Abstract
The objective of the present work was to study the effect of liquid whey from the cheese production process on the gels developed by high hydrostatic pressure from whey protein isolate powder (WPI). Changes in pH, color, textural parameters, and water retention capacity of the gels obtained were analyzed during storage for 28 days at refrigeration temperature (4 °C). Mixtures of liquid whey from cheese making processes and different WPI percentages gave gels with different characteristics after being processed by high hydrostatic pressures. The pH values and color parameters (L*, a*, b*) varied slightly, depending directly on WPI concentration and storage time. The values of hardness, elasticity, and cohesiveness were dependent on the liquid medium used to dissolve the WPI (liquid cheese whey or distilled water), WPI concentration, and storage time. The use of liquid cheese whey for gel formation favored water retention, reducing the appearance of syneresis (exudation). The results obtained in the present study indicated a possible use and revalorization of cheese whey obtained in cheese production to obtain WPI gels with improved physicochemical properties, using high hydrostatic pressure as technology for their production.
Keywords: High hydrostatic pressure, Gels, Cheese whey, Off streams, WPI
Introduction
In recent years there has been a great boom in interest in protein-enriched products, due to the high biological value of proteins. The development of new gel-based products that meet the high demands of consumers is now an important activity in many companies. A simplified definition of gel is “a gel is an intermediate between a solid and liquid possessing both elastic (solid) and viscous (liquid) characteristics (Banerjee and Bhattacharya 2011). There are in the market different gel-based products like jams, jellies, confectionary, desserts, yogurt, etc., which are composed of biopolymers. The commonly used biopolymers are polysaccharides and proteins that confer semisolid characteristics in a range of food products (Einhorn-Stoll and Drusch 2015). High hydrostatic pressure has been used as an effective technique for altering the surface functional properties of water-soluble proteins. Treatment by high hydrostatic pressure (HHP) modifies protein structure, destabilizes cell membranes, and eliminates pathogenic and spoilage microorganisms commonly present in foods (Totosaus et al. 2002, 2015; Krešić et al 2006). As a consequence of these changes, there is great potential in HHP to obtain new protein based products. Whey protein isolates (WPI), and whey protein concentrates (WPC) modified by high pressure have been developed and their functionality studied (Krešić et al 2006). According to those authors more studies are needed to raise the full potential of these food ingredients in industrial application. Several authors have studied the effect of HHP on whey protein concentrate solutions using different matrices with variations in protein concentrations (Patel et al. 2005). It has been reported that pressurizing treatments significantly alter the tertiary structure of proteins, allowing direct interaction of the proteins present in whey (Patel and Huppertz 2014). Other studies have indicated that the effect of HHP on proteins is altered by process factors such as time, pH, temperature, and type of protein (Huppertz et al. 2004; López-Fandiño 2006)
Proteins derived from milk and dairy products can be divided into two broad categories: casein, accounting for 80%, and whey proteins, representing around 20%. Casein is traditionally used in the food industry as a food aid or for manufacturing cheeses by separation of casein/whey fractions by modifying the pH to the isoelectric points of the caseins or by the addition of enzymes. The whey fraction is mainly composed of various globular proteins, the most common ones, with the highest percentages, being α-lactalbumin, β-lactoglobulin, and immunoglobulins. It should be noted that the whey composition varies depending on the process used in cheesemaking, because acid whey contains lower percentages of protein, lactose, and lipids than sweet whey, but it has higher levels of sodium, potassium, calcium, and magnesium (Lucey 2014; Oakenfull et al. 1997).
Lactoglobulin appears to be the main protein involved in gel formation when isolated whey protein is subjected to HHP (Considine, et al. 2007). α-Lactalbumin has a higher resistance to protein denaturation by HHP treatments than β-lactoglobulin (McGuffey et al. 2005; Patel and Huppertz 2014).
Whey is the liquid remaining after milk has been curdled and strained. It is a byproduct of the manufacture of cheese or casein. Liquid whey from cheesemakers companies represents a major environmental problem owing to its high content of soluble solids and the great number of cheese companies around the world. According to Barukčić et al. (2014), the production of 1 kg of hard/semi-hard cheese generates 9 L of whey. Previous studies by Baldasso et al. (2011) and OECD-FAO (2012) estimated that whey production ranges around 160–190 million tonnes/year, and only 50% is reused worldwide. Biological oxygen demand (BOD) and chemical oxygen demand (COD) in the range of 40,000–60,000 and 50,000–80,000 ppm, respectively, have been estimated (Saddoud et al. 2007).
