Abstract
Increase in grain nitrogen concentration (GNC), which is directly affected by nitrogen (N) application, can help overcome the issues of malnutrition. Here, the effects of urea type (polyaspartic acid (PASP) urea and conventional urea) and N management method (two splits and four splits) on GNC and N concentration of head rice were investigated in field experiments conducted in Sichuan, China, in 2014 and 2015. N concentration of grain and head rice were significantly (P < 0.05) increased by N redistribution from the leaf lamina, activities of glutamine synthetase (GS), and glutamate synthase (GOGAT) at the heading stage, and N concentration and GOGAT activity in the leaf lamina at the maturity stage. Compared to conventional urea, PASP-urea significantly improved N concentration of grain and head rice by improving the activities of GS and GOGAT, thereby increasing N distribution in the leaf lamina. The four splits method, unlike the two splits method, enhanced N concentration and activities of key N metabolism enzymes of leaf lamina, leading to increased GNC and N concentration in head rice too. Overall, four splits is a feasible method for using PASP-urea and improving GNC.
Introduction
Currently, approximately one-eighth of the world population is food deprived and more than 10% is chronically malnourished1. Crops are one of the basic sources of energy and protein in human and animal diets. Although crop production increased by 0.5–2% annually over the second half of the last century, sustained increase in production and improvement in quality are still required to meet the future needs1–3.
Rice is one of the most widely grown cereals in the world and plays a critical role in food security, especially in Asia4. However, modern rice production is associated with concerns regarding both yield and quality of grain, as well as the impact of the environment5,6. Nutritional quality, which is one of the key components of grain quality, is considered as an essential target for rice development, whereas the improvement in protein concentration of grain is important to improve the nutritional quality4,7. Protein is the second most abundant component in rice grain, which provides 29% of the source of daily protein intake for humans in developing countries4,8. In China, rice accounts for 41–49% of the protein intake of the population for which rice is the staple food9. Nitrogen (N) is one of the fundamental components of proteins, which is crucial to all forms of life, and usually has a key influence on growth and grain yield of crops10,11. More than 90% of accumulated N in rice grain is protein N at maturity12,13. Therefore, an increase in grain N concentration (GNC) of crops provides a feasible way to combat malnutrition14.
Grain N concentration of crops differs with environmental variations, genotype, and agronomic practices3,14–16. Of all the agronomic practices, N application is the most direct method to affect GNC of crops. Grain N concentration is associated with N application rate, timing and method of N application, N fertiliser type, as well as synchronisation between N requirement and supply14,17,18. Increasing the N application rate increases N uptake; however, the N redistribution efficiency in vegetative organs might be reduced19–21. These finally lead to various changes in GNC of crops.
Split N application, a simple and practical N management method, is widely used for increasing crop yield and N use efficiency22,23. It has also been confirmed as a feasible method for improving GNC of maize14. Polyaspartic acid, a polymer with free carboxylic amide groups, exhibits good adsorbability on cations and anions, is widely used as superabsorbent material and fertiliser synergist24–26. PASP-urea is produced by mixing a certain amount of PASP with conventional urea, and is widely used in crop production in China. Previous studies have demonstrated that PASP-urea increases the N balance in paddy fields, leading to improvement in grain yield, N uptake, and N use efficiency of rice, as well as the accumulation of N in the panicle21,25,27. However, there exists limited knowledge of the effectiveness of split N application and PASP-urea on GNC of rice.
It is known that both redistribution of N, accumulated in the vegetative organs before the heading stage, and uptake of N, after the heading stage, contribute to GNC of crops19,20,28. Mae and Ohira29 demonstrated that approximately 80% of the total N in the panicle is contributed by the redistributed N from vegetative organs, especially, from the leaf lamina28–30. During the reproductive period, accumulated N in the vegetative organs remobilises to the grains. The reutilisation of N from the senescent organs is controlled by a series of enzymes. NR, GS, and GOGAT are the three key enzymes that regulate the assimilation and recycling of N28,31,32. Both application time of N and N fertiliser type have an influence on N metabolism enzymes and N reutilisation21,33. However, the relationship between GNC and N redistribution from the leaf lamina using different urea types as fertiliser is not fully understood.
The hypothesis of the present study is that PASP-urea and split N application could improve GNC of rice. Therefore, in this study, a two-year field experiment involving two urea types and two N management methods was conducted in 2014 and 2015. The specific objectives of this study were to (a) investigate the effectiveness of split N application and PASP-urea on N concentration of grain and head rice and N redistribution from the leaf lamina; (b) examine the relationship between GNC and N redistribution characteristics of the leaf lamina; (c) determine the optimal technique for the application of PASP-urea focusing on the improvement of GNC.
Results
Analysis of variance
The effects of year, urea type, N management, and their interactions on the activities of NR, GS, and GOGAT in the leaf lamina, N concentration in the leaf lamina, grain, and head rice, and redistributed N from the leaf lamina are shown in Table 1. The effects of year on N concentration of leaf lamina, grain, and head rice, and redistribution of N from the leaf lamina were significant (P < 0.01), mostly due to the variation in weather and soil properties. Urea type and N management method had a significant (P < 0.01) influence on all the tested variables. The interaction between year and N management significantly affected N concentration in the leaf lamina, grain, and head rice, and redistributed N of the leaf lamina, whereas no significant effect of the interaction between year and urea type on redistributed N of the leaf lamina was observed. The interaction between urea type and N management significantly influenced NR activity of the leaf lamina, and N concentration of leaf lamina, grain, and head rice. A significant effect of the interaction between year, urea type, and N management on N concentration of the leaf lamina, grain, and head rice was recorded.
