Abstract
The present study aims to develop a novel process for improving the functional properties of beef. For this purpose, the effect of high-intensity ultrasound, alone and in combination with papain was investigated on pH, water-holding capacity (WHC), emulsion capacity and stability, cooking loss, and gelling property of Longissimus lumborum muscle. Meat samples were subjected to sonication (20 kHz, 100 and 300 W) for 10, 20 and 30 min in the presence and absence of papain solution. Results indicated that the application of ultrasound individually or in combination with papain significantly influenced on the functional properties of beef. For all tested samples compared with the untreated meat, there was a significant increase in the WHC, emulsion capacity and stability and a significant decrease in cooking loss. Generally, our findings suggest that the combination of ultrasound and papain was more beneficial for improving functional properties of meat compared with the individual treatment.
Keywords: Functional property, Papain, Ultrasound, Longissimus lumborum
Introduction
The functional properties of proteins compromise all physico-chemical properties affecting the behavior of such proteins during food processing (Colmenero and Borderias 1983). Functional properties which greatly influence on quality of meat products are water-holding and -binding capacity, protein extractability and gelation (Kurt and Kılınççeker 2011). In addition, emulsion capacity and stability are two other functional properties consider during production of meat products.
During rigor mortis, the myofibrillar proteins (especially actin and myosin), which play an important role in functional properties, permanently interact with each other. As a result of this phenomenon, the solubility of myofibrillar proteins decreases significantly (Feiner 2006). The less than desirable solubility has adverse effect on their functional properties. Therefore, the improvement of solubility and functional properties are highly desirable. One way of improving the functional properties of proteins and tailoring the functionality of certain proteins to achieve specific needs is enzymatic modifications (Smith and Brekke 1985). Proteases are the most common enzymes applied for improving functional properties of meat by partial hydrolysis. Except endogenous proteases (like cathepsins and calpains), meat industry now benefits from popular plant proteases such as ficin, bromelain, papain and actinidin (Aminlari et al. 2009).
Papain is a heat-stable cysteine protease with the wide range of application in pharmaceutics, cosmetics and food industries. In meat industry, papain is used for increasing tenderness due to hydrolysis of myofibrillar and collagen proteins (Ashie et al. 2002). Since the breakdown of myofibrillar proteins is associated with improvement of functional properties, papain can also be applied for this purpose. However, one problem associated with enzyme application is finding an effective way for increasing enzyme diffusion and distribution in the meat cuts. Traditional methods involve dipping of meat in a protease solution, pumping the solution containing enzyme into meat and rehydration of the freeze-dried meat in an enzyme solution. These methods are somewhat unsatisfactory because of low penetration depth (Gerelt et al. 2000). Therefore, the industry is seeking to find a new method for accelerating enzyme penetration.
Ultrasound is a safe, non-invasive and environmentally friendly technology that has been mainly used for improving the physical, chemical and functional properties of meat (Jayasooriya et al. 2004; Turantaş et al. 2015). Ultrasound refers to mechanical vibrations with a frequency greater than 20 kHz. The ranges of it can be basically divided into low energy (low power, low intensity) with frequency higher than 100 kHz and intensity lower than 1 W/cm2, and high energy (high power, high intensity) with frequency between 20 and 100 kHz and intensity higher than 1 W/cm2. Both types of this sound have been used as an efficient tool for analysis and modification of the protein’s behavior in food products (Turantaş et al. 2015). This technology with creating temperature gradient, cavitation and mechanical phenomena enhances the mass transfer, and creates structural changes (Ozuna et al. 2013). In several studies, it has been demonstrated that ultrasound treatment improves the functional properties of meat and poultry products (Jayasooriya et al. 2007). Stadnik et al. (2008) noted that ultrasound treatment (2 min, 2 W/cm2, 45 kHz) significantly increased water-holding capacity of beef muscle. Li et al. (2014a) explained that high-intensity ultrasound (20 kHz, 450 W, and 6 min) modified the protein structure of batter suspension prepared from chicken breast meat and increased its gel strength.
Ultrasound in combination with the other methods is reported to be effective for improving the general quality such as marination, tenderness and modifying the functional properties in meat and poultry products (Turantaş et al. 2015). Xiong et al. (2012) stated that contribution of ultrasound treatment (24 kHz, 12 W/cm2 for 4 min) along with endogenous proteolytic enzymes significantly increased tenderness and decreased cooking loss of hen muscle. In addition, the results of our recent study indicated that high-intensity ultrasound (20 kHz, 100 and 300 W for 10, 20 and 30 min) coupled with papain treatment increased tenderness and proteolytic activity of Longissimus lumborum muscle (Barekat and Soltanizadeh 2017).
Functional properties of the meat proteins influence on the physical appearance and behavior of food products during preparation, storage and distribution. In spite of the importance of this issue, very few novel methods have been reported for improving functional properties of myofibrillar proteins. Therefore, the present study was conducted to evaluate the effects of ultrasound alone and in combination with papain on the functional properties of beef Longissimus lumborum (LL) muscle in an attempt to develop a new method for improving the functionality of beef proteins.
Material and method
Materials
Papain extracted from papaya latex (EC 3.4.22.2), k-casein of bovine milk, cysteine, l-tyrosine and Tris–HCl buffer were obtained from Sigma Chemical Company, USA. All other chemicals and reagents used in this work were analytical grade.
