Abstract
Oxidative stability and loss of nutritional values during storage are the major problems that are encountered in the nut spreads and nut pastes affecting the commercial value. In this study, kinetic behavior of lipid oxidation and depletion of the phenolic antioxidants in the black carrot juice supplemented almond pastes stored at the temperature range of 4–60 °C were studied. Kinetic models were employed to quantify the observations. Lipid oxidation was modeled with the logistic equation. Addition of black carrot juice delayed lipid oxidation, and decreased the maximum peroxide value attained. Being different than the results of the previous studies performed with the similar pastes, the rate constants of peroxide formation reactions in the black carrot juice supplemented pastes decreased with increasing temperature (from 0.60 to 0.27 d−1); possibly due to capturing of the lipid oxidation intermediaries by the antioxidants at higher rates at higher temperatures. Depletion of phenolics agreed with a unimolecular first order apparent kinetic model. At the end of the storage period, phenolic losses in the pastes were 5.4, 31.8, 36.9 and 38.2% at 4, 20, 30 and 60 °C, respectively. The results showed that incorporation of the black carrot juice might have an effect on the mechanism of the lipid oxidation and its temperature dependency, and improve the shelf life of the almond pastes.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3510-6) contains supplementary material, which is available to authorized users.
Keywords: Kinetic models, Lipid oxidation, Natural antioxidants, Total phenolics, Black carrot, Almond
Introduction
Nuts such as cashew, peanut, hazelnut, and almond gained attention of many researchers, and recently by food marketers because of the health benefits (such as lowering blood pressure, decreasing the risk of heart attacks and diabetes) related to their high unsaturated fatty acid contents. They are widely used in the food industry raw, roasted, and as an ingredient in chocolates, cakes, ice creams, desserts, spreads and so on for their ability to develop organoleptic features (Balta 2013; Beltrán et al. 2009; King et al. 2008; Lin et al. 2012; Piedrahita et al. 2015; Shakerardekani and Karim 2013). Difficulty of their consumption by children and elderly people, and also microbial contamination and mycotoxin production risks during storage and handling limit the consumption of nuts in raw, salted, and roasted forms. Using nuts in spreads (such as peanut butter) and pastes reduces the risk of quality loss due to microbial toxin production, and also increases nut consumption by the consumers who experience chewing difficulties, such as children (Shakerardekani et al. 2013). On the other hand, large proportions of unsaturated fatty acids in nuts make their spreads and pastes highly susceptible to lipid oxidation during preparation, processing, storage, and distribution. Lipid oxidation decreases the consumer acceptability by producing compounds causing off-flavors, bitter taste, as well as by decreasing the nutritional value (Capanoglu and Boyacioglu 2008; Martin et al. 2001; Ozilgen 2014). In order to promote consumption of these products, their unsaturated fatty acids must be protected against oxidative deterioration and formation of secondary products causing quality loss. Exclusion of oxygen, low-temperature storage, avoiding metals, heat, and light, and addition of antioxidants into foods are the main precautions that can be taken to prevent or slow down lipid oxidation in foods (Capanoglu and Boyacioglu 2008; Choe and Min 2009; Lin et al. 2012; Martin et al. 2001; Shakerardekani and Karim 2013). Among these methods, use of natural or synthetic antioxidants is usually preferred since it is the easiest, effective, and the cheapest method. Antioxidants delay or prevent lipid oxidation by inhibiting free radical formation, and/or inhibiting the accumulation of free radicals in the propagation stage by capturing the free radicals, quenching oxygen, and reducing localized oxygen concentration. In recent years, foods that have been known for their antioxidant properties, are used in the food industry as “natural antioxidants” due to consumer anxiety over the safety of synthetic food additives (Algarra et al. 2014; Brewer 2011; Gök and Serteser 2003; Khandare et al. 2011; Larrauri et al. 2016; Martin et al. 2001; Ozen et al. 2011; Schevey and Brewer 2015). The structural characteristics of the phenolics in black carrot, which contribute to its antioxidant capacity, make them more stable to heat, light and pH changes in comparison to the phenolics from other food sources. Acylated phenolics in black carrots primarily act as free radical scavengers as they interrupt the lipid oxidation radical chain reaction at the propagation stage by donating hydrogen to free radicals. Therefore, they produce relatively stable antioxidant radicals and thus terminate the lipid oxidation. The mechanisms of action ultimately vary from one antioxidant to another, depending on the composition and structure of the compounds. Although the antioxidant components of variety of foods and their mechanisms of action have been investigated by many researchers, there is limited information on the effects of natural antioxidants on the kinetics of lipid oxidation in foods (Erkan et al. 2009; Gómez-Alonso et al. 2004; Lin et al. 2012; Nichenametla et al. 2006; Ozen et al. 2011).