In general, studies in which HHP has been used to produce gels have been carried out using protein isolate powder from whey (WPI), obtained by some concentration process such as ultrafiltration, suspended in water, which requires percentages of powder around 12%. The objective of this study was to assess the effect of using liquid whey from the cheese production process with lower levels of whey protein isolate powder (WPI) to obtain gels through the use of HHP that could be used as ingredients for industrial food production. . This practice could contribute to reduce the cost of making gels from WPI and the ecological impact of liquid whey produced by cheese making companies.
Materials and methods
Liquid cheese whey was obtained from a local supplier in Valencia (Spain) Calero S.L. The company employs an acid production process of cheese. This type of whey has around 0.84% (w/v) of protein, 5.57% (w/v) of total soluble solids and acidity of 0.61% (w/v, in lactic acid) (Lievore et al. 2015). The liquid whey samples were refrigerated at 4 ± 2 °C during transport from the cheese making factory to the laboratory, where they were frozen at − 40 °C until used. Whey protein isolate (WPI) was obtained from Best Protein S.L. (Barcelona, Spain); it contained 43–48% of β-lactoglobulin and 14–18% of α-lactalbumin.
Sample preparation
Frozen liquid whey was thawed and maintained under refrigeration at 4 ± 2 °C. It was mixed with the WPI at various concentrations (5, 7, 11, 15% w/v of WPI). The mixtures obtained were homogenized at room temperature (25 °C) for 10 min using a magnetic stirrer. After dispersion of the WPI in liquid cheese whey, 0.25% w/v sodium chloride (NaCl) and 0.15% w/v calcium chloride (CaCl2) were added in order to increase the gel ionic strength, and the pH of the liquid medium was adjusted to a final value of 8 because to the denaturation, as a result of the HHP treatment of milk, increases considerably at alkaline pH (López-Fandiño 2006). Distilled water instead of liquid cheese whey was used as a control, following the same preparation procedure as described above. In all cases pH was controlled by means of a pH-meter (Crisson Basic 20+, Barcelona, Spain) calibrated at pH 4 and 7.
HHP treatments
Three independent falcon tubes containing samples were packaged in thermo-sealed polyethylene bags (MULTIVAC, Thermosealer) and used in each HHP treatment (EPSI NV equipment, Temse, Belgium). Pressurized samples were stored under refrigeration and their physical properties (pH, texture, color, and syneresis) were analyzed. HHP treatments were carried out using a mixture of water with ethylene glycol as a pressurizing agent, with temperature control. The initial temperature of the HHP treatment was 20 °C, reaching a maximum final temperature of 38 °C at a pressure of 550 MPa for 10 min. The depressurizing time was less than 1 min in all the experiments. Those conditions were chosen considering the work carried out by Kanno et al. (1998).
Physicochemical evaluation of gels
Gels were monitored and evaluated for various physical/chemical properties during storage for 28 days, considered as the shelf life for those products, at refrigerated temperature (4 °C), taking 5 control points at different days (days 1, 7, 14, 21, and 28). The properties monitored were pH, color, instrumental texture parameters (TPA), and syneresis. The pH was measured following the same protocol as described in sample preparation.
The color of the gel was determined by a Konica Minolta CM-3500d colorimeter (Konica Minolta Sensing Inc., Japan). This equipment provides the reflectance values, as well as the coordinates of the CIELab uniform color system: L* = 0 (black), L* = 100 (white), a* = color from red (positive) to green (negative), and b* = color from yellow (positive) to blue (negative), the center being achromatic. The illuminant was D65 and the visual angle 10°. The colorimeter was standardized using a white calibration plate before measurement of the gels obtained by HHP, both for the liquid whey and for the control (distilled water) as suspension media.
The color difference (ΔE*) between the control and each of the cheese whey gels was calculated using Eq. 1 proposed by Bodart et al. (2008)
| 1 |
For ΔE* < 1 the color differences are not perceived by the human eye, for 1 < ΔE* < 3 the human eye can see minimal color differences, depending on the angle, and for ΔE* > 3 color differences are obvious for the human eye (Martínez-Cervera et al. 2011).
For texture analysis a TA-XTplus texture analyzer (Stable Micro Systems, Godalming, England) was employed. Samples were cut into 2 cm cubes (2 cm per side). A double compression test was performed to 40% deformation with a 75 mm diameter flat-ended cylindrical aluminum probe (P/75). The probe speed was 1 mm/s and a time delay of 5 s was employed between each of the two cycles. The primary texture parameters hardness, cohesiveness, and elasticity were calculated. Hardness was considered as the peak force during the first compression cycle, cohesiveness as the ratio of the positive force area during the second compression to the positive force area during the first compression and elasticity as the height recovered between the end of the first compression and the start of the second compression. Five replicates were used for each sample.