Table 1.
Analysis of variance for the activities of nitrate reductase (NR), glutamine synthetase (GS), and glutamate synthase (GOGAT) in the leaf lamina, nitrogen concentration of leaf lamina, grain, and head rice, and redistributed nitrogen from the leaf lamina in response to year, urea type, N management, and their interactions.
| Source | Nitrate reductase activity | Glutamine synthetase activity | Glutamate synthase activity | Nitrogen concentration in the leaf lamina | Redistributed nitrogen in the leaf lamina | Grain nitrogen concentration | Head rice Nitrogen concentration |
|---|---|---|---|---|---|---|---|
| Year | — | — | — | ** | ** | ** | ** |
| Urea type | ** | ** | ** | ** | ** | ** | ** |
| N management | ** | ** | ** | ** | ** | ** | ** |
| Y × U | — | — | — | * | NS | ** | ** |
| Y × N | — | — | — | ** | ** | ** | ** |
| U × N | ** | NS | NS | ** | NS | ** | ** |
| Y × U × N | — | — | — | ** | NS | ** | ** |
*** and NS indicate significance at P ≤ 0.05, P ≤ 0.01, and no significance, respectively.
Activities of nitrate reductase, glutamine synthetase, and glutamate synthase
Except for GS activity of CK, the activities of NR, GS, and GOGAT in all the treatments were found to decrease from the heading stage to the maturity stage (Fig. 1). With the exception of GS activity in two splits1 and two splits2 and NR activity in four splits1 at the maturity stag, N application improved the activities of NR, GS, and GOGAT at both the stages. Compared to the treatment with conventional urea, treatments with PASP-urea increased the activities of NR, GS, and GOGAT by 13.2%, 13.1%, and 16.1%, respectively, at the heading stage, and by 3.4%, 23.5%, and 5.4%, respectively, at the maturity stage. Compared to two splits, four splits significantly improved the activity of NR at the heading stage, as well as the activities of GS and GOGAT at both the stages. Among the four N treatments, the activity of NR in the heading stage, and activities of GS and GOGAT at both the stages were in the order, four splits2> four splits1> two splits2> two splits1. The use of PASP-urea with four splits obviously enhanced the activities of NR, GS, and GOGAT in the leaf lamina, indicating a higher N metabolism in four splits2.
Figure 1.
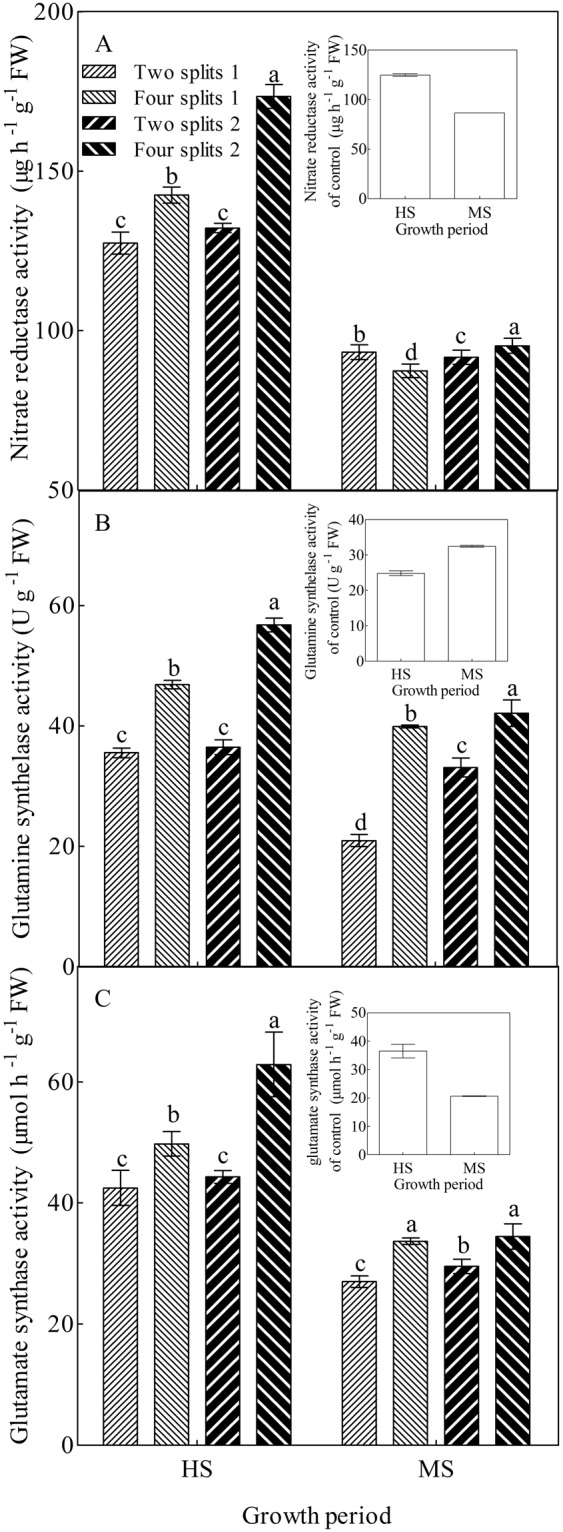
Activities of nitrate reductase (A), glutamine synthetase (B), and glutamate synthase (C) in the leaf lamina in response to urea type and N management in 2014. Different lowercase letters within a group of treatment represent significant (P < 0.05) differences among treatments. The control was not included in the analysis of variance and multiple comparisons. The bar with each column indicates the range of the standard error (SE) of the mean. HS, heading stage; MS, maturity stage.
