Abstract
Background
Visceral obesity is strongly associated with atherosclerosis. Even though waist circumference (WC) is the most common assessment method of total visceral adipose tissue and cardiometabolic risk, this method lacks direct measurement of adipose tissue and has better correlation to subcutaneous fat rather than visceral fat. We intended to investigate whether epicardial adipose tissue (EAT) is clinically superior to body mass index (BMI) and WC in predicting Framingham risk score (FRS) and carotid intima-media thickness (CIMT).
Methods
Our study included 331 patients who were admitted to our outpatient clinic for risk factor assessment. We calculated BMI, FRS, and WC, and the patients underwent echocardiographic and carotid examinations to measure EAT and CIMT. The metabolic syndrome (MS) score was calculated by summing the MS risk factor scores.
Results
The area under the curve values of EAT were similar to FRS and higher than those of weight, BMI, and WC for both increased CIMT and the presence of carotid plaque. Male gender, age, low-density lipoprotein-cholesterol level, and EAT thickness were independent predictors of CIMT, whereas male gender, age, WC, uric acid concentration, and EAT significantly predicted the presence of carotid plaque.
Conclusions
This study demonstrated that epicardial adipose tissue (EAT) has a stronger correlation with CIMT than BMI and WC, and it was a significant predictor of increased CIMT and the presence of carotid plaque. Additional data are required to clarify the diagnostic and therapeutic role of EAT in managing obese patients, and to decrease their cardiometabolic risk.
Keywords: Carotid intima-media thickness, Epicardial adipose tissue, Framingham risk score, Metabolic syndrome, Obesity, Waist circumference
INTRODUCTION
Coronary heart disease (CHD) is a major cause of death and disability in developed countries. Obesity, besides being a major modifiable risk factor of CHD, also plays an important role in the development of hypertension, diabetes mellitus and metabolic syndrome (MS), namely other cardiovascular risk factors.1-3 An increase in adipose tissue and also the localization of obesity can be used to predict increased cardiovascular risk. Several studies have identified abdominal (visceral) obesity, accumulated fat around the visceral organs, to be strongly associated with cardiovascular risk factors and atherosclerosis.4-6 Waist circumference (WC) is a widely utilized assessment method of total visceral adipose tissue and cardiometabolic risk.7 However, this method lacks direct measurement of adipose tissue and cannot provide adequate data for the severely obese, especially in men.8-10 Another factor limiting the widespread utilization of WC is considerable variability in its measurement. In addition, WC seems to have a better correlation to subcutaneous fat rather than visceral fat,11 and it might be less reliable in older than in younger individuals.12 Therefore, there is considerable interest in developing reliable measures of visceral obesity.8
Echocardiographic measurement of epicardial adipose tissue (EAT) may be an alternative to WC. Echocardiographic measurements of EAT is an objective, readily available and cheaper method compared to gold standard techniques of magnetic resonance imaging (MRI) and computed tomography (CT); thus may be favored in routine clinical practice.13 EAT, a fat tissue closely related to visceral adiposity of the embryological abdominal organs, is a true visceral fat,14 and secretes several pro-atherogenic mediators similar to visceral adipose tissues.15-17 Iacobellis and coworkers reported that echocardiographic measurements of EAT were strongly associated with anthropometric measures and total visceral adipose tissue as assessed by MRI.18
Several studies have investigated the clinical association of EAT with various parameters, however information regarding the direct relationship of EAT and anthropometric parameters with global risk predictors such as Framingham risk score,7 carotid-intima media thickness,19 and other risk parameters20,21 is lacking. Therefore, we investigated whether EAT is superior to body mass index (BMI) and WC in predicting global cardiovascular risk.
METHODS
Patient population and study protocol
This cross-sectional and observational study included 331 patients who were admitted to our outpatient clinic. The patient group included healthy people who received preventive evaluations, and patients with hypertension, diabetes mellitus and hyperlipidemia who received risk factor management. Patients with coronary artery disease (CAD), left ventricular systolic dysfunction, moderate-severe valvular disease, symptoms of CAD and equivalent findings on exercise electrocardiography and perfusion scans were excluded.
The patients were evaluated in terms of age, demographic data and cardiovascular risk factors. Hypertension was defined as the active use of antihypertensive drugs or documentation of blood pressure more than 140/90 mmHg. Diabetes mellitus was defined as fasting plasma glucose (FPG) levels over 126 mg/dl or glucose level over 200 mg/dl at any measurement or the active use of antidiabetic treatment. Patients who were using tobacco products on admission to our hospital and those who had quit smoking within the last year were considered as smokers. A family history of CAD was defined as a history of CAD or sudden death in a first-degree relative before the age of 55 years for men and 65 years for women.
This study was performed in accordance with the principles stated in the Declaration of Helsinki, and it was approved by the local Ethics Committee. All subjects provided informed consent before participation.
Routine measurements
Blood samples were drawn by venipuncture to measure routine blood chemistry parameters after fasting for at least 8 hours. Fasting blood glucose, serum creatinine, uric acid, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglyceride levels were recorded. Glucose, creatinine, and lipid profile were determined by standard methods. Serum C-reactive protein (CRP) was analyzed using a nephelometric technique (Beckman Coulter Immage 800; Fullerton, CA, USA; normal range 0-0.8 mg/dL).