Syneresis was measured by a centrifugation test (Ribotta et al. 2007, 2012) using a Beckman J2–MI centrifuge (Beckman Instruments, USA). At each selected storage time, gel samples were removed from the refrigerator, tempered at 25 °C (room temperature), and centrifuged at 1500 g for 15 min at 25 °C. After centrifugation the free water was separated and weighed, and the result was expressed as a percentage of the total water present in the gels. This procedure was done in triplicate for each of the WPI concentrations for both liquids: whey cheese sample and the control made by using distilled water.
Statistical analysis
The results obtained were analyzed calculating the mean and standard deviation. In addition, ANOVA was performed to detect significant differences between samples (p value < 0.05). All statistical analyses were done using Statgraphics Centurion XII software (StatPoint Technologies, Inc., Warrenton, VA, USA).
Results and discussion
The use of cheese whey in this work was focused on reducing the amount of whey powder isolated needed to produce the gels by high pressure. Isolated whey powder is expensive, while the cheese whey is a byproduct from cheese making factories that have a great impact on environment. Consequently, the use of this byproduct to reduce the amount of whey powder isolated could be a good choice for both, decrease the environmental impact and produce cheapest gels by high pressure technology. With this idea in mind physicochemical test on gels obtained with cheese whey were performed and results are discussed below.
Changes in pH during storage
The effect of type of sample (control or cheese whey), WPI concentration, and storage time on pH values was studied. The pH values were similar for all storage days, with small variations depending on the WPI percentage added, but no significant differences (p > 0.05) were observed between the concentrations of WPI studied. At day 1, pH values in the whey cheese sample varied from 8.03 (5% WPI) to 7.97 (15%), and similarly after 28 days, values varied from 8.01 (5% WPI) to 7.94 (15%). Results indicated that neither composition nor storage time significantly modified the initial pH values of the gels.
Evaluation of color
Color is an important attribute and could affect consumer acceptation of a product. Table 1 shows the effect of composition and storage time on the instrumental color parameters L*, a*, and b* of the gels. The L* values were slightly higher in the control gels (distilled water as suspension liquid) than in the gels obtained using liquid whey from cheese, which indicates a higher white component for control samples. In the case of parameter a* (green–red component), the values were higher in the gels containing the liquid whey from cheese, resulting, therefore, in greener tones, in comparison with the control gels. The parameter b* (yellow–blue component) indicated higher yellow tonalities in the gel with liquid whey from cheese as compared with the control samples. With regard to the effect of storage time on the L* value, no trend was observed, although it should be noted that the color parameters of the gels made with liquid cheese whey were more stable than those of the control gels, revealing an increase in color stability during storage with incorporation of liquid cheese whey cheese.
Table 1.
Evaluation of color parameters for gels at different WPI concentrations processed by HHP during 28 days of storage
| Time (days) | Sample | % WPI | L* | a* | b* |
|---|---|---|---|---|---|
| 1 | WC | 5 | 52.55 (0.37)aA | − 0.45 (0.04)aA | 0.74 (0.23)aA |
| LW | 48.82 (0.28)bA | − 1.08 (0.02)bA | 1.22 (0.16)bA | ||
| WC | 7 | 51.32 (0.21)aA | − 0.57 (0.04)aA | 0.83 (0.15)aA | |
| LW | 49.37 (0.90)bA | − 1.07 (0.06)bA | 1.69 (0.20)bA | ||