Nitrogen concentration and redistribution from the leaf lamina
Nitrogen concentration and redistribution in the leaf lamina varied with year, urea type, and N management (Fig. 2). N application significantly increased N concentration and redistribution in the leaf lamina. No significant difference in N concentration in the leaf lamina at the maturity stage was observed between 2014 and 2015 (Fig. 2B). However, N concentration in the leaf lamina at the heading stage was remarkably higher in 2014, which contributed to a significant enrichment in N redistribution from the leaf lamina (Fig. 2A,C). Furthermore, N concentration and redistribution in the leaf lamina were significantly improved by PASP–urea and four splits. With the interaction between year and urea type, N concentrations in the leaf lamina at the heading and maturity stages were increased by PASP-urea-2014 and PASP-urea-2015, respectively. Nitrogen concentration in the leaf lamina at the heading stage and N redistribution from the leaf lamina were improved by four splits-2014, whereas N concentration in the leaf lamina at the maturity stage was increased by four splits-2015. Furthermore, both N concentration and redistribution from the leaf lamina were in the order, four splits2 > four splits1 > two splits 2 > two splits1, and four splits2-2014 and four splits2-2015 possessed the highest N concentration in the leaf lamina at the heading and maturity stages, respectively. These results suggest the potential of using PASP-urea with the four splits method for improving N redistribution from the leaf lamina.
Figure 2.
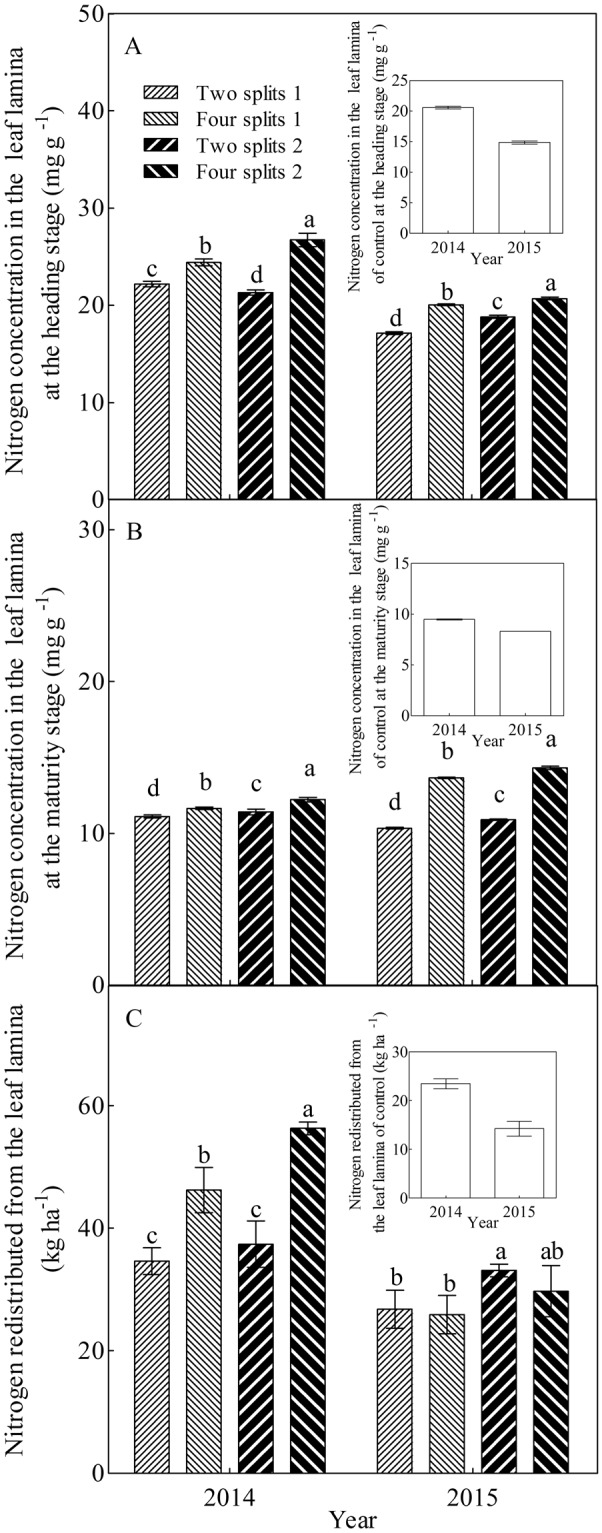
Nitrogen concentration in the leaf lamina at the heading stage (A) and maturity stage (B), and the nitrogen redistributed from the leaf lamina (C) in response to year, urea type, and N management. Different lowercase letters within a group of treatment represent significant (P < 0.05) differences among treatments. The control was not included in the analysis of variance and multiple comparisons. The bar with each column indicates the range of the standard error (SE) of the mean.