Kinetic models are useful tools to quantify and describe the changes in quality parameters governed by chemical, biochemical, microbial, and physical reactions, as a function of conditions in the food chain. This paper employs the logistic equation to model the effect of natural antioxidant addition on the kinetics of lipid oxidation in model almond pastes stored at temperatures ranging from 4 to 60 °C. In conjunction with lipid oxidation kinetics, the kinetic of natural antioxidant consumption during storage in the same model pastes was also undertaken in this study. The main purpose was to suggest a model to improve the quality factors by the increasing the knowledge of kinetics of quality deterioration.
Materials and methods
Almond paste preparation
Almonds and black carrots used in this study were obtained from the local bazaar. Almonds used for paste making were native to Datca, Turkey, they all came from the same batch.
Fresh black carrots (Daucus carota L. ssp sativus var. atrorunebs Alef.) used in this study were cultivated in Konya, Turkey. Carrots were stored at − 25 °C until the experiments were carried out. Fresh carrots were cut into small pieces, pressed, and filtered to produce the black carrot juice.
Two different model pastes, the reference (almond paste) and the almond-black carrot paste, were prepared using traditional Turkish almond paste production technique. Although the recipes may show slight differences from region to region, almond flour, water, and sugar are the main ingredients of traditional Turkish almond paste. The recipe used in this study was provided by the professional chef. Almonds were blanched in boiling water for 1 min., shocked, dried in between tissue papers, and then their thin skins were removed by hands. De-skinned almonds were ground into small pieces. During grinding, temperature of the samples were controlled using ice jacket to prevent thermal damage from friction. Ground almonds were mixed with 40 g/100 g confectioner’s sugar and 10 g/100 g water to prepare the reference samples. In almond-black carrot pastes, water was replaced with the same amount of black carrot juice; the rest of the recipe was the same. The pastes were kneaded, rolled into 2.5 cm diameter cylinders, and cut into 1 cm thick pieces. Samples were arranged on stainless steel trays in a single layer, tightly covered, and stored immediately. Both black carrot-almond and reference pastes were stored up to 31 days at 4 °C, 20 °C, 30 °C, and 60 °C. During storage, the samples were removed at different intervals for the analysis. All preparation steps were carried out in dark and temperature controlled cold rooms, and preparation time kept at a minimum.
Almond paste oil extraction
Ten grams of the paste were transferred to dark-colored flasks and mixed with 200 mL of petroleum ether and left stirring at room temperature for 24 h. The extracts were filtered through 20–25 micron filter paper, and then the residue was re-extracted. The total petroleum ether was evaporated in a rotary evaporator (Min and Ellefson 2010). Obtained almond extract was kept in sterile sample tubes and stored in at 4 °C in the dark. Further analyses were carried out within 24 h. The same procedure was applied to both the black carrot-almond and the reference pastes.
Peroxide value
The peroxide values were determined following the standard procedures of determination given in the official methods and recommended practices of American Oil Chemists’ Society (Walker 1989). In a glass-stoppered flask, 5.00 ± 0.05 g of a sample was dissolved in 30 mL of acetic acid- chloroform (60:40, v/v) solvent mixture. Subsequently, 0.5 mL of saturated potassium iodide solution was added to the solution, and then the solution was mixed constantly. After 1 min, the solution was diluted with 30 mL distilled water and then titrated with 0.01 mol/L sodium thiosulphate solution. The peroxide values were calculated as:
| 1 |
where S was the volume of titrant for the sample, B was the volume of titrant for blank, N was normality of sodium thiosulphate solution, W was the sample mass in Eq. 1. All samples were analyzed in triplicate.
Total phenolic content
The oil extracts were treated with methanol and hexane to extract the bioactive phytochemicals before total phenolic content analysis (Janu et al. 2014).