| WC | 11 | 53.42 (0.31)aA | − 0.49 (0.02)aA | 1.49 (0.09)aA | |
| LW | 47.06 (1.10)bA | − 1.29 (0.08)bA | 1.54 (0.21)aA | ||
| WC | 15 | 50.79 (0.57)aA | − 0.53 (0.02)aA | 1.89 (0.22)aA | |
| LW | 50.83 (0.39)aA | − 0.89 (0.03)bA | 2.64 (0.05)bA | ||
| 7 | WC | 5 | 49.91 (0.45)aB | − 0.47 (0.04)aA | 0.92 (0.13)aA |
| LW | 51.11 (0.82)aB | − 1.01 (0.01)bB | 1.80 (0.18)bB | ||
| WC | 7 | 52.52 (0.12)aB | − 0.49 (0.02)aB | 0.93 (0.01)aA | |
| LW | 46.95 (0.36)bB | − 1.08 (0.03)bA | 1.86 (0.13)bA | ||
| WC | 11 | 52.38 (0.10)aB | − 0.56 (0.05)aB | 1.59 (0.02)aA | |
| LW | 51.93 (0.24)bB | − 0.91 (0.02)bB | 2.58 (0.10)bB | ||
| WC | 15 | 51.98 (0.28)aB | − 0.69 (0.02)aB | 2.12 (0.22)aA | |
| LW | 50.19 (0.05)bB | − 0.93 (0.01)bA | 2.46 (0.01)bB | ||
| 14 | WC | 5 | 53.67 (0.05)aC | − 0.56 (0.04)aB | 1.40 (0.17)aB |
| LW | 49.55 (0.09)bC | − 1.08 (0.02)bA | 1.66 (0.06)aC | ||
| WC | 7 | 51.41 (0.13)aA | − 0.57 (0.02)aA | 0.95 (0.02)aA | |
| LW | 46.15 (0.24)bC | − 1.34 (0.01)bB | 1.01 (0.02)aB | ||
| WC | 11 | 51.40 (0.11)aC | − 0.61 (0.02)aC | 1.02 (0.02)aC | |
| LW | 51.10 (0.13)bC | − 0.96 (0.02)bC | 2.59 (0.25)bC | ||
| WC | 15 | 52.57 (0.28)aC | − 0.81 (0.03)aC | 1.81 (0.06)aA | |
| LW | 48.33 (0.33)bC | − 0.91 (0.01)bA | 2.94 (0.27)bA | ||
| 21 | WC | 5 | 52.34 (0.28)aA | − 0.51 (0.03)aA | 0.66 (0.08)aA |
| LW | 48.74 (0.32)bA | − 1.17 (0.05)bC | 1.03 (0.09)bA | ||
| WC | 7 | 52.15 (0.16)aC | − 0.52 (0.01)aA | 0.73 (0.05)aA | |
| LW | 50.34 (0.08)bA | − 1.04 (0.01)bA | 1.80 (0.01)bA | ||
| WC | 11 | 50.69 (0.15)aD | − 0.56 (0.02)aD | 1.05 (0.02)aD | |
| LW | 45.66 (0.42)bA | − 0.96 (0.01)bD | 2.36 (0.05)bD | ||
| WC | 15 | 50.43 (0.43)aA | − 0.57 (0.02)aA | 1.53 (0.12)aB | |
| LW | 50.01 (0.14)aD | − 0.98 (0.02)bB | 2.63 (0.11)bA | ||
| 28 | WC | 5 | 50.66 (0.15)aD | − 0.54 (0.05)aA | 0.53 (0.13)aA |
| LW | 49.09 (0.60)bA | − 1.06 (0.02)bA | 1.39 (0.30)bA | ||
| WC | 7 | 50.86 (0.53)aA | − 0.53 (0.02)aA | 0.89 (0.08)aA | |
| LW | 48.92 (0.45)bA | − 0.96 (0.08)bA | 1.45 (0.16)bA | ||
| WC | 11 | 48.80 (0.46)aE | − 0.71 (0.03)aE | 0.80 (0.06)aE | |
| LW | 49.60 (0.61)aD | − 1.05 (0.04)bE | 1.93 (0.19)bE | ||
| WC | 15 | 48.92 (0.29)aD | − 0.67 (0.03)aD | 1.44 (0.07)aC | |
| LW | 48.83 (0.34)aE | − 1.04 (0.03)bC | 2.62 (0.29)bA |
Values in parentheses are the standard deviation. Three replications per data
For the same storage time and WPI concentration, lowercase letters indicate significant differences among samples (p < 0.05) according to the LSD test
For the same storage time and the same sample type (WC or LW), capital letters indicate significant differences among WPI concentrations (p < 0.05) according to the LSD test
Control with water (WC) and sample with liquid cheese whey (LW)
In order to determine whether the differences observed in the instrumental color parameters would be perceptible to the human eye, the parameter ΔE* was calculated. Values of ΔE* > 3 indicate that the differences are perceptible to the human eye. ΔE* was calculated, comparing the control and the liquid cheese whey samples for each specific WPI concentration and for each specific time. After 28 days of storage ΔE was always lower than 3 (for all the WPI values), indicating that there were no differences perceptible to the human eye between the control and the cheese whey sample. From day 1 until 21 days of storage, no general pattern of behavior was observed regarding the influence of WPI and time on the color differences; in some cases ΔE* > 3 and in others < 3. The highest ΔE value (6.45) was found after day 1 with 11% WPI.
Texture analysis
The influence of WPI concentration and storage time on the primary textural parameters hardness, elasticity, and cohesiveness are shown in Tables 2, 3, and 4, respectively.
Table 2.