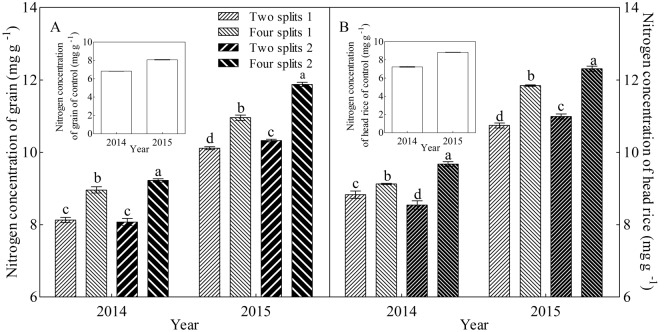
Nitrogen concentration of grain and head rice
N application significantly increased both GNC and N concentrations of head rice (Fig. 3). Compared to those in 2014, GNC and N concentrations of head rice were increased in 2015. Compared to conventional urea and two splits, PASP-urea and four splits significantly improved N concentration of grain and head rice. In 2015, with two splits, both GNC and N concentration of head rice were higher with PASP-urea compared with conventional urea, in contrast to 2014, in which there was no increase in GNC and a decrease in N concentration of head rice with PASP-urea when there were only two splits. With the interaction between year and N management, the effect of four splits compared with two splits was greater in 2015, at 11.3% increase in N concentration of head rice, compared with 2014, at 8.1% increase. Moreover, using PASP-urea with four splits significantly increased N concentration of grain and head rice. Compared to two splits and four splits1, splits2-2014 and four splits2-2015 led to 2.9–14.3% and 8.4–15.0% increases in GNC and 6.0–13.2% and 3.9–14.6% increases in N concentration of head rice were, respectively.
Figure 3.
Nitrogen concentration (A) and accumulation (B) in grain in response to urea type and N management in 2014 and 2015. Different lowercase letters within a group of treatment represent significant (P < 0.05) differences among treatments. The control was not included in the analysis of variance and multiple comparisons. The bar with each column indicates the range of the standard error (SE) of the mean.
Relationships between grain nitrogen and leaf lamina nitrogen
The relationships between N concentration of grain and head rice with leaf lamina N concentration, redistribution, and key enzymes activities of nitrogen metabolism are shown in Table 2. Both GNC and N concentration of head rice were significantly (P < 0.01) improved with increased N concentration in the leaf lamina at the maturity stage and redistributed N from the leaf lamina in both the years. Similar results were recorded between N concentration of grain and head rice with N concentration in the leaf lamina at the heading stage in 2014. The N concentration of grain and head rice also significantly (P < 0.01) increased with enhanced activity of GOGAT at both the stages, as well as with the activity of GS at the heading stage in 2014.
Table 2.
Correlation coefficients (r) between the nitrogen concentration of grain and head rice with the activities of nitrate reductase (NR), glutamine synthetase (GS), and glutamate synthase (GOGAT) in the leaf lamina, nitrogen concentration in the leaf lamina, and redistributed nitrogen from the leaf lamina.
| Index | Nitrogen concentration of grain | Nitrogen concentration of head rice |
|---|---|---|
| 2014 | ||
| Nitrogen concentration in the leaf lamina at the heading stage | 0.890* | 0.947** |
| Nitrogen concentration in the leaf lamina at the maturity stage | 0.969** | 0.882* |
| Redistributed nitrogen from the leaf lamina | 0.964** | 0.971** |
| Nitrate reductase activity in the leaf lamina at the heading stage | 0.783 | 0.786 |
| Nitrate reductase activity in the leaf lamina at the maturity stage | 0.514 | 0.686 |
| Glutamine synthetase activity in the leaf lamina at the heading stage | 0.960** | 0.945** |
| Glutamine synthetase activity in the leaf lamina at the maturity stage | 0.528 | 0.382 |
| Glutamate synthase activity in the leaf lamina at the heading stage | 0.885* | 0.890* |
| Glutamate synthase activity in the leaf lamina at the maturity stage | 0.987** | 0.939** |
| 2015 | ||
| Nitrogen concentration in the leaf lamina at the heading stage | 0.788 | 0.789 |
| Nitrogen concentration in the leaf lamina at the maturity stage | 0.966** | 0.979** |
| Redistributed nitrogen from the leaf lamina | 0.949** | 0.959** |
*Significant at the 0.05 probability level;**Significant at the 0.01 probability level.
Discussion
Effect of PASP-urea and N management method on N characteristics of the leaf lamina
Application of N fertilisers is a common practice in field crop management. However, the effect of N fertilisers on crops is influenced by fertiliser type, N rate, and application time14,19,22. PASP-urea, one of the high efficiency fertiliser, is demonstrated to increase grain yield and N use efficiency of crops21,24. By using PASP-urea, better synchronisation between N requirement and supply was realised, which contributed to an increase in photosynthetic capacity, dry matter accumulation, as well as effective panicles, grain filling percentage, and grain weight, and thereby significantly improved the grain yield21,25,27. It is well demonstrated that increasing the N rate and optimising the timing of N supply usually result in higher N concentration and accumulation in the rice organs34,35. Because of the improved balance between N requirement and supply in the paddy field caused by PASP-urea and four splits21, a significant improvement in N concentration of the leaf lamina was observed in the present study (Fig. 2). Furthermore, PASP-urea also significantly increased N redistribution from the leaf lamina. Thus, the use of PASP-urea with the four splits method might be a feasible way to enhance N redistribution from the leaf lamina.
