Abstract
Exopolysaccharide (EPS) produced from Leuconostoc lactis KC117496 isolated from the naturally fermented idli batter has been reported earlier. Here, study aimed to optimize the carbon source to enhance the yield of EPS production, partial characterization and antioxidant activity of α-d-glucan EPS. Among different disaccharides (sucrose, maltose, lactose) and monosaccharides (glucose, galactose and fructose), combination of sucrose and glucose at 2% showed highest EPS production of 4.55 g/L in MRS medium. The molecular weight was identified as ~ 4.428 × 103 kDa with MALDI-TOF mass spectroscopy. 1D and 2D NMR results exhibited the presence of α-1-6 and α-1-3 linked glucose revealed EPS as a glucan. Antioxidant properties of glucan (500 µg/mL) revealed the significant oxidation alleviation potential such as DPPH (74%) and Hydroxyl radical activity (97.8%), whereas metal chelating activity (70%) was lower as compare to control standard. These characteristics of glucan EPS reveals its potential application in food and pharmaceutical industry.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3469-3) contains supplementary material, which is available to authorized users.
Keywords: Exopolysaccharide, Optimization, Characterization, NMR, MALDI-TOF/TOF, Antioxidant activity
Introduction
Indian traditional fermented foods are rich source of Lactic Acid Bacteria (LAB) being widely consumed as a healthy diet with numerous benefits such as increased digestibility, enhanced flavour, improved nutritional and pharmacological values. Idli is one of the well-known, easily digestible, Indian traditional fermented food prepared from rice and black gram dhal. Most of the LAB involved in fermentation of idli batter are capable of producing exopolysaccharide (EPS) that are known to improve the texture and sensory properties of idli (Patel and Prajapati 2013; Patel et al. 2014; Saravanan et al. 2015). Furthermore, the EPS has wide application as bio-flocculants, stabilizers, emulsifiers, bio-absorbents, drug delivery agents and heavy metal removing agents (Liu et al. 2010). Dextran is a homopolysaccharide which is produced by various LAB, especially Leuconostoc and Streptococcus sp. in sucrose containing media (Kim et al. 2003). Glucan EPS produced by Leuconostoc garlicum PR encompasses 95% α-1 → 6 glucopyranose linkage along with low content of α-1 → 2, α-1 → 3 and α-1 → 4 linkages (Capek et al. 2011). Leuconostoc pseudomesenteroides XG5 produced water soluble EPS composed of glucose subunits with α-(1-6) linkage (Dextran) and the molecular mass was 2.6 × 103 kDa (Zhou et al. 2017). Molecular weight of EPS produced by Leuconostoc citreum-BMS, L. mesenteroides-TMS, Pediococcus pentosaceus-DPS and L. pseudomesenteroides-CM were around 106 Da (Abid et al. 2017). Joshi and Koijam (2014) isolated L. lactis from ethnically fermented beverage (Kyiad pyrsi) that yields EPS at a maximum of 0.340 g/L. Under normal conditions, EPS produced from LAB ranged between 100 and 196 mg/L, while homopolysaccharides produced are less than 1 g/L. EPS produced by L. lactis and L. mesenteroides isolated from raw milk ranges between 0.8 and 0.9 g/L (Van der Meulen et al. 2007). Under optimized conditions, glucan production by L. dextranicum NRRL B-1146 reached a maximum of 1.063 g/L (Majumder et al. 2009). EPS produced by Bacillus tequilensis FR9 showed potential strong antioxidant effects through scavenging reactive oxygen species (ROS) (Rani et al. 2017). The commercial glucan with different molecular weight (1, 4, and 14.7 × 104 Da) synthesized by L. mesenteroides exhibited strong antioxidant, anticoagulant and immunomodulatory activities (Soeiro et al. 2016). The in vitro antioxidant properties of the EPS showed remarkable scavenging effects on superoxide anion radical and hydroxyl radical (Adesulu-Dahunsi et al. 2018). Furthermore, EPS has been intensively employed as a food additive in texture improvement which impart the development of innovative food products with enhanced appearance, mouth feel, firmness and rheological properties (De Vuyst et al. 2001).