Anthropometric measurements
Weight and height were measured while the subjects were fasting and wearing only their undergarments. BMI was determined as weight (kg) / height2 (m). Waist circumference (in cm; defined as the circumference between the lower rib margin and the iliac crest, mid waist) was measured while the subjects were standing with their heels together.
Definition of metabolic syndrome score
The metabolic syndrome score (MS score) was calculated by summing the following MS risk factor scores: 1) Raised blood pressure (BP): systolic BP > 130 or diastolic BP > 85 mmHg, or treatment for previously diagnosed hypertension. 2) Raised FPG > 100 mg/dL (5.6 mmol/L), or previously diagnosed type 2 diabetes. 3) Central obesity: WC ≥ 102 cm or 40 inches (male), ≥ 88 cm or 36 inches (female). 4) Reduced high-density lipoprotein (HDL) cholesterol: < 40 mg/dL (1.03 mmol/L) in males, < 50 mg/dL (1.29 mmol/L) in females, or specific treatment for this lipid abnormality. 5) Raised triglycerides: > 150 mg/dL (1.7 mmol/L), or specific treatment for this lipid abnormality.22
Echocardiography
Patients were imaged in the left lateral decubitus position by two experienced cardiologists using commercially available systems with a GE-Vingmed Vivid S5 (GE-Vingmed Ultrasound AS, Horten, Norway) system according to echocardiography guidelines.23 Left ventricular mass (LVM) was calculated using the Devereux formula.24
Evaluation of epicardial adipose tissue
EAT was evaluated on the free wall of the right ventricle from the parasternal long-axis view, using the aortic annulus as an anatomic reference. We preferred the area above the right ventricle to measure EAT thickness, because this area is known to have the thickest EAT layer. EAT, identified as an echo-free space under the pericardial layer on two-dimensional echocardiography, was measured perpendicularly in front of the right ventricular free wall at end-systole.18,25 We magnified each still image for better visualization and accurate measurement of EAT thickness, and measured the thickest point of EAT in each cycle (Figure 1). To standardize the measuring axis, we used the aortic annulus as an anatomical reference. The measurements were performed at a point on the free wall of the right ventricle along the midline of the ultrasound beam, perpendicular to the aortic annulus. The average value comprising three cardiac cycles of each echocardiographic view was used for the statistical analysis. Inter-observer and intra-observer variability in epicardial fat thickness measurements were excellent (coefficients of correlation 0.94 and 0.98, respectively).
Figure 1.
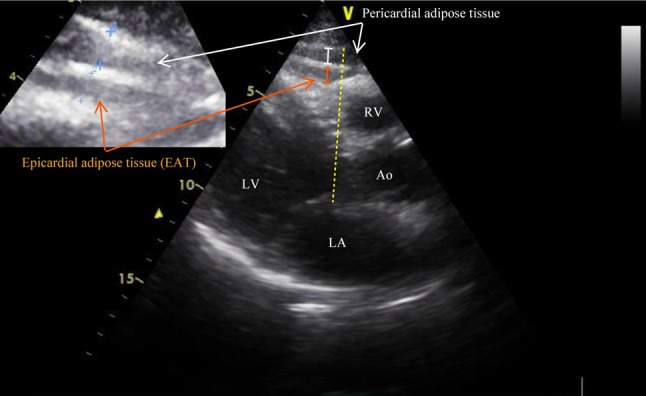
Evaluation of epicardial adipose tissue. EAT, identified as an echo-free space between the myocardium and visceral pericardium from the parasternal long-axis view on 2-dimensional echocardiography, was measured perpendicularly in front of the right ventricular free wall at end-systole.
Measurement of carotid intima-media thickness
Ultrasonography was performed on all patients using a high-resolution ultrasonography scanner (VingMed Vivid 3, GE Medical System, Horten, Norway) with a 7.0-MHz linear array transducer. Measurements were performed on the right and left carotid arteries.26 Each patient was asked to lie in the supine position with their head directed away from the side of interest and their neck slightly extended. The transducer was manipulated so that the near and far walls of the common carotid artery were parallel, and the lumen diameter was maximized in the longitudinal plane. The region 1 cm proximal to the carotid bifurcation was identified, and the carotid intima-media thickness (CIMT) of the far wall was eva-luated as the distance between the lumen-intima interface and the media-adventitia interface. The CIMT was measured on the frozen frame of a suitable longitudinal image, with the image magnified to achieve a higher resolution of detail. The CIMT measurement was obtained from 4 contiguous sites at 1-mm intervals, and the average of all 8 measurements was used for analysis. The same investigator who was blinded to patient data performed all measurements. The intra-observer mean absolute difference in measuring the common carotid intima-media thickness was 0.026 ± 0.043 mm (coefficient of variation: 1.6%, intra-class correlation: 0.95).
A mean carotid intima-media thickness lower than 0.9 mm was considered to be normal; a thickness higher than 0.9 mm but lower than 1.3 was considered to be increased; a thickness equal to or higher than 1.3 mm in any measurement site was defined as a plaque.