Effect of storage time in hardness (g) in gels made with water or liquid cheese whey at different WPI concentrations
| Type of sample | WPI concentration (%) | Day 1 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|---|
| Control Water | 5 | 346.94aA (41.80) |
240.22aB (34.72) |
234.51aC (22.20) |
219.67aD (27.51) |
182.89aE (26.84) |
| Whey | 5 | 266.97aA (23.21) |
186.93aB (8.25) |
225.48aA (36.65) |
207.92aC (20.98) |
195.77aD (31.98) |
| Control Water | 7 | 337.26aA (51.55) |
187.20aB (27.41) |
267.52aC (32.95) |
277.69aD (30.37) |
204.12aE (32.89) |
| Whey | 7 | 318.32aA (41.18) |
329.27bA (15.16) |
291.83aA (48.59) |
335.79aA (25.46) |
359.57bA (32.67) |
| Control Water | 11 | 518.98aA (68.94) |
457.97aA (88.81) |
341.93aB (44.93) |
397.62aC (18.74) |
291.85aD (26.49) |
| Whey | 11 | 500.31aA (56.46) |
824.73bB (132.78) |
924.13bC (79.71) |
834.97bD (77.08) |
898.14bE (97.50) |
| Control Water | 15 | 1338.65aA (62.19) |
1480.20aB (87.82) |
821.15aC (32.86) |
973.64aD (47.45) |
858.90aE (94.02) |
| Whey | 15 | 1012.67aA (65.98) |
1214.66bB (81.42) |
1346.00bC (128.21) |
1172.55bD (99.64) |
1237.36bE (97.11) |
Numbers between brackets are Standard Deviation. Three replicates per data
(ab) For the same storage time and WPI concentration, different lowercase letters indicate significant differences among samples (p < 0.05) according to the LSD test
(ABC) For the same storage time and the same sample type (control water or whey), different capital letters indicate significant differences among WPI concentrations (p < 0.05) according to the LSD test
Table 3.
Elasticity evolution for gels made with water or liquid cheese whey
| Type of sample | WPI concentration (%) | Day 1 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|---|
| Control Water | 5 | 0.82aA (0.01) |
0.90aB (0.02) |
0.85aA (0.03) |
0.92aC (0.05) |
0.89 (0.02)aD |
| Whey | 5 | 0.89bA (0.05) |
0.94aA (0.01) |
0.86aA (0.07) |
0.93aA (0.01) |
0.83aA (0.05) |
| Control Water | 7 | 0.84aA (0.04) |
0.84aA (0.06) |
0.90aA (0.05) |
0.92aA (0.04) |
0.90aA (0.03) |
| Whey | 7 | 0.87aA (0.04) |
0.92bA (0.01) |
0.89aA (0.02) |
0.93aA (0.02) |
0.93aB (0.01) |
| Control Water | 11 | 0.85aA (0.06) |
0.86aA (0.03) |
0.93aA (0.06) |
0.98aB (0.03) |
0.93aB (0.03) |
| Whey | 11 | 0.87aA (0.02) |
0.94bB (0.02) |
0.89aA (0.03) |
0.97 (0.02)aC |
0.93aD (0.03) |
| Control Water | 15 | 0.89aA (0.02) |
0.86aA (0.03) |
0.92aA (0.07) |
0.95aA (0.01) |
0.85aA (0.04) |
| Whey | 15 | 0.91aA (0.05) |
0.93aA (0.02) |
0.93aA (0.06) |
0.92aA (0.02) |
0.92aA (0.01) |
Numbers between brackets are the Standard Deviations. Three replications per data
(ab) For the same storage time and WPI concentration, different lowercase letters indicate significant differences among samples (p < 0.05) according to the LSD test
(ABCD) For the same storage time and the same sample type (control water or whey), different capital letters indicate significant differences among WPI concentrations (*p < 0.05) according to the LSD test
Table 4.
Effect of storage time in cohesiveness in gels made with water or liquid cheese whey at different WPI concentration
| Type of sample | WPI concentration (%) | Day 1 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|---|
| Control Water | 5 | 0.71aA (0.04) |
0.78aB (0.01) |
0.75aA (0.01) |
0.77aA (0.02) |
0.74aA (0.02) |
| Whey | 5 | 0.73aA (0.03) |
0.73bA (0.01) |
0.72aA (0.03) |
0.73aA (0.01) |
0.72aA (0.02) |
| Control Water | 7 | 0.71aA (0.03) |
0.72aA (0.01) |
0.77aB (0.01) |
0.78aB (0.02) |
0.77aB (0.01) |
| Whey | 7 | 0.75aA (0.02) |
0.80bB (0.01) |
0.76aA (0.01) |
0.81aB (0.02) |
0.80bB (0.01) |
| Control Water | 11 | 0.77aA (0.02) |
0.77aA (0.03) |
0.79aA (0.03) |
0.81aA (0.02) |
0.82aB (0.02) |
| Whey | 11 | 0.78aA (0.02) |
0.83bB (0.01) |
0.84bB (0.01) |
0.86aC (0.01) |
0.85aC (0.01) |
| Control Water | 15 | 0.82aA (0.03) |
0.85aA (0.01) |
0.86aA (0.03) |
0.87aB (0.01) |
0.86aB (0.01) |
| Whey | 15 | 0.82aA (0.01) |
0.85aB (0.01) |
0.85aA (0.02) |
0.84aA (0.01) |
0.85aB (0.01) |
Numbers between brackets are the Standard Deviations. Three replications per data
(ab) For the same storage time and WPI concentration, different lowercase letters indicate significant differences among samples (p < 0.05) according to the LSD test
(ABC) For the same storage time and the same sample type (control water or whey), different capital letters indicate significant differences among WPI concentrations (p < 0.05) according to the LSD test
At day 1 force values were slightly higher for the control than for the liquid cheese whey gels, but no significant differences were found. The increase in WPI concentration increased hardness in gels made using water and those made using liquid cheese whey.