Nitrate reductase is the first rate-limiting enzyme that controls nitrate assimilation in the leaf lamina of most crops31,36. In addition, the GS/GOGAT cycle, discovered by Lea and Miflin37, is the only route for primary assimilation of ammonium in plants and the essential step for N recycling in senescent vegetative organs28,30,32. Many studies have demonstrated the effect of N application on N metabolism in rice36,38–40. In the present study, both PASP-urea and four splits could enhance N metabolism in the leaf lamina. Similar to the results of Sun et al.33, four splits significantly improved the GS and GOGAT activities in the leaf lamina at the heading and maturity stages, as well as NR activity in the leaf lamina at the heading stage (Fig. 1). In addition, PASP-urea also significantly increased the activities of NR, GS, and GOGAT in the leaf lamina, which contributed to the obvious increase in N concentration and N redistribution in the leaf lamina (Figs 1 and 2).
Effect of PASP-urea and N management method on N concentration of grain and head rice
The GNC of crops has an important nutritional value, because crop grains or seeds are the primary source of proteins in the diet of humans and animals8,41,42. Application of N fertiliser is one of the major field management practices that play an important regulatory role on the concentrations of GNC or protein in crops14,18,43. In the present study, four splits significantly increased GNC of grain and N concentration of head rice (Fig. 3). This is in agreement with the results of Ning et al.7, which indicate that N fertiliser has a remarkable effect on protein concentration in rice grain, whereas topdressing N has a larger influence. Furthermore, PASP-urea also had a positive effect on N concentration of grain and head rice. These results demonstrated that the hypotheses of the present study were correct.
It has been clearly demonstrated that approximately 80% of N in the panicle is remobilised through the phloem from senescing organs28–30. Indeed, the leaf lamina is considered to be the main source of assimilates and N for grain filling of rice44. Approximately half of the accumulated N in the panicle is contributed by the leaf lamina21. In the present study, significant positive relationships were observed between N concentration of grain and head rice with N concentration and redistribution of the leaf lamina (Table 2). Furthermore, both N concentration of grain and head rice were increased with enhanced activities of GOGAT and GS (Table 2). This corroborates the findings of Tabuchi et al.28 and Tamura et al.30, who suggested that since the major form of N in the phloem sap is Gln, synthesis of Gln controlled by the GS/GOGAT cycle is the essential step for the reutilisation of N in senescing organs. During the senescence progress of the leaf lamina, both PASP-urea and four splits could increase the activities of GS and GOGAT in the leaf lamina at the heading and maturity stages; this promoted the remobilisation of N from the leaf lamina, and contributed to the significant increase in GNC and N concentrations of head rice (Figs 1–3). Therefore, using PASP-urea together with the four splits method is a feasible way to improve N concentration of grain and head rice.
Conclusions
The increase in crop yield and GNC provides a promising strategy to overcome hunger and malnutrition. Rational N application is a feasible method for improving the N concentration and protein in grains. The present study investigated the effectiveness of PASP-urea and N management method on N concentration and accumulation in rice grain, and their relationships with the N characteristics of the leaf lamina. Taken together, our findings support the strategy for increasing GNC of rice by using PASP-urea. N concentration of grain and head rice, and N redistribution characteristics of the leaf lamina were significantly influenced by urea type and N management method. Both PASP-urea and split N application could improve GNC of rice. With the use of PASP-urea, the activities of NR, GS, and GOGAT, and N concentration and redistribution of leaf lamina were increased, contributing to the improvement in N concentration of grain and head rice. Overall, using PASP-urea with four splits is a feasible method for increasing GNC and N concentration of head rice.
Materials and Methods
Study site and materials
The field experiment was conducted at the same field of Huihe farm of Sichuan Agricultural University in Wenjiang (30°43′N, 103°52′E), Sichuan, China, in 2014 and 2015. The previous crop in both years was wheat. Wenjiang had a subtropical humid monsoon climate, with an average air temperature and precipitation of 23.9 °C and 715.0 mm, respectively, during the period from transplantation to harvest (May to September) in 2014, and 24.0 °C and 568 mm, respectively, in 201525. The properties of the top soil layer (0–30 cm) are shown in Table 3. Fyou-498, a mid-late indica hybrid rice variety, bred by Sichuan Agricultural University, was used in the study. Conventional urea and PASP-urea with 46% N were supplied by Sichuan Meiqing Cyanamide Co., Ltd., China.
Table 3.
Properties of the top soil layer (0–30 cm) in the experimental field in 2014 and 2015.
| Year | Soil texture | pH | Organic matter (g kg−1) | Total N (g kg−1) | Alkali hydrolysable N (mg kg−1) | Olsen-P (mg kg−1) | Exchangeable K (mg kg−1) |
|---|---|---|---|---|---|---|---|
| 2014 | medium loam | 5.1 | 37.6 | 2.2 | 130.0 | 12.2 | 119.5 |
| 2015 | medium loam | 5.2 | 28.9 | 1.9 | 135.5 | 19.8 | 105.7 |
Experimental design
A two-factor, randomised block experiment, with three replicates, was conducted in 2014 and 2015. The complete experimental details were described previously by Deng et al.25,27. In brief, the treatments were as follows: (1) two urea types, conventional urea and PASP-urea were used; (2) two N management methods, two splits N application (180 kg N ha−1, by which 70% and 30% of N were applied at the basal and tillering stages, respectively) and four splits N application (180 kg N ha−1, split-applied as 35% at the basal, 15% at the tillering, 30% at the panicle initiation, and 20% at the spikelet differentiation stages); zero-N treatment (CK) with three replicates was set as the control. The management methods using conventional urea were designated as 1 (e.g. two splits1 and four splits1), whereas those using PASP-urea were designated as 2 (e.g. two splits2 and four splits2).
