Abstract
Olive fruit is very rich in terms of phenolic compounds. Antimicrobial activities of various phenolic compounds against bacteria and fungi are well established; however, their effects on yeasts have not been examined. Aim of this study was to investigate the antimicrobial effects induced by olive phenolic compounds, including tyrosol, hydroxytyrosol, oleuropein, luteolin and apigenin against two yeast species, Aureobasidium pullulans and Saccharomyces cerevisiae. For this purpose, yeasts were treated with various concentrations (12.5–1000 ppm) of phenolic compounds and reduction in yeast population was followed with optical density measurements with microplate reader, yeast colony forming units and mid-infrared spectroscopy. All phenolic compounds were effective on both yeasts, especially 200 ppm and higher concentrations have significant antimicrobial activity; however, effects of lower levels depend on the type of phenolic compound. According to mid-infrared spectral data, significant changes were observed in 1200–900 cm−1 range corresponding to carbohydrates of yeast structure as a result of exposure to all phenolic compounds except tyrosol. Spectra of tyrosol and luteolin treated yeasts also showed changes in 1750–1500 cm−1 related to amide section and 3600–3000 cm−1 fatty acid region. Since phenolic compounds from olives were effective against yeasts, they could be used in food applications where yeast growth showed problem. In addition, FTIR spectroscopy could be successfully used to monitor and characterize antimicrobial activity of phenolic compounds on yeasts as complementary to conventional microbiological methods.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3468-4) contains supplementary material, which is available to authorized users.
Keywords: Antimicrobial activity, Yeast, Olive, Phenolic compounds, Mid-infrared spectroscopy
Introduction
The phenolic fraction of olive fruit is very complex and can vary both in quality and quantity depending on agronomical practices and process parameters. One of the main phenolic compounds responsible for bitterness in unripe olives is oleuropein. This phenolic compound belongs to a specific group of coumarin-like compounds, the secoiridoids, which are abundant in olives (Bendini et al. 2007). Secoiridoids have been shown to inhibit or delay the rate of growth of a range of bacteria and microfungi; therefore, they were suggested to be used as alternative food additives or as agents in integrated pest-management programs (Bisignano et al. 1999). Other phenolic compounds, hydroxytyrosol and tyrosol (p-hydroxyphenylethanol), are the most abundant phenolic alcohols in olives (Jemai et al. 2009) and hydroxytyrosol is one of the principal degradation products of oleuropein. Flavonoids are also widespread secondary plant metabolites in olives. During the past decade, an increasing number of publications on the beneficial health effects of flavonoids on cancer and coronary heart diseases have been reported (Xiao et al. 2011; Shahidi and Ambigaipalan 2015; Mozaffarian and Wu 2018). Luteolin and apigenin are the most common flavonoids of olive oils and they may originate from rutin or luteolin-7-glucoside, and apigenin glucosides, respectively.
As a mid-infrared spectroscopic technique, Fourier transform infrared (FTIR) spectroscopy has been widely applied to the assessment of the mechanisms of bacterial inactivation by various food processing techniques and antimicrobial compounds (Alvarez-Ordonez et al. 2011; Zoumpopoulou et al. 2010; Schleicher et al. 2005), the monitoring of membrane properties in changing environments (Ami et al. 2009), the detection of stress-injured microorganisms in food-related environments (Al-Qadiri et al. 2008a, b), the evaluation of dynamic changes in bacterial populations (Ngo-Thi et al. 2003), the investigation of bacterial tolerance responses (Alvarez-Ordonez et al. 2010) and the study of spore ecology in foodborne pathogenic bacteria (Subramanian et al. 2007). These studies have been particularly focused on the effects of stress conditions on the cytoplasmic membrane composition and structure. This technique has been also used to determine the presence and quantity of injured vegetative cells in food products as a result of exposure to different food processing treatments (Alvarez-Ordonez et al. 2011). Besides bacteria, FTIR spectroscopy has also been used for the assessment of stress response in yeasts, e.g. Saccharomyces cerevisiae. This yeast can be considered as a valid model due to its eukaryotic nature, which allows extending the bioassay results to other eukaryotes, including human (Corte et al. 2010). Although there are many studies monitoring the stress response of bacterial cells by FTIR spectroscopy, only a few researches have been published about yeasts (Corte et al. 2010, 2014; Saharan and Sharma 2011).
Phenolic compounds have well-documented antimicrobial activities against bacteria and fungi. However, their effects on yeasts have not been investigated. In general, conventional approaches such as microbial counting are used to study the antimicrobial action of compounds that are desired to be tested. However, untraditional methods such as infrared (IR) spectroscopy have been also used to investigate the antimicrobial action of several compounds and technologies and may provide further information regarding the effect of antimicrobial compounds. In this study, two yeast species, Aureobasidium pullulans and Saccharomyces cerevisiae were selected to investigate the antimicrobial effects induced by olive phenolic compounds, including tyrosol (T), hydroxytyrosol (HT), oleuropein (O), luteolin (L) and apigenin (A) using FTIR spectroscopy as well as optical density (OD) measurements with microplate reader and yeast colony forming units. This study would allow the evaluation of antimicrobial activity of olive phenolic compounds on yeasts through the use of various techniques. Therefore, in the long term, it would be possible to control the growth of these yeasts using natural antimicrobial compounds.