Previously, we have reported this EPS as α-d-glucan along with production of 0.360 g/L from Leuconostoc lactis KC117496 isolated from fermented idli batter (Saravanan and Shetty 2016). In the present study, optimization of carbon source, partial characterization and antioxidant property of α-d-glucan EPS has been studied to improve its production and bioactive potential.
Materials and methods
Microbial culture and chemicals
The EPS producing strain Leuconostoc lactis KC117496 used in this study was isolated from naturally fermented idli batter. All reagents used were of analytical grade.
Growth condition of Leuconostoc lactis KC117496
For EPS production, 10 mL of L. lactis grown in MRS broth for 24 h (1x106 cfu/mL) was used as an inoculum in 100 mL MRS broth supplemented with 2% sucrose. Flasks were kept for incubation at 30 °C under different shaking conditions (100, 125 and 150 rpm) up to 48 h. At every 8 h intervals, optical density (O.D.) was assessed at 600 nm by using spectrophotometer (UV-1800, Shimadzu, North America) and growth curves were plotted.
Extraction and purification of EPS
At the time of harvesting, 48 h grown culture broth was subjected to 100 °C for 10 min for enzyme inactivation by heating the cell suspension. After cooling to room temperature, the biomass was removed by centrifugation at 4100×g for 20 min. The resulting supernatant was treated with Sevage reagent (chloroform:n-butanol at 5:1, v/v) three times to remove the proteinaceous materials by centrifugation. Then EPS was precipitated using thrice the volume of ice-cold ethanol and left overnight. The precipitated EPS was separated via centrifugation (19,200×g, 15 min) and dissolved in Milli Q water. For further purification, EPS was encased in a dialysis bag (12–14 kDa) and dialysed until 48 h at 4 °C in Milli Q water (Saravanan and Shetty 2016). Dialyzed EPS was subjected to lyophilization. Quantification of EPS was done using phenol sulphuric acid method (Dubois et al. 1956).
Optimization kinetics of L. lactis KC117496 to increase EPS production
The batch fermentation was tested initially for EPS production using different disaccharides such as sucrose, lactose and maltose as sole carbon source (2% w/v). Subsequently, the disaccharide that yields the highest EPS was further combined with monosaccharide (2% w/v) such as glucose, mannose and galactose after 8 h of incubation. Freshly prepared 10% inoculum (24 h) of L. lactis was added into each flask. EPS production was observed at every 8 h interval up to 48 h under shaking conditions.
Nuclear magnetic resonance spectroscopy analysis
In structural analysis, 1H and 13C NMR spectrum was measured using the partially purified EPS (10 mg) dissolved in 99.96% D2O as per the method described by Liu et al. (2007). 1H NMR spectra was carried out at 1.00 delay (D1) and 3.17 s acquisition time (AQ), while for 13C NMR the D1 and AQ was 2.0 and 1.1 s, respectively. 1H–1H correlation spectroscopy (COSY), 1H–13C heteronuclear single quantum coherence spectroscopy (HSQC) and 1H–13C heteronuclear multiple bond correlation spectroscopy (HMBC) were also done by using Bruker DRX Advance 400 MHz spectrometer.
MALDI-TOF/TOF mass spectrometry
Matrix-assisted laser desorption ionization (Ultraflex TOF/TOF) mass spectrometry was executed in positive and negative modes, with an alpha-cyano-4-hydroxycinnamic acid (10 mg/mL in 50% acetonitrile, 0.1% TFA) matrix (Ismail and Nampoothiri 2010). EPS (0.5 µL) was taken up with a nitrogen laser (k 330 nm) via a detector. Mass spectra were detailed over a range of 0–16,000 m/z and minimum 5000 laser shots were taken per spectrum with the laser repetition rate 2000 Hz.