In both the years, 0.2-m-wide low banks, covered with a plastic film inserted at a depth of 0.3 m in the soil, were used to prevent leakage of fertiliser to the adjacent plots. The dimension of each plot was 10 × 3 m and 9 × 3 m in 2014 and 2015, respectively. In all the treatments, 90 kg·ha−1 of P2O5 (as a single superphosphate) was applied at the basal stage, and 180 kg·ha−1 of K2O (as potassium chloride) was equally divided at the basal and panicle initiation stages. Seedlings were raised in upland nursery conditions, with 75 kg N ha−1 as seed fertilizer. On May 24, 2014, and May 31, 2015, 35-day-old seedlings were transplanted at 26.7 cm × 16.7 cm spacing. Standard chemicals, such as avermectin, validamycin, cyhalofop-butyl were used to control insects (Chilo suppressalis), pathogens (false smut), and weeds (barnyard grass) to avoid yield losses. Water management was conducted using a high-efficiency irrigation technique: damp irrigation before the panicle initiation stage, saturated irrigation from the panicle initiation to heading, and alternate wetting and drying irrigation after the heading stage21,25. Each plot was irrigated before fertiliser application.
Redistribution of N from the leaf lamina and N concentration of grain and head rice
At the heading and maturity stages, five representative hills were sampled from the middle of each plot. The leaf lamina from each sample was oven-dried at 105 °C for 1 h and then at 80 °C until obtaining a constant weight. On September 11, 2014, and September 15, 2015, rice plants from each plot were hand-harvested. Approximately 2 kg of hand-harvested grains were sampled. Grains were stored under ventilated conditions for 3 months, and then they were divided into two parts, one part was used to determine the GNC, and another part was shelled and milled to produce head rice. Samples were crushed and sifted through a 0.5-mm screen, and then the N concentration of the leaf lamina, grain, and head rice was measured by the Kjeldahl method. Redistribution of N from the leaf lamina was calculated as the difference in N weight between the heading and maturity stages21.
Activities of nitrate reductase, glutamine synthetase, and glutamate synthase in the leaf lamina
Ten flag leaves were sampled from each plot at the heading and maturity stages, in 2014. The midrib of the leaf was removed and its lamina was sliced and frozen in liquid N, and kept at −80 °C, until further analysis. Nitrate reductase, GS, and GOGAT activities in the flag leaves were determined using commercial chemical assay kits (Jiangsu Keming Biotechnology Institute, Suzhou, China). For the measurement of NR activity, frozen leaf tissues were soaked in inducing medium for 2 h, and then blotted moist tissues were stored at -20 °C for 20 min. Approximately 0.1 g (fresh weight) leaf tissue was homogenised in a triturator with 1.0 mL prechilled extraction medium and then centrifuged at 4000 × g for 10 min at 4 °C. The supernatant was used for the NR activity analysis according to the manufacturer’s instructions. The activity of NR (g h−1 g−1 FW) was determined as the production of NaNO2 per hour per gram fresh sample. For the measurement of GS and GOGAT activities, approximately 0.1 g leaf tissues was homogenised with 1.0 mL prechilled extraction medium and centrifuged at 8000 × g for 10 min at 4 °C, and then the supernatant was used for the analysis of GS and GOGAT activities according to the manufacturer’s instructions. The activity of GS (U g−1 FW) was defined as the quantity of 0.01 variation in the absorbance at 540 nm per minute per gram fresh sample. The activity of GOGAT (μmol h−1 g−1 FW) was described as the micromole of NADH oxidised per hour per gram fresh sample.
Data analysis
Analysis of variance of all the data, except the control, was performed using the general linear model of SPSS version 18.0 (IBM, Inc., Chicago, IL, USA). Nitrogen concentration in the leaf lamina and activities of NR, GS, and GOGAT were analysed using the repeated measures analysis model. For the analysis, year, urea type, N management, and sampling time were considered as the fixed effects, and the replicates were considered as the random effects. The LSD test (P < 0.05) was used to determine the differences among the treatments.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (31871564), the Ministry of Science and Technology of the People’s Republic of China (2016YFD0300506), and the Science and Technology Department of Sichuan Province (2016NYZ0051). We thank the students and farmers involved in the experiments.
Author Contributions
F.D., L.W. and W.R. conceived and designed the research; F.D., L.W., X.M., S.L. and S.P. performed the experiment; F.D. and Q.L. analysed data; F.D. and L.W. wrote the main manuscript; W.R. revised the manuscript.
Data Availability
No datasets were generated or analysed during the current study.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fei Deng and Li Wang contributed equally.