Materials and methods
Preparation of phenolic compounds
Phenolic compounds, tyrosol (Cat. No: 188255) (T), oleuropein (Cat. No: 12247) (O), luteolin (Cat. No: L9283) (L), apigenin (Cat. No: A3145) (A) were obtained from Sigma (Germany) and hydroxytyrosol (Cat. No: 4999S) (HT) was supplied by Extrasynthese (France). The solutions of each phenolic compound were prepared in various concentrations, while an extra concentration was tested for hydroxytyrosol due to its high amounts in olives (Aktas et al. 2014). Each compound was dissolved in ethanol (96%) and then diluted with yeast extract peptone dextrose (YEPD) (yeast extract 1%, peptone 2%, dextrose 2%) (BD Difco, USA) broth to the target concentrations (400, 200, 100, 50, 25 and 12.5 ppm for each phenolic compound and additionally 1000 ppm only for hydroxytyrosol). Ethanol content of all solutions was decreased below 1% (v/v) during dilutions. It was previously determined that ethanol concentration below this level does not have antimicrobial activity (Karaosmanoglu et al. 2010).
Construction of growth curves and determination of antimicrobial activity by optical density (OD) measurement
S. cerevisiae (NRRL Y-139, ATCC 2366, GenBank accession no: FJ238322.1) (Sc) and A. pullulans (GenBank accession no: KU240612) (Ap) as the most commonly isolated strain from a naturally black olive type were used for the characterization of antimicrobial activities of phenolic compounds. Both microorganisms were sub-cultured in YEPD agar medium (10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose and 20 g/L agar, pH = 6.5) (BD Difco, USA) at 28–30 °C for 48 h before construction of growth curves. Fresh yeast colony suspensions were prepared corresponding to McFarland 0.5, 1 and 2 using a densitometer (HVD, Den1, Grant Instruments, England); and growth curve of each yeast cell suspension was constructed using a microplate reader (Varioskan Flash Multimode Reader, Thermo, USA). Besides absorbance measurements at 600 nm in every hour during incubation at 30 °C for 48 h without shaking, cultural cell counts were also obtained during the growth of yeasts to monitor the growth phases. A specific incubation time was determined for further analysis representing early exponential growth phase of these two yeasts as 6 h, when S. cerevisiae and A. pullulans could reach to 3.8 × 106 and 2.7 × 105 CFU/ml at 30 °C, respectively.
Antimicrobial activities of phenolic compounds were first determined spectrophotometrically with a microtiter plate method (Dufour et al. 2003). 100 µl from each concentration of each phenolic compound was dispensed into a well of flat bottom 96 well microtiter plate (TPP, Sigma, Germany). Then, 100 µl of 1 × 104 CFU/ml yeast culture in early exponential growth phase was added to each well. OD measurements of each plate were obtained with 1 h intervals at 600 nm during incubation at 30 °C for 48 h. Viable cell count was also obtained at critical points (0, 18, 24, 48 h) on YEPD agar media using the same conditions. Both OD measurements and cultural yeast counts were performed with two replicates.
Determination of antimicrobial activity using FTIR spectrophotometer
First, the growth curves of yeasts treated with various concentrations of phenolic compounds were obtained with UV–Vis spectrophotometric measurements, then concentrations as the most (400 and 200 ppm for all phenolics and additionally 1000 ppm for hydroxytyrosol), moderately (50 ppm) and the least effective (12.5 ppm) ones were chosen for further investigation of their antimicrobial effects on both yeasts using FTIR spectroscopy. Cell growth for FTIR biological assay was performed as previously reported (Corte et al. 2014) with some modifications. Briefly, yeast suspensions and the same volume of phenolic standards were placed into the same test tubes as it was performed in OD measurements. Final yeast populations were also decreased to half to approximately 1.8x 104 CFU/ml for S. cerevisiae and ~ 1.2 × 104 CFU/ml for A. pullulans. Each tube containing phenolic compounds and control with only yeast suspension were centrifuged at 5300 g (7000 rpm) for 3 min (2–16 KC Sigma centrifuge, Germany), washed twice with distilled sterile water and re-suspended in the same amount of distilled water. Procedure explained by Kümmerle et al. (1998) was used in the preparation of a ZnSe plate for FTIR analysis and only the yeast cells were extracted after the application of this procedure. Briefly, 70 µl of each suspension was transferred to a ZnSe optical sample carrier and dried at 42 ± 2 °C for 20 min to yield transparent films. All spectra were recorded through a horizontal attenuated total reflectance (HATR) accessory of a FTIR spectrometer (Perkin Elmer Spectrum 100, Wellesley, MA) with deuterated triglycine sulfate (DTGS) detector within the range of 4000–650 cm−1. The spectrum was obtained at 4 cm−1 resolution with 64 scans and 1 cm/s scan speed. The sampling crystal was cleaned with ethanol and distilled water after each measurement and dried under nitrogen flow.