Antioxidant properties of EPS
2,2-diphenyl-1-picrylhydrazyl radicals scavenging activity
The assay was executed as per the method described by Yang et al. (2006) along with slight modification. For reaction, 100 µL of 0.2 mM DPPH (95% ethanol v/v) was added to 100 µL EPS solution at various concentrations (100–500 µg) and incubated in dark room for 30 min at room temperature. The absorbance (517 nm) was compared with control solution of DPPH without EPS and a positive control (Ascorbic acid).
whereas, AControl is the absorbance with only DPPH solution, ATest is the absorbance with DPPH and EPS.
Hydroxyl radical (OH) scavenging activity
Hydroxyl radical scavenging activity of EPS was evaluated as explained by Ye et al. (2012). The reaction mixture consists of 2.0 mL of 0.15 mM PBS (pH 7.4), 0.2 mL safranin T (0.52 mg/mL), 1.0 mL of 6 mM EDTA-Fe(II), 0.8 mL of 6% (v/v) H2O2 and 1.0 mL EPS at various concentrations. After incubation for 30 min at 40 °C, the absorbance (520 nm) was evaluated against Ascorbic acid as a positive control.
where, Asample—absorbance of the reagent mixture with EPS, Ablank—absorbance of the reagent mixture without EPS, and Acontrol—absorbance of the reagent mixture without EPS and H2O2.
Fe-chelating activity
Fe2+ chelating activity of EPS was investigated as described by Decker and Welch (1990) with slight modifications. Briefly, 10 µL of EPS at various concentrations was mixed with 0.5 mL FeCl2 (2 mM) and 1 mL ferrozine solution (5 mM) and kept for 20 min under dark conditions. The absorbance (562 nm) of the reactant solution was recorded along with distilled water as a control. Na2EDTA was used as a positive control.
where, Acontrol was the absorbance of the reagent without EPS, and Atest was the absorbance of the EPS.
Statistical analysis
All experiments were done in triplicates and the results were expressed as mean ± SD. Data processing was done using Microsoft office excel 2016 with Windows (2010) computer software.
Results and discussion
Growth kinetics of L. lactis KC117496
To optimize the growth condition of L. lactis KC117496, various trials was carried out. Figure 1 shows the growth pattern of L. lactis KC117496 at different shaking conditions (100, 125 and 150 rpm). The optical density (O.D) values of 125 rpm (1.59) was higher compared to 100 rpm (1.31) and 150 rpm (1.22), due to shear effect of bacterial cells in shaker flask at 30 °C up to 48 h.
Fig. 1.
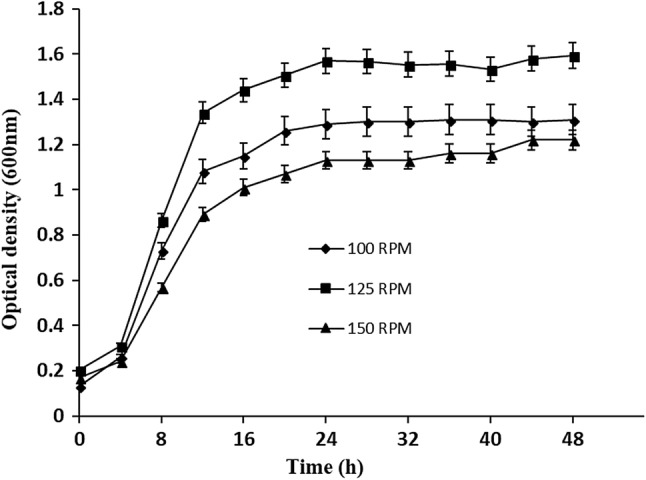
Growth curve of Leuconostoc lactis at different RPM (100,125,150)
The exponential growth rate (0.082, 0.087, 0.076) and doubling time or generation time (8.44, 7.87, 9.05 h) of L. lactis was observed at 100, 125 and 150 rpm, respectively. The highest EPS production was recorded at 125 rpm (1.80 g/L) rather than 100 (0.740 g/L) and 150 rpm (0.340 g/L). In an earlier report, Leuconostoc mesenteroides NCIM 2947 isolated from idli batter showed a growth rate of 0.2 and doubling time of 5 h (Subathra Devi et al. 2014).