Meat preparation
Meat preparation was performed according to Barekat and Soltanizadeh, (2017). Longissimus lumborum muscle of seven Holstein bulls (average age of 2.5 years and live weight of 500 ± 50 kg) was obtained from a local slaughterhouse immediately after slaughter. The muscles were transported to the laboratory and ultimate pH was measured after 12 h storage at 16 °C (rigor mortis was completed) by a Consort C831 pH-meter (Consort N.V., Turnhout, Belgium) and only five muscles with an ultimate pH in the range of 5.5–5.8 were selected for further treatment. The visible surface fat, silver skin and external connective tissues were carefully trimmed off, and samples were sliced into cubes with dimensions of 3 × 3×3 cm. The meat pieces from each muscle was finally sealed in individual plastic bags, labeled, and kept frozen at − 18 °C. Following one-week frozen storage, samples were completely thawed at 25 °C for 2 h in control conditions and were used for experiments.
Meat treatment
For evaluation of the effect of ultrasonic radiation alone and in combination with the papain enzyme (0.1 g/100 ml), the samples were divided in two groups: those treated with ultrasound (group 1), and those treated with the enzyme and ultrasound, simultaneously (group 2).
In group 1, the meat pieces were freely immersed in 100 ml deionized water and sonicated using a 20 kHz probe system (Adeeco, Iran). Ultrasound was applied at power of 100 and 300 W (ultrasound intensity of 69 and 208 W/cm2, respectively) for 10, 20 and 30 min, and a constant amplitude of 100%. During the sonication process, the ultrasound probe was retained 1 cm above the meat surface and temperature was maintained at 11–17 °C by application of water bath. A control sample was considered for this group, which only immersed in deionized water for 3 s, and did not treated with ultrasound.
In group 2, the samples immersed in papain solution (0.1% w/v) and exposed to ultrasound waves as described above. The control sample for this group was only immersed in papain solution for 3 s without ultrasound application.
Since the optimum temperature for papain activity is 65 °C, samples in group 2 were removed from papain solution after ultrasound treatment and incubated at 65 °C for 30 min. For comparing all samples in the same conditions; the samples in group 1 were also removed from water and incubated at that temperature and time.
During sonication, samples were rotated so that all faces of meat pieces were equally subjected to the ultrasonic waves. All treatments were done in five replicates on each muscle.
pH
Two grams of the meat samples were homogenized with 8 ml of distilled water using IKA homogenizer (ULTRA-TURRAX, Germany). The pH of homogenized sample was measured with a digital pH meter (JENWAY 3330, USA).
Water holding capacity (WHC)
Water holding capacity (WHC) was measured according to a simple centrifugal procedure as described by Jauregui et al. (1981) with some modification. The meat samples weighing 0.2 g were placed into a thimble shape filter paper. Each thimble was inserted inside a 2.0 ml centrifuge tube and centrifuged (Z36 HK, Hermle, Germany) at 14,000 rpm for 20 min. The weight of the filter paper was measured before insertion of sample and after centrifuge to determine the ability of muscle for retention of water against external forces.
Cooking loss
The method of Li et al. (2014b) was used to determine cooking loss of meat samples. The beef samples (8.5 g) were transferred into plastic bags and held in water bath at 85 °C until the internal temperature reached to 75 °C. After cooking, samples were removed, cooled at room temperature and surface water was removed using the paper towel. Cooking loss was calculated from a difference between the weight of sample before and after cooking treatment.
Emulsion capacity (EC)
For determining the emulsifying capacity, the electrical end point method of Von Seggern et al. (2005) was used with some modification. A 10 g meat sample was homogenized with 20 ml cold 1 M NaCl solution for 2 min at 13,000 rpm. A 6.25 g of the slurry was transferred into another glass jar and homogenized 10 s at 10,000 rpm. The aliquot of the homogenate was mixed with 25 ml of the Corn oil. Then, the addition of oil was continued at a rate of 0.8 ml/s until a sudden increase in electrical resistance happened. The EC was expressed as the total amount of oil emulsified (ml) per 1 g of meat.
Emulsion stability (ES)
Twenty grams of the emulsion prepared above were weighed and capped in a 50 ml centrifuge tube. The tube immersed in water bath at 85 °C until the central temperature reached to 75 °C. After the tube was cooled to room temperature, it centrifuged at 1200 rpm for 15 min. Total volume, fat volume, and volume of water, released by the broken emulsion were recorded to determining emulsion stability of the meat samples (Karakaya et al. 1997).
Gelling property
In this experiment, the gelling property of extracted myofibrillar protein from Longissimus lumborum muscle after the treatment was evaluated. Extraction of myofibrillar proteins was carried out according to the method of Li et al. (2014b). Each sample was mixed with four volumes (w/v) of a cold phosphate buffer (20 mM, pH 7.5) and homogenized at 16,000 rpm for 30 s. The homogenate was placed into a 50 ml test tube and centrifuged for 15 min at 2000 g. After removal of the supernatant, pellet was treated again as described above. Then, the pellet was transferred to another centrifuge tube and was homogenized (1 min) with four volumes (w/v) of a 0.5 M NaCl solution. Finally, for isolation of stroma protein, myofibrillar proteins suspension was filtered by cheese cloth filter. After measurement of protein concentration using Biuret method (Gornall et al. 1949), the isolated myofibrillar protein was diluted into 3% protein by addition of 0.5 M NaCl solution. The solution was then held in a water bath at 80 °C for 20 min and cooled to 4 °C for 24 h. The gel strength was tested using Instron Universal Testing Machine equipped with a 5 kg load cell and a round needle type probe (1.3 cm in diameter). Data were expressed as the maximum force required for 1 cm penetration of a probe into the samples (Li et al. 2014b).