The method for total phenolic content of almond extracts was based on Folin-Ciocalteou’s reactive reagent, using as an ultraviolet spectrophotometer (Singleton et al. 1999). In a test-tube, 20 μL of extract, 1580 μL of water, and 100 μL of Folin-Ciocalteu reactive reagent were mixed. After 3 min, a volume of 300 μL of 2% sodium carbonate was added, and the solution was mixed thoroughly. The mixture was allowed to stand at 25 °C in the dark for 120 min. The absorbance was then read at 750 nm using spectrophotometer. The total phenolic concentration was calculated from a calibration curve, and the results were expressed in terms of gallic acid equivalent per 100 g of extract (mg GAE/100 g extract). All samples were analyzed in triplicate. The same procedure was followed for the both black carrot-almond and the reference pastes.
Kinetics of the almond oil oxidation
The logistic equation, which was based on a free radical chain mechanism proposed by Ozilgen and Ozilgen (1990) was applied for quantification of the effect of the added natural antioxidant on the kinetics of lipid oxidation in model almond pastes stored at temperatures ranging from 4 to 60 °C. The logistic equation was stated as:
| 2 |
where c was the concentration of the total oxidation products, was the maximum attainable value of parameter c at the end of the lipid oxidation process, k was the reaction rate constant, and t was time. The logistic equation was integrated and linearized to evaluate the rate of peroxide formation in the pastes at the propagation stage as:
| 3 |
where x is , k is the slope of the line and the is the intercept with t = 0
Kinetics of total phenolic compounds
First order reaction rate kinetic model was employed for total phenolic compound consumption in the model almond pastes stored at temperatures ranging from 4 to 60 °C. The first order reaction rate equation was stated as:
| 4 |
where M was the concentration of total phenolics in terms of gallic acid concentration, t was time, and ka was the reaction rate constant. In kinetic studies, Mmax values were considered as the initial phenolic concentrations.
Statistical analysis
All experiments were done in triplicate. All values were averaged and expressed as mean ± standard deviation. Peroxide values were subjected to analysis of variance (ANOVA) for each storage temperature to test for differences arising from black carrot juice addition. Confidence limits used in this study were based on p < 0.01.
Results and discussion
In the present study, the lipid content was 25.5 ± 0.05 g/100 g for both formulations of the almond pastes, and this was in agreement with Capanoglu and Boyacioglu (2008). Fatty acid profiles for almond genotypes from different parts of Turkey have been extensively studied. An almond variety grown in Datca was reported to have approximately 52.32% of total fat. Oleic acid was major fatty acid (76.11%) in Datca almond followed by linoleic (17.11%), palmitic (6.14%), and palmitoleic acids (0.04%) (Nizamoglu 2015). In the literature, small differences in the composition of the same almond genotype explained by differences in varieties and the geographical conditions of the regions where they were grown (Capanoglu and Boyacioglu 2008). Based on data adopted from the earlier study, the oleic acid, linoleic acid, palmitic acid, and palmitoleic acid contents were calculated as 19.40 g, 4.36 g, 1.57 g, and 0.01 g/100 g of the almond pastes (wet basis), respectively (Nizamoglu 2015). Antioxidant capacity, oxidative stability, and content of phenolic compounds of black carrots harvested from Konya, a city in Central Anatolia in Turkey, have previously been studied in detail (Kamiloglu et al. 2015; Ozen et al. 2011; Oztan 2006). In black carrots, the phenolic concentrations are reported to be approximately 102–108 mg/100 g, and they are primarily acylated anthocyanins (Oztan 2006). In the present study, replacing water with black carrot juice in formulations increased the initial total phenolic content of the almond pastes from 95.90 to 187.29 mg/100 g (by 48.79%) (Table 1).
Table 1.