Calculation of cardiovascular risk
Baseline characteristics of the patients were recorded. The Framingham risk score was calculated for every participant based on the current version of the Framingham Risk Score that was published in 20027 (http://www.mdcalc.com/framingham-coronary-heart-disease-risk-score-si-units). The classical risk factors included age, gender, systolic BP, total cholesterol, HDL-cholesterol concentration, and smoking.
Statistical analysis
Continuous variables were given as mean ± SD, and categorical variables as percentages. Data were tested for normal distribution using the Kolmogorov-Smirnov test. Spearman’s rank correlation coefficient was used to analyze relationships between variables. Optimal cut-off values for the detection of increased CIMT (≥ 0.9 mm) and presence of carotid plaque by EAT and the other study parameters were determined using receiver operating characteristic (ROC) curve analysis and area under the curve (AUC) values. Linear and logistic regression analyses with the enter method were used for all relevant independent variables, which were included if they were significantly different in the univariate analysis. In addition, the analysis was repeated after pre-elimination with the stepwise method for the independent variables. Statistical significance was defined as p < 0.05. SPSS statistical software (SPSS for Windows, version 15.0, Chicago, IL, USA) was used for all statistical calculations.
RESULTS
The clinical characteristics of the patients are presented in Table 1. Our study included 331 patients (mean age: 46 ± 8 years) with a male preponderance (80%). We divided the patients into two groups according to the presence of carotid plaque. The patients with carotid plaque were older with a higher WC, and they were more likely to be male, hypertensive and smokers. Moreover, these patients had elevated plasma glucose, uric acid, total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride concentrations, and higher EAT, FRS, and MS scores compared to the patients with CIMT < 1.3 mm.
Table 1. Demographic characteristics, standard cardiovascular risk factors, and laboratory findings in patients with or without carotid plaque.
| Variables (N = 331) | Without carotid plaque (N = 254) | Carotid plaque (N = 77) | p value |
| Age (years) | 45 ± 8 | 51 ± 9 | < 0.001 |
| Gender (male) | 68% | 82% | 0.027 |
| BMI (kg/m2) | 31 ± 4.7 | 30 ± 4.2 | 0.836 |
| Waist circumference (cm) | 100 ± 110 | 104 ± 13 | 0.038 |
| Hypertension % | 61% | 75% | 0.019 |
| Diabetes mellitus % | 7% | 12% | 0.124 |
| Smoking status % | 42% | 60% | 0.026 |
| Hyperlipidemia % | 34% | 45% | 0.053 |
| Family history of CAD % | 41% | 56% | 0.066 |
| Glucose (mg/dL) | 102 ± 28 | 111 ± 46 | 0.032 |
| Creatinine (mg/dL) | 0.83 ± 0.16 | 0.85 ± 0.13 | 0.258 |
| Uric acid (mg/dL) | 5.2 ± 1.4 | 5.9 ± 1.5 | < 0.001 |
| Total cholesterol (mg/dL) | 213 ± 40 | 228 ± 42 | 0.003 |
| LDL-cholesterol (mg/dL) | 134 ± 34 | 146 ± 39 | 0.009 |
| HDL-cholesterol (mg/dL) | 45 ± 12 | 44 ± 13 | 0.370 |
| Triglycerides (mg/dL) | 171 ± 109 | 201 ± 158 | 0.049 |
| CRP (mg/dL) | 0.48 ± 0.52 | 0.54 ± 0.56 | 0.387 |
| Epicardial adipose tissue thickness (mm) | 5.4 ± 2.3 | 7.7 ± 2.6 | < 0.001 |
| CIMT (mean, mm) | 0.78 ± 0.15 | 0.98 ± 0.12 | < 0.001 |
| Framingham risk score | 6.6 ± 6.5 | 12.6 ± 9.3 | < 0.001 |
| Metabolic syndrome score | 2.4 ± 1.6 | 3.0 ± 1.6 | 0.022 |
BMI, body mass index; CAD, coronary artery disease; CIMT, carotid intima-media thickness; CRP, C-reactive protein; HDL, high density lipoprotein; LDL, low density lipoprotein; SD, standard deviation.
Correlations of anthropometric measures, EAT, and CRP to metabolic parameters, metabolic syndrome (MS) score, CIMT and FRS are detailed in Table 2. EAT was significantly correlated with age (r = 0.231, p < 0.001), plasma glucose (r = 0.175, p = 0.028), uric acid (r = 0.256, p = 0.002), CRP (r = 0.435, p < 0.001), LDL-cholesterol (r = 0.236, p = 0.003), CIMT (r = 0.596, p < 0.001), FRS (r = 0.414, p < 0.001), and MS score (r = 0.393, p < 0.001). BMI was correlated with age (r = 0.127, p = 0.008), creatinine (r = -0.214, p < 0.001), uric acid (r = 0.146, p = 0.001), CRP (r = 0.410, p < 0.001), LDL-cholesterol (r = 0.132, p = 0.024), CIMT (r = 0.237, p < 0.001), FRS (r = 0.191, p = 0.001) and MS score (r = 0.589, p < 0.001). The correlations between metabolic parameters and WC were significant for age (r = 0.107, p = 0.050), glucose (r = 0.179, p = 0.008), uric acid (r = 0.231, p = 0.001), CRP (r = 0.337, p < 0.001), triglycerides (r = 0.258, p < 0.001), CIMT (r = 0.378, p < 0.001), and FRS (r = 0.212, p = 0.002). MS score was well correlated with BMI, whereas FRS and CIMT had strong correlations with age and EAT.