Statistical analysis
The data were analyzed using linear mixed model (LMM) methods. The animals and samples within animals (assigned the ultrasound and enzyme treatments) which are associated with the split-plot nature of the experimental designs included in LMM as random effects. The fixed terms in the model were effects (main and interactions) associated to ultrasound power, ultrasound time, and enzyme treatment. The confidence interval was set for a level of significance at p < 0.05 using the Statistical Analysis System (SAS) version 13.1 to evaluate the significance level of differences between the mean values.
Result and discussion
pH
pH is one of the most important attributes of meat and has an increasing effect on water-holding capacity, enzyme activity and tenderness of meat. Figure 1 indicates the effect of ultrasound alone and in combination with enzyme treatment on the pH values of meat. The raw meat before any treatment has pH value of ~ 5.69 and its treatment with ultrasound at power of 100 W for 10, 20 and 30 min did not have any significant effect on pH value. However, when power increased to 300 W, pH of the samples was first (at 10 min) decreased and then increased (20 and 30 min) with increasing the duration of ultrasound radiation, as it can be seen in Fig. 1. Compared to treatment at the power of 100 W, sonication at 300 W for 10 and 20 min significantly decreased the pH values of samples. This is while after 30 min sonication, there was not significant difference between pH value of samples treated at 100 and 300 W. This result could probably be due to sonolysis process. Torres et al. (2008) explained that ultrasonic process which leads to the acoustic cavitation is following three stages of formation, growth and collapse of microbubbles, periodically. The adiabatic compression of gases and vapors entrapped in bubbles after the fast collapse of bubbles can produce short and local hot spots. The temperature in this bubbles or surrounding environment is too high; so that water and dissolved gases can dissociate. The result of this reaction is production of ·OH and ·OOH radicals after dissociation of oxygen and water. The breakdown of ·OOH to O2− and H+ is a source of proton for the pH reduction. Power intensity influences on size of cavitation bubbles, time of bubble collapse, the transient temperature and the internal pressure during the collapse in the cavitation bubbles (Barekat and Soltanizadeh 2017). Thus, the different results in 100 and 300 W may be due to the effect of power intensity on the severity of the cavitation process.
Fig. 1.
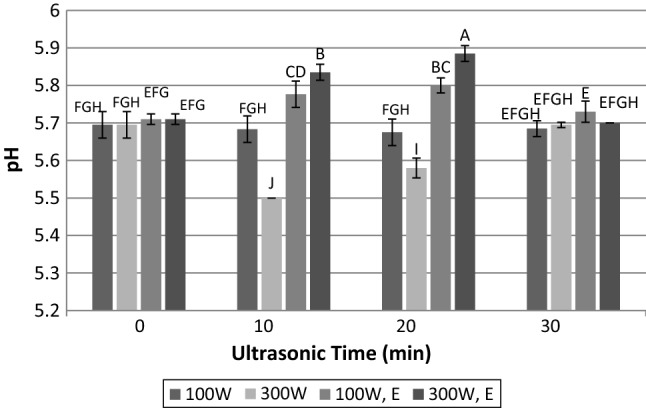
Influence of time and power of ultrasound radiation and papain hydrolysis on pH value. 100 W: ultrasonic power of 100 W; 300 W: ultrasonic power of 300 W; 100 W, E: simultaneous treatment with papain enzyme and ultrasonic power of 100 W; 300 W, E: simultaneous treatment with papain enzyme and ultrasonic power of 300 W. Dissimilar letters indicate significant difference (p < 0.05)
Immersion of meat in papain solution did not haveh significant effect on pH value of meat. Although when enzyme treatment and ultrasonic waves applied with each other, pH of meat was increased till 20 min sonication, and longer sonication time decreased the pH of meat so that it did not have significant difference with raw and enzyme treated samples (Fig. 1). It is obvious that ultrasound (for 20 min) and papain treatment could not separately have positive effect on enhancement of pH value but when they applied with each other, pH increased significantly. In similar circumstances to this test, our recent study indicated that ultrasound treatment could increase protease activity (Barekat and Soltanizadeh 2017). So, it seems that degradation of muscle fiber, which related to higher proteolytic activity, increased pH value of meat. The decrease in pH after 30 min sonication of meat immersed in papain solution could be due to a change in the location of ionic groups resulted from conformational changes in proteins. These ionic groups then participate in buffering capacity of meat and prevent the pH changes (Got et al. 1999). Figure 1, shows that samples after 30 min sonication in different power, and in the presence and absence of papain did not have significant (p > 0.05) difference with the unexposed ultrasonic meat. This could be related to enhancement of buffering properties of meat achieved after 30 min sonication.