Addition of sugars to increase the yield of EPS by L. lactis KC117496
To improve the EPS production, L. lactis KC117496 was grown in MRS medium with 2% sucrose at 125 rpm and 30 °C. Addition of various disaccharides (sucrose, maltose and lactose) at 2% induced EPS production of L. lactis from 8 h onwards (Fig. 2). Highest EPS production was observed with sucrose at 40 h of incubation. The yield of EPS is correlated with specific gravity (S.G.) of the broth at each 8 h time interval. The S.G. and yield of EPS at 40 h was improved more with sucrose (1.043; 1.94 g/L) compared to maltose (1.036; 1.32 g/L) and lactose (1.028; 1.11 g/L).
Fig. 2.
Optimization of different sugars to increase the yield of EPS from Leuconostoc lactis. EPS production (a) and the specific gravity (b) from different diasaccharides. Whereas, EPS production (c) and the specific gravity (d) using combination of sucrose and other monosaccharides. All the experiment was done in triplicate and results expressed as mean ± SD
Furthermore, supplementation of monosaccharides (glucose, fructose and galactose) with 2% sucrose was carried out for EPS production and S.G. The specific gravity (1.066) and yield of EPS (4.55 g/L) with glucose addition was higher compared to fructose (1.053; 3.47 g/L) and galactose (1.060; 3.60 g/L). Under optimized conditions, the EPS production was found to be increased compared to normal conditions (0.360 g/L) (Saravanan and Shetty 2016). Similarly, the yield of EPS from Weissella cibaria GA44 was improved through media optimization up to 4.80 g/L (Adesulu-Dahunsi et al. 2018). Different sucrose concentration (2, 5, 10, 15, 20, 25 and 30%) was also tested for growth and EPS production. The effect of increasing sucrose concentration was directly proportional to EPS production whereas inversely proportional to the growth of the culture due to substrate inhibitory effect (Supplement 1).
Nuclear magnetic resonance spectroscopy analysis
NMR is a spectroscopic technique to determine the structure of organic molecules. The influence of structural character on chemical shifts and coupling constants is obtained from correlation spectroscopy (COSY), hetero-nuclear single quantum coherence (HSQC) and hetero-nuclear multiple bond correlation (HMBC) (Bubb 2003). Earlier studies on H1 and 13C NMR showed the EPS is a glucan comprises α-1 → 6 and α-1 → 3 linkages (Saravanan and Shetty 2016). Though heteronuclear, HSQC spectra (Fig. 3) showed the back bone as (H1/C1) δ 5/98, (H2/C2) δ 3.6/72, (H3/C3) δ 3.7/73, (H4/C4) δ 3.5/70, (H5/C5) δ 3.9/71 and (H6/C6) δ 4,3.75/66. These results correlate well with data published by Capek et al. (2011) for Leuconostoc garlicum PR. Hetero-nuclear multiple bond correlation (HMBC) spectrum displayed the cross signal between C1/H6 (98/3.8) and C6/H1 (66/5) signifying the existence of α-1 → 6 linkage and cross signal between C1/H3 (98/3.7) and C3/H1 (73/5) indicating the presence of α-1 → 6 linkage (Fig. 4). Similar, α-1 → 6 linkage was observed with galactan EPS of Weissella confusa KR 780676 from an acidic fermented food (Kavitake et al. 2016).
Fig. 3.
COSY spectrum indicating the cross signal between the protons and HSQC spectra showed the distribution of carbon and hydrogen in pyranose ring of monosaccharide units
Fig. 4.
HMBC spectra revealed the α-1-6 linkage through the cross signal between H1/C6 and C1/H6
MALDI-TOF–MS analysis
The glucan molecular weight was revealed through MALDI-TOF analysis (Fig. 5). Depending upon the calibration curve of elution retention time of several standard polysaccharides with glucose subunits, the molecular mass of glucan was estimated to be ~ 4.428 × 103 kDa. Similarly, Liu et al. (2017) also stated EPS from Lactobacillus plantarum WLPL04 with a molecular weight of 6.61 × 104 Da.
Fig. 5.