Results
Antimicrobial activities of phenolics
Effect of tyrosol on yeasts
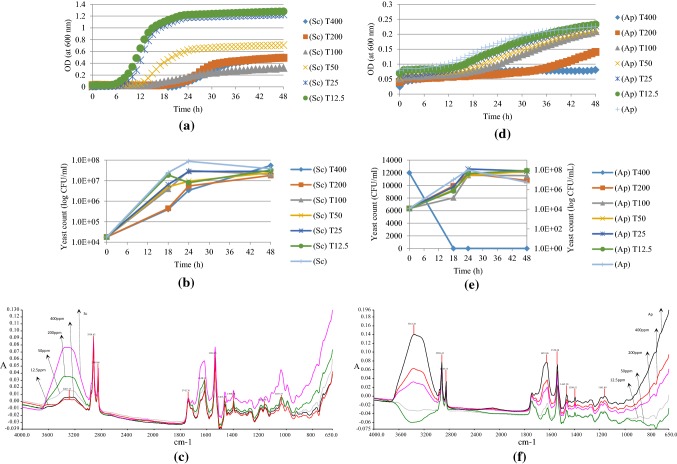
The effects of various tyrosol concentrations (12.5–400 ppm) on determined level of S. cerevisiae (~ 1.8 × 104 CFU/ml) and A. pullulans (1.2 × 104 CFU/ml) as OD measurements, yeast colony forming units (CFU/ml) and mid-IR spectroscopic data are shown in Fig. 1. As it could be observed from Fig. 1a, 12.5 and 25 ppm tyrosol concentrations were the least effective concentrations on S. cerevisiae, whereas 100, 200 and 400 ppm were the most effective and 50 ppm had moderate effect. 200 and 400 ppm tyrosol caused almost 2 log reduction of yeast count at 18 h, which corresponds to exponential phase for S. cerevisiae (Fig. 1b). This reduction in number was 1 log in 24 h and the numbers were almost the same for each tyrosol concentration at 48 h. Except for 12.5 ppm, effect of tyrosol concentration on S. cerevisiae until 18 h was similar, and these concentrations caused almost 1 log reduction of the yeast count at 18 h. Figure 1c shows infrared spectral differences between yeast itself and yeast treated with various tyrosol concentrations. According to visual examination of FTIR spectral data, it was observed that peak shoulder at 1700–1600 cm−1 in amide region became more visible for treated samples. Peak at 3600–3000 cm−1 region (–OH groups) was flattened and twin peaks at 900–800 cm−1 (DNA fingerprint) became clearer and separated.
Fig. 1.
Antimicrobial effects of tyrosol (T) (18 h treatment time) as spectroscopic measurements (a, d), yeast colony forming units (CFU/ml) (b, e) and FTIR spectral data (c, f) at different concentrations on the growth of S. cerevisiae (Sc) and A. pullulans (Ap)
Figure 1d shows that only the highest tyrosol concentration (400 ppm) had a bactericidal activity on A. pullulans; whereas the yeasts exposed to other concentrations have similar growth curves as the control yeast itself. Same conclusion could also be reached in terms of yeast count (Fig. 1e) which indicates that 400 ppm tyrosol caused 4 log decrease in the number of A. pullulans as CFU/ml. This result showed that the same concentration of tyrosol was more effective on this yeast than S. cerevisiae. According to FTIR spectral data (Fig. 1f), it could also be observed that peaks in DNA fingerprint region [900–700 cm−1] were separated better, shoulder at 1622 cm−1 in amide region [1750–1500 cm−1] became more obvious after tyrosol treatment of A. pullulans.
Effect of hydroxytyrosol on yeasts
Figure 2 shows the effects of various hydroxytyrosol concentrations (12.5–1000 ppm) on S. cerevisiae (~ 1.8 × 104 CFU/ml) and A. pullulans (1.2 × 104 CFU/ml) as spectroscopic measurements, yeast colony forming units (CFU/ml) and mid-IR spectroscopic measurements. According to Fig. 2a, the strongest effect of hydroxytyrosol was observed with 1000 and 400 ppm concentrations and 50 ppm of hydroxytyrosol still appeared to be effective on the yeast S. cerevisiae. According to Fig. 2b, 200 ppm hydroxytyrosol caused almost 3 log reduction of yeast count at 18 h which corresponds to exponential phase for S. cerevisiae. Figure 2c indicates that the major difference between control and treated samples is the disappearance of peaks at 1040 and 1000 cm−1 in carbohydrate region [1200–900 cm−1] especially at concentrations above 12.5 ppm.
Fig. 2.