Numerical values for parameters in lipid oxidation and total phenolics consumption models. Values are means ± standard deviation (n = 3)
| Logistic equation parameters for lipid oxidation | Kinetic model parameters for total phenolics consumption | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Sample | Onset of lipid oxidation time (d) | camax | Time for cmax (d) | kb (d−1) | r2,c | Md0 (mg/100 g) | Memax (mg/100 g) | Mfmin (mg/100 g) | Time for Mmin (d) | k (d−1) | r2 |
| 4 | Plain almond paste | 11 | 0.64 ± 0.01 | 21 | 0.34 | 0.99 | 95.9 ± 1.68 | 95.9 ± 1.68 | 69.8 ± 0.81 | 15 | 0.018 | 0.95 |
| Almond paste with black carrot | 13 | 0.36 ± 0.01 | 21 | 0.60 | 0.95 | 187.3 ± 2.17 | 191.8 ± 1.48 | 181.5 ± 0.54 | 15 | 0.004 | 0.99 | |
| 20 | Plain almond paste | 9 | 1.35 ± 0.01 | 26 | 0.24 | 0.98 | 95.9 ± 1.68 | 95.9 ± 1.68 | 59.0 ± 0.89 | 21 | 0.021 | 0.96 |
| Almond paste with black carrot | 11 | 0.59 ± 0.01 | 24 | 0.48 | 0.98 | 187.3 ± 2.17 | 252.8 ± 4.38 | 172.4 ± 2.79 | 17 | 0.025 | 0.93 | |
| 30 | Plain almond paste | 9 | 3.50 ± 0.01 | 24 | 0.34 | 0.96 | 95.9 ± 1.68 | 98.6 ± 2.23 | 51.5 ± 1.89 | 21 | 0.032 | 0.98 |
| Almond paste with black carrot | 11 | 1.58 ± 0.06 | 24 | 0.36 | 0.97 | 187.3 ± 2.17 | 270.5 ± 6.32 | 170.5 ± 2.85 | 21 | 0.026 | 0.97 | |
| 60 | Plain almond paste | 6 | 4.03 ± 0.06 | 21 | 0.38 | 0.98 | 95.9 ± 1.68 | 106.9 ± 1.68 | 48.6 ± 0.54 | 21 | 0.034 | 0.96 |
| Almond paste with black carrot | 9 | 2.00 ± 0.01 | 24 | 0.27 | 0.94 | 187.3 ± 2.17 | 272.4 ± 0.66 | 168.3 ± 0.43 | 17 | 0.026 | 0.95 | |
aThe maximum attainable concentration of the total oxidation products at the end of the lipid oxidation process
bThe reaction rate constant
cRegression coefficient
dThe initial concentration of total phenolics
eThe maximum attainable concentration of total phenolics
fThe minimum attainable concentration of total phenolics
The initial, maximum, and minimum total phenolic contents and the maximum peroxide values of the model pastes are presented in Table 1. Figure 1S shows the changes in the peroxide values, and the total phenolic concentrations with time in model pastes stored at 4 °C, 20 °C, 30 °C, and 60 °C, respectively. Lipid oxidation data followed sigmoidal curves for all model pastes (Fig. 2S). The peroxide values stayed almost constant at the early stages of storage and increased exponentially as the storage time progressed, at all storage temperatures. Clearly, increase in storage temperatures resulted in higher peroxide values in all samples, as reported by Capanoglu and Boyacioglu (2008). Almond contains natural antioxidants, such as flavonoids. The content, mechanism of action, and stability of antioxidants in almonds depend on the genetic and environmental factors, and the processing and storage conditions, and might be adequate to protect the lipids against lipid oxidation only for a certain period (Bolling et al. 2010). Black-carrot addition to the recipe delayed the exponential increase, from 13 to 9 days to 11–6 days, with respect to the reference samples stored at the same temperatures (Fig. 1S, Table 1). For all formulations, black carrot added pastes had the lowest peroxide values than the corresponding reference samples throughout the storage periods (Fig. 2S) (p < 0.01). This might be due to the inhibitory effect of antioxidants in black carrot on peroxide formation in foods that are rich in lipids (Assous et al. 2014). Peroxide formation continued until there were no free fatty acid radicals available in the samples for further oxidation reactions (termination) (Fig. 2S). In general, protective effect of the natural antioxidant in combination with the low storage temperature decreased the peroxide values of the almond pastes, as reported by Assous et al. (2014), Capanoglu and Boyacioglu (2008), and Larrauri et al. (2016) (Figures 1S and 2S).