Water holding capacity (WHC)
Water-holding capacity is known as an important organoleptic property in muscle foods and processed meats. It was evident that water holding capacity increased as a function of ultrasonic power (p < 0.05), time (p < 0.05) and enzyme treatment (p < 0.05). Application of ultrasound treatment at 100 and 300 W for 10, 20 and 30 min leads to a low increase in WHC compared to untreated meat samples (Fig. 2). This increase was not affected by time and power of ultrasound. The findings about WHC are in agreement with those of Stadnik et al. (2008) who reported the enhancement of WHC after sonication within 2 min with frequency of 45 kHz. While few studies have reported that WHC will increase under ultrasound treatment, others have found no effect of ultrasound on WHC. Li et al. (2015) indicated that ultrasound treatment (40 kHz, 300 W) for 20 min with reduced-salt (1.5%) did not have the significant effect on WHC of reduced-salt chicken breast meat batter. As stated in previous studies, the most volume of water in meat has been placed between thin and thick filaments. McDonnell et al. (2014) indicated the increase in the water amount in this region after sonication at frequency of 20 kHz and ultrasonic intensity of 19 W cm−2. The increase in myofibrillar diameter could be another reason of WHC improvement in meat samples exposed to ultrasonic waves, which has been reported by some researchers (Got et al. 1999; Stadnik et al. 2008).
Fig. 2.
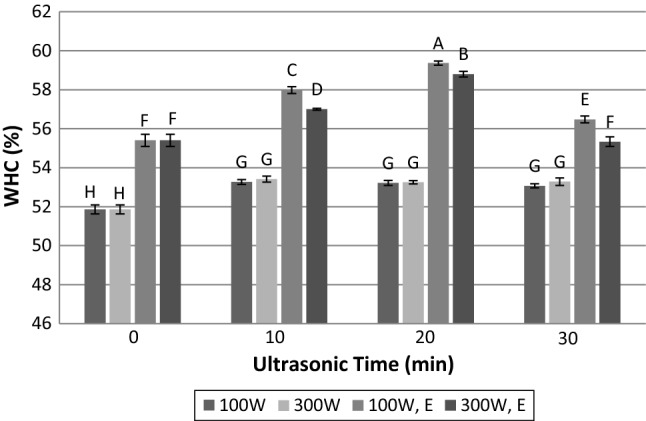
Influence of time and power of ultrasound radiation and papain hydrolysis on water-holding capacity. 100 W: ultrasonic power of 100 W; 300 W: ultrasonic power of 300 W; 100 W, E: Simultaneous treatment with papain enzyme and ultrasonic power of 100 W; 300 W, E: simultaneous treatment with papain enzyme and ultrasonic power of 300 W. Dissimilar letters indicate significant difference (p < 0.05)
While enzyme treatment could significantly increase WHC (p < 0.05), the simultaneous immersion of meat in papain solution and ultrasound treatment for 10 and 20 min caused a great improvement in WHC of meat (Fig. 2). The pH, onset of rigor mortis and protein fragmentation are three main factors involved in swelling of myofibrils and elevation of WHC (Huff-Lonergan and Lonergan 2005). Because of no significant difference in pH value of untreated and papain treated meat; therefore, the increase in WHC for immersed meat in papain could be explained by the enzymatic hydrolysis and protein fragmentation (Demirhan and Özbek 2013). WHC is affected by the number of hydrogen binding between polypeptide groups of protein. The change in pH influenced the ionization of amino acid groups, increased polarity of proteins and caused a higher number of hydrogen bonding to the surrounding water. Therefore, WHC generally increased with increasing pH. This relationship was observable when pH changes (Fig. 1) and WHC (Fig. 2) compared for samples exposed to ultrasound and papain, simultaneously.
Cooking loss
The ultrasound treatment at 100 and 300 W for 10, 20 and 30 min significantly affected the cooking loss of meat (Fig. 3). For all tested samples (ultrasound, alone and in combination with papain), cooking loss was significantly (p < 0.05) lower than untreated meat. The comparison of cooking loss and WHC curves (Figs. 2, 3) indicated an inverse relationship between these two parameters; so that the enhancement of WHC leads to the reduction of cooking loss.
Fig. 3.
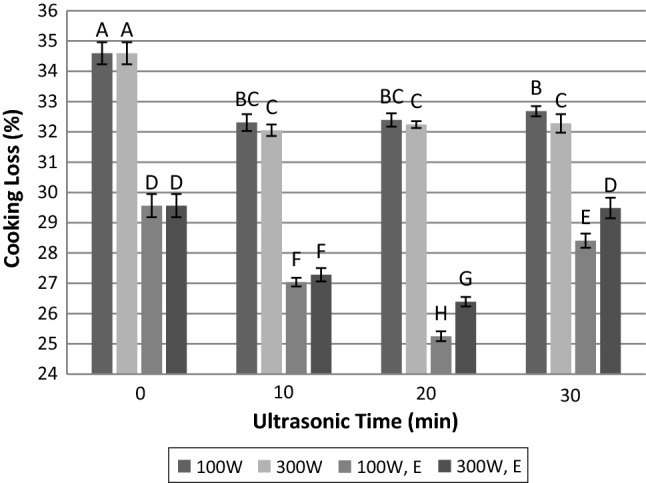
Influence of time and power of ultrasound radiation and papain hydrolysis on cooking loss. 100 W: ultrasonic power of 100 W; 300 W: ultrasonic power of 300 W; 100 W, E: simultaneous treatment with papain enzyme and ultrasonic power of 100 W; 300 W, E: simultaneous treatment with papain enzyme and ultrasonic power of 300 W. Dissimilar letters indicate significant difference (p < 0.05)
As it can be seen in Fig. 3, regardless of ultrasound power and duration, sonication resulted in retention of more water in meat during cooking compared with untreated one. This result is in agreement with Reynolds et al. (1978) who reported the reduction of cooking loss after ultrasound treatment. This may be due to the role of solubilized protein after sonication for binding of released moisture. In contrast, there were some reports about negative or lack of ultrasound effect on cooking loss (Jayasooriya et al. 2007).