Table 2. Correlations of different body fat indexes with metabolic parameters.
| Parameters | Weight (kg) | BMI (kg/m2) | Waist circumference (cm) | EAT (mm) | CRP | CIMT (mm) | FRS |
| Age (years) | r = -0.041, | r = 0.127, | r = 0.107, | r = 0.231, | r = 0.146, | r = 0.473, | r = 0.642, |
| p = 0.393 | p = 0.008 | p = 0.050 | p < 0.001 | p = 0.006 | p < 0.001 | p < 0.001 | |
| Glucose (mg/dL) | r = 0.113, | r = 0.080, | r = 0.179, | r = 0.175, | r = 0.174, | r = 0.178, | r = 0.111, |
| p = 0.044 | p = 0.156 | p = 0.008 | p = 0.028 | p = 0.006 | p = 0.002 | p = 0.055 | |
| Creatinine (mg/dL) | r = 0.030, | r = -0.214, | r = -0.075, | r = -0.026, | r = -0.184, | r = 0.005, | r = 0.013, |
| p = 0.599 | p < 0.001 | p = 0.270 | p = 0.742 | p = 0.003 | p = 0.934 | p = 0.824 | |
| Uric acid (mg/dL) | r = 0.236, | r = 0.146, | r = 0.231, | r = 0.256, | r = 0.206, | r = 0.240, | r = 0.204, |
| p < 0.001 | p = 0.013 | p = 0.001 | p = 0.002 | p = 0.001 | p < 0.001 | p = 0.001 | |
| CRP (mg/dL) | r = 0.238, | r = 0.410, | r = 0.337, | r = 0.435, | - | r = 0.274, | r = 0.263, |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| LDL (mg/dL) | r = 0.026, | r = 0.132, | r = 0.044, | r = 0.236, | r = 0.312, | r = 0.166, | r = 0.427, |
| p = 0.663 | p = 0.024 | p = 0.531 | p = 0.003 | p < 0.001 | p = 0.005 | p < 0.001 | |
| HDL (mg/dL) | r = -0.126, | r = -0.014, | r = -0.132, | r = -0.012, | r = -0.025, | r = -0.048, | r = -0.158, |
| p = 0.028 | p = 0.809 | p = 0.056 | p = 0.883 | p = 0.693 | p = 0.407 | p = 0.006 | |
| Triglyceride (mg/dL) | r = 0.249, | r = 0.203, | r = 0.258, | r = 0.156, | r = 0.138, | r = 0.001, | r = 0,296 |
| p < 0.001 | p < 0.001 | p < 0.001 | p = 0.053 | p = 0.030 | p = 0.988 | p < 0.001 | |
| CIMT (mm) | r = 0.166, | r = 0.237, | r = 0.378, | r = 0.596, | r = 0.274, | - | r = 0.480, |
| p = 0.003 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| FRS | r = 0.129, | r = 0.191, | r = 0.212, | r = 0.414, | r = 0.263, | r = 0.480, | - |
| p = 0.025 | p = 0.001 | p = 0.002 | p < 0.001 | p < 0.001 | p < 0.001 | ||
| MS score | r = 0.414, | r = 0.589, | r = 0.698, | r = 0.393, | r = 0.321, | r = 0.344, | r = 0.470, |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Leukocytes (/mm3) | r = 0.060, | r = 0.070, | r = 0.073, | r = 0.017, | r = 0.336, | r = 0.003, | r = 0.028, |
| p = 0.312 | p = 0.236 | p = 0.297 | p = 0.834 | p < 0.001 | p = 0.966 | p = 0.642 | |
| Neutrophil (/mm3) | r = -0.070, | r = -0.022, | r = -0.066, | r = -0.055, | r = 0.316, | r = -0.003, | r = -0.015, |
| p = 0.227 | p = 0.711 | p = 0.349 | p = 0.507 | p < 0.001 | p = 0.961 | p = 0.803 | |
| Monocyte (/mm3) | r = 0.114, | r = 0.017, | r = 0.070, | r = 0.031, | r = 0.131, | r = 0.057, | r = -0.006, |
| p = 0.053 | p = 0.779 | p = 0.317 | p = 0.708 | p = 0.042 | p = 0.340 | p = 0.916 | |
| Lymphocyte (/mm3) | r = 0.217, | r = 0.235, | r = 0.231, | r = 0.108, | r = 0.147, | r = 0.015, | r = 0.115, |
| p < 0.001 | p < 0.001 | p = 0.001 | p = 0.188 | p = 0.022 | p = 0.805 | p = 0.057 | |
| EAT (mm) | r = 0.389, | r = 0.453, | r = 0.566, | - | r = 0.435, | r = 0.596, | r = 0.414, |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
BMI, body mass index, CIMT, carotid intima-media thickness; CRP, C-reactive protein; EAT, epicardial adipose tissue; FRS, Framingham risk score; HDL, high density lipoprotein; LDL, low density lipoprotein; MS, metabolic syndrome; SD, standard deviation.