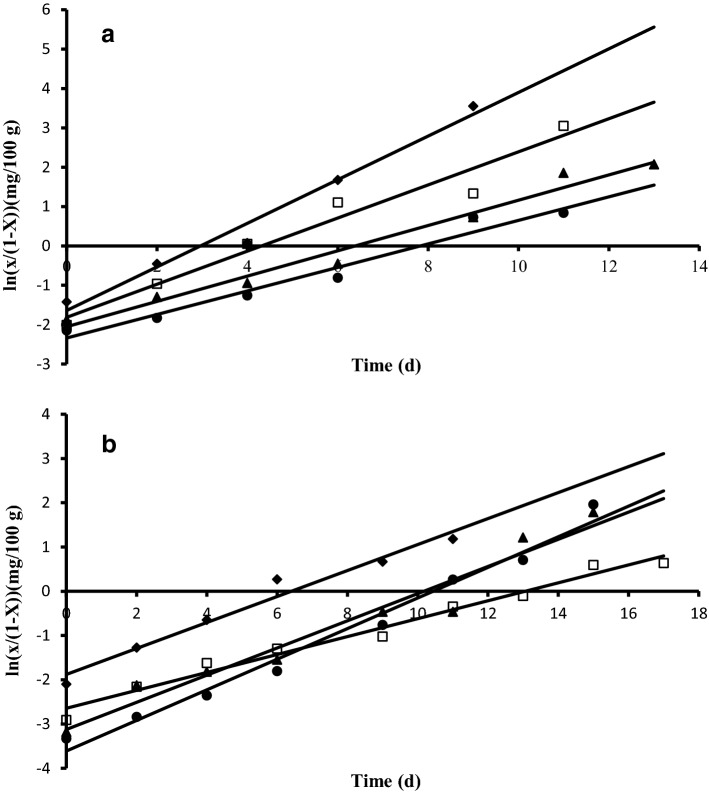
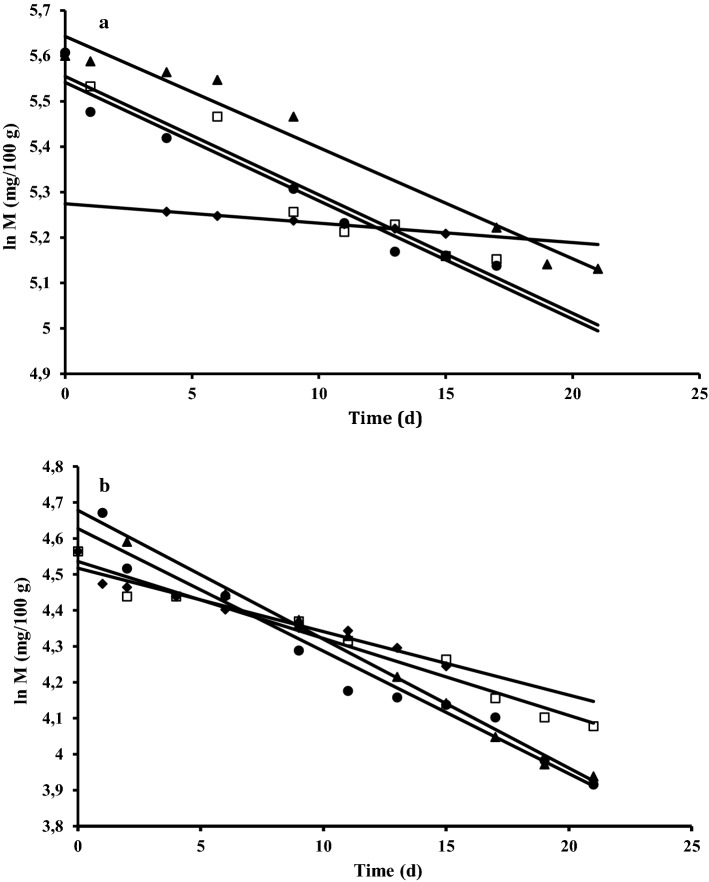
The logistic equation (Eqs. 2 and 3) was in good agreement with the experimental data (; Fig. 1). Logistic equation implies that when the concentration of the oxidation products reaches to its maximum, , the lipid oxidation reaction terminates. The values increased with temperature both in the reference and the almond-black carrot pastes (Table 1). This observation may be directly related to decomposition of more fatty acids into oxidation products at higher temperatures (Labuza 1982). Replacing water with the black carrot juice noticeably decreased the maximum peroxide value attained,, in the paste compared to the reference samples stored at the same temperature (Table 1). On the other hand, the rate of peroxide formation in the almond-black carrot samples decreased with increasing temperature (slopes were changing from 0.60 to 0.27 d−1), unexpectedly (Table 1). The relation agreed with the Arrhenius equation (r2 = 0.98) (Lin et al. 2012). These results were different from those obtained with the reference samples, and also the lipid oxidation studies carried out with almonds and the other types of nuts (Capanoglu and Boyacioglu 2008; Lin et al. 2012). Replacing water with the black carrot juice possibly changed the mechanism of lipid oxidation in the almond pastes (Piedrahita et al. 2015). In those samples, the rate to achieve the cmax value was higher at lower temperatures as a result of less free radical formation; hence the free radicals scavenged by the black carrot phenolic compounds more efficiently. Possibly, it was also the main reason for the lowest phenolic consumption rates at 4 °C. At the early stages of the storage periods, increase was observed in the total phenolic contents of the model pastes as a result of increase in solubility of the phenolic compounds with increasing temperature (Fig. 1S) (Algarra et al. 2014). At the end of the storage periods, phenolic consumptions were 5.4% at 4 °C, 31.8% at 20 °C, 36.9% at 30 °C, and 38.2% at 60 °C in the almond-black carrot pastes. In general, consumption of phenolic compounds during storage followed a first order kinetic reaction (Eq. 4) in all model pastes (r2 > 0.93) (Fig. 2), agreeing with the results of previous studies that employed the same model during storage of products containing black carrot juice (Kırca et al. 2007; Ozen et al. 2011; Patras et al. 2010). Although the phenolic consumptions increased with storage temperature (Table 1, Fig. 1S), the rate of concentration change did not show an Arrhenius-type temperature dependency in the black carrot-almond pastes. High amount of acylated cyanidin derivatives in the structures of black carrots phenolic compounds reported to give stability to heat, light, and pH changes (Kamiloglu et al. 2015). Heat stability of black carrots at 40–70 °C was reported in one of the recent studies (Assous et al. 2014). Therefore, the effect of storage temperature on the structure of black carrot phenolic compounds was neglected in the present study. There were also no correlation between the peroxide formation rate and the rate of phenolic consumption (r2 = 0.66). It is evident from all these results that, in combination with consumption in lipid oxidation reactions some other factors, such as solubility of phenolic compounds, water activity of the food, self-oxidation, and sugar crystallization during storage might also had an effect on the phenolic concentrations (Kamiloglu et al. 2015; Kırca et al. 2006; Patras et al. 2010; Rhim 2002).