During storage time, some changes were observed in the parameter hardness. In samples prepared with water, storage time reduced the hardness values, at all WPI concentrations. For samples made with liquid cheese whey the concentration of WPI affected the evaluation of hardness during storage. While at the lower WPI concentrations (5 and 7%) no effect was observed in force values, this parameter remained stable during storage, for the higher WPI concentrations (11 and 15%) an increase in force was found with storage time. Kanno et al. (1998) found that gels formed by using WPI and WPC dissolved in phosphate buffer at a pH of 6.8 and treated at 600 or 400 MPa were too soft to measure their textural properties with a rheometer. In the present work all the samples had enough consistency to measure their texture instrumentally, the reason of the different in the gel texture could be attributed to extrinsic factors, for example the differences in the pH values. López-Fandiño (2006) indicated that alkaline pH values increase considerably the denaturation of proteins, as a result of the HHP treatment of milk. The presence of Ca2Cl and NaCl as indicated by Kinsella et al. (1994) are factors that could contribute in giving harder gels when treated by high pressure at the conditions of the present work (500 MPa for 10 min).
The elasticity values are shown in Table 3. Elasticity is a measure of the ability of the gel structure to recover its initial height after being compressed. A system that completely recovers its initial height will have an elasticity value of 1. In general no significant differences were found in elasticity values among the samples made by using liquid cheese whey and the control samples made with water. Values closer to 1 were found in both systems revealing the spongy characteristics of the obtained gels. In both systems an increase in WPI increased elasticity. The increase in elasticity indicates a reinforcing of the gel structure. Storage time also increased elasticity values, especially at the higher WPI levels.
The cohesiveness (Table 4) was dependent on WPI concentration. At the lower WPI concentrations (5 and 7%) cohesiveness was higher for the liquid cheese whey samples, but, no statistical significance was observed. For the higher levels (11 and 15% WPI) no significant differences in cohesiveness were observed between the control and the liquid cheese whey gels. In both systems, the increase in WPI increased cohesiveness. In the control sample cohesiveness increased with time, while in the liquid cheese whey samples no change was observed for the lowest WPI, although for the highest WPI an increase with time was observed.
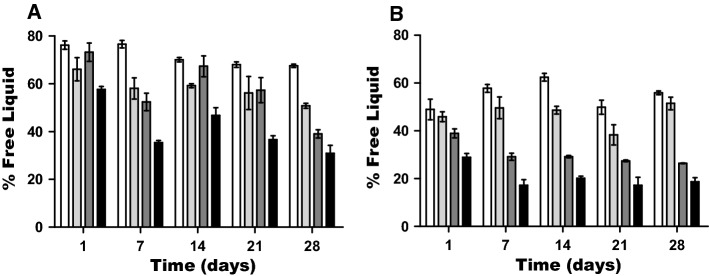
Evaluation of syneresis
Evaluation of exudate (Syneresis) from the gels is of great importance since it establishes the stability of the gel during diverse preservation processes or changes of temperature during storage. Syneresis values expressed as percentage of exudate released for the control gels (made with water) and the cheese whey gels (made with liquid cheese whey) are shown in Fig. 1a, b. Increasing the WPI concentrations resulted in a reduction of exudate released in both control and liquid cheese whey gels. After 28 days of storage, a lower release of exudate was found as the percentage of WPI was increased for both liquid whey gels and control sample gels (Fig. 1a, b). Comparison of figures A and B shows that in the cheese whey gels the exudate released was in general lower than in the control samples. Thus, the addition of cheese whey appears to favor water retention in the gel structure, which is a positive point in favor of cheese way incorporation. As expected, for both the control and the cheese whey the lowest syneresis occurred for the highest WPI concentration independently of the sampling day. According to studies carried out by other authors (Ribotta and Rosell, 2010; Zheng and Sosulski, 1998), the loss of water of gels is mainly caused by spontaneous contraction of the gel network, which leads to a reorganization of water retained in the interior. The results obtained by Kanno et al. (1998), with WPI in sodium phosphate buffer medium (pH 6.8) and pressures of 400–1000 MPa, suggest that S–S bridges between β-lactoglobulin and α-lactalbumin are mainly responsible for the formation of gels when subject to such pressures. Future work with different cheese whey solutions and their chemical characterization would be necessary to fully understand the existing chemical interactions responsible for the increase in water retention capacity and the resulting gel texture.