MALDI-TOF–MS analysis of exopolysaccharide produced by Leuconostoc lactis
DPPH radical scavenging activity
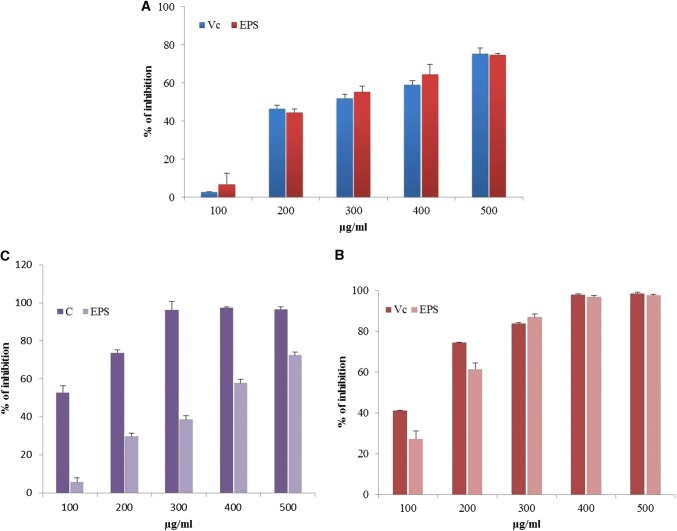
The DPPH free radical acts as an extensively proven tool for assessing the free radical scavenging activities of antioxidants. DPPH free radical scavenging activity was resolved through spectrophotometric analysis in which the antioxidants transfers an electron or a hydrogen atom to DPPH, thereby neutralizing its free radical characteristic to form colour solution (Liu et al. 2011). In this present work, the scavenging activity of glucan was comparably in increasing drift with concentration (Fig. 6a). The scavenging activity at various concentrations (100–500 µg/mL) of Vc (ascorbic acid) and glucan showed an increasing trend of 2.8–75% and 6.8–74%, respectively. Previously, Li et al. (2014) and Zhang et al. (2013) reported glucan EPS with high radical scavenging activity which was 41.92% and 52.23%, respectively.
Fig. 6.
DPPH radical scavenging activity of EPS (a), hydroxyl radical scavenging activity of EPS (b), metal chelating activity of EPS (c). Vc-Ascorbic acid, C = Na2EDTA, EPS-Exopolysaccharide. Results are expressed as means ± standard deviations (n = 3)
Hydroxyl radical (OH) scavenging activity
Hydroxyl radicals are extremely strong oxidants that easily react with bio-molecules of living cells and creates destruction to the contiguous macromolecules in biological system (Valko et al. 2016). The hydroxyl radical scavenging effect of both ascorbic acid and glucan were increased with increasing concentrations from 100 to 500 µg/mL (Fig. 6b). The hydroxyl radical scavenging activity of Vc and glucan were 41.16–98.63% and 27.5–97.8%, respectively. The scavenging activities of crude and purified fraction of EPS were 31.67%, 38.61, and 43.17% at 4.0 mg/mL (Liu et al. 2010).
Fe-chelating activity
The metal chelating activity plays a critical function as an antioxidant in the biological system. The transition Fe2+ can influence lipid peroxidation through producing hydroxyl radicals by fenton reaction and speed up lipid peroxidation through disintegrating lipid hydroperoxides into alkoxyl and peroxyl radicals (Benedet and Shibamoto 2008). In this study, Fe2+ chelating activity was observed from 100 to 500 µg/mL for both control (EDTA) and glucan (Fig. 6c). Increase in chelating ability was increased with concentrations and activity of glucan was less (5.8–72.5%) than control. Similar results were observed with crude and two EPS fractions were 92.4%, 81.1% and 86.5% (Liu et al. 2011).