Fig. 1.
Modeling of lipid oxidation in a almond-black carrot pastes and in b plain almond pastes stored at, (♦) 4 °C, (□) 20 °C, (▲) 30 °C, (●) 60 °C.—Logistic model fit. Regression coefficient (r2) values were better than 0.96 and the sum of square error was 0.076 and 0.04 in Fig. 1a and b, respectively
Fig. 2.
Changes in concentration of total phenolics in a almond-black carrot pastes and in b plain almond pastes stored at, (♦) 4 °C, (□) 20 °C, (▲) 30 °C, (●) 60 °C.—First order model fit. Regression coefficient (r2) values were better than 0.93 and the sum of square error was 0.21 and 0.06 in Fig. 2a and b, respectively
The results of this study may help food industry professionals, including professional chefs, for quantification of the effects of black carrot juice on controlling lipid oxidation in foods that have similar recipes to traditional Turkish almond paste recipe.
Conclusion
Logistic equation simulated all the experimental patterns obtained from the peroxide measurements in model almond pastes. Black carrot juice, when substituted for water in the almond paste recipe increased the oxidative stability of almond pastes, delayed the initiation of lipid oxidation, and reduced the maximum attainable peroxide values. From the kinetic study, it also decreased the rates of the lipid oxidation reactions at increasing storage temperatures. This was the major difference from the previous studies, and pointed as the major impact of this study. Thus, the kinetic models can be helpful in controlling lipid oxidation in foods that share similar recipes with traditional Turkish almond paste during storage for confectionary industry and professional chefs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Changes in total phenolic contents (■) and peroxide value (♦) of (a) almond-black carrot pastes and (b) plain almond pastes stored at different temperatures. The values are mean±standard deviation (n=3) (PDF 99 kb)
Comparison of changes in peroxide value of plain almond pastes (♦) and almond black-carrot pastes (●) stored at different temperatures. The values are means±standard deviation (n=3) (PDF 53 kb)
References
- Algarra M, Fernandes A, Mateus N, de Freitas V, Esteves da Silva JCG, Casado J. Anthocyanin profile and antioxidant capacity of blackcarrots (Daucus carota ssp. sativus var. atrorubens Alef.) from Cuevas Bajas, Spain. J Food Compos Anal. 2014;33:71–76. doi: 10.1016/j.jfca.2013.11.005. [DOI] [Google Scholar]
- Assous MTM, Abdel-Hady MM, Medany GM. Evaluation of red pigment extracted from purple carrots and its utilization as antioxidant and natural food colorants. Ann Agric Sci. 2014;59:1–7. [Google Scholar]
- Balta MF. Fatty acid profiles for almond (Prunus amygdalusBatsch) genotypes with different kernel taste and formation. Iğdır Univ J Inst Sci Tech. 2013;3:17–24. [Google Scholar]
- Beltrán A, Prats MS, Maestre SE, Grané N, Martín ML. Classification of four almond cultivars using oil degradation parameters based on FTIR and GC data. J Am Oil Chem Soc. 2009;86:51–58. doi: 10.1007/s11746-008-1323-x. [DOI] [Google Scholar]