Immersion of meat in papain solution was found to have a considerable effect on reducing cooking loss, particularly when the treatment was accompanied by ultrasound radiation (Fig. 3). So that, the lowest value is related to the sample immersed in papain and exposed to 100 W ultrasound for 20 min. Decrease in cooking loss is probably due to the effect of enzyme on muscle structure and an increase in the solubility of protein hydrolysates. When the longer times (30 min) applied for sonication of meat, cooking loss increased again. It may be as a result of the increasing gaps between muscle fibres and the connective tissue and also the denaturation of myofibrillar proteins (Jayasooriya et al. 2007). In addition, the role of pH in this increase should not be ignored.
Emulsion capacity and emulsion stability
The emulsion capacity of meat proteins and the emulsion stability of comminuted meat products are so important for production of emulsion type meat products (Aminlari et al. 2009). Results indicated that time and power of ultrasound as well as enzyme treatment, and their interactions have significant effects on emulsion capacity and stability (p < 0.05). It can be seen in Figs. 4 and 5 that both emulsion capacity and emulsion stability follow the same trend. After ultrasonic radiation of meat at 100 and 300 W, the emulsion capacity and stability increased as a function of time. Results also show that application of ultrasound at 300 W has more influence on emulsion capacity and stability than 100 W. This is according to Zou et al. (2018) who reported the effect of ultrasound power and duration on droplet size of emulsion. Krasulya et al. (2016) indicated that with enhancement of sonication time and power intensity, the droplet size in emulsion reduced. In fact, collapsing of cavitation bubble in the vicinity of two immiscible liquid boundary layers produces the shock wave which in turn can mix two phases, efficiently. Very fine emulsions with high stability will form as a result of this energy input, and only a little emulsifier is needed to stabilize the emulsion (Krasulya et al. 2016). Indeed, high intensity ultrasound can influence on flocculation of droplets in these emulsions and reduces it (Soria and Villamiel 2010). Since the increasing of ultrasound power could produce larger microbubbles, which are able to release higher energy during collapsing, emulsion droplets will be smaller with enhancement of power and time of ultrasound, and the stability of them will increase. Jambrak et al. (2009) stated that the highest emulsion activity of sonicated samples could be due to the decrease of droplet size and the increase of a percentage of adsorbed proteins to the binary layer. On the other hand, at lower power, cavitation has lower contribution in drop breakage, and coalescence is more significant. Marginal drop coalescence is a consequence of the increase in both the acoustic streaming velocity and the number of droplets, which enhanced collision frequency (Soria and Villamiel 2010).
Fig. 4.
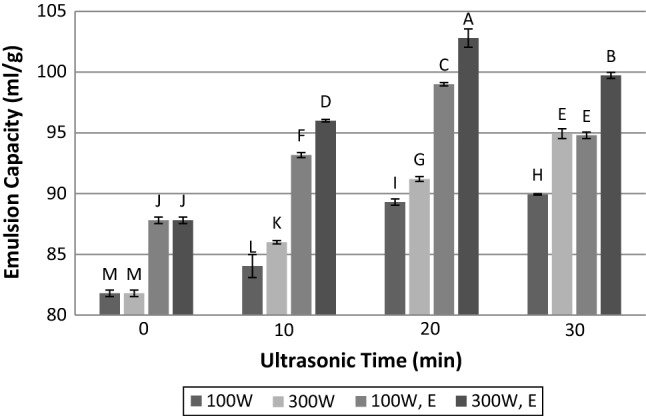
Influence of time and power of ultrasound radiation and papain hydrolysis on emulsion capacity. 100 W: ultrasonic power of 100 W; 300 W: ultrasonic power of 300 W; 100 W, E: simultaneous treatment with papain enzyme and ultrasonic power of 100 W; 300 W, E: simultaneous treatment with papain enzyme and ultrasonic power of 300 W. Dissimilar letters indicate significant difference (p < 0.05)
Fig. 5.