Fig. 1.
Syneresis evaluation at different WPI concentrations (□) 5; (
 ) 7; (
) 7; (
 ) 11; (■) 15% w/v processed by HHP during 28 days of storage at 4 °C. Control with water (a) and Sample with liquid cheese whey (b). Three replications per data set
) 11; (■) 15% w/v processed by HHP during 28 days of storage at 4 °C. Control with water (a) and Sample with liquid cheese whey (b). Three replications per data set
Conclusion
Cheese whey solutions with addition of WPI processed under HHP gave gels with physicochemical characteristics that may be applied in the food industry, in a variety of applications including desserts, or confectionery products, among others. Neither composition nor storage time significantly modified the initial pH values of the gels, while color values (L*, a*, b*) varied depending directly on the WPI concentration and storage time. Textural properties of the cheese whey gels can be modulated by varying the WPI concentration. In general those gels presented different texture attending to primary parameters than gels made using water, and can enlarge the number of food applications. The use of cheese whey for gel formation favored water retention, reducing the appearance of syneresis (exudate) under refrigeration conditions. Nevertheless, it is well known that cheese whey has an unpleasant taste; therefore, it is very important to perform sensory evaluations before they can be used by the food industry. The present study offers a possible reuse and revalorization of cheese whey added directly to WPI to produce gels with a high biological value and improved physicochemical properties, using high hydrostatic pressures as the production technology.
Acknowledgements
We would like to thank the Instituto Alpina de Investigación (IAI) contract number 19-017 R001 for the funds needed to carry out this work and the IATA (CSIC) for the use of its facilities.
Contributor Information
Edwin F. Torres, Email: fabiantorresbello@gmail.com
Gerardo González, Email: gerardo.gonzalez@alpina.com.
Bernadette Klotz, Email: bernadette.klotz@alpina.com.
Teresa Sanz, Email: tesanz@iata.csic.es.
Dolores Rodrigo, Email: lolesra@iata.csic.es.
Antonio Martínez, Email: amartinez@iata.csic.es.
References
- Baldasso C, Barros TC, Tessaro IC. Concentration and purification of whey proteins by ultrafiltration. Desalination. 2011;278(1):381–386. doi: 10.1016/j.desal.2011.05.055. [DOI] [Google Scholar]
- Banerjee S, Bhattacharya S. Food gels: gelling process and new applications. Crit Rev Food Sci Nutr. 2011;52:334–346. doi: 10.1080/10408398.2010.500234. [DOI] [PubMed] [Google Scholar]
- Barukčić I, Božanić R, Jakopović K, Tratnik L. Possibilities of whey utilisation. Austin J Nutr Food Sci. 2014;2(7):1–7. [Google Scholar]
- Bodart M, De Peñaranda R, Deneyer A, Flamant G. Photometry and colorimetry characterisation of materials in daylighting evaluation tools. Build Environ. 2008;43(12):2046–2058. doi: 10.1016/j.buildenv.2007.12.006. [DOI] [Google Scholar]
- Considine T, Patel HA, Anema SG, Singh H, Creamer LK. Interactions of milk proteins during heat and high hydrostatic pressure treatments—a review. Innov Food Sci Emerg Technol. 2007;8(1):1–23. doi: 10.1016/j.ifset.2006.08.003. [DOI] [Google Scholar]
- Einhorn-Stoll U, Drusch S. Methods for investigation of diffusion processes and biopolymer physics in food gels. Curr Opin Food Sci. 2015;3:118–124. doi: 10.1016/j.cofs.2015.07.002. [DOI] [Google Scholar]
- Huppertz T, Fox PF, Kelly AL. High pressure treatment of bovine milk: effects of casein micelles and whey proteins. J Dairy Res. 2004;71:97–106. doi: 10.1017/S002202990300640X. [DOI] [PubMed] [Google Scholar]
- Kanno C, Mu TH, Hagiwara T, Ametani M, Azuma N. Gel formation from industrial milk whey proteins under hydrostatic pressure: effect of hydrostatic pressure and protein concentration. J Agric Food Chem. 1998;46(2):417–424. doi: 10.1021/jf970652f. [DOI] [PubMed] [Google Scholar]