- Bolling BW, Dolnikowski G, Blumberg JB, Chen CO. Polyphenol content and antioxidant activity of California almonds depend on cultivar and harvest year. Food Chem. 2010;122:819–825. doi: 10.1016/j.foodchem.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10:221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- Capanoglu E, Boyacioglu D. Improving the quality and shelf life of Turkish almond paste. J Food Qual. 2008;31:429–445. doi: 10.1111/j.1745-4557.2008.00210.x. [DOI] [Google Scholar]
- Choe E, Min DB. Mechanisms of antioxidants in the oxidation of foods. Compr Rev Food Sci Food Saf. 2009;8:345–358. doi: 10.1111/j.1541-4337.2009.00085.x. [DOI] [Google Scholar]
- Erkan N, Ayranci G, Ayranci E. A kinetic study of oxidation development in sunflower oil under microwave heating: effect of natural antioxidants. Food Res Int. 2009;42:1171–1177. doi: 10.1016/j.foodres.2009.06.003. [DOI] [Google Scholar]
- Gök V, Serteser A (2003) Dogal Antioksidanların Biyo yararlılıgı. 3. Gıda Mühendisligi Kongresi, Ankara, 2–4 Ekim 2003
- Gómez-Alonso S, Mancebo-Campos V, Salvador DM, Fregapane G. Oxidation kinetics in olive-oil triacylglycerols under accelerated shelf-life testing (25–75 °C) Eur J Lipid Sci Technol. 2004;106:369–375. doi: 10.1002/ejlt.200300921. [DOI] [Google Scholar]
- Janu C, Kumar DR, Reshma MV, Jayamurthy P, Sundaresan A, Nisha P. Comparative study on the total phenolic content and radical scavenging activity of common edible vegetable oils. J Food Biochem. 2014;38(1):38–49. doi: 10.1111/jfbc.12023. [DOI] [Google Scholar]
- Kamiloglu S, Pasli AA, Ozcelik B, Van Camp J, Capanoglu E. Colour retention, anthocyanin stability and antioxidant capacity in blackcarrot (Daucus carota) jams and marmalades: effect of processing, storage conditions and in vitro gastrointestinal digestion. J Funct Foods. 2015;13:1–10. doi: 10.1016/j.jff.2014.12.021. [DOI] [Google Scholar]
- Khandare V, Walia S, Singh M, Kaur C. Black carrot (Daucus carota ssp. sativus) juice: processing effects on antioxidant composition and color. Food Bioprod Process. 2011;89:482–486. doi: 10.1016/j.fbp.2010.07.007. [DOI] [Google Scholar]
- King J, Blumberg J, Ingwersen L, Jenab M, Tucker K. Treenuts and peanuts as components of a healthy diet. J Nutr. 2008;138:1736–1740. doi: 10.1093/jn/138.9.1736S. [DOI] [PubMed] [Google Scholar]
- Kırca A, Özkan M, Cemeroglu B. Stability of blackcarrot anthocyanins in various fruit juices and nectars. Food Chem. 2006;97:598–605. doi: 10.1016/j.foodchem.2005.05.036. [DOI] [Google Scholar]
- Kırca A, Özkan M, Cemeroglu B. Storage stability of strawberry jam color enhanced with black carrot juice concentrate. J Food Process Preserv. 2007;31:531–545. doi: 10.1111/j.1745-4549.2007.00140.x. [DOI] [Google Scholar]
- Labuza TP. Shelf life dating of foods. New York: Food and Nutrition Press Inc.; 1982. [Google Scholar]
- Larrauri M, Demaría MG, Ryan LC, Asensio CM, Grosso NR, Nepote V. Chemical and sensory quality preservation in coated almonds with the addition of antioxidants. J Food Sci. 2016;81:208–215. doi: 10.1111/1750-3841.13164. [DOI] [PubMed] [Google Scholar]
- Lin X, Wu J, Zhu R, Chen P, Huang G, Li Y, Ruan R. California almond shelf life: lipid deterioration during storage. J Food Sci. 2012;77:583–593. doi: 10.1111/j.1750-3841.2012.02706.x. [DOI] [PubMed] [Google Scholar]
- Martin MBS, Fernández-García T, Romero A, López A. Effect of modified atmosphere storage on hazelnut quality. J Food Process Preserv. 2001;25:309–321. doi: 10.1111/j.1745-4549.2001.tb00463.x. [DOI] [Google Scholar]