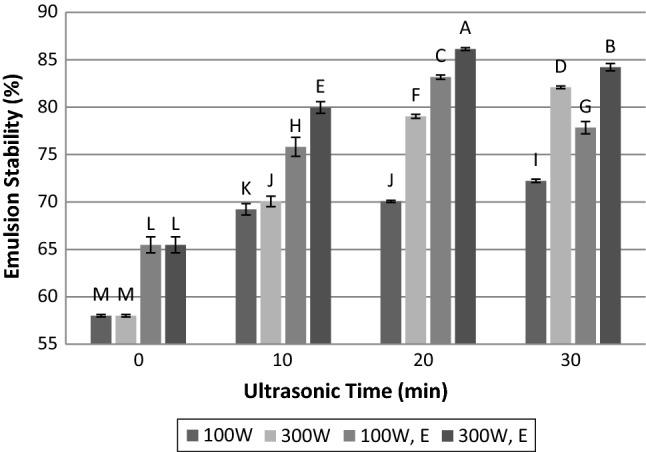
Influence of time and power of ultrasound radiation and papain hydrolysis on emulsion stability. 100 W: ultrasonic power of 100 W; 300 W: ultrasonic power of 300 W; 100 W, E: simultaneous treatment with papain enzyme and ultrasonic power of 100 W; 300 W, E: simultaneous treatment with papain enzyme and ultrasonic power of 300 W. Dissimilar letters indicate significant difference (p < 0.05)
In addition to paramount effect of enzyme hydrolysis on improvement of emulsion capacity and stability of meat, the simultaneous application of enzyme and ultrasound treatment also significantly increased these characteristics, and this effect is considerable in comparison with untreated meat. At these treatments, the emulsion capacity and stability of the samples were first increased, and then with increasing the time of sonication from 20 to 30 min; they were diminished. It has been reported that proteolysis of beef proteins increases the number of degraded protein molecules (Aminlari et al. 2009). The hydrolysates due to higher solubility and flexibility adsorb to the surface of bindery layer between water and oil droplets during homogenization and constitute a protective layer which prevented the coalescence of droplets (Gbogouri et al. 2004). When ultrasound accompanied by enzyme treatment, the synergistic effect between these two factors is observable. Sonication could produce smaller oil droplets with higher stability, and papain provides protein hydrolysate for covering the surface of oil droplets. In these conditions, both treatments lead to the enhancement of emulsion capacity and stability. It was assumed that because of more coalescence of oil droplets at lower ultrasonic power, the emulsion capacity and stability in power of 100 W are lower than 300 W even if combined with enzyme treatment. When ultrasound duration increased to 30 min, a considerable decrease happened in emulsion capacity and stability. This event may be affected by three factors: (1) the production of smaller oil droplets by sonication for 30 min and lack of sufficient protein hydrolysate for adsorption to water–oil interface; (2) the effect of ultrasound treatment on the enzymatic activity and reduction of it which in turn produces inadequate protein hydrolysate for generation and stabilization of emulsion; and (3) the lower pH of meat treated with an enzyme and ultrasound that can diminish protein solubility; hence, reduce emulsion capacity and stability.
Gelling property
Gel formation is another critical aspect of muscle proteins which plays an important role in fat–water stabilization and binding in meat products such as emulsion type sausage (Samejima et al. 1969). Gel forming ability of myofibrillar protein isolates is presented in Fig. 6. As can be seen, enzyme treatment significantly decreased the gel strength of myofibrillar protein (p < 0.05). The lower gel strength in enzyme treated samples might be due to the effect of enzyme and enzymatic hydrolysis. Protein gel formation is the result of protein–protein and protein-solvent (water) cross-linking at junction zones in which mainly hydrogen bonds, ionic and hydrophobic interactions create areas where free water can be trapped into the protein network. The growing of these junctions is related to chemical and molecular structures of the polypeptides in the gel network. The enzymatic treatment alters the chemical or/and molecular structures, and with reduction of molecule length decreases the formation of junction zones, and consequently, reduces the gelling capacity of enzyme treated samples (Yu and Perret 2003).
Fig. 6.
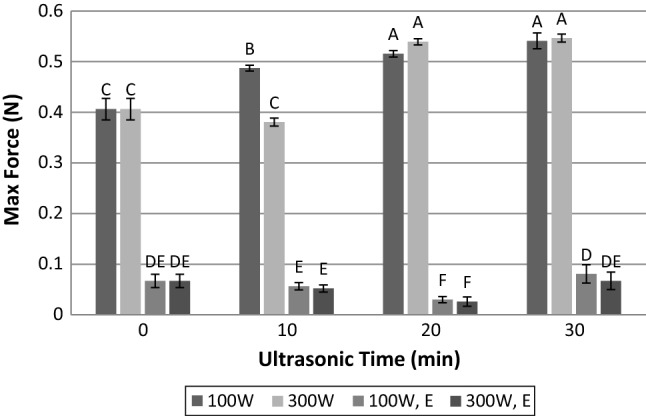
Influence of time and power of ultrasound radiation and papain hydrolysis on gel strength. 100 W: ultrasonic power of 100 W; 300 W: ultrasonic power of 300 W; 100 W, E: simultaneous treatment with papain enzyme and ultrasonic power of 100 W; 300 W, E: simultaneous treatment with papain enzyme and ultrasonic power of 300 W. Dissimilar letters indicate significant difference (p < 0.05)
When meat samples were only exposed to ultrasound treatment, the gelling properties of myofibrillar protein improved considerably (Fig. 6). These findings are in agreement with those of Li et al. (2014a) who explained that high-intensity ultrasound (20 kHz, 450 W, and 6 min) modifies the protein structure and increases its gel strength. Malik and Saini (2018) also reported the significant enhancement of gel strength in the sunflower protein isolates after ultrasound treatment for a short time. This could be due to better solubility of protein, and somewhat reduction of particle size, results in production of uniform and dense gel network. Also, ultrasonic radiation induces exposition of hydrophobic residue of protein, which might facilitate the formation of protein–protein aggregates during heating process and leads to a gel with higher strength (Hu et al. 2013).