- Kinsella JE, Rector DJ, Phillips LG. Physicochemical properties of proteins: texturization via gelation, glass and film formation. In: Yada RY, Jackman RL, Smith JL, editors. Protein structure-function relationships in foods. London: Blackie Academic & Professional; 1994. pp. 1–21. [Google Scholar]
- Krešić G, Vesna L, Zoran H, Režek A. Effects of high pressure on functionality of whey protein concentrate and whey protein isolate. Lait. 2006;86:303–315. doi: 10.1051/lait:2006012. [DOI] [Google Scholar]
- Lievore P, Simões D, Silva KM, Drunkler NL, Barana AC, Nogueira A, Demiate IN. Chemical characterisation and application of acid whey in fermented milk. J Food Sci Technol. 2015;52(4):2083–2092. doi: 10.1007/s13197-013-1244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Fandiño R. High pressure-induced changes in milk proteins and possible applications in dairy technology. Int Dairy J. 2006;16:1119–1131. doi: 10.1016/j.idairyj.2005.11.007. [DOI] [Google Scholar]
- Lucey JA. Milk protein gels. In: Boland M, Singh H, Thompson A, editors. Milk proteins:from expression to food, chapter 17. New York: Academic Press; 2014. pp. 493–523. [Google Scholar]
- McGuffey MK, Epting KL, Kelly RM, Foegeding EA. Denaturation and aggregation of three α-lactalbumin preparations at neutral pH. J Agric Food Chem. 2005;53:3182–3190. doi: 10.1021/jf048863p. [DOI] [PubMed] [Google Scholar]
- Oakenfull D, Pearce J, Burley RW. Protein gelation. In: Damodaran S, Paraf A, editors. Food proteins and their applications. New York: Marcel Dekker; 1997. pp. 111–142. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD) and the Food and Agriculture Organization (FAO) of the United Nations (2012) Agricultural Outlook 2012–2021. Accessed 2 July, 2015
- Patel HA, Huppertz T. Effects of high-pressure processing on structure and interactions of milk proteins. In: Boland M, Singh H, Thompson A, editors. Milk proteins: from expression to food, chapter 8. New York: Academic Press; 2014. pp. 243–267. [Google Scholar]
- Patel HA, Singh H, Havea P, Considine T, Creamer LK. Pressure-induced unfolding and aggregation of the proteins in whey protein concentrate solutions. J Agric Food Chem. 2005;53:9590–9601. doi: 10.1021/jf0508403. [DOI] [PubMed] [Google Scholar]
- Ribotta PD, Rosell CM. Effects of enzymatic modification of soybean protein on the pasting and rheological profile of starch–protein systems. Starch-Stärke. 2010;62(7):373–383. doi: 10.1002/star.200900259. [DOI] [Google Scholar]
- Ribotta PD, Colombo A, León AE, Añón MC. Effects of soy protein on physical and rheological properties of wheat starch. Starch-Stärke. 2007;59(12):614–623. doi: 10.1002/star.200700650. [DOI] [Google Scholar]
- Ribotta PD, Colombo A, Rosell CM. Enzymatic modifications of pea protein and its application in protein–cassava and corn starch gels. Food Hydrocoll. 2012;27(1):185–190. doi: 10.1016/j.foodhyd.2011.07.006. [DOI] [Google Scholar]
- Saddoud A, Hassaïri I, Sayadi S. Anaerobic membrane reactor with phase separation for the treatment of cheese whey. Bioresour Technol. 2007;98:2102–2108. doi: 10.1016/j.biortech.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Salvador A, Muguerza B, Moulay L, Fiszman SM. Cocoa fibre and its application as a fat replacer in chocolate muffins. LWT-Food Sci and Technol. 2011;44(3):729–736. doi: 10.1016/j.lwt.2010.06.035. [DOI] [Google Scholar]
- Torres EF, González-M G, Klotz B, Rodrigo D. Effects of high hydrostatic pressure and temperature increase on Escherichia coli spp. and pectin methyl esterase inactivation in orange juice. Food Sci Technol Int. 2015;22:173–180. doi: 10.1177/1082013215582107. [DOI] [PubMed] [Google Scholar]
- Totosaus A, Montejano JG, Salazar JA, Guerrero I. A review of physical and chemical protein-gel induction. Int J Food Sci Tech. 2002;37:589–601. doi: 10.1046/j.1365-2621.2002.00623.x. [DOI] [Google Scholar]
- Zheng GH, Sosulski FW. Determination of water separation from cooked starch and flour pastes after refrigeration and freeze-thaw. J Food Sci. 1998;63:134–139. doi: 10.1111/j.1365-2621.1998.tb15693.x. [DOI] [Google Scholar]