- Min DB, Ellefson WC. Fat analysis. In: Nielsen SS, editor. Food analysis. 4. New York: Springer; 2010. pp. 117–136. [Google Scholar]
- Nichenametla SN, Taruscio TG, Barney DL, Exon JH. A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr. 2006;46:161–183. doi: 10.1080/10408390591000541. [DOI] [PubMed] [Google Scholar]
- Nizamoglu NM (2015) The effects of roasting and storage conditions on some physical, chemical and sensory properties of almond kernel. Institute of science. http://acikerisim.pau.edu.tr/xmlui/bitstream/handle/11499/657/Nizam%20Mustafa%20Nizaml%C4%B1o%C4%9Flu.pdf?sequence=1&isAllowed=y. Accessed 6 July 2018
- Ozen G, Akbulut M, Artık N. Stability of black carrot anthocyanins in the turkish delight (Lokum) during storage. J Food Process Eng. 2011;34:1282–1297. doi: 10.1111/j.1745-4530.2009.00412.x. [DOI] [Google Scholar]
- Ozilgen S. Cooking as a chemical reaction: culinary science with experiments. Boca Raton: CRC Press, Taylor and Francis Group; 2014. [Google Scholar]
- Ozilgen S, Ozilgen M. Kinetic model of lipid oxidation in foods. J Food Sci. 1990;55:498. doi: 10.1111/j.1365-2621.1990.tb06795.x. [DOI] [Google Scholar]
- Oztan T (2006) Antioxidant activities and phenolic substance profile of purple carrot, its concentrate, shalgam beverage, pomegranate juice and sour pomegranate concentrate products. Institute of science and technology. https://polen.itu.edu.tr/handle/11527/2203. Accessed 30 June 2018
- Patras A, Brunton NP, O’Donnell C, Tiwari BK. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci Technol. 2010;21:3–11. doi: 10.1016/j.tifs.2009.07.004. [DOI] [Google Scholar]
- Piedrahita AM, Peñaloza J, Cogollo Á, Rojano BA. Kinetic study of the oxidative degradation of Choibáoil (Dipteryx oleifera Benth) with addition of rosemary extract (Rosmarinusofficinalis L.) Food Nutr Sci. 2015;6:466–479. [Google Scholar]
- Rhim JW. Kinetics of thermal degradation of anthocyanin pigment solutions driven from red flower cabbage. Food Sci Biotechnol. 2002;11:361–364. [Google Scholar]
- Schevey CT, Brewer MS. Effect of natural antioxidants and lipid model system on lipid oxidation. J Food Qual. 2015;38:40–52. doi: 10.1111/jfq.12119. [DOI] [Google Scholar]
- Shakerardekani A, Karim R. Effect of different types of plastic packaging films on the moisture and aflatoxin contents of pistachio nuts during storage. J Food Sci Technol. 2013;50:409–411. doi: 10.1007/s13197-012-0624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakerardekani A, Karim R, Ghazali HM, Chin NL. Textural, rheological and sensory properties and oxidative stability of nut spreads—a review. Int J Mol Sci. 2013;14:4223–4241. doi: 10.3390/ijms14024223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteure agent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Walker RO, editor. Official methods and recommended practices of American oil chemists’ society, Method Cd 8-53. 4. Champaign: J Am Oil Chem Soc; 1989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in total phenolic contents (■) and peroxide value (♦) of (a) almond-black carrot pastes and (b) plain almond pastes stored at different temperatures. The values are mean±standard deviation (n=3) (PDF 99 kb)
Comparison of changes in peroxide value of plain almond pastes (♦) and almond black-carrot pastes (●) stored at different temperatures. The values are means±standard deviation (n=3) (PDF 53 kb)