Conclusion
In the present paper, the effects of ultrasonic radiation, enzyme treatment, and simultaneous application of ultrasound and papain on the functional properties of beef (Longissimus lumborum muscle) were investigated. Based on the results, we could state that combined utilization of exogenous enzymes and ultrasonic radiation rapidly improves functional properties of beef without need to any additional treatment or ingredients. In fact, advanced technologies gained more attention recently because of perform the process at short time which can be accompanied by economic benefits. In addition, application of ultrasound during immersion in papain solution reduce demand for enzyme. This method required very low financial investment and little training. So, it may be concluded that the combination of ultrasound radiation and papain treatment could be used as a promising technique for the production of beef pieces that might have a high potential application in meat processed industries. However, further studies are still needed in order to optimize the conditions and analysis financial aspects before applying this technology in a wider range of industrial sectors. Our work will continue to reveal the microstructural changes and protein hydrolysis during enzyme treatment under sound waves.
References
- Aminlari M, Shekarforoush S, Gheisari H, Golestan L. Effect of actinidin on the protein solubility, water holding capacity, texture, electrophoretic pattern of beef, and on the quality attributes of a sausage product. J Food Sci. 2009;74(3):C221–C226. doi: 10.1111/j.1750-3841.2009.01087.x. [DOI] [PubMed] [Google Scholar]
- Ashie I, Sorensen T, Nielsen P. Effects of papain and a microbial enzyme on meat proteins and beef tenderness. J Food Sci. 2002;67(6):2138–2142. doi: 10.1111/j.1365-2621.2002.tb09516.x. [DOI] [Google Scholar]
- Barekat S, Soltanizadeh N. Improvement of meat tenderness by simultaneous application of high-intensity ultrasonic radiation and papain treatment. Innov Food Sci Emerg Technol. 2017;39:223–229. doi: 10.1016/j.ifset.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenero FJ, Borderias A. A study of the effects of frozen storage on certain functional properties of meat and fish protein. Int J Food Sci Technol. 1983;18(6):731–737. doi: 10.1111/j.1365-2621.1983.tb00311.x. [DOI] [Google Scholar]
- Demirhan E, Özbek B. Influence of enzymatic hydrolysis on the functional properties of sesame cake protein. Chem Eng Commun. 2013;200(5):655–666. doi: 10.1080/00986445.2012.717316. [DOI] [Google Scholar]
- Feiner G. Handbook of meat products. Boca Raton: CRC Press; 2006. [Google Scholar]
- Gbogouri G, Linder M, Fanni J, Parmentier M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J Food Sci. 2004;69(8):C615–C622. doi: 10.1111/j.1365-2621.2004.tb09909.x. [DOI] [Google Scholar]
- Gerelt B, Ikeuchi Y, Suzuki A. Meat tenderization by proteolytic enzymes after osmotic dehydration. Meat Sci. 2000;56(3):311–318. doi: 10.1016/S0309-1740(00)00060-7. [DOI] [PubMed] [Google Scholar]
- Gornall AG, Baradawill CJ, David MM. Determination of serum protein by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- Got F, Culioli J, Berge P, Vignon X, Astruc T, Quideau J. Effects of high-intensity high-frequency ultrasound on ageing rate, ultrastructure and some physico-chemical properties of beef. Meat Sci. 1999;51(1):35–42. doi: 10.1016/S0309-1740(98)00094-1. [DOI] [PubMed] [Google Scholar]
- Hu H, Fan X, Zhou Z, Xu X, Fan G, Wang L, Zhu L. Acid-induced gelation behavior of soybean protein isolate with high intensity ultrasonic pre-treatments. Ultrason Sonochem. 2013;20(1):187–195. doi: 10.1016/j.ultsonch.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Huff-Lonergan E, Lonergan SM. Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci. 2005;71(1):194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Jambrak AR, Lelas V, Mason TJ, Krešić G, Badanjak M. Physical properties of ultrasound treated soy proteins. J Food Eng. 2009;93(4):386–393. doi: 10.1016/j.jfoodeng.2009.02.001. [DOI] [Google Scholar]
- Jauregui CA, Regenstein JM, Baker RC. A simple centrifugal method for measuring expressible moisture, a water-binding property of muscle foods. J Food Sci. 1981;46(4):1271. doi: 10.1111/j.1365-2621.1981.tb03038.x. [DOI] [Google Scholar]
- Jayasooriya S, Bhandari B, Torley P, D’arcy B. Effect of high power ultrasound waves on properties of meat: a review. Int J Food Prop. 2004;7(2):301–319. doi: 10.1081/JFP-120030039. [DOI] [Google Scholar]
- Jayasooriya SD, Torley P, D’arcy BR, Bhandari BR. Effect of high power ultrasound and ageing on the physical properties of bovine semitendinosus and longissimus muscles. Meat Sci. 2007;75(4):628–639. doi: 10.1016/j.meatsci.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Karakaya M, Gökalp HY, Yetim H. Model system evaluations of meat emulsions prepared with different edible beef by products and fats and oil. Pamukkale Univ J Eng Sci. 1997;3(2):347–352. [Google Scholar]