Antimicrobial effects of hydroxytyrosol (HT) (18 h treatment time) as spectroscopic measurements (a, d), yeast colony forming units (CFU/ml) (b, e) and FTIR spectral data (c, f) at different concentrations on the growth of S. cerevisiae (Sc) and A. pullulans (Ap)
The growth of A. pullulans exposed to various concentrations of hydroxytyrosol showed an inhibition pattern with increased concentration (Fig. 2d). Figure 2e shows that both 1000 ppm and 400 ppm of hydroxytyrosol had bactericidal activities against A. pullulans at 18 h; whereas 200 ppm had the same effect on this yeast after 24 h. Even 12.5 ppm of hydroxytyrosol caused 2 log reduction at 18 h and 50 ppm of hydroxytyrosol resulted in 3 log reduction on yeast count. The results of FTIR spectral data (Fig. 2f) showed that the important changes in spectra caused by hydroxytyrosol are in carbohydrate region, and this observation is similar with S. cerevisiae results. Disappearance of double peaks around 1050 cm−1 was again observed for this yeast.
Effect of oleuropein on yeasts
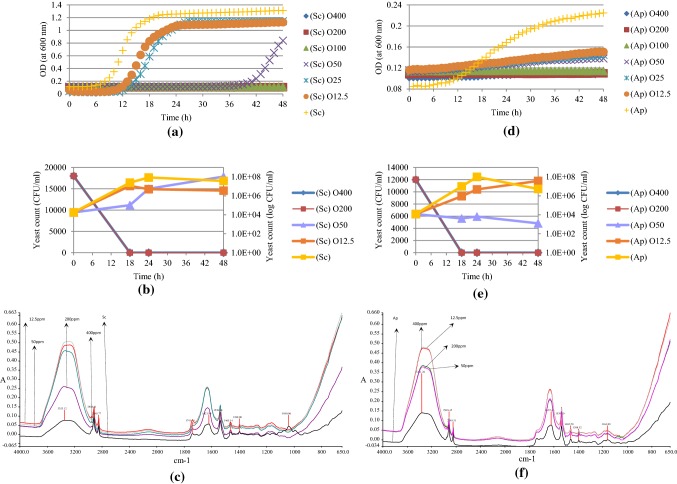
Spectroscopic measurements, yeast colony forming units (CFU/ml) and mid-IR spectroscopic measurements for S. cerevisiae (~ 1.8 × 104 CFU/ml) and A. pullulans (1.2 × 104 CFU/ml) which are treated with oleuropein at various concentrations (12.5–400 ppm) for 48 h are shown in Fig. 3. As it could be observed from Fig. 3a, the most effective concentrations of oleuropein on S. cerevisiae are 400, 200 and 100 ppm. In addition, 50 ppm of oleuropein extends the lag phase of this yeast from 6 h to approximately 40 h. At 18 h which corresponds to exponential phase for S. cerevisiae, 200 and 400 ppm oleuropein caused bactericidal effect and 50 ppm oleuropein resulted in 2 log reduction in number (Fig. 3b). However, this reduction in number was almost 1 log for the same concentration at 24 h. Figure 3c shows that the most significant change was the disappearance of the peaks in 1050–950 cm−1 region.
Fig. 3.
Antimicrobial effects of oleuropein (O) (18 h treatment time) as spectroscopic measurements (a, d), yeast colony forming units (CFU/ml) (b, e) and FTIR spectral data (c, f) at different concentrations on the growth of S. cerevisiae (Sc) and A. pullulans (Ap)
The growth of A. pullulans exposed to various concentrations of oleuropein also showed a decreasing pattern with increased concentrations of oleuropein (Fig. 3d). As it could be seen in this figure, even 100 ppm of oleuropein still had significant antimicrobial effect against A. pullulans as higher concentrations. Figure 3e shows that this phenolic compound is effective on A. pullulans at both 18 h and 24 h with 4 log reduction on yeast count with 50 ppm concentration, and this result matched with OD measurements results. According to FTIR spectra (Fig. 3f), peaks around 1050 cm−1 region were not present after treatment with oleuropein.
Effect of luteolin on yeasts
The effects of various luteolin concentrations (12.5–400 ppm) on determined level of S. cerevisiae (~ 1.8 × 104 CFU/ml) and A. pullulans (1.2 × 104 CFU/ml) as spectroscopic measurements, yeast colony forming units (CFU/ml) and mid-IR spectroscopic measurements are shown in Fig. 4. As it could be observed from Fig. 4a, luteolin has the highest antimicrobial activity on S. cerevisiae with the concentrations of 400, 200, 100 and 50 ppm. Even 12.5 ppm of luteolin extended the lag phase of S. cerevisiae (104 CFU/ml) to 16 h. Figure 4b shows that both 400 and 200 ppm of luteolin had the highest activity that led to 4 log reduction of S. cerevisiae after 18 h. In addition, even 50 ppm luteolin concentration decreased the yeast count 2 log after 18 h and 24 h. Significant changes in FTIR spectra (Fig. 4c) were observed in carbohydrate region with disappearance of two peaks (1050–950 cm−1). In addition, broad peak corresponding to –OH groups in 3600–3000 cm−1 fatty acid region became triple peak with flattening trend. Shoulder of the peak at 1622 cm−1 in amide region were also separated after treatment.