Conclusion
The L. lactis KC117496 produced glucan homopolysaccharide in MRS medium supplemented with sucrose, having a molecular weight of ~ 4.428 × 103 kDa. Addition of glucose along with sucrose at 2% increased the yield up to 4.55 g/L. Structural investigation of EPS through HMBC showed presence of α-1 → 6 and α-1 → 3 linked glucose subunits. Antioxidant properties of glucan EPS exhibited an increasing trend at higher concentrations for DPPH and Hydroxyl radical activity, while a lower metal chelating activity. These results suggest that the glucan have potent antioxidant activities that could contribute as a natural agent for possible application in functional foods and therapeutics. Further studies can be done to prove the biological potential of this EPS through in vivo studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Pondicherry University for providing Sophisticated Analytical Instrumental Facility.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abid Y, Casillo A, Gharsallah H, Joulak I, Lanzetta R, Corsaro MM, Azabou S. Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. Int J Biol Macromol. 2017;108:719–728. doi: 10.1016/j.ijbiomac.2017.10.155. [DOI] [PubMed] [Google Scholar]
- Adesulu-Dahunsi AT, Sanni AI, Jeyaram K. Production, characterization and In vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44. LWT Food Sci Technol. 2018;87:432–442. doi: 10.1016/j.lwt.2017.09.013. [DOI] [Google Scholar]
- Benedet JA, Shibamoto T. Role of transition metals, Fe(II), Cr(II), Pb(II), and Cd(II) in lipid peroxidation. Food Chem. 2008;107:165–168. doi: 10.1016/j.foodchem.2007.07.076. [DOI] [Google Scholar]
- Bubb WA. NMR spectroscopy in the study of carbohydrates: characterizing the structural complexity. Concepts Magn Reson Part A Bridg Educ Res. 2003;19:1–19. [Google Scholar]
- Capek P, Hlavoňová E, Matulová M, Mislovicová D, Růžička J, Koutný M, Keprdová L. Isolation and characterization of an extracellular glucan produced by Leuconostoc garlicum PR. Carbohydr Polym. 2011;83:88–93. doi: 10.1016/j.carbpol.2010.07.024. [DOI] [Google Scholar]
- De Vuyst L, De Vin F, Vaningelgem F, Degeest B. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int Dairy J. 2001;11:687–707. doi: 10.1016/S0958-6946(01)00114-5. [DOI] [Google Scholar]
- Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 1990;38:674–677. doi: 10.1021/jf00093a019. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Ismail B, Nampoothiri KM. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch Microbiol. 2010;192:1049–1057. doi: 10.1007/s00203-010-0636-y. [DOI] [PubMed] [Google Scholar]
- Joshi SR, Koijam K. Exopolysaccharide production by a lactic acid bacteria, Leuconostoc lactis isolated from ethnically fermented beverage. Natl Acad Sci Lett. 2014;37:59–64. doi: 10.1007/s40009-013-0203-6. [DOI] [Google Scholar]
- Kavitake D, Devi PB, Singh SP, Shetty PH. Characterization of a novel galactan produced by Weissella confusa KR780676 from an acidic fermented food. Int J Biol Macromol. 2016;86:681–689. doi: 10.1016/j.ijbiomac.2016.01.099. [DOI] [PubMed] [Google Scholar]
- Kim D, Robyt JF, Lee SY, Lee JH, Kim YM. Dextran molecular size and degree of branching as a function of sucrose concentration, pH, and temperature of reaction of Leuconostoc mesenteroides B-512FMCM dextransucrase. Carbohydr Res. 2003;338:1183–1189. doi: 10.1016/S0008-6215(03)00148-4. [DOI] [PubMed] [Google Scholar]
- Li W, Ji J, Chen X, Jiang M, Rui X, Dong M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr Polym. 2014;102:351–359. doi: 10.1016/j.carbpol.2013.11.053. [DOI] [PubMed] [Google Scholar]
- Liu C, Lin Q, Gao Y, Ye L, Xing Y, Xi T. Characterization and antitumor activity of a polysaccharide from Strongylocentrotus nudus eggs. Carbohydr Polym. 2007;67:313–318. doi: 10.1016/j.carbpol.2006.05.024. [DOI] [Google Scholar]