- Krasulya O, Bogush V, Trishina V, Potoroko I, Khmelev S, Sivashanmugam P. Impact of acoustic cavitation on food emulsions. Ultrason Sonochem. 2016;30:98–102. doi: 10.1016/j.ultsonch.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Kurt Ş, Kılınççeker O. Mixture optimization of beef, turkey, and chicken meat for some of the physical, chemical, and sensory properties of meat patties. Poult Sci. 2011;90(8):1809–1816. doi: 10.3382/ps.2010-01306. [DOI] [PubMed] [Google Scholar]
- Li K, Kang ZL, Zhao YY, Xu XL, Zhou GH. Use of high-intensity ultrasound to improve functional properties of batter suspensions prepared from PSE-like chicken breast meat. Food Bioprocess Technol. 2014;7(12):3466–3477. doi: 10.1007/s11947-014-1358-y. [DOI] [Google Scholar]
- Li Y, Jia W, Zhang CH, Li X, Wang JZ, Zhang DQ, Mu GF. Fluctuated low temperature combined with high-humidity thawing to reduce physicochemical quality deterioration of beef. Food Bioprocess Technol. 2014;7(12):3370–3380. doi: 10.1007/s11947-014-1337-3. [DOI] [Google Scholar]
- Li K, Kang ZL, Zou YF, Xu XL, Zhou GH. Effect of ultrasound treatment on functional properties of reduced-salt chicken breast meat batter. J Food Sci Technol. 2015;52(5):2622–2633. doi: 10.1007/s13197-014-1356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik MA, Saini CS. Rheological and structural properties of protein isolates extracted from dephenolized sunflower meal: effect of high intensity ultrasound. Food Hydrocoll. 2018;81:229–241. doi: 10.1016/j.foodhyd.2018.02.052. [DOI] [Google Scholar]
- McDonnell C, Allen P, Morin C, Lyng J. The effect of ultrasonic salting on protein and water–protein interactions in meat. Food Chem. 2014;147:245–251. doi: 10.1016/j.foodchem.2013.09.125. [DOI] [PubMed] [Google Scholar]
- Ozuna C, Puig A, García-Pérez JV, Mulet A, Cárcel JA. Influence of high intensity ultrasound application on mass transport, microstructure and textural properties of pork meat (longissimus dorsi) brined at different NaCl concentrations. J Food Eng. 2013;119(1):84–93. doi: 10.1016/j.jfoodeng.2013.05.016. [DOI] [Google Scholar]
- Reynolds J, Anderson D, Schmidt G, Theno D, Siegel D. Effects of ultrasonic treatment on binding strength in cured ham rolls. J Food Sci. 1978;43(3):866–869. doi: 10.1111/j.1365-2621.1978.tb02442.x. [DOI] [Google Scholar]
- Samejima K, Hashimoto Y, Yasui T, Fukazawa TT. Heat gelling properties of myosin, actin, actomyosin and myosin-subunits in a saline model system. J Food Sci. 1969;34(3):242–245. doi: 10.1111/j.1365-2621.1969.tb10331.x. [DOI] [Google Scholar]
- Smith DM, Brekke CJ. Enzymic modification of the structure and functional properties of mechanically deboned fowl proteins. J Agric Food Chem. 1985;33(4):631–637. doi: 10.1021/jf00064a016. [DOI] [Google Scholar]
- Soria AC, Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci Technol. 2010;21(7):323–331. doi: 10.1016/j.tifs.2010.04.003. [DOI] [Google Scholar]
- Stadnik J, Dolatowski ZJ, Baranowska HM. Effect of ultrasound treatment on water holding properties and microstructure of beef (m. semimembranosus) during ageing. LWT Food Sci Technol. 2008;41(10):2151–2158. doi: 10.1016/j.lwt.2007.12.003. [DOI] [Google Scholar]
- Torres RA, Pétrier C, Combet E, Carrier M, Pulgarin C. Ultrasonic cavitation applied to the treatment of bisphenol A. Effect of sonochemical parameters and analysis of BPA by-products. Ultrason Sonochem. 2008;15(4):605–611. doi: 10.1016/j.ultsonch.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Turantaş F, Kılıç GB, Kılıç B. Ultrasound in the meat industry: general applications and decontamination efficiency. Int J Food Microbiol. 2015;198:59–69. doi: 10.1016/j.ijfoodmicro.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Von Seggern D, Calkins C, Johnson D, Brickler J, Gwartney B. Muscle profiling: characterizing the muscles of the beef chuck and round. Meat Sci. 2005;71(1):39–51. doi: 10.1016/j.meatsci.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Xiong G, Zhang L, ZhanG W, Wu J. Influence of ultrasound and proteolytic enzyme inhibitors on muscle degradation, tenderness, and cooking loss of hens during aging. Czech J Food Sci. 2012;30(3):195–205. doi: 10.17221/136/2011-CJFS. [DOI] [Google Scholar]
- Yu L, Perret J. Effects of xylanase treatments on gelling and water-uptaking properties of psyllium. J Agric Food Chem. 2003;51(2):492–495. doi: 10.1021/jf020644l. [DOI] [PubMed] [Google Scholar]
- Zou Y, Xu P, Wu H, Zhang M, Sun Z, Sun C, Xu W. Effects of different ultrasound power on physicochemical property and functional performance of chicken actomyosin. Int J Biol Macromol. 2018;113:640–647. doi: 10.1016/j.ijbiomac.2018.02.039. [DOI] [PubMed] [Google Scholar]