References
- 1.Zhao GM, et al. A preliminary precision rice management system for increasing both grain yield and nitrogen use efficiency. Field Crop Res. 2013;154:23–30. doi: 10.1016/j.fcr.2013.07.019. [DOI] [Google Scholar]
- 2.Cassman KG. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA. 1999;96:5952–5959. doi: 10.1073/pnas.96.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Triboi E, Martre P, Girousse C, Ravel C, Triboi-Blondel A. Unravelling environmental and genetic relationships between grain yield and nitrogen concentration for wheat. Eur J Agron. 2016;25:108–118. doi: 10.1016/j.eja.2006.04.004. [DOI] [Google Scholar]
- 4.Ning HF, et al. Effect of nitrogen fertilizer application on grain phytic acid and protein concentrations in japonica rice and its variations with genotypes. J Cereal Sci. 2009;50:49–55. doi: 10.1016/j.jcs.2009.02.005. [DOI] [Google Scholar]
- 5.Lin ZM, et al. Effects of nitrogen fertilization and genotype on rice grain macronutrients and micronutrients. Rice Sci. 2014;21:233–242. doi: 10.1016/S1672-6308(13)60178-X. [DOI] [Google Scholar]
- 6.Koutroubas SD, Katsantonis D, Ntanos DA, Lupotto E. Nitrogen utilisation efficiency and grain yield components of rice varieties grown under blast disease stress. Australas Plant Path. 2008;37:53–59. doi: 10.1071/AP07078. [DOI] [Google Scholar]
- 7.Ning HF, et al. Distribution of proteins and amino acids in milled and brown rice as affected by nitrogen fertilization and genotype. J Cereal Sci. 2010;52:90–95. doi: 10.1016/j.jcs.2010.03.009. [DOI] [Google Scholar]
- 8.Sautter C, Poletti S, Zhang P, Gruissem W. Biofortification of essential nutritional compounds and trace elements in rice and cassava. P Nutr Soc. 2006;65:153–159. doi: 10.1079/PNS2006488. [DOI] [PubMed] [Google Scholar]
- 9.Wu, D. X. & Shu, X. L. Research and utilization of rice protein China Agriculture Press, Beijing (2008).
- 10.Ma WQ, et al. Nitrogen flow and use efficiency in production and utilization of wheat, rice, and maize in China. Agr Syst. 2009;99:53–63. doi: 10.1016/j.agsy.2008.10.001. [DOI] [Google Scholar]
- 11.Porto RDA, Koetz M, Bonfim-Silva EM, Polizel AC, Silva TJAD. Effects of water replacement levels and nitrogen fertilization on growth and production of gladiolus in a greenhouse. Agr Water Manage. 2014;131:50–56. doi: 10.1016/j.agwat.2013.09.007. [DOI] [Google Scholar]
- 12.Peng, J. W. et al. Effect of combined application of N, P, K fertilizers and N rate on N uptake and grain quality of rice. China Rice. 23–25 (2004).
- 13.Chen JB, et al. Effects of several plant growth regulators on accumulation, transportation, and storage of nitrogen in rice. J Hunan Agr Uni (Natural Sciences) 2003;29:99–102. [Google Scholar]
- 14.Yan P, et al. Using maize hybrids and in-season nitrogen management to improve grain yield and grain nitrogen concentrations. Field Crop Res. 2014;166:38–45. doi: 10.1016/j.fcr.2014.06.012. [DOI] [Google Scholar]
- 15.Gholamhoseini M, AghaAlikhani M, Modarres-Sanavy SAM, Mirlatifi SM. Interactions of irrigation, weed and nitrogen on corn yield, nitrogen use efficiency and nitrate leaching. Agr Water Manage. 2013;126:9–18. doi: 10.1016/j.agwat.2013.05.002. [DOI] [Google Scholar]
- 16.Lieffering M, Kim HY, Kobayashi K, Okada M. The impact of elevated CO on the elemental concentrations of field-grown rice grains. Field Crop Res. 2004;88:279–286. doi: 10.1016/j.fcr.2004.01.004. [DOI] [Google Scholar]
- 17.Gao XP, Lukow OM, Grant CA. Grain concentrations of protein, iron and zinc and bread making quality in spring wheat as affected by seeding date and nitrogen fertilizer management. J Geochem Explor. 2012;121:36–44. doi: 10.1016/j.gexplo.2012.02.005. [DOI] [Google Scholar]
- 18.Gaju O, et al. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars. Field Crop Res. 2014;155:213–223. doi: 10.1016/j.fcr.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song GY, Xu ZJ, Yang HS. Effects of N rates on N uptake and yield in erect panicle rice. Agr Sci. 2013;4:499–508. [Google Scholar]
- 20.Chen YL, et al. Effects of nitrogen application rate on grain yield and grain nitrogen concentration in two maize hybrids with contrasting nitrogen remobilization efficiency. Eur J Agron. 2015;62:79–89. doi: 10.1016/j.eja.2014.09.008. [DOI] [Google Scholar]
- 21.Deng F, Wang L, Ren WJ, Mei XF. Enhancing nitrogen utilization and soil nitrogen balance in paddy fields by optimizing nitrogen management and using polyaspartic acid urea. Field Crop Res. 2014;169:30–38. doi: 10.1016/j.fcr.2014.08.015. [DOI] [Google Scholar]
- 22.Zhang ZJ, et al. Mid-season nitrogen application strategies for rice varieties differing in panicle size. Field Crop Res. 2013;150:9–18. doi: 10.1016/j.fcr.2013.06.002. [DOI] [Google Scholar]
- 23.Pang SG, et al. Effects of N management on yield and N uptake of rice in central China. J Integr Agr. 2012;12:1993–2000. [Google Scholar]
- 24.Du ZJ, et al. Advance of homologous polypeptides polyaspartic acids for agriculture. Chinese J Tro Crop. 2011;12:2381–2384. [Google Scholar]
- 25.Deng F, et al. Polyaspartate urea and nitrogen management affect nonstructural carbohydrates and yield of rice. Crop Sci. 2016;56:3272–3285. doi: 10.2135/cropsci2016.02.0130. [DOI] [Google Scholar]
- 26.King, W. E., Fister, R. P. & Norris, S. J. Slow-release fertilizer and method of making and using same. http://www.google.com/patents/US20120222170 (2008).