Fig. 4.
Antimicrobial effects of luteolin (L) (18 h treatment time) as spectroscopic measurements (a, d), yeast colony forming units (CFU/ml) (b, e) and FTIR spectral data (c, f) at different concentrations on the growth of S. cerevisiae (Sc) and A. pullulans (Ap)
All of the luteolin concentrations have significant antimicrobial activity on A. pullulans according to spectrophotometric measurements (Fig. 4d). When it was compared with the yeast count graphics, it could be concluded that 400 and 200 ppm luteolin had the highest activity beginning from 18 h (Fig. 4e). The other two concentrations showed 3 log reduction on the count of A. pullulans at the same incubation time. Visually, FTIR spectra (Fig. 4e) also show that the biggest changes are in DNA fingerprint region [900–600 cm−1], carbohydrate region [1200–900 cm−1] and –OH groups [3600 cm−1] of A. pullulans.
Effect of apigenin on yeasts
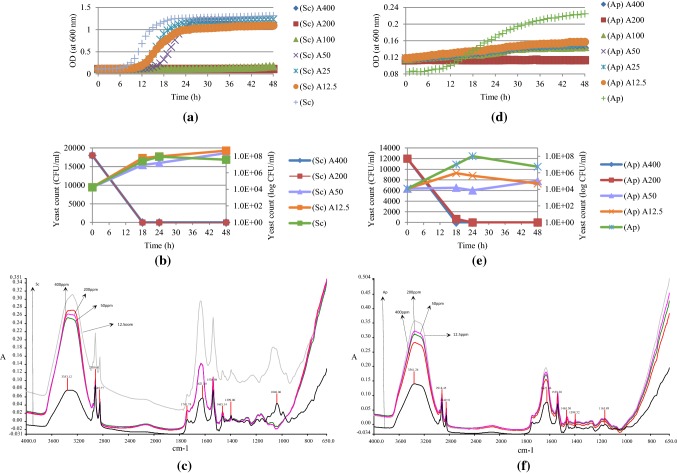
The effects of various apigenin concentrations (12.5–400 ppm) on S. cerevisiae (~ 1.8 × 104 CFU/ml) and A. pullulans (1.2 × 104 CFU/ml) as spectroscopic measurements, yeast colony forming units (CFU/ml) and mid-IR spectroscopic measurements are shown in Fig. 5. Apigenin has the highest antimicrobial activity on S. cerevisiae in the concentration range of 100–400 ppm (Fig. 5a). As seen in Fig. 5b, 200 and 400 ppm of apigenin had bactericidal activity at 18 h which corresponds to exponential phase for S. cerevisiae. 50 ppm of apigenin caused 1 log reduction in number at 18 h. FTIR spectra (Fig. 5c) indicate that the most obvious change in FTIR spectra was due to the disappearance of peaks in 1040 and 1000 cm−1.
Fig. 5.
Antimicrobial effects of apigenin (A) (18 h treatment time) as spectroscopic measurements (a, d), yeast colony forming units (CFU/ml) (b, e) and FTIR spectral data (c, f) at different concentrations (400, 200, 100, 50, 25, 12.5 ppm) on the growth of S. cerevisiae (Sc) and A. pullulans (Ap)
The growth of A. pullulans exposed to various concentrations of apigenin showed a decreasing pattern with increased concentration (Fig. 5d). In addition, 200 and 400 ppm of apigenin showed significant antimicrobial effect against A. pullulans. According to Fig. 5e, 50 ppm of apigenin resulted 3 log reduction at 18 h; and further decrease in yeast count to 4 log was observed at 24 h. FTIR spectra of the yeast (Fig. 5f) show that the changes due to exposure to apigenin are similar with S. cerevisiae and peaks around 1050 cm−1 in carbohydrate region were not present after apigenin exposure.
Discussion
There is not any study in the literature related with the effects of phenolic compounds in olives on yeasts that could be used as a reference to choose the types of phenolics and their amounts; therefore, a previous chemical characterization investigation (Aktas et al. 2014) was taken as a reference to choose the typical concentrations of phenolic compounds in olives in order to apply on yeasts. The most common phenolic compounds found in olives with the highest concentrations were used in the current research and it was aimed to investigate the antimicrobial effects of each type and class of phenols including tyrosol, hydroxytyrosol, oleuropein, luteolin and apigenin on the yeasts. While tyrosol and hydroxytyrosol belong to phenolic alcohol group luteolin and apigenin are the flavonoids found in the olives. Oleuropein, on the other hand, is classified as a secoiridoid. Antimicrobial activities of five phenolic compounds on two yeasts, S. cerevisiae and A. pullulans, were investigated through conventional microbiological methods and mid-IR spectroscopy. A. pullulans is a yeast from the natural microflora of the olive itself while S. cerevisiae could be considered as a valid yeast model having the advantage of eukaryotic nature. Therefore, investigation of antimicrobial activity against these two yeasts would allow comparison with respect to the source of the yeast and activity of phenolics. In addition, mid-IR spectroscopy would provide extra information with regard to the type of effect of these phenolic compounds.