We then performed ROC analysis to determine the sensitivity and specificity of FRS, MS score, weight, BMI, WC, EAT, age, and CRP in order to detect increased CIMT and the presence of carotid plaque (Figures 2-3, and Table 3). The AUC values of EAT were similar to FRS and higher than those of weight, BMI, and WC for both CIMT and carotid plaque.
Figure 2.
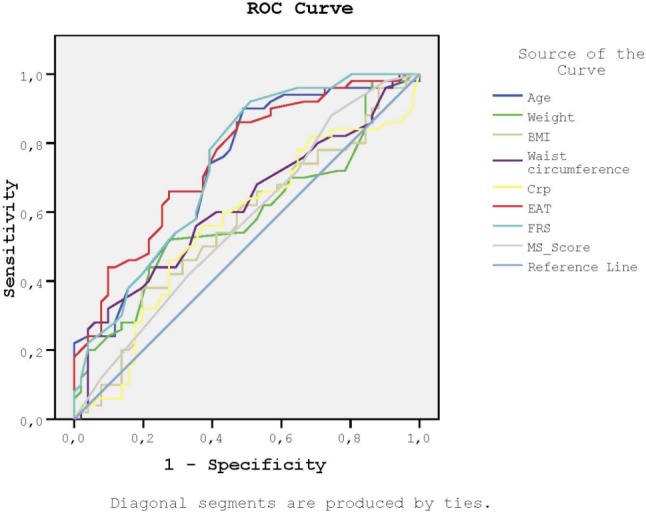
Receiver-operating characteristic curves for the sensitivity and the specificity of FRS, MS score, weight, BMI, waist circumference, EAT, age and CRP to detect increased CIMT. BMI, body mass index; CIMT, carotid intima-media thickness; CRP, C-reactive protein; EAT, epicardial adipose tissue; FRS, Framingham risk score; MS score, metabolic syndrome score.
Figure 3.
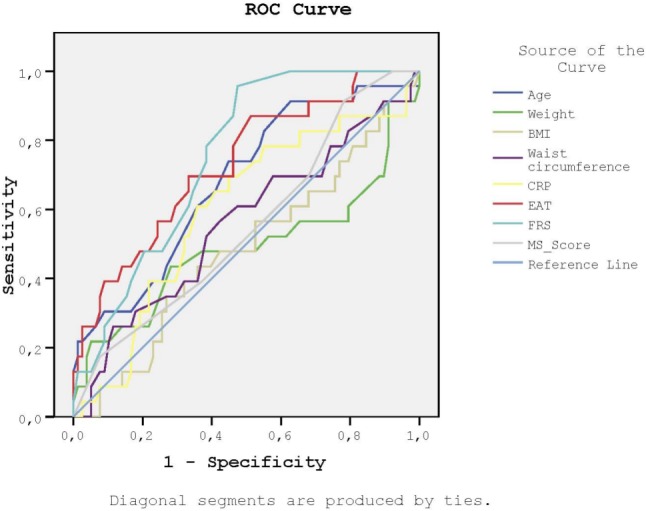
Receiver-operating characteristic curves for the sensitivity and the specificity of FRS, MS score, weight, BMI, waist circumference, EAT, age and CRP to detect the presence of carotid plaque. BMI, body mass index; CIMT, carotid intima-media thickness; CRP, C-reactive protein; EAT, epicardial adipose tissue; FRS, Framingham risk score; MS score, metabolic syndrome score.
Table 3. Receiver-operating characteristic curves for the sensitivity and the specificity of FRS, MS score, weight, BMI, waist circumference, EAT, age and CRP to detect the presence of carotid plaque.
| Parameters | AUC | SE | p | 95% CI |
| Age | 0.680 | 0.064 | 0.009 | 0.555-0.804 |
| Weight | 0.488 | 0.081 | 0.862 | 0.329-0.648 |
| BMI | 0.483 | 0.070 | 0.805 | 0.346-0.620 |
| Waist circumference | 0.558 | 0.071 | 0.397 | 0.418-0.698 |
| CRP | 0.598 | 0.068 | 0.154 | 0.465-0.731 |
| EAT | 0.730 | 0.060 | 0.001 | 0.613-0.847 |
| FRS | 0.753 | 0.049 | 0.000 | 0.656-0.849 |
| MS score | 0.552 | 0.067 | 0.454 | 0.419-0.684 |
AUC, area under the curve; BMI, body mass index; CI, confidence interval. CRP, C-reactive protein; EAT, epicardial adipose tissue; FRS, Framingham risk score; MS score, metabolic syndrome score; SE, standard error.