- 27.Deng F, Wang L, Mei XF, Li SX, Pu SL. Morphological and physiological characteristics of rice leaves in response to PASP-urea and optimized nitrogen management. Arch Agron. Soil Sci. 2017;11:1582–1596. [Google Scholar]
- 28.Tabuchi M, Abiko T, Yamaya T. Assimilation of ammonium ions and reutilization of nitrogen in rice (Oryza sativa, L.) J Exp Bot. 2007;59:2319–2327. doi: 10.1093/jxb/erm016. [DOI] [PubMed] [Google Scholar]
- 29.Mae T, Ohira K. The remobilization of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa, L.) Plant Cell Physiol. 1981;22:1067–1074. [Google Scholar]
- 30.Tamura W, et al. Disruption of a novel NADH-glutamate synthase2 gene caused mark ed reduction in spik elet number of rice. Front Plant Sci. 2011;2:1–9. doi: 10.3389/fpls.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali A, Sivakami S, Raghuram N. Regulation of activity and transcript levels of NR in rice (Oryza sativa): Roles of protein kinase and G-proteins. Plant Sci. 2007;172:406–413. doi: 10.1016/j.plantsci.2006.10.003. [DOI] [Google Scholar]
- 32.Kamachi K, Yamaya T, Mae T, Ojima K. A role for glutamine synthetase in the remobilization of leaf nitrogen during natural senescence in rice leaves. Plant Physiol. 1991;96:411–417. doi: 10.1104/pp.96.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun, Y. J. et al. The effects of different water and nitrogen managements on yield and nitrogen use efficiency in hybrid rice of China. Field Crop Res. 85–98 (2012).
- 34.Sui B, et al. Optimizing nitrogen supply increases rice yield and nitrogen use efficiency by regulating yield formation factors. Field Crop Res. 2013;150:99–107. doi: 10.1016/j.fcr.2013.06.012. [DOI] [Google Scholar]
- 35.Wang SH, Zhu Y, Jiang HD, Cao WX. Positional differences in nitrogen and sugar concentrations of upper leaves relate to plant N status in rice under different N rates. Field Crop Res. 2006;96:224–234. doi: 10.1016/j.fcr.2005.07.008. [DOI] [Google Scholar]
- 36.Santos LA, Bucher CA, Souza SRD, Fernandes MS. Effects of nitrogen stress on proton-pumping and nitrogen metabolism in rice. J Plant Nutr. 2009;32:549–564. doi: 10.1080/01904160802714953. [DOI] [Google Scholar]
- 37.Lea PJ, Miflin BJ. Alternative route for nitrogen assimilation in higher plants. Nature. 1974;251:614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- 38.Gerendás J, Zhu Z, Sattelmacher B. Influence of N and Ni supply on nitrogen metabolism and urease activity in rice (Oryza sativa, L.) J Exp Bot. 1998;49:1545–1554. doi: 10.1093/jxb/49.326.1545. [DOI] [Google Scholar]
- 39.Liang ZJ, Bao AL, Li HX, Cai HM. The effect of nitrogen level on rice growth, carbon-nitrogen metabolism and gene expression. Biologia. 2015;70:1340–1350. doi: 10.1515/biolog-2015-0148. [DOI] [Google Scholar]
- 40.Luo LF, et al. Effect of controlled release nitrogen fertilizer on protein quality of brown rice and key enzyme activity involved in nitrogen metabolism in hybrid rice. Chinese J Rice Sci. 2007;21:403–410. [Google Scholar]
- 41.Zhao YL, et al. Nitrogen effect on amino acid composition in leaf and grain of japonica rice during grain filling stage. J Cereal Sci. 2015;64:29–33. doi: 10.1016/j.jcs.2015.03.011. [DOI] [Google Scholar]
- 42.Galili G, Amir R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol J. 2013;11:211–222. doi: 10.1111/pbi.12025. [DOI] [PubMed] [Google Scholar]
- 43.Jaksomsak P, Rerkasem B, Prom-U-Thai C. Responses of grain zinc and nitrogen concentration to nitrogen fertilizer application in rice varieties with high-yielding low-grain zinc and low-yielding high grain zinc concentration. Plant & Soil. 2017;411:101–109. doi: 10.1007/s11104-016-3056-1. [DOI] [Google Scholar]
- 44.Zhang, X. C. et al. Amino acid composition of leaf, grain and bracts of japonica rice (Oryza Sativa ssp. Japonica) and its response to nitrogen fertilization. Plant Growth Regul. 1–9 (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.