:61–69. doi: 10.1016/S0925-5214(00)00181-2. [DOI] [Google Scholar]
- Onursal CE, Bayındır D, Celepaksoy F, Koyuncu MA. Combined effects of MAP and postharvest Putrescine treatment on storage life and quality of ‘Alyanak’ apricot. Acta Hortic. 2015;1071:165–172. doi: 10.17660/ActaHortic.2015.1071.17. [DOI] [Google Scholar]
- Parmar N, Singh N, Kaur A, Thakur S. Comparison of color, anti-nutritional factors, minerals, phenolic profile and protein digestibility between hard-to-cook and easy-to-cook grains from different kidney bean (Phaseolus vulgaris) accessions. JFST. 2017;54(4):1023–1034. doi: 10.1007/s13197-017-2538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Vicente A, Martinez-Romero D, Carbonell A, Serrano M, Riquelme F, Guillen F, Valero D. Role of polyamines in extending shelf life and the reduction of mechanical damage during plum (Prunus salicina Lindl.) storage. Postharvest Biol and Technol. 2002;25(1):25–32. doi: 10.1016/S0925-5214(01)00146-6. [DOI] [Google Scholar]
- Porat R, Lichter A, Terry LA, Harker R, Buzby J. Postharvest losses of fruit and vegetables during retail and in consumers’ homes: quantifications, causes, and means of prevention. Postharvest Biol Technol. 2018;139(2018):135–149. doi: 10.1016/j.postharvbio.2017.11.019. [DOI] [Google Scholar]
- Ramesh NK, Monahar PD, Veena J, Padmavathamma AS, Syamraj NC. Effect of Antioxidants on shelf life and quality minimally processed pomegranate arils cv. Bhagwa. Adv. Life Sci. 2016;5:4678–4682. [Google Scholar]
- Raskin I. Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:439–463. doi: 10.1146/annurev.pp.43.060192.002255. [DOI] [Google Scholar]
- Sayyari M, Babalar M, Kalantari S, Serrano M, Valero D. Effect of salicylic acid treatment on reducing chilling injury in stored pomegranates. Postharvest Biol Technol. 2009;53:152–154. doi: 10.1016/j.postharvbio.2009.03.005. [DOI] [Google Scholar]
- Sayyari M, Valero D, Babalar M, Kalantari S, Zapata PJ, Serrano M. Prestorage oxalic acid treatment maintained visual quality, bioactive compounds, and antioxidant potential of pomegranate after long-term storage at 2 °C. J Agric Food Chem. 2010 doi: 10.1021/jf100196h. [DOI] [PubMed] [Google Scholar]
- Sayyari M, Castillo S, Valero D, Diaz-Mula HM, Serrano M. Acetyl salicylic acid alleviates chilling injury and maintains nutritive and bioactive compounds and antioxidant activity during postharvest storage of pomegranates. Postharvest Biol Technol. 2011;60:136–142. doi: 10.1016/j.postharvbio.2010.12.012. [DOI] [Google Scholar]
- Selçuk N, Erkan M. Changes in antioxidant activity and postharvest quality of sweet pomegranates cv. Hicrannar under modified atmosphere packaging. Postharvest Biol Technol. 2014;9:29–36. doi: 10.1016/j.postharvbio.2014.01.007. [DOI] [Google Scholar]
- Selçuk N, Erkan M. Changes in phenolic compounds and antioxidant activity of sour–sweet pomegranates cv. ‘Hicaznar’ during long-term storage under modified atmosphere packaging. Postharvest Biol Technol. 2015;109:30–39. doi: 10.1016/j.postharvbio.2015.05.018. [DOI] [Google Scholar]
- Shaarawi SA, Nagy KS. Effect of modified atmosphere packaging on fruit quality of “Wonderful” pomegranate under cold storage conditions. Middle East J Agric Res. 2017;6(2):495–505. [Google Scholar]
- Sharma S, Pareek S, Sagar NA, Valero D, Serrano M. Modulatory effects of exogenously applied polyamines on postharvest physiology, antioxidant system and shelf life of fruits: a review. Int J Mol Sci. 2017;18(8):1789. doi: 10.3390/ijms18081789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TA. Polyamines. Annu Rev Plant Physiol. 1985;36(1):117–143. doi: 10.1146/annurev.pp.36.060185.001001. [DOI] [Google Scholar]
- Swain T, Hillis WE. The phenolic constituents of Prunus domestica. I.—the quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compost Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Valero D, Diaz-Mula HM, Zapata PJ, Castillo S, Guillen F, Martinez-Romero D, Serrano M. Postharvest treatments with salicylic acid, acetylsalicylic acid or oxalic acid delayed ripening and enhanced bioactive compounds and antioxidant capacity in sweet cherry. J Agric Food Chem. 2011;59:5483–5489. doi: 10.1021/jf200873j. [DOI] [PubMed] [Google Scholar]
- Varasteh F, Arzani K, Barzegar M, Zamani Z. Changes in anthocyanins in arils of chitosan-coated pomegranate (Punica granatum L. cv. Rabbab-e-Neyriz) fruit during cold storage. Food Chem. 2012;130:267–272. doi: 10.1016/j.foodchem.2011.07.031. [DOI] [Google Scholar]
- Watada AE. A high-performance liquid chromatography method for determining ascorbic acid content of fresh fruits and vegetables. Hort Science. 1982;17:334–335. [Google Scholar]
- Zheng XL, Tian SP, Xu Y, Li BQ. Effects of exogenous oxalic acid on ripening and decay incidence in mango fruit during storage at controlled atmosphere. J Fruit Sci. 2005;22(4):351–355. [Google Scholar]
- Zheng X, Tian S, Gidley MJ, Yue H, Li B. Effects of exogenous oxalic acid on ripening and decay incidence in mango fruit during storage at room temperature. Postharvest Biol Technol. 2007;45:281–284. doi: 10.1016/j.postharvbio.2007.01.016. [DOI] [Google Scholar]
- Zokaee Khosroshahi MR, Esna-Ashari M. Post-harvest putrescine treatments extend the storage-life of apricot (Prunus armeniaca L.) ‘Tokhm-sefid’ fruit. J Hortic Sci Biotechnol. 2007;82:986–990. doi: 10.1080/14620316.2007.11512337. [DOI] [Google Scholar]