Finally, we performed multiple regression analyses in order to identify the independent predictors of increased CIMT and the presence of carotid plaque. Male gender, age, LDL-cholesterol level, and EAT thickness were independent predictors of CIMT, whereas male gender, age, WC, uric acid concentration, and EAT significantly predicted the presence of carotid plaque (Tables 4 and 5).
Table 4. Multivariate analyses for prediction of CIMT.
| Linear regression analysis | Dependent variable: carotid intima-media thickness | |||
| Independent variables | p value* | Beta (standardized) | p value# | Beta (standardized) |
| Age, years | < 0.001 | 0.494 | < 0.001 | 0.391 |
| Gender, male | < 0.001 | 0.467 | < 0.001 | 0.258 |
| Family history of CAD | 0.090 | 0.122 | ||
| Glucose (mg/dL) | 0.706 | -0.025 | ||
| Creatinine (mg/dL) | 0.353 | -0.070 | ||
| Uric acid (mg/dL) | 0.587 | -0.043 | ||
| LDL, mg/dL | 0.019 | 0.155 | 0.257 | 0.054 |
| EAT, mm | < 0.001 | 0.312 | < 0.001 | 0.441 |
| CRP (mg/dL) | 0.644 | 0.029 | ||
| Constant | 0.871 | 0.002 | ||
| Adjusted R2 | 0.554 | 0.519 |
CAD, coronary artery disease; CRP, C-reactive protein; EAT, epicardial adipose tissue thickness; HDL, high density lipoprotein; LDL, low density lipoprotein; SE, standard error.
* Linear and logistic regression analyses with enter method were used for all relevant independent variables which were included if they were significantly different in the univariate analyses. # In addition, the analysis was repeated after a pre-elimination with stepwise method for the independent variables.
Table 5. Multivariate analyses for prediction of carotid plaque presence.
| Logistic regression analysis | Dependent variable: Presence of carotid plaque | |||||
| Independent variables | p value* | Wald | OR (95% CI) | p value# | Wald | OR (95% CI) |
| Age, years | 0.019 | 5.5 | 1.065 (1.011-1.122) | 0.005 | 8.1 | 1.075 (1.023-1.130) |
| Gender, male | 0.016 | 5.8 | 4.427 (1.316-14.9) | 0.033 | 4.5 | 3.183 (1.098-9.229) |
| Waist circumference | 0.012 | 6.3 | 0.930 (0.879-0.984) | 0.008 | 7.1 | 0.927 (0.877-0.980) |
| Hypertension | 0.391 | 0.7 | 2.043 (0.400-10.4) | |||
| Smoking | 0.961 | 0.2 | 1.028 (0.336-3.145) | |||
| HDL-C, mg/dL | 0.857 | 0.3 | 1.005 (0.956-1.056) | |||
| LDL, mg/dL | 0.412 | 0.7 | 1.006 (0.992-1.020) | |||
| Glucose (mg/dL) | 0.931 | 0.1 | 0.999 (0.970-1.028) | |||
| Uric acid (mg/dL) | 0.042 | 4.1 | 1.456 (1.014-2.091) | 0.015 | 5.9 | 1.536 (1.088-2.167) |
| EAT, mm | < 0.001 | 13.9 | 1.649 (1.267-2.149) | < 0.001 | 13.8 | 1.635 (1.262-2.118) |
| Constant | 0.161 | 1.9 | 0.193 | 1.7 | ||
| Nagelkerke R square | 0.394 | 0.371 |
CAD, coronary artery disease; EAT, epicardial adipose tissue thickness; HDL, high density lipoprotein; LDL, low density lipoprotein; SE, standard error.
* Linear and logistic regression analyses with enter method were used for all relevant independent variables which were included if they were significantly different in the univariate analyses. # In addition, the analysis was repeated after a pre-elimination with stepwise method for the independent variables.
DISCUSSION
In this study, we aimed to investigate whether EAT is superior to anthropometric measures in predicting cardiovascular risk. We found that EAT had a stronger association with FRS and CIMT than to BMI and WC. Moreover, EAT was a significant predictor of increased CIMT and the presence of carotid plaque. To the best of our knowledge, this is the first study to compare EAT with BMI and WC, and to show that EAT has a stronger correlation with CIMT than BMI and WC in a clinical setting.
Even though there are validated clinical risk identification algorithms for the prediction of cardiovascular events, several biochemical biomarkers and novel imaging methods have been investigated as alternatives. Obesity is an important health problem in developed and developing countries. This problem is mainly due to the endocrine and paracrine effects of visceral adipose tissue.8,27 Although subcutaneous fat has also been related to increased cardiovascular risk, this effect is not as prominent as with visceral adipose tissue.27
Since there is no real fascia between EAT and myocardium, EAT may influence the myocardium and coronary arteries directly via hormonal and inflammatory mediators, in addition to systemic effects. This mechanism may be especially important in CAD and left ventricular hypertrophy. An autopsy study by Corradi et al. revealed that EAT was more strongly correlated to increased left ventricular mass than BMI.28 Recently, Greif et al. reported a direct association between epicardial adipose tissue volume and the extent of CAD.29 The Heinz-Nixdorf Recall Study reported that epicardial fat was associated with fatal and nonfatal coronary events in the general population independently of traditional cardiovascular risk factors.30 Park et al. demonstrated that increased epicardial fat was independently associated with plaque vulnerability in patients with severe CAD.31 These studies support our findings, however the exact pathophysiologic mechanisms remain unknown. We know for sure that EAT has both local and systemic pro-atherogenic effects similar to visceral adipose tissues. It may therefore be possible that EAT has a stronger local action than other visceral adipose tissues due to a lack of a real fascia between the myocardium and EAT, and that mediators may impair the coronary arteries directly or systemically through the coronary microcirculation. A recent study demonstrated that EAT stimulates myocardial fibrosis through the secretion of adipo-fibrokines.32 Moreover, supporting our hypothesis, the myocardium was very close to adipose tissue in sections of atrial and ventricular myocardium.32 Thus, echocardiographic measurements of EAT thickness may reflect the strong local effect of several adipokines.