- Liu C, Lu J, Lu L, Liu Y, Wang F, Xiao M. Isolation, structural characterization and immunological activity of an exopolysaccharide produced by Bacillus licheniformis 8-37-0-1. Bioresour Technol. 2010;101:5528–5533. doi: 10.1016/j.biortech.2010.01.151. [DOI] [PubMed] [Google Scholar]
- Liu CF, Tseng KC, Chiang SS, Lee BH, Hsu WH, Pan TM. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J Sci Food Agric. 2011;9:2284–2291. doi: 10.1002/jsfa.4456. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang Z, Qiu L, Zhang F, Xu X, Wei H, Tao X. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J Dairy Sci. 2017;100:6895–6905. doi: 10.3168/jds.2016-11944. [DOI] [PubMed] [Google Scholar]
- Majumder A, Singh A, Goyal A. Application of response surface methodology for glucan production from Leuconostoc dextranicum and its structural characterization. Carbohydr Polym. 2009;75:150–156. doi: 10.1016/j.carbpol.2008.07.014. [DOI] [Google Scholar]
- Patel A, Prajapati JB. Food and health applications of exopolysaccharides produced by lactic acid bacteria. Adv Dairy Res. 2013;1:107. [Google Scholar]
- Patel A, Prajapati JB, Holst O, Ljungh A. Determining probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from vegetables and traditional Indian fermented food products. Food Biosci. 2014;5:27–33. doi: 10.1016/j.fbio.2013.10.002. [DOI] [Google Scholar]
- Rani RP, Anandharaj M, Sabhapathy P, Ravindran AD. Physiochemical and biological characterization of novel exopolysaccharide produced by Bacillus tequilensis FR9 isolated from chicken. Int J Biol Macromol. 2017;96:1–10. doi: 10.1016/j.ijbiomac.2016.11.122. [DOI] [PubMed] [Google Scholar]
- Saravanan C, Shetty PKH. Isolation and characterization of exopolysaccharide from Leuconostoc lactis KC117496 isolated from idli batter. Int J Biol Macromol. 2016;90:100–106. doi: 10.1016/j.ijbiomac.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Saravanan C, Gopu V, Shetty PKH. Diversity and functional characterization of microflora isolated from traditional fermented food idli. J Food Sci Technol. 2015;52:7425–7432. doi: 10.1007/s13197-015-1791-6. [DOI] [Google Scholar]
- Soeiro VC, Melo KRT, Alves MGCF, Medeiros MJC, Grilo MLPM, Almeida-Lima J, Pontes DL, Costa LS, Rocha HAO. Dextran: influence of molecular weight in antioxidant properties and immunomodulatory potential. Int J Mol Sci. 2016;17:1340. doi: 10.3390/ijms17081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subathra Devi C, Reddy S, Mohanasrinivasan V. Fermentative production of dextran using Leuconostoc spp. isolated from fermented food products. Front Biol. 2014;9:244–253. doi: 10.1007/s11515-014-1303-5. [DOI] [Google Scholar]
- Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K. Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016;90:1–37. doi: 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- Van der Meulen R, Grosu-Tudor S, Mozzi F, Vaningelgem F, Zamfir M, de Valdez GF, De Vuyst L. Screening of lactic acid bacteria isolates from dairy and cereal products for exopolysaccharide production and genes involved. Int J Food Microbiol. 2007;118:250–258. doi: 10.1016/j.ijfoodmicro.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Yang B, Wang J, Zhao M, Liu Y, Wang W, Jiang Y. Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities. Carbohydr Res. 2006;341:634–638. doi: 10.1016/j.carres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Ye S, Liu F, Wang J, Wang H, Zhang M. Antioxidant activities of an exopolysaccharide isolated and purified from marine Pseudomonas PF-6. Carbohydr Polym. 2012;87:764–770. doi: 10.1016/j.carbpol.2011.08.057. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tang X, Wang F, Zhang Q, Zhang Z. Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int J Biol Macromol. 2013;61:270–275. doi: 10.1016/j.ijbiomac.2013.06.031. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Feng F, Yang Y, Zhao F, Du R, Zhou Z, Han Y. Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. Int J Biol. 2017 doi: 10.1016/j.ijbiomac.2017.10.098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.