Results indicated that all three methods used to investigate the antimicrobial activity showed the same type of trends regarding the effect of phenolic compounds on yeasts. When bactericidal activity was observed in OD measurements it was also confirmed by the yeast counts. Changes that took place in the yeast structure due to treatment of yeasts with phenolic compounds were observed through the alterations in FTIR spectra.
As a result of application of several techniques to investigate the effect of phenolic compounds, tyrosol, hydroxytyrosol, oleuropein, luteolin, and apigenin on two yeasts, S. cerevisiae and A. pullulans, it was found out that all of these compounds have concentration dependent antimicrobial activities against the tested yeasts. To compare the antimicrobial activities of phenolic compounds only the concentrations causing bactericidal effect are considered since ups and downs or re-growth in microbial population was observed at concentrations below that level. Oleuropein, luteolin and apigenin have lethal effect on concentrations at and above 200 ppm. Phenolic alcohol, hydroxytyrosol, had bactericidal activity at and above 200 ppm against A. pullulans; however, 400 ppm caused bactericidal effect on S. cerevisiae. The other phenolic alcohol, tyrosol, had a lethal effect on A. pullulans population at 400 ppm while this concentration did not result in bactericidal effect for S. cerevisiae. Hence, tyrosol was the least effective of the phenolic compounds tested followed by hydroxytyrosol for S. cerevisiae. Other three phenolic compounds having flavonoid (luteolin and apigenin) and secoiridioid (oleuropein) structure have similar antimicrobial activities against both yeasts. Chemical structure of tyrosol is consisted of a phenolic ring which carries a –OH group while hydroxytyrosol having the same chemical structure differs from tyrosol by the presence of another –OH group. Consequently, difference between antimicrobial activities of tyrosol and hydroxytyrosol may originate from this additional –OH group. It is known that increase in the number of –OH groups result in increased biological activity of phenolic compounds which confirms our observations (Rice-Evans et al. 1996). Other tested phenolic compounds used in this study, on the other hand, carry multiple phenolic rings and multiple –OH groups in their structure. Thus, their antimicrobial activities at lower concentrations could be associated with these differences in chemical structures explained above (Rice-Evans et al. 1996). In addition, A. pullulans is more sensitive to exposure to phenolic alcohols, tyrosol and hydroxytyrosol, compared to S. cerevisiae since lower concentrations of these phenolic compounds caused death of this yeast. A. pullulans is a yeast that comes from the natural flora of the olive itself.
Main difference between FTIR spectra of both yeasts which are untreated to and treated with hydroxytyrosol, oleuropein, luteolin and apigenin is in the form of disappearance of a major peak in 1050–1000 cm−1. Differences in the spectra of yeasts in contact with tyrosol, on the other hand, took place in 1700–1500, 3600–3000 and 900–700 cm−1 regions (Online Resource). Therefore, it is likely that interaction between phenolic compounds and yeasts resulted in a major change in yeast structure which could be associated with a change in 1050–1000 cm−1. Several authors have shown the use of FTIR spectroscopy as a tool to determine the cellular target mechanism of different antimicrobial compounds and food processing technologies (Alvarez-Ordonez et al. 2011; Zoumpopoulou et al. 2010; Schleicher et al. 2005). A correlation was established between the chemical structures of the molecules and IR band positions. In terms of wavenumbers; w1 is defined as the fatty acid region (3050–2800 cm−1), where peaks correspond to the vibrations of the -CH2 and -CH3 groups of fatty acids. w2 region is the amide part (1750–1500 cm−1), where protein and peptide bands exist. w3, which ranges from 1500 to 1200 cm−1, is a mixed region including vibrations of fatty acids, proteins, and polysaccharide. w4 (1200–900 cm−1) is dominated by the peaks belonging to polysaccharides. 900–700 cm−1 region (w5) is called as the fingerprint region. This part contains bands which are the most characteristic at the species level (Kümmerle et al. 1998). Similar to our study, the most important differences were generally in w4 spectral region (1200–900 cm−1) in previous researches, indicating that some of the IR spectral changes were due to the damage or conformational/compositional alterations in the components of the cell wall and membrane. In other studies, spectral variations observed between 1300 and 900 cm−1 were also linked to the damage of cell walls and membranes (Alvarez-Ordonez et al. 2011). Although there are many studies monitoring the stress response of bacterial cells by FTIR spectroscopy, only a few researches have been published about yeasts. In the study of Corte et al. (2010), S. cerevisiae cells were exposed to different chemical compounds at various concentrations in order to analyze the effect of the stress induced by these substances on the cells using FTIR analysis. The spectral areas in which the more intense variations took place were identified in the aforementioned study. Region corresponding to amides were found as the most reactive part. In addition, the typing DNA fingerprint and the carbohydrate regions were the significant parts of the spectra. The same research group (Corte et al. 2014) conducted a more recent study based on the measurement of metabolomic stress response induced by a surfactant (N-alkyltropinium) on the yeasts S. cerevisiae and C. albicans. In that study, FTIR spectroscopic analysis revealed that S. cerevisiae cells showed the maximum stress response in the regions of amides (w2) and fatty acids (w1); whereas C. albicans cells were most affected in amides (w2) and mixed (w3) regions.