Systemic inflammation and subclinical atherosclerosis, which are closely related to long-term mortality and morbidity, may stem from obesity. Two recent studies by Natale and Sengul et al. demonstrated a positive association between EAT and increased CIMT.33,34 Furthermore, Natale and coworkers also showed that the association between CIMT and EAT was stronger than anthropometric measurements, and reported a significant relationship between EAT and hsCRP. Our study showed that EAT was more significantly related to systemic inflammation and subclinical atherosclerosis than BMI and WC. Even though EAT and anthropometric measures had similar relationships with metabolic parameters including glucose intolerance, and dyslipidemia, EAT had a stronger association with subclinical atherosclerosis and systemic inflammation. This association might be due to the fact that EAT, besides being a marker of visceral adipose tissue, also has a significant influence on inflammation and atherosclerosis. Therefore, echocardiographic measurements of EAT not only provide information regarding the systemic influence of obesity but also its local effects. Due to this ability, EAT may be a better predictor of cardiovascular risk than BMI and WC.
Another systemic target of adipose tissue-related derangement is metabolism. Several studies have demonstrated that increased adiposity leads to insulin resistance and atherogenic lipid profile.35,36 These findings are also supported by our results. However, all three parameters used to assess cardiovascular risk had similar relationships with deranged metabolic parameters. On the other hand, compared to BMI and WC, EAT had a stronger correlation to FRS, which includes several metabolic parameters in order to calculate 10-year cardiovascular risk.19
An important aspect in obese patients is monitoring weight loss. Decreased visceral adipose tissue leads to lower cardiovascular risk,37 however, the amount of visceral adipose tissue loss that is required to induce favorable metabolic changes is currently unknown. Moreover, it has yet to be clarified which parameter is better to monitor weight loss. Even though anthropometric measures are still routinely used in monitoring weight loss, there is considerable data supporting the notion that EAT may be a better predictor of decreased cardiovascular risk. Additionally, recent evidence has shown that EAT thickness, besides significantly lowering with weight loss, is more closely related to decreased LVM and improved diastolic function than BMI.38 A recent study performed in a group of asymptomatic morbidly obese patients also demonstrated decreased EAT and CIMT thickness 6 months after laparoscopic sleeve gastrectomy.39 Interestingly, a change in EAT was the only predictor of CIMT independently of BMI, which is similar to our results.
Limitations
The study group included patients who were admitted to our outpatient clinic. Therefore, our population is not homogenous and may not reflect the entire population. BMI and WC are simple and convenient measures which do not require special personnel. However, EAT requires an echocardiography scanner and experienced operators. MRI and CT are currently the gold standard diagnostic methods for measuring epicardial fat thickness, and not using MRI or CT is a limitation of this study. Although epicardial fat is readily visualized with high-speed CT and MRI, the widespread use of these methods to assess EAT is not practical. Echocardiography provides an objective, noninvasive, readily available method and is less expensive than MRI or CT for measuring epicardial fat. The measurement of CIMT involves both the intimal and medial layer of the arterial wall, whereas the atherosclerotic process is restricted to the intimal layer. Furthermore, CIMT is an indirect method to assess the possible atherosclerotic burden in the coronary arteries. Lastly, CIMT not only reflects atherosclerotic burden but also age-related changes.
CONCLUSIONS
Our study demonstrated that EAT has a stronger correlation with CIMT than BMI and WC, and that it was a significant predictor of increased CIMT and the presence of carotid plaque. Additional data are required in order to clarify the diagnostic and therapeutic role of EAT in managing obese patients, and to decrease their cardiometabolic risk.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
SOURCE FUNDING
None declared.
REFERENCES
- 1.Wilson PW, D'Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 2.Mandviwala T, Khalid U, Deswal A. Obesity and cardiovascular disease: a risk factor or a risk marker? Curr Atheroscler Rep. 2016;18:21. doi: 10.1007/s11883-016-0575-4. [DOI] [PubMed] [Google Scholar]
- 3.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 4.Wei M, Gaskill SP, Haffner SM, et al. Waist circumference as the best predictor of noninsulin dependent diabetes mellitus (NIDDM) compared to body mass index, waist/hip ratio and other anthropometric measurements in Mexican Americans--a 7-year prospective study. Obes Res. 1997;5:16–23. doi: 10.1002/j.1550-8528.1997.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 6.Dhaliwal SS, Welborn TA. Central obesity and multivariable cardiovascular risk as assessed by the Framingham prediction scores. Am J Cardiol. 2009;103:1403–1407. doi: 10.1016/j.amjcard.2008.12.048. [DOI] [PubMed] [Google Scholar]
- 7.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 8.Shuster A, Patlas M, Pinthus JH, et al. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross R, Shaw KD, Rissanen J, et al. Sex differences in lean and adipose tissue distribution by magnetic resonance imaging: anthropometric relationships. Am J Clin Nutr. 1994;59:1277–1285. doi: 10.1093/ajcn/59.6.1277. [DOI] [PubMed] [Google Scholar]
- 10.Ross R, Léger L, Morris D, et al. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol. 1992;72:787–795. doi: 10.1152/jappl.1992.72.2.787. [DOI] [PubMed] [Google Scholar]
- 11.Bonora E, Micciolo R, Ghiatas AA, et al. Is it possible to derive a reliable estimate of human visceral and subcutaneous abdominal adipose tissue from simple anthropometric measurements? Metabolism. 1995;44:1617–1625. doi: 10.1016/0026-0495(95)90084-5. [DOI] [PubMed] [Google Scholar]
- 12.Iwao S, Iwao N, Muller DC, et al. Does waist circumference add to the predictive power of the body mass index for coronary risk? Obes Res. 2001;9:685–695. doi: 10.1038/oby.2001.93. [DOI] [PubMed] [Google Scholar]
- 13.Gorter PM, van Lindert AS, de Vos AM, et al. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 15.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–1319; quiz 417-8. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Payne GA, Kohr MC, Tune JD. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. Br J Pharmacol. 2012;165:659–669. doi: 10.1111/j.1476-5381.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 18.Iacobellis G, Assael F, Ribaudo MC, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379:2053–2062. doi: 10.1016/S0140-6736(12)60441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuller LH, Tracy RP, Shaten J, et al. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;144:537–547. doi: 10.1093/oxfordjournals.aje.a008963. [DOI] [PubMed] [Google Scholar]
- 21.Ozturk MT, Ebinc FA, Okyay GU, et al. Epicardial adiposity is associated with microalbuminuria in patients with essential hypertension. Acta Cardiol Sin. 2017;33:74–80. doi: 10.6515/ACS20160418A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 23.Galderisi M, Cosyns B, Edvardsen T, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017;18:1301–1310. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 24.Marwick TH, Gillebert TC, Aurigemma G, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). Eur Heart J Cardiovasc Imaging. 2015;28:727–754. doi: 10.1016/j.echo.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Bettencourt N, Toschke AM, Leite D, et al. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol. 2012;158:26–32. doi: 10.1016/j.ijcard.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 26.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 27.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 28.Corradi D, Maestri R, Callegari S, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004;13:313–316. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Greif M, Becker A, von Ziegler F, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:781–786. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 30.Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 31.Park JS, Choi SY, Zheng M, et al. Epicardial adipose tissue thickness is a predictor for plaque vulnerability in patients with significant coronary artery disease. Atherosclerosis. 2013;226:134–139. doi: 10.1016/j.atherosclerosis.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Venteclef N, Guglielmi V, Balse E, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. 2015;36:795–805. doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 33.Natale F, Tedesco MA, Mocerino R, et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr. 2009;10:549–555. doi: 10.1093/ejechocard/jep002. [DOI] [PubMed] [Google Scholar]
- 34.Sengul C, Cevik C, Ozveren O, et al. Echocardiographic epicardial fat thickness is associated with carotid intima-media thickness in patients with metabolic syndrome. Echocardiography. 2011;28:853–858. doi: 10.1111/j.1540-8175.2011.01471.x. [DOI] [PubMed] [Google Scholar]
- 35.Nieves DJ, Cnop M, Retzlaff B, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes. 2003;52:172–179. doi: 10.2337/diabetes.52.1.172. [DOI] [PubMed] [Google Scholar]
- 36.Haffner SM, D'Agostino Jr, Jr., Mykkanen L, et al. Insulin sensitivity in subjects with type 2 diabetes. Relationship to cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 1999;22:562–568. doi: 10.2337/diacare.22.4.562. [DOI] [PubMed] [Google Scholar]
- 37.Okauchi Y, Nishizawa H, Funahashi T, et al. Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care. 2007;30:2392–2394. doi: 10.2337/dc07-0218. [DOI] [PubMed] [Google Scholar]
- 38.Iacobellis G, Singh N, Wharton S, et al. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) 2008;16:1693–1697. doi: 10.1038/oby.2008.251. [DOI] [PubMed] [Google Scholar]
- 39.Altin C, Erol V, Aydin E, et al. Impact of weight loss on epicardial fat and carotid intima media thickness after laparoscopic sleeve gastrectomy: a prospective study. Nutr Metab Cardiovasc Dis. 2018;28:501–509. doi: 10.1016/j.numecd.2018.02.001. [DOI] [PubMed] [Google Scholar]