FTIR spectroscopy provides information regarding the molecular structure of compounds with the peaks in the spectra corresponding to frequencies of vibration between the bonds of the atoms. Sample preparation method for FTIR analysis of yeasts allow harvesting of only yeast cells after their treatment with phenolic compounds. Harvesting of cells in sample preparation is necessary to obtain a clear spectra of yeasts since absorbance peaks in the spectra of media in which the yeasts were grown could block the peaks in the spectra belonging to yeast structure (Kümmerle et al. 1998). As a result, any change in yeast cell structure due to treatment with phenolic compounds could be observed from obtained FTIR spectra. In this study, the main differences between the spectra of yeast itself and yeast treated with phenol solutions could be clearly observed for each yeast in FTIR spectral data (Online Resource). According to FTIR spectral data obtained in this study, the significant changes were observed in w4 region [1200–900 cm−1], corresponding to carbohydrates in the yeast structure exposed to all phenolics except tyrosol. However, w2 region [1750–1500 cm−1], corresponding to amide section was also important for tyrosol and luteolin treated yeasts. The changes in carbohydrate region were associated with the changes in the cell wall or cell membrane (Al-Qadiri et al. 2008a); whereas changes in amide region were associated with the protein degradation involving the cell wall (Corte et al. 2014; Saharan and Sharma 2011). Changes in amide regions might be also related with changes in secondary and tertiary structure of proteins; however, this needs to be confirmed by further analysis. Characteristic bands found in the infrared spectra of proteins include the Amide I and Amide II due to the amide bonds that link the amino acids. The absorption related with the Amide I band leads to stretching vibrations of the C=O bond of the amide, while absorption related with the Amide II band leads to bending vibrations of the N–H bond. The changes in carbohydrate region, which involves disappearance of carbohydrate peaks, might be due to the complex formation between phenolic compounds and carbohydrates.
Conclusion
In this study, two yeast species, S. cerevisiae and A. pullulans were used to investigate the antimicrobial effects induced by olive phenolic compounds, including tyrosol, hydroxytyrosol, oleuropein, luteolin and apigenin using FTIR spectroscopy besides absorbance measurements with microplate reader and yeast colony forming units. As a result, all phenolic compounds were found effective on both yeasts, especially 200 ppm and higher concentrations have more significant effect; however, antimicrobial activity at lower levels depends on the type of phenolic compounds. When the two yeasts were compared, A. pullulans was observed to be more sensitive to phenolic compounds than S. cerevisiae and tyrosol was the least effective of tested phenolic compounds. Shifts in the infrared spectra of the both yeasts treated with phenolic compounds indicated possible changes in the cell wall and/or membrane structures. Other analysis techniques such as microscopic analysis could be used for better understanding of the mechanism of interaction between phenolic compounds and yeasts. It was concluded that FTIR spectroscopy could successfully be used to monitor and characterize antimicrobial activity of phenolic compounds on yeasts as complementary to microbiological methods.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financially supported by Izmir Institute of Technology Scientific Research Projects (IYTE SRP) Programme (Project No: 2014-IYTE-4 and 2015-IYTE-14).
References
- Aktas AB, Ozen B, Tokatli F, Sen I. Comparison of some chemical parameters of a naturally debittered olive. Food Chem. 2014;161:104–111. doi: 10.1016/j.foodchem.2014.03.116. [DOI] [PubMed] [Google Scholar]
- Al-Qadiri HM, Al-Alami NI, Al-Holy MA, Rasco BA. Using Fourier transform infrared (FT-IR) absorbance spectroscopy and multivariate analysis to study the effect of chlorine-induced bacterial injury in water. J Agric Food Chem. 2008;56:8992–8997. doi: 10.1021/jf801604p. [DOI] [PubMed] [Google Scholar]
- Al-Qadiri HM, Lin M, Al-Holy MA, Cavinato AG, Rasco BA. Detection of sublethal thermal injury in Salmonella enterica serotype Typhimurium and Listeria monocytogenes using Fourier transform infrared (FT-IR) spectroscopy (4000–600 cm−1) J Food Sci. 2008;73:M54–M61. doi: 10.1111/j.1750-3841.2007.00640.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Ordonez A, Halisch J, Prieto M. Changes in Fourier transform infrared spectra of Salmonella enterica serovars Typhimurium and Enteritidis after adaptation to stressful growth conditions. Int J Food Microbiol. 2010;142:97–105. doi: 10.1016/j.ijfoodmicro.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Alvarez-Ordonez A, Mouwen DJM, Lopez M, Prieto M. Fourier transform infrared spectroscopy as a tool to characterize molecular composition and stress response in foodborne pathogenic bacteria. J Microbiol Meth. 2011;84:369–378. doi: 10.1016/j.mimet.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Ami D, Natalello A, Schultz T, Gatti-Lafranconi P, Lotti M, Doglia SM, De Marco A. Effects of recombinant protein misfolding and aggregation on bacterial membranes. BBA Proteins Proteom. 2009;1794:263–269. doi: 10.1016/j.bbapap.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Bendini A, Cerretani L, Carrasco-Pancorbo A, Gómez-Caravaca AM, Segura-Carretero A, Fernández-Gutiérrez A, Lercker G. Phenolic molecules in virgin olive oils: a survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade Alessandra. Molecules. 2007;12:1679–1719. doi: 10.3390/12081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisignano G, Tomaino A, Cascio RL, Crisafi G, Uccella N, Saija A. On the in vitro antimicrobial activity of oleuropein and hydroxytyrosol. J Pharm Pharmacol. 1999;51:971–974. doi: 10.1211/0022357991773258. [DOI] [PubMed] [Google Scholar]
- Corte L, Rellini P, Roscini L, Fatichenti F, Cardinali G. Development of a novel, FTIR (Fourier transform infrared spectroscopy) based, yeast bioassay for toxicity testing and stress response study. Anal Chim Acta. 2010;659:258–265. doi: 10.1016/j.aca.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Corte L, Tiecco M, Roscini L, Germani R, Cardinali G. FTIR analysis of the metabolomic stress response induced by N-alkyltropinium bromide surfactants in the yeasts Saccharomyces cerevisiae and Candida albicans. Colloid Surface B. 2014;116:761–771. doi: 10.1016/j.colsurfb.2014.01.054. [DOI] [PubMed] [Google Scholar]
- Dufour M, Simmonds RS, Bremer PJ. Development of a method to quantify in vitro the synergistic activity of “natural” antimicrobials. Int J Food Microbiol. 2003;85:249–258. doi: 10.1016/S0168-1605(02)00544-5. [DOI] [PubMed] [Google Scholar]
- Jemai H, Bouaziz M, Sayadi S. Phenolic composition, sugar contents and antioxidant activity of Tunisian sweet olive cultivar with regard to fruit ripening. J Agric Food Chem. 2009;57:2961–2968. doi: 10.1021/jf8034176. [DOI] [PubMed] [Google Scholar]
- Karaosmanoglu H, Soyer F, Ozen B, Tokatli F. Antimicrobial and antioxidant activities of Turkish extra virgin olive oils. J Agric Food Chem. 2010;58:8238–8245. doi: 10.1021/jf1012105. [DOI] [PubMed] [Google Scholar]
- Kümmerle M, Scherer S, Seiler H. Rapid and reliable identification of food-borne yeasts by Fourier-transform infrared spectroscopy. Appl Environ Microbiol. 1998;64:2207–2214. doi: 10.1128/aem.64.6.2207-2214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Wu JH. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ Res. 2018;122:369–384. doi: 10.1161/CIRCRESAHA.117.309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo-Thi NA, Kirschner C, Naumann D. Characterization and identification of microorganisms by FT-IR microspectrometry. J Mol Struct. 2003;661:371–380. doi: 10.1016/j.molstruc.2003.08.012. [DOI] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Bio Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Saharan RK, Sharma SC. FTIR spectroscopy and biochemical investigation of ethanol stressed yeast Pachysolen tannophilus. Vib Spectrosc. 2011;55:85–89. doi: 10.1016/j.vibspec.2010.08.003. [DOI] [Google Scholar]
- Schleicher E, Heßling B, Illarionova V, Bacher A, Weber S, Richter G, Gerwert K. Light-induced reactions of Escherichia coli DNA photolyase monitored by Fourier transform infrared spectroscopy. FEBS J. 2005;272:1855–1866. doi: 10.1111/j.1742-4658.2005.04617.x. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects—A review. J Funct Foods. 2015;18:820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- Subramanian A, Ahn J, Balasubramaniam VM, Rodriguez-Saona L. Monitoring biochemical changes in bacterial spore during thermal and pressure-assisted thermal processing using FT-IR spectroscopy. J Agric Food Chem. 2007;55:9311–9317. doi: 10.1021/jf0708241. [DOI] [PubMed] [Google Scholar]
- Xiao ZP, Peng ZY, Peng MJ, Yan WB, Ouyang YZ, Zhu HL. Flavonoids health benefits and their molecular mechanism. Mini-Rev Med Chem. 2011;11:169–177. doi: 10.2174/138955711794519546. [DOI] [PubMed] [Google Scholar]
- Zoumpopoulou G, Papadimitriou K, Polissiou MG, Tarantilis PA, Tsakalidou E. Detection of changes in the cellular composition of Salmonella enterica serovar Typhimurium in the presence of antimicrobial compound (s) of Lactobacillus strains using Fourier transform infrared spectroscopy. Int J Food Microbiol. 2010;144:202–207. doi: 10.1016/j.ijfoodmicro.2010.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.